Published online May 27, 2014. doi: 10.4254/wjh.v6.i5.293
Revised: January 22, 2014
Accepted: March 17, 2014
Published online: May 27, 2014
Processing time: 195 Days and 19.6 Hours
Cystic echinococcosis (CE) is a complex, chronic and neglected disease with a worldwide distribution. The liver is the most frequent location of parasitic cysts. In humans, its clinical spectrum ranges from asymptomatic infection to severe, potentially fatal disease. Four approaches exist in the clinical management of CE: surgery, percutaneous techniques and drug treatment for active cysts, and the ”watch and wait” approach for inactive cysts. Allocation of patients to these treatments should be based on cyst stage, size and location, available clinical expertise, and comorbidities. However, clinical decision algorithms, efficacy, relapse rates, and costs have never been properly evaluated. This paper reviews recent advances in classification and diagnosis and the currently available evidence for clinical decision-making in cystic echinococcosis of the liver.
Core tip: Cystic echinococcosis (CE) is a neglected parasitic disease and echinococcal cysts are mostly located in the liver. Therefore, CE should always be included in the differential diagnosis of cystic lesions of the liver. However, diagnosis and clinical management can be difficult because of the combination of clinical variables (cysts stage, size, presence of complications, available expertise and three different treatments that have never been systematically compared). This review summarizes current knowledge and open issues in this field for those hepatologists who have limited or no experience with this complex condition.
- Citation: Rinaldi F, Brunetti E, Neumayr A, Maestri M, Goblirsch S, Tamarozzi F. Cystic echinococcosis of the liver: A primer for hepatologists. World J Hepatol 2014; 6(5): 293-305
- URL: https://www.wjgnet.com/1948-5182/full/v6/i5/293.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i5.293
Hepatologists may encounter cystic echinococcosis (CE) in their practice. However, due to its relatively low prevalence in many Western countries, this infection is poorly characterized and its complex management can be difficult for clinicians unfamiliar with this condition. Moreover, hepatic CE should be included in the differential diagnosis of focal liver lesions. In this paper, we summarize the current knowledge on clinical management of hepatic CE to increase hepatologists’ awareness of this complex condition.
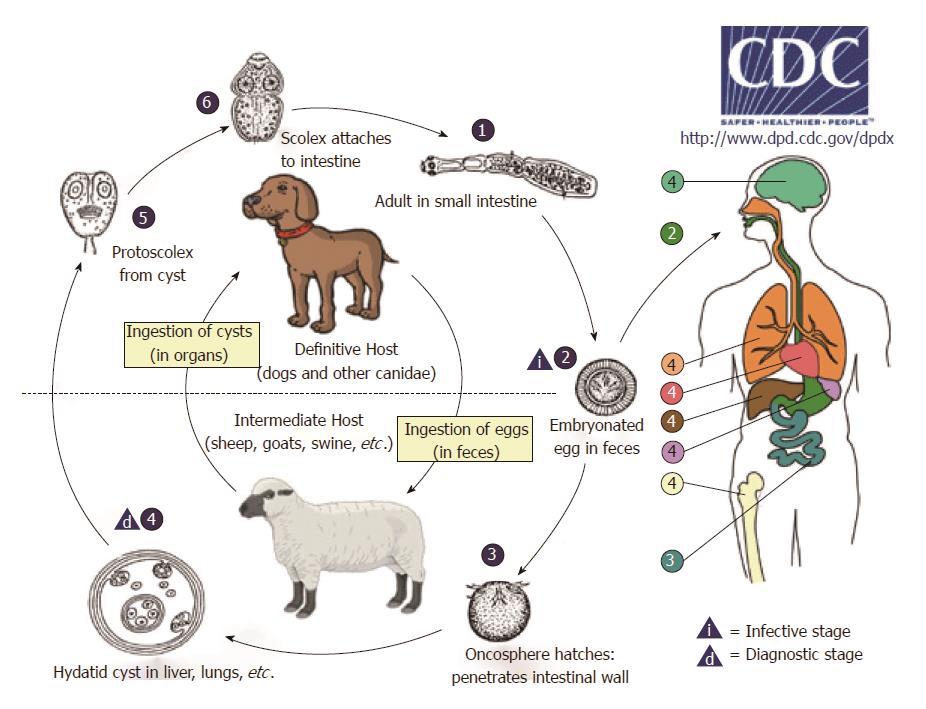
CE, or hydatidosis, is caused by the larval stage (metacestode) of Echinococcus granulosus (E. granulosus). Its life cycle develops in dogs and other canids, which harbor the adult tapeworm in the intestine, and herbivores (or humans as dead-end occasional host) as intermediate hosts, where the larval metacestode form develops in different organs (Figure 1).
Once eggs are ingested by the intermediate host, the oncosphere (also named exacanth larva), is released from the keratinized embryophore in the stomach and intestine where it penetrates the small intestine wall via its hook movements. The oncosphere is then carried via portal flow to the liver and other organs where the metacestode implants. Organs may also be reached through the lymphatic system[1]. This process results in primary echinococcosis, while secondary echinococcosis follows the spillage of protoscoleces (tapeworm heads) or small daughter cysts from the original cyst that ruptures following trauma or surgery and their seeding, primarily in the peritoneum for abdominal cysts[2].
The impact of CE on human health is significant, with an estimated 1.2 million people affected and 3.6 million DALYs (Disability Adjusted Life Years) lost globally[3]. Despite the low mortality rate (0.2/100000 population with a case fatality rate of 2.2%) morbidity is high[4]. Moreover, it has a major economic impact with an estimated annual livestock production loss of up to 2190 million US$[5].
Despite these figures, the infection is still under-reported and has received to date much less attention than infections of comparing burden[5]. In humans, its clinical manifestations range from asymptomatic infection to severe, potentially fatal disease.
The liver is the most frequent location of echinococcal cysts, representing approximately 70% of cases[4]. The lungs are the second most common location; however, CE can present in virtually any other organ, although this rarely occurs[1,2].
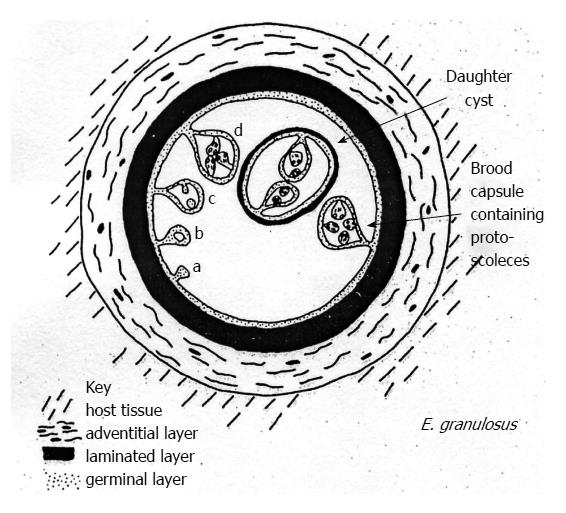
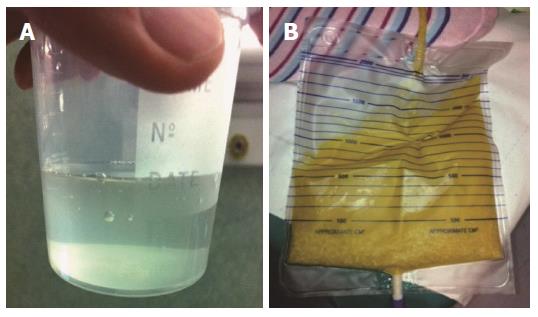
Echinococcal cysts consist of a periparasitic host tissue (pericyst or adventitia), which surrounds the larval endocyst, and an endocyst itself. The endocyst is composed of an outer, acellular laminated layer and an inner layer, the germinal layer, which gives rise, in fertile cysts, to brood capsules and protoscoleces[6]. Each protoscolex may develop into an adult tapeworm if ingested by a suitable definitive host. The cyst is filled with clear fluid containing molecules of both parasite and host origin, numerous brood capsules, and protoscoleces. Some cysts may also harbor daughter cysts of variable size (Figure 2). The fluid is clear in the early stages (Figure 3A), but can be yellowish and turbid, with fragments of endocyst in advanced stages (e.g., in CE3b cysts) or after months of treatment with albendazole (Figure 3B).
E. granulosus occurs in a broad range of geographic areas and can be found on all continents except Antarctica, and in circumpolar, temperate, subtropical, and tropical zones. Eurasia, Africa, Australia, and South America show the highest prevalence[7]. Within endemic zones, the prevalence varies from sporadic to high, with recent studies showing an higher prevalence among females and with increasing age[8]. Only a few countries can be regarded as free of E. granulosus infection[3].
E. granulosus parasites from different hosts show considerable phenotypic variation in terms of morphology, larval growth in vivo and in vitro, range of host infectivity, and biochemical features. Currently, 10 genotypic strains of E. granulosus have been identified (G1-G10), and the impact of these variations on CE epidemiology, pathology and control is being investigated. Genotypes are grouped into 4 species that constitute the E. granulosus complex: E. Granulosus sensu strictu (G1-G3), E. equinus (G4), E. ortleppi (G5) and E. canadensis (G6-G10). The great majority of E. granulosus isolates from humans thus far characterized have been of the sheep genotype (G1)[1,2].
Acute infection in humans has never been documented[9], thus all available data come from experimental studies in animal intermediate hosts. Cavity formation and the development of both germinal and laminated layers of the cyst wall occur 10 to 14 d post infection in the mouse model[10]. Formation of brood capsules and protoscoleces requires a longer time period in sheep, from 10 mo to 4 years[11].
Based on clinical observations using ultrasound (US), the cysts progress from a fluid-filled unilocular cavity to a pseudo-solid, eventually calcified lesion. The sequence of cyst development between these 2 stages is poorly understood[12]. Long-term clinical observation indicates that the early stages are CE1 and CE3a cysts, while final stages are represented by CE4 and CE5, referring to the standardized US classification (see “Imaging” below)[13]. Preliminary observations suggest that cysts that have reached the CE4 stage as a result of treatment may revert to CE3b more often than those reaching the inactive stage spontaneously; this may occur many years after apparently successful treatment[14] (Junghanss, personal communication). The origin and fate of CE2 and CE3b stages are less clear. CE2 may represent a relapsed CE3a, and CE3b a relapsed CE4, but long-term observations of large cohorts of patients are needed to confirm this hypothesis.
The growth rate of cysts is variable. The average increase in cyst diameter is thought to be 1 cm/year, but data on the natural history of CE are scarce. Cysts may behave differently in different subjects and their growth rate also depends on the surrounding host tissue, with growth rates up to 5 cm/year reported for brain cysts[15-19].
The presentation of human CE is protean. Patients come to the clinician’s attention for a variety of reasons. Potential presentations may be due to the mechanical effect of a large cyst on surrounding tissues, rupture of a cyst causing an acute hypersensitivity reaction, or complications such as biliary obstruction or embolism. The cyst is often asymptomatic and diagnosed accidentally during radiographic examination, surgery, or during evaluation of other clinical diagnoses.
Common symptoms are upper abdominal discomfort and pain and poor appetite. Physical findings are hepatomegaly, presence of an abdominal palpable mass and abdominal distension. Cysts in the liver should be included in the differential diagnosis of several conditions, such as jaundice, colicky pain, portal hypertension, ascites, compression of the inferior vena cava and Budd-Chiari syndrome and can be misdiagnosed as non-parasitic cysts, single or multiple hemangiomas, pyogenic or amebic liver abscess, hematoma, adenoma, adenocarcinoma, hepatocellular carcinoma, metastases, focal or diffuse lymphoma, alveolar echinococcosis, and textiloma[20,21].
As the infection may remain silent for years before the enlarging cysts cause symptoms, the clinical diagnosis of CE is often difficult and requires a combination of physical examination, imaging techniques, in particular US, and serology; the latter plays a supportive role in diagnosing CE despite the development of sensitive serodiagnostic tests and the use of different antigen sources.
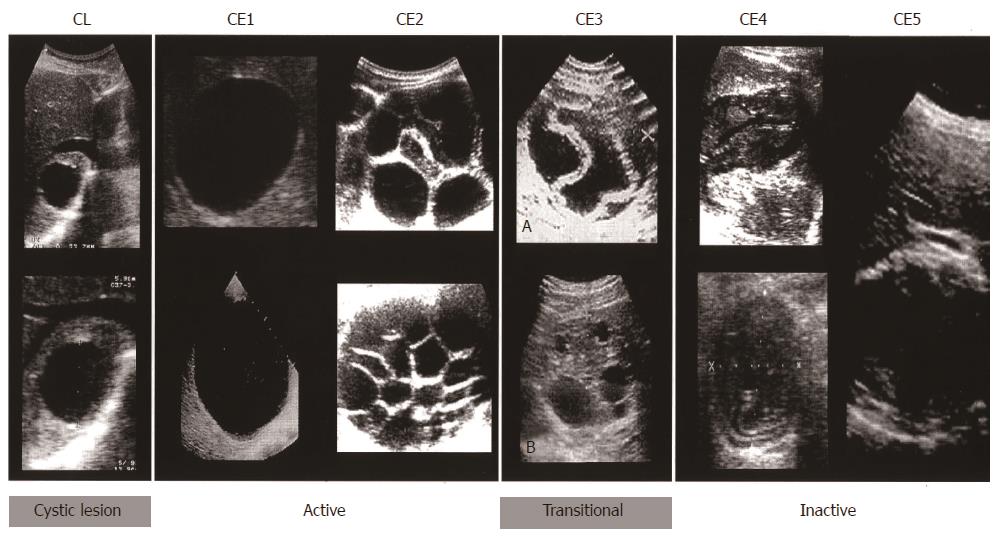
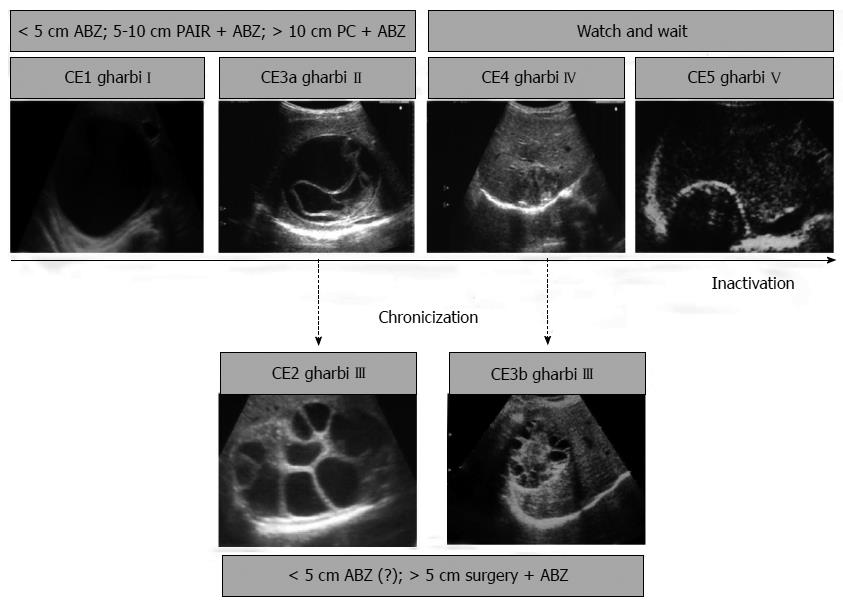
Imaging techniques have revolutionized the diagnosis and clinical management of CE. Gharbi et al[22] developed the first US classification for CE in 1981. Other classifications were subsequently produced but were not widely adopted. In 1995, the WHO Informal Working Group developed an international standardized US classification that could be universally applied to replace the plethora of classifications in use.
This classification, published in 2003[23], differs from Gharbi original classification by introducing a cystic lesion (CL) category to include cysts of unclear origin, and by reversing the order of CE types 2 and 3 (Figure 4). The number of cyst types remains unchanged from Gharbi’s classification and the types are categorized into active, transitional, and inactive stages. CL cysts are not included as a type of CE, as they require further evaluation before being classified as CE[24]. CE1 and 2 are active, usually fertile cysts containing viable protoscoleces. CE3 are cysts entering a transitional stage where the integrity of the cyst has been compromised either by the host or by chemotherapy. CE4 and CE5 are inactive cysts that have lost their fertility and are degenerating. A more recent amendment to the WHO classification clarifies that calcifications are not limited to CE5 cysts, but may be present to a various extent in all cystic stages and are therefore not indicative of cyst death[25].
Data on long-term follow-up of cysts treated with albendazole and percutaneous treatment provide ground for a further sub-classification of CE3 (transitional) cysts into CE3a (with detached endocyst) and CE3b (predominantly solid with daughter vesicles). This has important implications for clinical decision-making and prognosis[26]. The sub-classification of CE3 into CE3a and CE3b is supported a recent work using high-field 1H magnetic resonance spectroscopy evaluating the metabolic profile of cysts contents ex vivo[27]. This study confirmed findings from optical microscopy that CE3a are equally likely to be viable or non-viable, whereas CE3b are consistently viable. Of note, CE3a and CE3b also respond differently to non-surgical treatments[28,29]. In light of these features, CE3b cysts should be considered as active, while CE3a are the transitional cysts sensu stricto.
The same study confirmed the biological activity of CE1 and CE2 and the inactivity of CE4 and CE5. Another study showed how a CE1 brain cyst, in in vivo magnetic resonance spectroscopy matched the profile of an active stage before the medical treatment with albendazole (ABZ) and that of an inactive one after ABZ[30]. CE2 and CE3b cysts tend to relapse both after PAIR (puncture, aspiration, injection of a scolecidal agent, and reaspiration) and ABZ[26,28,29], and several studies suggest that a strong Th2 response correlates with susceptibility to disease (active cyst), whereas a Th1 response correlates with protective immunity (inactive cyst), however this is not clear cut[31-36].
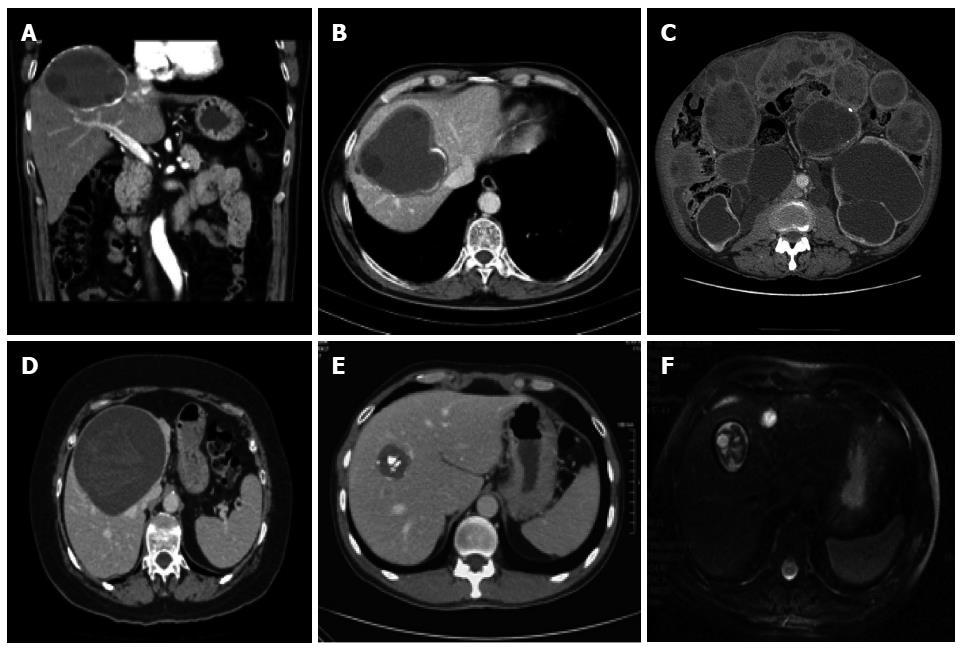
Computed tomography (CT), including spiral or multidetector CT, with multiplanar reformations, and magnetic resonance imaging (MRI), with at least a T2-weighted imaging sequence, and if necessary cholangiopancreatography, have distinct indications: (1) impaired US visualization due to obesity or subdiaphragmatic location of the cyst; (2) disseminated disease; (3) extra-abdominal location; (4) complications (cyst infection, cysto-biliary fistulae); and (5) pre-surgical evaluation and follow-up (Figure 5). Whenever possible, MRI is preferred to CT for pre-treatment assessment[37,38].
Routine blood tests are not specific for CE, and with liver involvement they can be normal or suggestive of cholestasis with or without hyperbilirubinaemia or raised transaminases or γ-glutamyltransferase (γ-GT)[1,39,40]. Transient elevation of γ-GT and alkaline phosphatase, in association with hyper-transaminasemia and eosinophilia, may indicate cyst rupture in the biliary tree. Despite CE being a helminthic infection, eosinophilia is usually moderate or absent.
Despite the development of sensitive laboratory tests and the use of different antigen sources, serology remains complementary to imaging in the diagnosis of CE. Currently, lipoprotein antigen B (AgB) and Ag5, the major components of cystic fluid, have received the most attention with regard to diagnosis, but purified cyst hydatid fluid is still the most widely used in current assays for immunodiagnosis of CE, which are not standardized[41,42]. In clinical practice, usually two tests are performed (for example ELISA and indirect hemagglutination (IHA), with immunoblotting (IB) as a confirmatory test. IHA and ELISA show sensitivity for hepatic cysts ranging between 85% and 98%[42-46]. The result of a single test is not considered diagnostic, and the two tests are generally run in parallel. IB is performed as a confirmatory test when ELISA and IHA are inconclusive or for the differential diagnosis with other infections, although E. granulosus specific bands may be also be detected in the serum of patients affected by E. multilocularis and rarely T. solium cysticercosis[47]. False positives result from cross-reactivity, most commonly with other cestode infections (E. multilocularis, Taenia solium cysticercosis) and some other parasitoses (schistosomiasis, liver flukes, filariasis), but also from non-infectious diseases such as malignancies and cirrhosis[1,41,48-50].
Serologic testing for CE is hampered by many problems[41-43,45]. These include low sensitivity, partially dependent upon the location of the cysts in the body and the cystic stage, and the inability of serology to clearly distinguish between active and inactive cysts when US is inconclusive[43]. Up to 20% of patients with single hepatic cysts and up to 50% of those with lung cysts may be seronegative at diagnosis, while patients with cysts in other locations are often seronegative. In addition, patients with multiple cysts are generally seropositive. In the case of hepatic cysts, patients with CE1 and CE4-CE5 cysts are often seronegative (30%-58% and 50%-87% respectively), while rates of negativity are lower in the presence of CE2 and CE3 cysts (5%-20%). It is also worth noting that serodiagnostic tests may be persistently positive for > 10 years even after radical surgical removal of the cysts, and are often positive in the presence of inactive cysts[17,51,52]. This may lead inexperienced clinicians to prescribe unnecessary treatment and cause unjustified anxiety to the patient. New antigens are under investigation which promise to have higher diagnostic performances in these situations[53].
When US and serology are inconclusive, a direct analysis of the material obtained by percutaneous aspiration is needed. The procedure must be performed with the assistance of an anesthesiologist because of the very low but nonetheless present risk of anaphylaxis[54]. The presence of protoscoleces or their components or of antigens specific to E. granulosus indicates the parasitic nature of the cyst[55].
There is no standard treatment for hepatic CE. The appropriate treatment depends on individual patient factors, cyst characteristics, the therapeutic resources available, and the physician’s preference[56]. Matters are further complicated by the dearth of randomized clinical trials evaluating treatment options, and the ensuing low level of evidence to support one therapeutic modality over another[57,58] .
Surgery has long been considered the best, if not the only, option in the treatment of CE. However, in the past two decades, medical treatment, percutaneous procedures, and a “watch and wait” approach have been successfully introduced and have replaced surgery as the treatment of choice in selected cases[59].
While surgery is increasingly being replaced by other options in uncomplicated cysts, it maintains a central role in complicated cysts (i.e., rupture, biliary fistula, compression of vital structures, superinfection, hemorrhage), cysts at high risk of rupture, or large cysts with many daughter vesicles that are not suitable for percutaneous treatments.
Surgery can be performed as an open procedure, with either radical or conservative techniques, or laparoscopically. There are still controversies as to the safest and most effective technique, and in which cases it should be applied[57,60,61]. As a rule, perioperative ABZ prophylaxis, from 1 wk prior to surgery until 4 wk postoperatively, is necessary to minimize the risk of secondary echinococcosis from seeding of protoscoleces in the abdominal cavity[59].
Radical surgery aims to remove the entire pericystic membrane and the parasitic contents with or without hepatic resection, and can be performed with either the “open-cyst” or “closed-cyst” method. In conservative procedures, only the parasitic material is removed while part or all of the pericyst is left in place and the residual cavity is managed with different techniques, such as omentoplasty, capitonnage, or external drainage.
A cleavage plan between the inner layer of the host’s reaction towards the parasite and the cyst outer layer, or “adventitia”, as described by Peng et al[62], limits damage to liver parenchyma when dissecting around the cyst and allows for safer removal. Based on these anatomical considerations, such an operation should be more adequately termed “total cystectomy.” Mortality ranges between 0.8% and 6.5%, morbidity between 12% and 84%, and relapse rate between 2% and 30%[39,60,63,64].
It is commonly perceived that the more radical the surgery, the higher the operative risk but the lower the risk of relapses and vice versa. However, results of meta-analyses and single center studies indicate that radical surgery is superior to conservative surgery, with lower morbidity (3%-24% vs 11%-25%), mortality (1%-1.8% vs 2%-5%) and recurrence rates (2%-6.4% vs 10.4%-40%)[61,64-66], although the type of surgery was not found to be a predictive factor of post-surgery complications in the study of El Malki et al[60]. Other factors associated with surgical outcome are large cyst size, more than 3 hepatic cysts, presence of biliary fistulae, age > 40 years, repeated surgery due to recurrence, capitonnage alone as a measure of residual cavity management, and cyst rupture during surgery[60,61,64,67,68].
Recurrence, both local and as secondary echinococcosis, is associated with spillage during removal of the cyst, incomplete removal of the endocyst, and possibly the presence of unnoticed exophytic cyst development[63,69]. For the latter, intraoperative US has been shown to be an important tool to improve the quality of hepatic surgery[70].
Infection and biliary communication with the cyst (i.e., leakage or rupture with cholestasis) are the most common complications of echinococcal cysts and can occur before or after surgical or percutaneous interventions[71,72]. Cyst diameter is a factor associated with a high risk of biliary-cyst communication in clinically asymptomatic patients. A recent study reported that cyst diameter > 7.5 cm had a specificity and sensitivity for biliary-cyst communication of 73% and 79%, respectively[73]. Thus, surgeons operating on cysts larger than 7.5 cm should be prepared to deal with this complication and should perform preoperative retrograde cholangiopancreatography or MR imaging[73,74].
Several methods have been proposed for the management of cyst-biliary communications. When intrabiliary rupture is diagnosed pre- or intra-operatively, a simple suture of the orifice is sufficient if there are no cystic contents in the biliary tree and the common bile duct has a normal caliber. When cyst contents are found in the biliary tree or the common bile duct has an abnormal caliber, evacuation of the cystic content and a T-tube drainage placement or even a coledochoduodenostomy are needed[71,75]. Alternatively, endoscopic treatment with sphincterotomy and placement of a nasobiliary catheter has been performed[76,77]. Postoperative bile leakage resulting in symptomatic bilomas or high-output biliary fistulae can be managed endoscopically by sphincterotomy with nasobiliary drainage or biliary stenting[78,79].
Surgical interventions other than segmentectomies can result in a number of residual cavities that may be mistaken for recurrences or other conditions[20]. Some groups have evaluated these findings and attempted to categorize them relative to the type of surgical procedure performed[80].
A recent review on management of post-surgical complications concluded that “ the evidence level is low” and that “there are many questions and few answers”[81].
Percutaneous treatments for abdominal CE were introduced in the mid-1980s, with the adoption of minimally invasive procedures made possible by new imaging tools, particularly CT and US[82-85]. These treatment modalities aim either to destroy the germinal layer with scolecidal agents or to evacuate the entire endocyst.
The most popular method is PAIR[13]. Several modified catheterization techniques are used to evacuate the endocyst, and are generally reserved for cysts which are difficult to drain or tend to relapse after PAIR, such as multivesiculated cysts or cysts with predominantly solid content and daughter cysts[26].
Catheterization techniques are based on the aspiration of the “solid” content of the cyst, the endocyst surrounded by pseudocaseous inflammatory material, through a large-bore catheter or other device. Several variants of these techniques have been proposed, in particular percutaneous evacuation (PEVAC)[86], a modified catheterization technique[87], and dilatable multi-function trocar[88].
Puncture of echinococcal cysts has long been discouraged because of the risk of anaphylactic shock and spillage of the fluid; however, as experience with US-guided interventional techniques has increased since the early 1980s, a growing number of articles have reported its safety in treating abdominal, especially liver, echinococcal cysts. In a recent systematic analysis on percutaneous aspiration of echinococcal cysts, only 2 cases of lethal anaphylaxis (0.04%) and 99 reversible anaphylactic reactions (1.8%) were reported[54]. This study divided the complications related to cyst puncture into major (0.5% of cases with anaphylactic shock and peritoneal liquid seeding, liver or intra-abdominal abscess, sepsis, biliary fistulas) and minor (10%-30% of patients with fever, hypotensive reactions, nausea, vomiting, skin rash, respiratory symptoms). Peritoneal seeding has never been reported, but it is difficult to assess the true rate because many reported series have a short follow-up time. Prophylactic administration of ABZ starting 4 h before the puncture and for at least 30 d after puncture is a cautionary measure that should always accompany PAIR[59].
PAIR is performed with several variants of the standard protocol and is generally successful at inducing permanent solidification of medium-sized CE1 and CE3a cysts[13]. A few reports with long-term follow-up indicate that multivesiculated cysts (i.e., CE2 and CE3b) tend to relapse repeatedly after PAIR[26,29,89,90]. Reported morbidity and mortality range from 8.5%-32% and 0%-1% respectively[89,91-94]. Mean hospital stay is 1-4 d compared to 12 d in case of surgery[89,91,93]. PAIR has also been performed in remote, resource-poor areas using portable US machines[95]. Overall response rates range from 72%-97%, with relapse rates from 1.6%-5%[89,91,92,94,96]. However, these figures vary greatly when cyst stages are taken into account. Indeed, unilocular CE1 and CE3a cysts respond very well to percutaneous treatment (> 80% response), while multi-vesiculated CE2 and CE3b cysts have a success rate lower than 40%[29,89,90] . Giant CE1 and CE3a cysts of 10 cm or greater, should preferably be treated with a large catheter left in place until the daily drainage is less than 10 mL, on average 3 wk[97].
The experience with catheterization techniques in CE2 and CE3b cysts is more recent and less extensive than that with PAIR, and results from series with long-term follow-up are needed before their efficacy can be determined. Data available for PEVAC in cysts with cysto-biliary fistulas are less than satisfying, given the long hospitalization and catheter times, up to 128 and 55 d, respectively[86]; in these cases PEVAC does not compare favorably with surgery.
The use of percutaneous techniques should be reserved for referral or specialized centers where teams are prepared to deal with possible complications and an anesthesiologist should always be present during the procedure.
Use of scolecidal agents in surgery and percutaneous treatments: Scolecidal agents should be applied only after having excluded the presence of cysto-biliary fistulae, either with intraoperative cystoscopy or evaluating bilirubin content in the cyst fluid. Although chemical sclerosing cholangitis, due to contact of the scolecidal agent with the biliary ducts, has never been reported using PAIR, several reports are present in the literature after surgery[98-100] and damage to the biliary epithelium has been shown in animal models[101,102]. While hypertonic (15%-20%) saline and 95% ethanol are the most widely used scolecidal agents for percutaneous treatments, a range of other compounds have been tested or are being investigated in the attempt to find an agent that does not damage the biliary epithelium[103-106].
Direct intracystic injection of mebendazole (MBZ) has been successfully performed in animals and humans, and ABZ sulfoxide, the active metabolite of ABZ, has been successfully injected in cysts in animals, but not in humans[107-110]. However, little difference has been found in in vitro studies between the effect of hypertonic saline and that of ABZ sulfoxide or sulfone[103]. Unfortunately, ABZ sulfoxide is not available as an injectable formulation and this prevents its clinical use.
The use of benzimidazole (BZD) carbamates in the treatment of CE was introduced in the 1970s. While both albendazole and MBZ have been proven effective against the larval stage of E. granulosus, ABZ is the current treatment of choice due to better absorption[111]. ABZ is administered orally at a dose of 10-15 mg/kg per day generally for 3-6 mo; administration should be continuous without treatment interruptions, in contrast to the recommendation in the 1980s[26,112]. However, the optimal dose and duration of treatment with ABZ have not been formally assessed.
The comparative rarity of CE in many industrialized countries where BZD is available and affordable is such that only a few centers are able to follow sufficient numbers of patients within a reasonable period of time. Thus, most studies are small, and few have adequate controls.
In the largest series published thus far, 848 patients with 929 cysts received 3-6-mo continuous cycles of MBZ or ABZ treatment[113]. Long-term follow-up showed that 74.1% of the cysts developed degenerative changes. These were more frequent in ABZ-treated than in MBZ-treated cysts (82.2% vs 56.1%; P < 0.001). During follow-up, 104 cysts (22%) had degenerative changes, whereas 163 cysts (25%) relapsed. In other series, reported outcome rates for hepatic cysts are: 28.5%-58% cure/marked improvement, 10%-51% partial response, 13%-37% no change, and 4%-33% worsened[112,114-120]. Relapse rates range from 9%-25%[112,113,116,121], and, although responsive to subsequent treatments, cysts tend to relapse multiple times[28]. Factors associated with treatment outcome include cyst stage, size, and localization. Unilocular (CE1 and CE3a) cysts and small cysts (< 6 cm) respond better and faster to ABZ treatment compared with multivesciculated (CE2 and CE3b) and larger cysts, with a lower relapse rate[28,113,117,120,122], as clearly shown in the systematic review by Stojkovic et al[28]. A recent study highlighted the importance of at least 12 mo of follow-up, since it is difficult to predict cyst behavior after treatment[28].
Adverse effects of BMZ include headache (10% of cases), gastrointestinal symptoms (56%), hepatotoxicity, severe leukopenia, neutropenia or thrombocytopenia (< 1%), and alopecia (2%)[59,123]. Increases in aminotransferases (15% cases) may be due to drug-related efficacy or to real drug-related toxicity. Risks observed in laboratory animals include embryotoxicity and teratogenicity. While teratogenicity is theoretical, it is nonetheless good practice to avoid use during pregnancy whenever possible. Thus, the treatment should be delayed until after delivery[124]. Hospitalization is not necessary, but regular follow-up is required with a monthly check of the hemogram and liver enzymes.
If ABZ is not available or not tolerated, MBZ, the first BMZ tested against Echinococcus, may be used at a dosage of 40-50 mg/kg body weight, in three divided doses during fat-rich meals. Costs of BMZ and repeated examinations may be prohibitive in countries with limited resources. Praziquantel (PZQ) 40 mg/kg once a week in combination with ABZ seems more effective in killing protoscoleces than ABZ alone[125]. Other clinical studies evaluating this combination are available but they do not clarify whether PZQ has a pharmacological effect in its own right or acts only by enhancing ABZ absorption[126]. The usefulness of PZQ to avoid secondary echinococcosis needs confirmation[127].
Recent expert opinion recommends that inactive CE4-CE5 cysts that are asymptomatic and uncomplicated should be left untreated and monitored regularly by imaging techniques, using the so-called “watch-and-wait” approach[56,59]. The rationale of leaving uncomplicated, inactive cysts untreated and solely monitored over time follows the observation that up to 20% of cysts become spontaneously inactive without any treatment and such cysts are likely to remain stable over time[18,26,118,120,128-130].
In chronic conditions such as CE, follow-up is crucial in order to evaluate the efficacy of treatment. The follow-up should start with a short interval (every 6 mo for the first 2 years) and continue with a longer interval (once a year), but this needs to be adjusted to the patient’s setting. In referral centers, follow-up includes US imaging and serology; for specific patients (e.g., with abdominal gas, obesity, multiple cysts, and so on) it may also include CT or MRI.
Long-term follow-up, generally longer than 5 years, is required to evaluate local recurrences which have been reported up to 10 years after apparently successful treatment[14].
CE can be very difficult to treat and even more difficult to cure for a number of reasons. The disease is complex and dynamic, with an evolving phase and quietly growing cysts, followed by an involution process during which the parasite is gradually dying, leaving behind a solidified, often calcified cyst or a scar.
Each successive active cyst stage carries its own risks for serious and even life-threatening complications. This variation during the CE disease process leads to a wide range of treatment modalities with an equally wide range of technological and training backgrounds necessary for implementation and delivery. As a result of all of these issues, no “one size fits all” management approach is available, and a stage-specific approach currently appears to be the best way to manage this condition[26].
Technical and economic difficulties are encountered in countries with limited resources where the patient load is greatest: here CE is defined as a neglected disease. Problems in acquiring clinical competence in countries where few patients suffer from the disease are also an obstacle: in these settings CE is an orphan disease[26]. Further complicating matters is the fact that CE is a chronically neglected disease. Investment in research is very low compared to what is needed based on estimated burden of disease[5]. The latter is very difficult to gauge because the true incidence is unknown. Acute cases have never been recorded because they are clinically silent and only the prevalence can be assessed, although often with great difficulties due to poor access to healthcare and underreporting[3]. The best solution to this problem is likely the setting up of national CE registries modeled on the European Register for Alveolar Echinococcosis. We have recently set up the Italian Register for Cystic Echinococcosis: http://www.iss.it/riec/), and preliminary results of systematic enrolment of CE patients seen in Italian hospitals will be published in the near future. However, such initiatives require resources and funding, both difficult to come by when dealing with a neglected disease[5].
Although the evidence base for clinical decision-making is still at the level of expert opinion, clinical management of hepatic CE patients is facilitated by the standardization of US classification, enabling clinicians to identify the most rational option on the basis of cyst stage[26,131]. The stage-specific treatment approach for uncomplicated cysts of the liver can be summarized as follows (Figure 6).
Small (< 5 cm) univesicular CE1 and C3a cysts tend to respond well to ABZ treatment, while larger cysts are treated preferentially with PAIR plus ABZ. Giant cysts (> 10 cm) should be treated with a catheter left in place until the drainage is minimum, usually about 3 wk.
Surgery should be reserved for complicated cysts, including those with rupture or high risk of rupture, fistulization, compression of vital organs or vessels, hemorrhage, or bacterial infection. Surgery is also an option for cysts poorly responsive to medical or percutaneous treatment when a “watch and wait” approach is not viable because of poor access to healthcare.
| 1. | Eckert JGM, Meslin FX, Pawlowski ZS, editors . WHOI/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: World Health Organization for Animal Health 2001; . |
| 2. | Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1222] [Article Influence: 55.5] [Reference Citation Analysis (1)] |
| 3. | Craig PS, Budke CM, Schantz PM, Li T, Qiu J, Yang Y, Zeyhle E, Rogan MT, Ito A. Human Echinococcosis: A Neglected Disease? Trop Med Heal. 2007;35:283-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 737] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 5. | Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12:296-303. [PubMed] |
| 6. | Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20:795-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 501] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Schantz PM, Kern P, Brunetti E. Echinococcosis: Tropical Infectious Diseases. United Kingdom: Elsevier Saunders 2011; . |
| 8. | Budke CM, Carabin H, Ndimubanzi PC, Nguyen H, Rainwater E, Dickey M, Bhattarai R, Zeziulin O, Qian MB. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am J Trop Med Hyg. 2013;88:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Brunetti E, Garlaschelli AL, Filice C, Schantz P. Comment on “Acute echinococcosis: a case report”. J Clin Microbiol. 2003;41:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Ferreira AM, Irigoín F, Breijo M, Sim RB, Diáz A. How Echinococcus granulosus deals with complement. Parasitol Today. 2000;16:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Gemmell MA, Lawson JR, Roberts MG. Population dynamics in echinococcosis and cysticercosis: biological parameters of Echinococcus granulosus in dogs and sheep. Parasitology. 1986;92:599-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rogan MT, Hai WY, Richardson R, Zeyhle E, Craig PS. Hydatid cysts: does every picture tell a story? Trends Parasitol. 2006;22:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | WHO-IWGE, PAIR: Puncture, Aspiration, Injection, Re-Aspiration. An option for the treatment of Cystic echinococcosis. Vol. WHO/CDS/CSR/APH/2001.6 2003. Geneva: WHO; . |
| 14. | Trotta F, Prati U, Roveda L, Brunetti E, Filice C. Intra-operative PAIR of hepatic echinococcal cyst after cholecystectomy with laparoscopic approach. Liver Int. 2007;27:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Sierra J, Oviedo J, Berthier M, Leiguarda R. Growth rate of secondary hydatid cysts of the brain. Case report. J Neurosurg. 1985;62:781-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Romig T, Zeyhle E, Macpherson CN, Rees PH, Were JB. Cyst growth and spontaneous cure in hydatid disease. Lancet. 1986;1:861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Moro PL, Gilman RH, Verastegui M, Bern C, Silva B, Bonilla JJ. Human hydatidosis in the central Andes of Peru: evolution of the disease over 3 years. Clin Infect Dis. 1999;29:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Frider B, Larrieu E, Odriozola M. Long-term outcome of asymptomatic liver hydatidosis. J Hepatol. 1999;30:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Brunetti E, Gulizia R, Garlaschelli AL, Filice C. Cystic echinococcosis of the liver associated with repeated international travels to endemic areas. J Travel Med. 2005;12:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Cattaneo F, Graffeo M, Brunetti E. Extrahepatic textiloma long misdiagnosed as calcified echinococcal cyst. Case Rep Gastrointest Med. 2013;2013:261685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475-494; quiz 536-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 22. | Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459-463. [PubMed] |
| 23. | WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 516] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 24. | Grisolia A, Troìa G, Mariani G, Brunetti E, Filice C. A simple sonographic scoring system combined with routine serology is useful in differentiating parasitic from non-parasitic cysts of the liver(). J Ultrasound. 2009;12:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Hosch W, Stojkovic M, Jänisch T, Kauffmann GW, Junghanss T. The role of calcification for staging cystic echinococcosis (CE). Eur Radiol. 2007;17:2538-2545. [PubMed] |
| 26. | Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301-311. [PubMed] |
| 27. | Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann GW, Hull WE. Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed. 2008;21:734-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, Nicolaidou P, Cobanoglu N, Junghanss T. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3:e524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Kabaalioğlu A, Ceken K, Alimoglu E, Apaydin A. Percutaneous imaging-guided treatment of hydatid liver cysts: do long-term results make it a first choice? Eur J Radiol. 2006;59:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Seckin H, Yagmurlu B, Yigitkanli K, Kars HZ. Metabolic changes during successful medical therapy for brain hydatid cyst: case report. Surg Neurol. 2008;70:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Riganò R, Buttari B, De Falco E, Profumo E, Ortona E, Margutti P, Scottà C, Teggi A, Siracusano A. Echinococcus granulosus-specific T-cell lines derived from patients at various clinical stages of cystic echinococcosis. Parasite Immunol. 2004;26:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Tamarozzi F, Meroni V, Genco F, Piccoli L, Tinelli C, Filice C, Brunetti E. Ex vivo assessment of serum cytokines in patients with cystic echinococcosis of the liver. Parasite Immunol. 2010;32:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Riganò R, Profumo E, Bruschi F, Carulli G, Azzarà A, Ioppolo S, Buttari B, Ortona E, Margutti P, Teggi A. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect Immun. 2001;69:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Hernández-Pomi A, Borras-Salvador R, Mir-Gisbert A. Analysis of cytokine and specific antibody profiles in hydatid patients with primary infection and relapse of disease. Parasite Immunol. 1997;19:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Riganò R, Profumo E, Buttari B, Teggi A, Siracusano A. Cytokine gene expression in peripheral blood mononuclear cells (PBMC) from patients with pharmacologically treated cystic echinococcosis. Clin Exp Immunol. 1999;118:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Hosch W, Stojkovic M, Jänisch T, Heye T, Werner J, Friess H, Kauffmann GW, Junghanss T. MR imaging for diagnosing cysto-biliary fistulas in cystic echinococcosis. Eur J Radiol. 2008;66:262-267. [PubMed] |
| 38. | Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis. 2012;6:e1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Filippou D, Tselepis D, Filippou G, Papadopoulos V. Advances in liver echinococcosis: diagnosis and treatment. Clin Gastroenterol Hepatol. 2007;5:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 40. | Brunetti E, White AC. Cestode infestations: hydatid disease and cysticercosis. Infect Dis Clin North Am. 2012;26:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop. 2006;98:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 42. | Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 43. | Lorenzo C, Ferreira HB, Monteiro KM, Rosenzvit M, Kamenetzky L, García HH, Vasquez Y, Naquira C, Sánchez E, Lorca M. Comparative analysis of the diagnostic performance of six major Echinococcus granulosus antigens assessed in a double-blind, randomized multicenter study. J Clin Microbiol. 2005;43:2764-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Ito A, Craig PS. Immunodiagnostic and molecular approaches for the detection of taeniid cestode infections. Trends Parasitol. 2003;19:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Ortona E, Siracusano A, Castro A, Rigano R, Mühlschlegel F, Ioppolo S, Notargiacomo S, Frosch M. Use of a monoclonal antibody against the antigen B of Echinococcus granulosus for purification and detection of antigen B. Appl Parasitol. 1995;36:220-225. [PubMed] |
| 46. | Ortona E, Riganò R, Buttari B, Delunardo F, Ioppolo S, Margutti P, Profumo E, Teggi A, Vaccari S, Siracusano A. An update on immunodiagnosis of cystic echinococcosis. Acta Trop. 2003;85:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Pawlowski Z, Eckert J, Vuitton DA, Amman RWP, Kern P, Craig PS, Dar KF, De Rosa F, Filice C, Gottstein B. Echinococcosis in humans: clinical aspects, diagnosis and treatment, in WHO/OIE manual on echinococcosis in humans and animals: public health problem of global concern. Paris, France: WHO/OIE 2001; 20-66. |
| 48. | Iacona A, Pini C, Vicari G. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;29:95-102. [PubMed] |
| 49. | Dar FK, Buhidma MA, Kidwai SA. Hydatid false positive serological test results in malignancy. Br Med J (Clin Res Ed). 1984;288:1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Poretti D, Felleisen E, Grimm F, Pfister M, Teuscher F, Zuercher C, Reichen J, Gottstein B. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg. 1999;60:193-198. [PubMed] |
| 51. | Galitza Z, Bazarsky E, Sneier R, Peiser J, El-On J. Repeated treatment of cystic echinococcosis in patients with a long-term immunological response after successful surgical cyst removal. Trans R Soc Trop Med Hyg. 2006;100:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Hernández-González A, Muro A, Barrera I, Ramos G, Orduña A, Siles-Lucas M. Usefulness of four different Echinococcus granulosus recombinant antigens for serodiagnosis of unilocular hydatid disease (UHD) and postsurgical follow-up of patients treated for UHD. Clin Vaccine Immunol. 2008;15:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Hernández-González A, Santivañez S, García HH, Rodríguez S, Muñoz S, Ramos G, Orduña A, Siles-Lucas M. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis. 2012;6:e1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Neumayr A, Troia G, de Bernardis C, Tamarozzi F, Goblirsch S, Piccoli L, Hatz C, Filice C, Brunetti E. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis-a systematic literature review. PLoS Negl Trop Dis. 2011;5:e1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 55. | Stefaniak J. Fine needle aspiration biopsy in the differential diagnosis of the liver cystic echinococcosis. Acta Trop. 1997;67:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 56. | Menezes da Silva A. Hydatid cyst of the liver-criteria for the selection of appropriate treatment. Acta Trop. 2003;85:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Dziri C, Haouet K, Fingerhut A. Treatment of hydatid cyst of the liver: where is the evidence? World J Surg. 2004;28:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Brunetti E, Garcia HH, Junghanss T. Cystic echinococcosis: chronic, complex, and still neglected. PLoS Negl Trop Dis. 2011;5:e1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1416] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 60. | El Malki HO, El Mejdoubi Y, Souadka A, Mohsine R, Ifrine L, Abouqal R, Belkouchi A. Predictive factors of deep abdominal complications after operation for hydatid cyst of the liver: 15 years of experience with 672 patients. J Am Coll Surg. 2008;206:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Gollackner B, Längle F, Auer H, Maier A, Mittlböck M, Agstner I, Karner J, Langer F, Aspöck H, Loidolt H. Radical surgical therapy of abdominal cystic hydatid disease: factors of recurrence. World J Surg. 2000;24:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Peng X, Zhang S, Niu JH. Total subadventitial cystectomy for the treatment of 30 patients with hepatic hydatid cysts. Chin J Gen Surg. 2002;17:529-530. |
| 63. | Kapan M, Kapan S, Goksoy E, Perek S, Kol E. Postoperative recurrence in hepatic hydatid disease. J Gastrointest Surg. 2006;10:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Daradkeh S, El-Muhtaseb H, Farah G, Sroujieh AS, Abu-Khalaf M. Predictors of morbidity and mortality in the surgical management of hydatid cyst of the liver. Langenbecks Arch Surg. 2007;392:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Aydin U, Yazici P, Onen Z, Ozsoy M, Zeytunlu M, Kiliç M, Coker A. The optimal treatment of hydatid cyst of the liver: radical surgery with a significant reduced risk of recurrence. Turk J Gastroenterol. 2008;19:33-39. [PubMed] |
| 66. | Buttenschoen K, Carli Buttenschoen D. Echinococcus granulosus infection: the challenge of surgical treatment. Langenbecks Arch Surg. 2003;388:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Bedioui H, Bouslama K, Maghrebi H, Farah J, Ayari H, Hsairi H, Kacem M, Jouini M, Bensafta Z. Predictive factors of morbidity after surgical treatment of hepatic hydatid cyst. Pan Afr Med J. 2012;13:29. [PubMed] |
| 68. | Prousalidis J, Kosmidis C, Anthimidis G, Kapoutzis K, Karamanlis E, Fachantidis E. Postoperative recurrence of cystic hydatidosis. Can J Surg. 2012;55:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Lissandrin R, Agliata S, Brunetti E. Secondary peritoneal echinococcosis causing massive bilateral hydronephrosis and renal failure. Int J Infect Dis. 2013;17:e141-e142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Dervisoglu A, Erzurumlu K, Taç K, Arslan A, Gürsel M, Hökelek M. Should intraoperative ultrasonography be used routinely in hepatic hydatidosis? Hepatogastroenterology. 2002;49:1326-1328. [PubMed] |
| 71. | Bedirli A, Sakrak O, Sozuer EM, Kerek M, Ince O. Surgical management of spontaneous intrabiliary rupture of hydatid liver cysts. Surg Today. 2002;32:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Manouras A, Genetzakis M, Antonakis PT, Lagoudianakis E, Pattas M, Papadima A, Giannopoulos P, Menenakos E. Endoscopic management of a relapsing hepatic hydatid cyst with intrabiliary rupture: a case report and review of the literature. Can J Gastroenterol. 2007;21:249-253. [PubMed] |
| 73. | Kilic M, Yoldas O, Koc M, Keskek M, Karakose N, Ertan T, Gocmen E, Tez M. Can biliary-cyst communication be predicted before surgery for hepatic hydatid disease: does size matter? Am J Surg. 2008;196:732-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Unalp HR, Baydar B, Kamer E, Yilmaz Y, Issever H, Tarcan E. Asymptomatic occult cysto-biliary communication without bile into cavity of the liver hydatid cyst: a pitfall in conservative surgery. Int J Surg. 2009;7:387-391. [PubMed] |
| 75. | Erzurumlu K, Dervisoglu A, Polat C, Senyurek G, Yetim I, Hokelek M. Intrabiliary rupture: an algorithm in the treatment of controversial complication of hepatic hydatidosis. World J Gastroenterol. 2005;11:2472-2476. [PubMed] |
| 76. | Singh V, Reddy DC, Verma GR, Singh G. Endoscopic management of intrabiliary-ruptured hepatic hydatid cyst. Liver Int. 2006;26:621-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Galati G, Sterpetti AV, Caputo M, Adduci M, Lucandri G, Brozzetti S, Bolognese A, Cavallaro A. Endoscopic retrograde cholangiography for intrabiliary rupture of hydatid cyst. Am J Surg. 2006;191:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Chowbey PK, Shah S, Khullar R, Sharma A, Soni V, Baijal M, Vashistha A, Dhir A. Minimal access surgery for hydatid cyst disease: laparoscopic, thoracoscopic, and retroperitoneoscopic approach. J Laparoendosc Adv Surg Tech A. 2003;13:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Agarwal S, Sikora SS, Kumar A, Saxena R, Kapoor VK. Bile leaks following surgery for hepatic hydatid disease. Indian J Gastroenterol. 2005;24:55-58. [PubMed] |
| 80. | Ozturk A, Ozturk E, Zeyrek F, Sirmatel O. Late ultrasonographic findings in cases operated for hydatid cyst of the liver. Eur J Radiol. 2005;56:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Dziri C, Haouet K, Fingerhut A, Zaouche A. Management of cystic echinococcosis complications and dissemination: where is the evidence? World J Surg. 2009;33:1266-1273. [PubMed] |
| 82. | Ben Amor N, Gargouri M, Gharbi HA, Golvan YJ, Ayachi K, Kchouck H. Trial therapy of inoperable abdominal hydatid cysts by puncture. Ann Parasitol Hum Comp. 1986;61:689-692. [PubMed] |
| 83. | Gargouri M, Ben Amor N, Ben Chehida F, Hammou A, Gharbi HA, Ben Cheikh M, Kchouk H, Ayachi K, Golvan JY. Percutaneous treatment of hydatid cysts (Echinococcus granulosus). Cardiovasc Intervent Radiol. 1990;13:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Filice C, Pirola F, Brunetti E, Dughetti S, Strosselli M, Foglieni CS. A new therapeutic approach for hydatid liver cysts. Aspiration and alcohol injection under sonographic guidance. Gastroenterology. 1990;98:1366-1368. [PubMed] |
| 85. | Mueller PR, Dawson SL, Ferrucci JT, Nardi GL. Hepatic echinococcal cyst: successful percutaneous drainage. Radiology. 1985;155:627-628. [PubMed] |
| 86. | Schipper HG, Laméris JS, van Delden OM, Rauws EA, Kager PA. Percutaneous evacuation (PEVAC) of multivesicular echinococcal cysts with or without cystobiliary fistulas which contain non-drainable material: first results of a modified PAIR method. Gut. 2002;50:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Akhan O, Gumus B, Akinci D, Karcaaltincaba M, Ozmen M. Diagnosis and percutaneous treatment of soft-tissue hydatid cysts. Cardiovasc Intervent Radiol. 2007;30:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Vuitton DA, Wang XZ, Feng SL, Chen JS, Shou LY, Li SF, Ke TQ. PAIR-derived US-guided techniques for the treatment of cystic echinococcosis: a Chinese experience (e-letter). Gut. 2002;. |
| 89. | Giorgio A, de Stefano G, Esposito V, Liorre G, Di Sarno A, Giorgio V, Sangiovanni V, Iannece MD, Mariniello N. Long-term results of percutaneous treatment of hydatid liver cysts: a single center 17 years experience. Infection. 2008;36:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Golemanov B, Grigorov N, Mitova R, Genov J, Vuchev D, Tamarozzi F, Brunetti E. Efficacy and safety of PAIR for cystic echinococcosis: experience on a large series of patients from Bulgaria. Am J Trop Med Hyg. 2011;84:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Khuroo MS, Wani NA, Javid G, Khan BA, Yattoo GN, Shah AH, Jeelani SG. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 196] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Smego RA, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis. 2003;37:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Yagci G, Ustunsoz B, Kaymakcioglu N, Bozlar U, Gorgulu S, Simsek A, Akdeniz A, Cetiner S, Tufan T. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg. 2005;29:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 94. | Smego RA, Sebanego P. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005;9:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 95. | Filice C, Brunetti E. Use of PAIR in human cystic echinococcosis. Acta Trop. 1997;64:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Ustünsöz B, Akhan O, Kamiloğlu MA, Somuncu I, Uğurel MS, Cetiner S. Percutaneous treatment of hydatid cysts of the liver: long-term results. AJR Am J Roentgenol. 1999;172:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Men S, Yücesoy C, Edgüer TR, Hekimoğlu B. Percutaneous treatment of giant abdominal hydatid cysts: long-term results. Surg Endosc. 2006;20:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Taranto D, Beneduce F, Vitale LM, Loguercio C, Del Vecchio Blanco C. Chemical sclerosing cholangitis after injection of scolicidal solution. Ital J Gastroenterol. 1995;27:78-79. [PubMed] |
| 99. | Belghiti J, Benhamou JP, Houry S, Grenier P, Huguier M, Fékété F. Caustic sclerosing cholangitis. A complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Castellano G, Moreno-Sanchez D, Gutierrez J, Moreno-Gonzalez E, Colina F, Solis-Herruzo JA. Caustic sclerosing cholangitis. Report of four cases and a cumulative review of the literature. Hepatogastroenterology. 1994;41:458-470. [PubMed] |
| 101. | Sahin M, Eryilmaz R, Bulbuloglu E. The effect of scolicidal agents on liver and biliary tree (experimental study). J Invest Surg. 2004;17:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Houry S, Languille O, Huguier M, Benhamou JP, Belghiti J, Msika S. Sclerosing cholangitis induced by formaldehyde solution injected into the biliary tree of rats. Arch Surg. 1990;125:1059-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J Gastroenterol. 2009;15:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Kismet K, Kilicoglu SS, Kilicoglu B, Erel S, Gencay O, Sorkun K, Erdemli E, Akhan O, Akkus MA, Sayek I. The effects of scolicidal agent propolis on liver and biliary tree. J Gastrointest Surg. 2008;12:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 105. | Rouhani S, Salehi N, Kamalinejad M, Zayeri F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J Invest Surg. 2013;26:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Topcu O, Sumer Z, Tuncer E, Aydin C, Koyuncu A. Efficacy of chlorhexidine gluconate during surgery for hydatid cyst. World J Surg. 2009;33:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Paksoy Y, Odev K, Sahin M, Arslan A, Koç O. Percutaneous treatment of liver hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. AJR Am J Roentgenol. 2005;185:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Brunetti E, Filice C, Meroni V. Comment on percutaneous treatment of liver hydatid cysts. AJR Am J Roentgenol. 2006;186:1198-1199; author reply 1199-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 109. | Paksoy Y, Odev K, Sahin M, Dik B, Ergül R, Arslan A. Percutaneous sonographically guided treatment of hydatid cysts in sheep: direct injection of mebendazole and albendazole. J Ultrasound Med. 2003;22:797-803. [PubMed] |
| 110. | Deger E, Hokelek M, Deger BA, Tutar E, Asil M, Pakdemirli E. A new therapeutic approach for the treatment of cystic echinococcosis: percutaneous albendazole sulphoxide injection without reaspiration. Am J Gastroenterol. 2000;95:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Horton J. Albendazole for the treatment of echinococcosis. Fundam Clin Pharmacol. 2003;17:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 112. | Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Franchi C, Di Vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999;29:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Todorov T, Vutova K, Mechkov G, Georgiev P, Petkov D, Tonchev Z, Nedelkov G. Chemotherapy of human cystic echinococcosis: comparative efficacy of mebendazole and albendazole. Ann Trop Med Parasitol. 1992;86:59-66. [PubMed] |
| 115. | Nahmias J, Goldsmith R, Soibelman M, el-On J. Three- to 7-year follow-up after albendazole treatment of 68 patients with cystic echinococcosis (hydatid disease). Ann Trop Med Parasitol. 1994;88:295-304. [PubMed] |
| 116. | Horton RJ. Chemotherapy of Echinococcus infection in man with albendazole. Trans R Soc Trop Med Hyg. 1989;83:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Todorov T, Mechkov G, Vutova K, Georgiev P, Lazarova I, Tonchev Z, Nedelkov G. Factors influencing the response to chemotherapy in human cystic echinococcosis. Bull World Health Organ. 1992;70:347-358. [PubMed] |
| 118. | Wen H, Zou PF, Yang WG, Lu J, Wang YH, Zhang JH, New RR, Craig PS. Albendazole chemotherapy for human cystic and alveolar echinococcosis in north-western China. Trans R Soc Trop Med Hyg. 1994;88:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Salinas JL, Vildozola Gonzales H, Astuvilca J, Arce-Villavicencio Y, Carbajal-Gonzalez D, Talledo L, Willig JH. Long-term albendazole effectiveness for hepatic cystic echinococcosis. Am J Trop Med Hyg. 2011;85:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Li T, Ito A, Pengcuo R, Sako Y, Chen X, Qiu D, Xiao N, Craig PS. Post-treatment follow-up study of abdominal cystic echinococcosis in tibetan communities of northwest Sichuan Province, China. PLoS Negl Trop Dis. 2011;5:e1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 121. | el-Mufti M, Kamag A, Ibrahim H, Taktuk S, Swaisi I, Zaidan A, Sameen A, Shimbish F, Bouzghaiba W, Haasi S. Albendazole therapy of hydatid disease: 2-year follow-up of 40 cases. Ann Trop Med Parasitol. 1993;87:241-246. [PubMed] |
| 122. | Liu Y, Wang X, Wu J. Continuous long-term albendazole therapy in intraabdominal cystic echinococcosis. Chin Med J (Engl). 2000;113:827-832. [PubMed] |
| 123. | Gil-Grande LA, Rodriguez-Caabeiro F, Prieto JG, Sánchez-Ruano JJ, Brasa C, Aguilar L, García-Hoz F, Casado N, Bárcena R, Alvarez AI. Randomised controlled trial of efficacy of albendazole in intra-abdominal hydatid disease. Lancet. 1993;342:1269-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 124. | Bradley M, Horton J. Assessing the risk of benzimidazole therapy during pregnancy. Trans R Soc Trop Med Hyg. 2001;95:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Cobo F, Yarnoz C, Sesma B, Fraile P, Aizcorbe M, Trujillo R, Diaz-de-Liaño A, Ciga MA. Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatisosis caused by Echinococcus granulosus. Trop Med Int Health. 1998;3:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 126. | Mohamed AE, Yasawy MI, Al Karawi MA. Combined albendazole and praziquantel versus albendazole alone in the treatment of hydatid disease. Hepatogastroenterology. 1998;45:1690-1694. [PubMed] |
| 127. | Bygott JM, Chiodini PL. Praziquantel: neglected drug? Ineffective treatment? Or therapeutic choice in cystic hydatid disease? Acta Trop. 2009;111:95-101. [PubMed] |
| 128. | Larrieu E, Del Carpio M, Salvitti JC, Mercapide C, Sustersic J, Panomarenko H, Costa M, Bigatti R, Labanchi J, Herrero E. Ultrasonographic diagnosis and medical treatment of human cystic echinococcosis in asymptomatic school age carriers: 5 years of follow-up. Acta Trop. 2004;91:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Wang Y, He T, Wen X, Li T, Waili A, Zhang W, Xu X, Vuitton DA, Rogan MT, Wen H. Post-survey follow-up for human cystic echinococcosis in northwest China. Acta Trop. 2006;98:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H, Rajabzadeh F. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 131. | Kish MA. Guide to development of practice guidelines. Clin Infect Dis. 2001;32:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
P- Reviewers: Castiella A, Chetty R, Diamantis I, Tsuchiya A S- Editor: Gou SX L- Editor: Cant MR E- Editor: Wang CH