Peer-review started: June 8, 2014
First decision: July 10, 2014
Revised: July 20, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: January 27, 2015
Processing time: 219 Days and 1.2 Hours
Hepatic steatosis is commonly seen in the patients with chronic hepatitis C virus (HCV) infection. HCV is closely associated with lipid metabolism, and viral steatosis is more common in genotype 3 infection owing to a direct cytopathic effect of HCV core protein. In non-genotype 3 infection, hepatic steatosis is considered largely to be the result of the alterations in host metabolism; metabolic steatosis is primarily linked with HCV genotype 1. Adipose tissue secretes different hormones involved in glucose and lipid metabolisms. It has been demonstrated that adipocytokines are involved in the pathogenesis of non-alcoholic fatty liver disease, as the decreased plasma adiponectin levels, a soluble matrix protein expressed by adipoctyes and hepatocyte, are associated with liver steatosis. Various studies have shown that steatosis is strongly correlated negatively with adiponectin in the patients with HCV infection. The role of adiponectin in hepatitis C virus induced steatosis is still not completely understood, but the relationship between adiponectin low levels and liver steatosis is probably due to the ability of adiponectin to protect hepatocytes from triglyceride accumulation by increasing β-oxidation of free fatty acid and thus decreasing de novo free fatty acid production.
Core tip: Three main types of steatosis in the patients with hepatitis C virus (HCV) infection are known: a metabolic type associated with metabolic syndrome, viral steatosis directly triggered by the virus and a “middle ground” between metabolic and viral mechanisms. Liver steatosis is a common histological feature of chronic hepatitis C infection, and the recent studies have shown that it is strongly correlated negatively with adiponectin levels. This finding suggests that adiponectin may have a role in modulating the progression of hepatic steatosis in HCV infected patients.
- Citation: Peta V, Torti C, Milic N, Focà A, Abenavoli L. Adiponectin serum level in chronic hepatitis C infection and therapeutic profile. World J Hepatol 2015; 7(1): 44-52
- URL: https://www.wjgnet.com/1948-5182/full/v7/i1/44.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i1.44
Hepatitis C virus (HCV) infection is a common liver disease with an estimated 3% of the world’s population chronically infected with this viral pathogen. The majority of the infected individuals (60%-80%) develop chronic hepatitis C (CHC), which is associated with progressive liver fibrosis and a risk of cirrhosis after 20 years[1-3].
About 20%-30% of chronic HCV infections are associated with hepatic steatosis, type II diabetes[4], insulin resistance (IR) and cardiovascular diseases[5]. Hepatic steatosis, defined as an excessive lipid accumulation in the cytoplasm of hepatocytes, is a frequent histological feature in the patients chronically infected with HCV. However, the mechanisms that have induced hepatic steatosis in HCV-infected patients are difficult to understand, due to the possible co-existence of several factors[6]. Different studies have shown that there are three main types of steatosis defined in the HCV patients: metabolic steatosis, viral steatosis and a “middle ground” between metabolic and viral mechanisms[7,8]. The first type has been described in the patients infected with HCV who also suffer from other metabolic disorders such as obesity, dyslipidemia and diabetes mellitus[9]. Metabolic steatosis is primarily linked with HCV genotype 1, but one study has shown the absence of the relationship with viral load and the severity of steatosis in the patients infected by genotype 1[10]. Viral steatosis develops in the absence of other steatogenic co-factors and is linked with HCV genotype 3 infection[11]. In viral steatosis lipid accumulation in hepatocytes may be the result of a direct cytopathic effect of HCV core protein. Different experiments conducted in vitro and in transgenic mice, have suggested that the nucleocapsid protein of HCV may be involved in the pathogenesis of triglyceride accumulation in hepatocytes[12,13]. Some other experiments have provided a correlation between the level of intrahepatic HCV genotype 3 ribonucleic acid (RNA) and severity of the steatosis[14] and identified specific “steatogenic” sequences in HCV-3, particularly phenylalanine (F) has been shown to be specifically associated with higher levels of lipid accumulation in cellular models in vitro[15]. All these findings are also supported by the observation that the degree of liver steatosis is directly related to the level of HCV replication as measured by serum HCV RNA, at least in the patients with HCV-3 infection in the absence of confounding metabolic causes of steatosis[16].
The third type of steatosis can be considered a “middle ground” between the first and the second one. Undoubtedly, this kind of steatosis is a combination of viral and metabolic factors and is associated with a direct interference of HCV core protein in the intracellular, post-receptorial pathways of insulin. This evidence, mostly found in the HCV genotype 1b patients, has convinced some authors to coin the term virus associated steato-hepatitis[17,18]. Numerous studies have shown the involvement of HCV in steatosis. Some insights into the pathways of steato-hepatitis are defined by impaired lipid accumulation due to hepatic loss of adiponectin receptors, which play an important role in fatty acid accumulation by elevating the expression levels of the enzyme AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), fatty acid synthase, liver gluconeogenic enzyme and phosphoenol pyruvate carboxy kinase due to HCV infection[6,19-21]. Various studies have shown that steatosis is strongly correlated negatively with adiponectin in the patients with chronic HCV infection, and this finding suggests that adiponectin may have a role in modulating the progression of hepatic steatosis, fibrosis and inflammation[22]. The main objective of this review is to discuss the biological effect of adiponectin and its receptors in the progression of liver steatosis in the HCV-infected patients and the possible role of adiponectin as a therapeutic target for the treatment of fatty liver diseases.
Adipose tissue is an active endocrine organ which secretes a number of hormones involved in glucose/lipid metabolism. Adiponectin is a soluble matrix protein expressed exclusively by adipocytes and hepatocytes[23]. Recent studies have demonstrated adiponectin mRNA expression in liver after injury and skeletal muscle and that its expression and serum levels are reduced in humans and animals with obesity and insulin resistance[24,25]. Adiponectin exists in three forms: low molecular weight trimers, medium molecular weight hexamers, and high molecular weight (HMW) multimers.
HMW adiponectin is thought to have more biological activity than other two forms. Human adiponectin gene is located at chromosome 3q27, and it codes for a 244 amino acid polypeptide. The primary sequence of adiponectin contains a signal peptide at the N-terminus, short hypervariable region and C-terminal half of the protein with a globular domain[26].
Adiponectin expression is reduced in obesity[27], insulin resistance and type 2 diabetes, and the plasma concentrations are inversely related to body weight, especially visceral adiposity[27-29]. Adiponectin is also inversely associated with other traditional cardiovascular risk factors, such as blood pressure, low-density lipoprotein cholesterol and triglyceride levels[30,31], and is positively related to high-density lipoprotein cholesterol levels[32]. A Recent research has indicated that adiponectin has anti-inflammatory properties, producing the anti-inflammatory mediator interleukin (IL)-10 in primary human monocytes, monocyte-derived macrophages and dendritic cells. In addition, adiponectin significantly impaired the production of the pro-inflammatory cytokine interferon-γ in human macrophages[33].
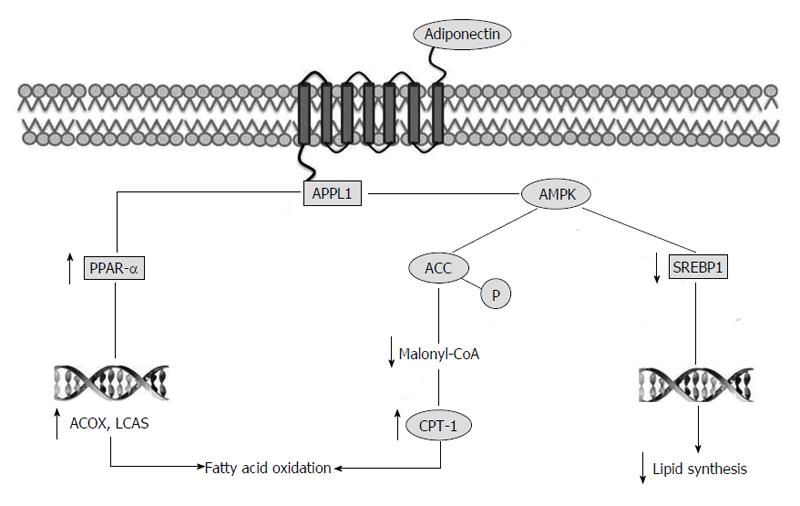
Adiponectin exerts its action via its two receptors, adiponectin receptor1 (Adipo R1) and Adipo R2. In mice, Adipo R1 is expressed abundantly in skeletal muscles, while Adipo R2 is considered as the primary transcript in liver. Adipo R1 and Adipo R2 are structurally related integral plasma membrane proteins with seven membrane-spanning domains. AdipoR1 possesses high affinity to the globular form of adiponectin and low affinity to full-length adiponectin, whereas Adipo R2 exhibits intermediate binding affinity to both the globular and the full-length adiponectin[34]. Adipo R1 and R2 mediate increased AMPK activities, peroxisome proliferator-activated receptor alpha (PPAR-α) activities, fatty-acid oxidation and glucose uptake[35]. To confirm the physiological role of these receptors, Adipo R knockout mice have been generated, and in wild-type mice, adiponectin have lowered plasma glucose levels, whereas this effect of adiponectin has completely been abrogated in Adipo R1 and R2 double knockout mice[36,37].
It is known that adiponectin and its receptors have hepato-protective role in fatty liver diseases and steatosis development[35,38]. Adiponectin is believed to protect hepatocytes from triglyceride accumulation by increasing β-oxidation of free fatty acid and/or decreasing de novo free fatty acid production in hepatocytes[39]. Indeed, it has been shown that adiponectin stimulates AMPK in different tissues including liver. The precise mechanisms whereby adiponectin activates AMPK remain to be determined. However, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1), an adaptor protein, appears to be the molecule that promotes the interaction between adiponectin and its receptors and the AMPK activation. The interaction between adiponectin receptor and APPL1 causes phosphorylation and activation of AMPK and AMPK phosphorylates ACC. The inhibition of ACC reduces lipid synthesis and increases fatty acid oxidation by blocking the production of malonyl-CoA, the allosteric inhibitor of carnitine palmitoyl transferase 1, which is the rate-limiting enzyme in fatty acid oxidation[40,41].
Moreover, AMPK downregulates the expression of sterol regulatory element-binding protein 1c, a transcription factor that regulates cholesterol and lipid synthesis[39,42]. Finally, adiponectin stimulates PPAR-α, a transcriptional factor controlling different genes involved in fat oxidation, such as acyl-CoA oxidase and long chain acyl-CoA synthetase[43] (Figure 1).
Recent data suggest that gut bacteria contribute to differences in body weight, insulin sensitivity, glucose metabolism and liver steatosis, in fact the imbalance of small intestinal bacterial overgrowth occurs in a large percentage of patients with chronic liver diseases, and has been associated with the severity of steatosis[44]. In particular some studies showed that the use of antibiotics to alter gut microbiota in obese mice reduces body weight, improves fasting glycaemia, glucose tolerance, and increases adiponectin levels. However, it is not clear how the gut microbiota plays a role in the production of adiponectin in adipose tissue, but this finding suggest that the gut microbiota could be a novel target for treating metabolic diseases, in fact high adiponectin levels enhance the insulin sensitivity and glycogen storage and decrease triglyceride accumulation[45,46].
Liver steatosis is a histological feature of CHC. CHC-related steatosis is chiefly virus-induced in HCV genotype 3 infection, while the host factors seem to play the major pathogenic role in HCV genotype non-3 infection. The evidence suggests that steatosis has an important role in the progression of liver fibrosis in CHC.
It has been demonstrated that adipocytokines are involved in the pathogenesis of non-alcoholic fatty liver disease, and the decreased plasma adiponectin levels are related to liver steatosis[47]. Hypoadiponectinemia has been implicated in the development of obesity-related morbidities such as dyslipidemia and cardiovascular diseases[48]. In addition, it is known that hypoadiponectinemia enhances hepatic steatosis, inflammation, fibrosis, and hepatocarcinogenesis in animal models of liver diseases[49]. Some studies have shown that steatosis is strongly correlated negatively with adiponectin in the patients with CHC infection[50-52]. These findings indicate a significant relationship between hepatic steatosis and adiponectin level. However, the role of adiponectin in HCV induced steatosis is still not completely understood, but the relationship between adiponectin low levels and liver steatosis is probably due to the ability of adiponectin to protect hepatocytes from triglyceride accumulation by increasing β-oxidation of free fatty acid and thus decreasing de novo free fatty acid production[40].
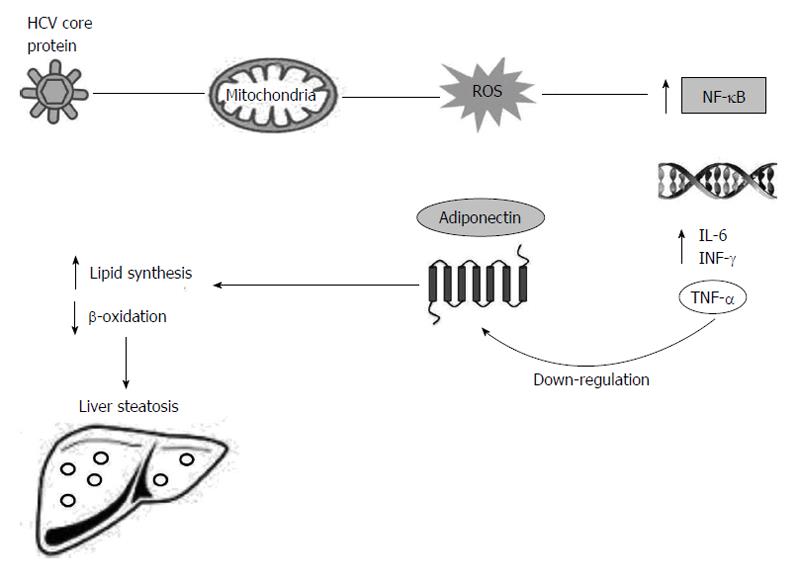
To clarify this point Durante-Mangoni et al[52] have found lower serum adiponectin levels and higher levels of tumour necrosis factor-α (TNF-α) in the chronic HCV patients. The higher tumour necrosis factor-α levels have particularly been observed in the patients with low adiponectin levels, and especially, in the patients infected whit HCV genotype-3. The extension of steatosis has inversely been correlated with adiponectin levels[52].
The substantial evidence in the available literature demonstrates that TNF-α inhibits adiponectin expression, and the decreased TNF-α level possibly contributes to the increased adiponectin level[53,54]. The expression level of adiponectin in cultured adipocytes has significantly been reduced by co-culture with macrophages or upon the exposure to the conditioned media from macrophages, suggesting that macrophage secreted factors, possibly TNF-α, are responsible for repressing adiponectin production[55]. Moreover, Bruun et al[56] have shown that the increase in TNF-α and IL-6 serum levels and decrease in adiponectin serum levels may be involved in insulin resistance. During the HCV infection, the immune response against HCV releases reactive oxygen species (ROS) from sequestered phagocytes and activates Kupffer cells in the liver[57]. High levels of ROS can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is a transcription factor. As a consequence of NF-κB activation, the expression of a variety of cytokines is increased, including tumour necrosis factors (TNF-α and TNF-β), IL-1, 6 and interferon-γ[58].
TNF-α modulates adipocytes and induces changes and reductions in the production of cytokines, adiponectin and leptin[52,59]. Thus, the reduced levels of adiponectin may increase influx and synthesis of free fatty acids into the liver of the patients with HCV infection, generating liver steatosis (Figure 2)[35,60].
Jonsson et al[22] have studied 194 patients with chronic HCV to assess the relationship between serum adiponectin levels and hepatic steatosis. The authors have found that a decreased serum level of adiponectin is associated with steatosis only in males, so they have suggested that the role of adiponectin in the HCV infected patients might be linked to gender[22]. Various studies hypothesized a possible interaction between HCV core protein-PPARs-adiponectin. It has been reported that the HCV core protein impairs the expression of the PPAR-α which plays an important role as a target of adiponectin in lipid metabolism[61]. Undoubtedly, the administration of recombinant adiponectin has been shown to increase PPAR-α ligand activity and the administration of a PPAR-γ agonist is associated with a significant increase of adiponectin levels and reversal of steatosis[62,63].
In another study, Ashour et al[64] have demonstrated that the Egyptian patients with HCV genotype-4 infection and with steatosis have shown the reduced serum levels of adiponectin, with a significant inverse correlation between adiponectin level and steatosis grade, homeostatic model assessments index, body mass index and fibrosis stage. Moreover, they have proved that serum levels of adiponectin and leptin show no significant differences between males and females[64].
Corbetta et al[65] have hypothesized that hyperadiponectinemia might be sustained by down-regulation of hepatic Adipo Rs. In order to test this hypothesis, they have assessed the expression levels of Adipo R1 and Adipo R2 in CHC biopsies and have shown that Adipo R2 mRNA levels are similar in normal liver and HCV-infected liver biopsies, but the Adipo R1 mRNA expression levels have been reduced in HCV-infected liver biopsies compared with normal liver biopsies. This reduction was also confirmed at protein level[65].
Tiftikci et al[66] have shown that the leptin-to-adiponectin ratio is significantly reduced in the HCV chronic patients. The increased leptin concentration, corrected by reduced adiponectin values (leptin-to-adiponectin ratio), has been linked to the development of metabolic abnormalities[67], so the data obtained by Tiftikci about a reduced leptin-to-adiponectin ratio in the chronic HCV patients, lend a new support to the argument that protein adiponectin may be involved in the pathogenesis of liver injury in the patients with HCV infection.
In contrast with these results, Aksõz et al[68] have suggested that a decrease in the level of adiponectin may be associated with metabolic disorders, independent from chronic HCV infection. In fact, they have tried to investigate the effects of the virus in the patients without visceral obesity and metabolic disorders, so they have suggested that a decrease in the level of adiponectin may be associated with metabolic disorders in association with HCV infection, but HCV virus alone has not altered adiponectin serum concentration[68].
Different studies showed the influence of genetic factors in the development of HCV-induced liver steatosis. In this contest Valenti et al[69] have suggested that rs738409 single nucleotide polymorphism of patatin-like phospholipase domain-containing 3 (PNPLA3), encoding for a protein variant (I148M) that influences hepatic triglycerides accumulation and the susceptibility to fibrosis and steatosis, may represent a genetic determinant of serum adiponectin levels in non-alcoholic fatty liver disease (NAFLD) and CHC patients. In another work Valenti et al[70] showed that this genetic variant in CHC patients affects steatosis development, is independently associated with fibrosis and cirrhosis, and may influence response to antiviral treatment. Finally Nakamura et al[71] showed that in Japanese CHC patients there is no association between PNPLA3 rs738409 genotype, hepatic steatosis or liver fibrosis, suggesting that in HCV infection the mechanism of hepatic steatosis might be different from that of NAFLD.
The biological effect of adiponectin and its receptors and their hepato-protective role in fatty liver diseases suggest that controlling the level of adiponectin receptors might be an important therapeutic target for the treatment of fatty liver diseases. There are no data on the potential therapeutic role of adiponectin in HCV chronic infection, but there are a lot of data showing that adiponectin is a therapeutic strategy for the treatment of insulin resistance, metabolic syndrome and steatosis that are common features of CHC, especially, in the patients infected with genotypes 3 and 1 virus. On the other hand, hepatic steatosis and IR reduce the probability of achieving a sustained virological response to pegylated interferon and ribavirin combination therapy[72], so reducing liver steatosis can be useful as a response to antiviral treatment in the patients with chronic HCV infection. Adiponectin replacement therapy is not yet available as a treatment option, but an alternative approach would be to identify and use the classes of the agents that can induce secretion or expression of adiponectin. In this context, some reports indicate that thiazolidinediones (TZDs) might up-regulate adiponectin, possibly, by increasing its rate of secretion[73,74], TZDs may up-regulate adiponectin by generating small adipocytes that express and secrete adiponectin and/or directly activating adiponectin gene transcription[75,76]. Other studies have shown that the inhibitors of the renin-angiotensin pathway, such as angiotensin converting enzyme inhibitor, increase serum adiponectin concentration, in fact, the blockers of the angiotensin pathway promote adipocyte differentiation[77].
Xu et al[78] have reported the identification of two structurally related natural compounds, astragaloside II and isoastragalosideI, from the medicinal herb Radix Astragali, that increase adiponectin secretion in primary adipocytes, without any effects on other adipokines. An alternative approach could be the design of the agents that serve as adiponectin mimetics. AMPK activators fall into such category because numerous adiponectin effects might be mediated via activation of AMPK. Moreover, the design of stable peptides or drugs, structurally and biologically simulating adiponectin production, could be another alternative[79].
In vitro studies have shown that adiponectin reduces free fatty acid-induced CD95/Fas expression and apoptosis of HepG2 hepatoma cells, which suggests that this hormone has a protective role with promising therapeutic implications, in fact, the receptor mediated apoptosis is a prominent feature in various liver diseases, including HCV chronic infection[80]. Finally, it is known that adiponectin has anti-inflammatory activity and this can be a promising therapeutic implication in numerous diseases including HCV. The anti-inflammatory effects attributed to adiponectin include the inhibition of TNF-α production and activity, inhibition of NF-κB activation and the induction of anti-inflammatory cytokines[81].
Steatosis development and CHC infection are clearly linked; about 20%-30% of chronic HCV infections are associated with hepatic steatosis. The biological mechanisms of the underlying steatosis occurrence and the progression to the liver disease are not entirely understood and are probably due to a number of factors: direct effect of the virus, genetic factors, metabolic syndrome and other unknown factors. The recent data suggest a significant link between hepatic steatosis and adiponectin low level. It is known that adiponectin and its receptors have hepato-protective role in fatty liver diseases and steatosis development. This relationship is probably due to the ability of adiponectin to increase β-oxidation of free fatty acid and to decrease de novo free fatty acid production. However, the role of adiponectin in HCV induced steatosis is still not completely understood. The biological effect, the hepato-protective role and the anti-inflammatory activity of adiponectin suggest that controlling the level of adiponectin, by increasing adiponectin production and using the drugs that structurally and biologically stimulate adiponectin, might be a potential therapeutic tool for the treatment of fatty liver diseases including steatosis induced by HCV chronic infection.
| 1. | Björnsson E, Angulo P. Hepatitis C and steatosis. Arch Med Res. 2007;38:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Torti C, Zazzi M, Abenavoli L, Trapasso F, Cesario F, Corigliano D, Cosco L, Costa C, Curia RL, De Rosa M. Future research and collaboration: the “SINERGIE” project on HCV (South Italian Network for Rational Guidelines and International Epidemiology). BMC Infect Dis. 2012;12 Suppl 2:S9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809-816. [PubMed] |
| 4. | Abenavoli L, Rouabhia S. Type 2 diabetes mellitus in chronic hepatitis C virus infection: risk factor or consequence? Expert Rev Gastroenterol Hepatol. 2013;7:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Abenavoli L, Almasio PL. Chronic hepatitis C infection and insulin resistance: two best friends. Expert Rev Anti Infect Ther. 2011;9:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Khan M, Jahan S, Khaliq S, Ijaz B, Ahmad W, Samreen B, Hassan S. Interaction of the hepatitis C virus (HCV) core with cellular genes in the development of HCV-induced steatosis. Arch Virol. 2010;155:1735-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Roingeard P. Hepatitis C virus diversity and hepatic steatosis. J Viral Hepat. 2013;20:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Solis-Herruzo JA, Pérez-Carreras M, Rivas E, Fernández-Vázquez I, Garfia C, Bernardos E, Castellano G, Colina F. Factors associated with the presence of nonalcoholic steatohepatitis in patients with chronic hepatitis C. Am J Gastroenterol. 2005;100:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Hézode C, Roudot-Thoraval F, Zafrani ES, Dhumeaux D, Pawlotsky JM. Different mechanisms of steatosis in hepatitis C virus genotypes 1 and 3 infections. J Viral Hepat. 2004;11:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Müllhaupt B, Borovicka J, Heim M, Moradpour D, Cerny A. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. [PubMed] |
| 13. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] |
| 14. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Hourioux C, Patient R, Morin A, Blanchard E, Moreau A, Trassard S, Giraudeau B, Roingeard P. The genotype 3-specific hepatitis C virus core protein residue phenylalanine 164 increases steatosis in an in vitro cellular model. Gut. 2007;56:1302-1308. [PubMed] |
| 16. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] |
| 17. | Koike K, Moriya K. Metabolic aspects of hepatitis C viral infection: steatohepatitis resembling but distinct from NASH. J Gastroenterol. 2005;40:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Masarone M, La Mura V, Bruno S, Gaeta GB, Vecchione R, Carrino F, Moschella F, Torella R, Persico M. Steatohepatitis is associated with diabetes and fibrosis in genotype 1b HCV-related chronic liver disease. J Viral Hepat. 2007;14:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 429] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 21. | Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473-7478. [PubMed] |
| 22. | Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, Powell EE, Tilg H. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol. 2005;43:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1451] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 24. | Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352-40363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 766] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 25. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Abenavoli L. Adiponectin levels in nonalcoholic fatty liver disease. Metabolism. 2011;60:e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Lautamäki R, Rönnemaa T, Huupponen R, Lehtimäki T, Iozzo P, Airaksinen KE, Knuuti J, Nuutila P. Low serum adiponectin is associated with high circulating oxidized low-density lipoprotein in patients with type 2 diabetes mellitus and coronary artery disease. Metabolism. 2007;56:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Kazumi T, Kawaguchi A, Hirano T, Yoshino G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metabolism. 2004;53:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Christou GA, Tellis KC, Elisaf MC, Tselepis AD, Kiortsis DN. High density lipoprotein is positively correlated with the changes in circulating total adiponectin and high molecular weight adiponectin during dietary and fenofibrate treatment. Hormones (Athens). 2012;11:178-188. [PubMed] |
| 33. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 608] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 34. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2341] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 35. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3091] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 36. | Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc Cc, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309-16313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 719] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 37. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1082] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 38. | Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1095] [Article Influence: 43.8] [Reference Citation Analysis (8)] |
| 39. | Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 661] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 40. | Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2105] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 41. | Liu Q, Gauthier MS, Sun L, Ruderman N, Lodish H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 2010;24:4229-4239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32 Suppl 7:S13-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 43. | Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 328] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 44. | Abenavoli L, Scarpellini E, Rouabhia S, Balsano C, Luzza F. Probiotics in non-alcoholic fatty liver disease: which and when. Ann Hepatol. 2013;12:357-363. [PubMed] |
| 45. | Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Macé K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 46. | Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Targher G, Bertolini L, Scala L, Poli F, Zenari L, Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf). 2004;61:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Im JA, Kim SH, Lee JW, Shim JY, Lee HR, Lee DC. Association between hypoadiponectinemia and cardiovascular risk factors in nonobese healthy adults. Metabolism. 2006;55:1546-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Asano T, Watanabe K, Kubota N, Gunji T, Omata M, Kadowaki T, Ohnishi S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol. 2009;24:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Petit JM, Minello A, Jooste V, Bour JB, Galland F, Duvillard L, Verges B, Olsson NO, Gambert P, Hillon P. Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J Clin Endocrinol Metab. 2005;90:2240-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Liu CJ, Chen PJ, Jeng YM, Huang WL, Yang WS, Lai MY, Kao JH, Chen DS. Serum adiponectin correlates with viral characteristics but not histologic features in patients with chronic hepatitis C. J Hepatol. 2005;43:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Zhang L, Sugiyama T, Murabayashi N, Umekawa T, Ma N, Kamimoto Y, Ogawa Y, Sagawa N. The inflammatory changes of adipose tissue in late pregnant mice. J Mol Endocrinol. 2011;47:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Lira FS, Rosa JC, Cunha CA, Ribeiro EB, do Nascimento CO, Oyama LM, Mota JF. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011;10:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One. 2011;6:e24358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527-E533. [PubMed] |
| 57. | De Maria N, Colantoni A, Fagiuoli S, Liu GJ, Rogers BK, Farinati F, Van Thiel DH, Floyd RA. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic Biol Med. 1996;21:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Wright E, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 338] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 59. | Neuman MG, Benhamou JP, Marcellin P, Valla D, Malkiewicz IM, Katz GG, Trepo C, Bourliere M, Cameron RG, Cohen L. Cytokine--chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073-9085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 826] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 61. | Dharancy S, Malapel M, Perlemuter G, Roskams T, Cheng Y, Dubuquoy L, Podevin P, Conti F, Canva V, Philippe D. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology. 2005;128:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 757] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 63. | Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 64. | Ashour E, Samy N, Sayed M, Imam A. The relationship between serum adiponectin and steatosis in patients with chronic hepatitis C genotype-4. Clin Lab. 2010;56:103-110. [PubMed] |
| 65. | Corbetta S, Redaelli A, Pozzi M, Bovo G, Ratti L, Redaelli E, Pellegrini C, Beck-Peccoz P, Spada A. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection. Eur J Clin Invest. 2011;41:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Tiftikci A, Atug O, Yilmaz Y, Eren F, Ozdemir FT, Yapali S, Ozdogan O, Celikel CA, Imeryuz N, Tozun N. Serum levels of adipokines in patients with chronic HCV infection: relationship with steatosis and fibrosis. Arch Med Res. 2009;40:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, Hayakawa N, Suzuki A, Takeda J, Horikawa Y. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 68. | Aksõz K, Unsal B, Kirci A, Alper E, Buyraç Z, Aslan F, Cekiç C, Cengiz O, Ozcan Ari F, Akpinar Z. The relationship between chronic HCV infection and the level of plasma adiponectin. Turk J Gastroenterol. 2008;19:254-257. [PubMed] |
| 69. | Valenti L, Rametta R, Ruscica M, Dongiovanni P, Steffani L, Motta BM, Canavesi E, Fracanzani AL, Mozzi E, Roviaro G. The I148M PNPLA3 polymorphism influences serum adiponectin in patients with fatty liver and healthy controls. BMC Gastroenterol. 2012;12:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, Dongiovanni P, Maggioni M, Fracanzani AL, Rametta R. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 71. | Nakamura M, Kanda T, Nakamoto S, Miyamura T, Jiang X, Wu S, Yokosuka O. No correlation between PNPLA3 rs738409 genotype and fatty liver and hepatic cirrhosis in Japanese patients with HCV. PLoS One. 2013;8:e81312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766-12771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 706] [Article Influence: 26.1] [Reference Citation Analysis (2)] |
| 73. | Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100-E1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab. 2006;290:E42-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 773] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 76. | Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 579] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 77. | Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 78. | Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, Wang Y, Wu D, Chu W, Qin G, Lam KS. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009;150:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 80. | Wedemeyer I, Bechmann LP, Odenthal M, Jochum C, Marquitan G, Drebber U, Gerken G, Gieseler RK, Dienes HP, Canbay A. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J Hepatol. 2009;50:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Palmer C, Hampartzoumian T, Lloyd A, Zekry A. A novel role for adiponectin in regulating the immune responses in chronic hepatitis C virus infection. Hepatology. 2008;48:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
P- Reviewer: Chen EQ, Higuera-de la Tijera MDF, Invernizzi P, Kanda T, Lisotti A
S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/