Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.230
Revised: January 9, 2014
Accepted: April 3, 2014
Published online: April 27, 2014
Processing time: 206 Days and 20.6 Hours
AIM: To elucidate the effects of melatonin on cisplatin-induced hepatocellular carcinoma (HepG2) cell death and to identify potential cross-talk pathways.
METHODS: Hepatocellular carcinoma HepG2 cells were treated with melatonin and/or cisplatin for 24 to 48 h. Cell viability and the 50% cytotoxic concentration (CC50) were calculated by MTT assays. The effects and intracellular events induced by the selected concentrations of melatonin (1 mmol/L) and cisplatin (20 μmol/L) were investigated. Cell death and survival detection were primarily evaluated using a fluorescence microscope to assess 4',6 diamideno-2-phenylindol DNA staining and acridine orange lysosome staining and then further analyzed with immunocytochemistry using an anti-LC3 antibody. The potential molecular responses mediated by melatonin against cisplatin after the combined treatment were investigated by reverse transcription-polymerase chains reaction and Western blot analyses of the genes and proteins associated with cell survival and death. A cell cycle analysis was performed using a flow cytometry assay.
RESULTS: Melatonin had a concentration-dependent effect on HepG2 cell viability. At 1 mmol/L, melatonin significantly increased the cell viability percentage and decreased reactive oxygen species production due to cisplatin. Melatonin reduced cisplatin-induced cell death, decreasing phosphorylated p53 apoptotic protein, cleaved caspase 3 and Bax levels but increasing anti-apoptotic Bcl-2 gene and protein expression. When combined with cisplatin, melatonin induced S phase (DNA synthesis) cell cycle arrest and promoted autophagic events in HepG2 cells. Melatonin also had a concentration-dependent effect on Beclin-1 and its autophagic regulator mammalian target of rapamycin (mTOR) as well as the DNA excision repair cross complementary 1 (ERCC1) protein. The expression levels of these proteins were altered in HepG2 cells during cisplatin or melatonin treatment alone. In the combination treatment, melatonin reversed the effects of cisplatin by suppressing the over-expression of mTOR and ERCC 1 and enhancing the expression levels of Beclin-1 and microtubule-associated protein-light chain3-II, leading to intracellular autophagosome progression.
CONCLUSION: Melatonin attenuated cisplatin-induced cell death in HepG2 cells via a counter-balance between the roles of apoptotic- and autophagy-related proteins.
Core tip: Melatonin has anti-oxidative stress and anti-proliferative effects on cisplatin-treated hepatocellular carcinoma cells through a counter-balance between the roles of apoptosis and autophagy proteins. Melatonin also reduced cisplatin-induced DNA damage by decreasing the activation of excision repair cross complementary 1 in the DNA repair system. Thus, co-treatment with melatonin to ameliorate cisplatin adverse effects might be beneficial for Hepatocellular carcinoma therapy.
-
Citation: Bennukul K, Numkliang S, Leardkamolkarn V. Melatonin attenuates cisplatin-induced HepG2 cell death
via the regulation of mTOR and ERCC1 expressions. World J Hepatol 2014; 6(4): 230-242 - URL: https://www.wjgnet.com/1948-5182/full/v6/i4/230.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i4.230
Hepatocellular carcinoma (HCC) is one of the leading causes of human death worldwide. Aberrant gene expression and mutations induced by any genotoxic agents that can cause DNA damage contribute to its development. Effective treatment for HCC is not yet available because all anticancer strategies have limitations associated with the tumor grade and stage of the disease. For example, chemotherapy is limited by the side effects of cytotoxic drugs that also kill normal cells and by the long-term side effects of these drugs, which are carcinogenic and can cause secondary cancers[1]. Moreover, the mechanisms controlling the responses of pro-oncogenes and tumor suppressor genes in the effected cells are not well defined. In cancer research, the human hepatocellular carcinoma (HepG2) cell line has been used as a model system for studies of hepatocarcinogenesis because it is a permanent cell line derived from a well-differentiated hepatocellular carcinoma patient. The HepG2 cell line has also been used as an in vitro model for studying liver metabolism and in drug targeting assays[2].
To date, several anticancer drugs have been used clinically. Cisplatin, a platinum-based drug that has a known molecular mechanism of action, is a drug of choice that has been widely chosen for the treatment of solid cancers[3,4]. Cisplatin platinum can form complexes with DNA, inducing DNA adducts and damage[5]. Once produced, the complex mediates a series of intracellular responses that lead to apoptosis and also activates the DNA repair system, which is capable of inducing recovery of the damaged DNA and participates in the restoration of normal cell systems. However, if the damage is too extensive, the repair mechanism fails, and the cell undergoing apoptosis dies[6]. Through this pathway, cisplatin has no selective effects on cancers or normal cells. When receiving chemotherapy with cisplatin, some individuals respond by showing excessive side effects, and some cancers develop resistance to cisplatin by mechanisms that are only partially known. To improve chemotherapeutic efficiency, investigators have emphasized the need to search for new drugs. An example would be to study the potency of cisplatin at low concentrations, which can protect against hepatotoxicity, and to study drug resistance mechanisms to identify a new drug to replace cisplatin.
Melatonin is a hormone that is produced in the pineal gland and has several normal physiological functions in the human body. Its known properties include circadian rhythm regulation[7], sleep induction[8], immunemodulation[9], neuroprotection[10], bone differentiation[11], and anti-microbial[12] and anti-oxidative effects[13]. Previous investigations have demonstrated that melatonin also possesses anti-proliferative effects, especially in cell lines derived from various malignancies such as lymphoma[14], prostate cancer[15], melanoma[16] and hepatocellular carcinoma[17]. Although most of the biological effects of melatonin are produced through the activation of melatonin receptors, some are due to its status as a powerful antioxidant that plays roles in the protection of nuclear and mitochondrial DNA[18]. The anti-proliferative effect of the human osteosarcoma cell line MG-63 was shown to be activated by melatonin if the concentration of melatonin reached an optimal value, which was a high concentration of 4-10 mol/L[19]. Further data have suggested that melatonin could be used as an adjuvant to increase responses to anticancer drugs and to ameliorate their side effects[20-22]. However, the effectiveness of melatonin as an adjuvant of chemotherapy most likely differs among cancer types, and the mechanisms of the action of melatonin are still unclear. Due to the selective effects of melatonin, the current oncology research aiming to enhance the apoptosis effect of cisplatin combined with melatonin has extended to alternative pathways in treated cells.
It is well accepted that cells under oxidative stress or exposed to DNA damage, hypoxia, nutrient deprivation, and intracellular pathogens can survive through an autophagy pathway[23]. This pathway involves the lysosomal degradation of cytoplasmic organelles or cytosolic components, allowing cells to eliminate damaged or harmful components and also to recycle the released amino acids and energy to maintain cellular homeostasis[24]. In mammalian cells, the pathway is regulated through a serine/threonine protein kinase called mammalian target of rapamycin (mTOR)[25]. Normally, mTOR is activated under nutrient-rich conditions and inhibits autophagy[26]. However, under stress conditions, mTOR plays a role in autophagy activation via an effector Beclin-1 that initiates core nucleation for autophagy formation[27]. Under the normal condition, Beclin-1 is inhibited by an interaction with the Bcl-2/Bcl-xL complex, but when p53 binds to Bcl-2, it frees Beclin-1, leading to autophagy[28]. In addition to losing collaborative proto-oncogenes and tumor-suppressor genes during DNA damage, the cells expressed several efficient DNA repair systems, such as the nucleotide excision repair (NER) pathway, to prevent cancer formation[29]. NER can eradicate a broad spectrum of DNA damage lesions through the action of a specific endonuclease enzyme named excision repair cross complementary 1 (ERCC1), which functions at the incision step of the NER pathway. ERCC1 cleaves damaged DNA at upstream sites, leading to DNA re-synthesis and ligation to return the damaged DNA to its native state and configuration[30]. Therefore, increased or decreased levels of ERCC1 expression should indicate efficiency of the DNA repair system.
In this study, we hypothesized that autophagy is an important pathway that plays roles in the outcome of cell death or survival in HepG2 cell during cisplatin and melatonin chemotherapy. We explored the expression of genes and proteins that regulate autophagy processes and lead to cell death and also identified the possible mechanism or cross-talk pathways mediated by melatonin and cisplatin.
A HepG2 cell line was purchased from the American Type Culture Collection (Rockville, MD, United States). The cells were cultured at 37 °C in a humidified 5% CO2 incubator and maintained in DMEM supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) non-essential amino acids, 1% (v/v) sodium pyruvate, and 100 Units/mL penicillin-streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, United States).
HepG2 cells were seeded onto 96-well plates (2 × 104 cells/well) for 24 h and then treated with 0.5-5.0 mmol/L melatonin (Merck, Frankfurter , Germany), 2.5-80.0 μmol/L cisplatin (Sigma Aldrich, St. Louis, MO, United States), or the combination of both for 24 and 48 h. In the combination treatment, the selected concentration was based on the minimal concentration that induced the anti-proliferative effect of melatonin and the most tolerable concentration that induced the cytotoxic effect of cisplatin. Cell viability was measured using an MTT colorimetric assay; MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Sigma Aldrich (St. Louis, MO, United States), a working solution was added to each well and incubated at 37 °C for 2 h. The optical density of each well was measured using a microplate reader at 570 nm and the reference wavelength of 690 nm. Cell viability was calculated as the percentage of viable cells in the drug-treated group versus the untreated control group. The concentration of the compound that decreased cell viability by 50% cytotoxic concentrations (CC50) was calculated. Each experiment was performed in triplicate, and each result was presented as the mean ± SE.
HepG2 cells were seeded onto 96-well black, flat, clear-bottom plates (2 × 104 cells/well). After treatment, the intracellular levels of ROS were measured by staining the treated and untreated cells with 50 μmol/L 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) that was purchased from Sigma Aldrich (St. Louis, MO, United States) after incubation in the dark at 37 °C for 30 min. The fluorescence intensity was quantified by the relative percentage of untreated cells using a fluorescence microplate reader and used 500 μmol/L H2O2-treated cells as the positive control. Each experiment was performed in triplicate, and each result was presented as the mean ± SE.
HepG2 cells were seeded onto 24-well plates (2 × 104 cells/well) for 24 h and then treated with 1 mmol/L melatonin, 20 μmol/L cisplatin, or both for 24 and 48 h.
DNA stain: Treated cells were fixed with cold methanol, air dried, and stained with 4’-6-diamidino-2-phenylindole, (DAPI) (Boehringer, Ingelheim, Germany) at 37 °C for 30 min. The primary signs of apoptosis induction, namely, chromatin condensation, fragmented DNA, and/or apoptotic body formation, were evaluated under an inverted fluorescent microscope.
Acridine orange stain: Treated cells were stained with 5 μg/mL acridine orange (Sigma Aldrich, St. Louis, MO, United States) in serum-free medium at 37 °C for 15 min. The cells were examined under an inverted fluorescence microscope. Positive acidic vacuoles or stained lysosomes were observed as orange or red foci in the cytoplasm, and DNA bound-acridine orange was detected as a green signal.
To determine an autophagic event, treated cells grown on coverslips were fixed in 4% paraformaldehyde for 5 min, permeabilized with methanol, and blocked with 3% bovine serum albumin in phosphate buffer saline (PBS) for 1 h at room temperature. The cells were then incubated with an indicator of autophagy, an anti-LC3 antibody was purchased from Cell Signaling Technology (Danvers, MA, United States) and was diluted 1:400 in PBS, overnight at 4 °C. After incubation, the cells were washed with PBS and incubated with an Alexa Fluor 555-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA, United States) for 2 h at room temperature, washed with PBS, and counter-stained with DAPI. The immunofluorescently labeled cells were visualized under a confocal laser-scanning microscope (Olympus, Tokyo, Japan).
HepG2 cells were seeded onto 6-well plates (4 × 105 cells/well). After treatment with 1 mM melatonin, 20 μmol/L cisplatin, or both for 24 h, total RNA was extracted with TRI Reagent (Molecular Research Center Inc; Cincinnati, OH, United States). cDNAs were synthesized by RevertAid™ M-MuLV Reverse Transcriptase and amplified by polymerase chain reaction (PCR) with specific primer pairs, as indicated in Table 1, using a High Fidelity PCR kit (Thermo Fisher Scientific, Waltham, MA, United States). The PCR cycles were adjusted accordingly to primer annealing. The PCR products were analyzed by 1.5% agarose gel electrophoresis and gel staining with ethidium bromide for 30 min. The gels were photographed using gel documentation (UVP Bioimaging System, United States). The experiments were performed in triplicate, and the relative band densities of cDNA from the treated cells were compared to the untreated cells.
| Genes | Function | Primers sequence (5'-3') | Size (bp) |
| Bax | Pro-apoptosis | F- AAAGCTAGCGAGTGTCTCAAGCGC | 366 |
| R-TCCCGCCACAAAGATGGTCACG | |||
| Bcl-2 | Anti-apoptosis | F-TTGTGGCCTTCTTTGAGTTCG | 332 |
| R-TACTGCTTTAGTGAACCTTTT | |||
| mTOR | Autophagic inhibition | F-TCTCATGGGCTTCGGAACAA R-GTGAAGGCAGAAGGTCGGAA | 318 |
| ERCC1 | DNA repair | F-CCCTGGGAATTTGGCGACGTAA R-CTCCAGGTACCGCCCAGCTTCC | 273 |
| GAPDH | House keeping | F-CATCACCATCTTCCAGGAGC | 307 |
| R-CATGAGTCCTTCCACGATACC |
The treated cells were lysed in RIPA buffer (25 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail were purchased from Merck (Frankfurter, Germany) on ice for 30 min. The samples were then centrifuged at 24000 g at 4 °C for 15 min. The cell lysates were collected, and the total protein concentrations were determined by a BCA assay (Merck, Frankfurter, Germany). Equal amounts of protein (30 μg) from each sample were separated by 8%-15% SDS-polyacrylamide gel electrophoresis at 100 V and transferred onto nitrocellulose membranes (GE Healthcare, Buckinghamshire, United Kingdom). The membranes were blocked with 3% w/v bovine serum albumin (Sigma Aldrich, St. Louis, MO, United States) in Tris-buffered saline containing 0.1% Tween-20 for 2 h at room temperature. Then, the membranes were incubated with a 1:1000 dilution of primary antibodies against Bax (Santa Cruz, CA, United States), p53, phospho-p53, Bcl-2, pro-caspase3, cleaved-caspase3, p-mTOR, ERCC1, Beclin-1 or LC3 (Cell Signaling Technology, Danvers, MA, United States) overnight at 4 °C, followed by two extensive washings with TBST. The membranes were incubated with a 1:2000 dilution of horseradish peroxidase-conjugated secondary antibody for 2 h. The specific bands corresponding to the investigated proteins were visualized using enhanced chemiluminescence (ECL reagents, GE Healthcare, Buckinghamshire, United Kingdom). The signal intensities were determined by densitometry using Image-J software (National Institutes of Health, Bethesda, MD, United States). After stripping off the first probe, each membrane was re-probed with a β-actin antibody (Santa Cruz, CA, United States) to confirm the equal loading of protein in each experiment. The levels of protein expression were presented in relation to β-actin.
HepG2 cells were seeded onto 6-well plates (4 × 105 cells/well). After treatment with 1 mmol/L melatonin, 20 μmol/L cisplatin, or a combination of both for 24 h, the cells were trypsinized, centrifuged, washed twice with cold PBS, and fixed overnight with 70% ethanol at -20 °C. The cells were resuspended in staining buffer containing 50 μg/mL propidium iodide (Merck, Frankfurter, Germany), 3.8 mmol/L sodium citrate, and 50 μg/mL RNase A (Sigma Aldrich, St. Louis, MO, United States) and incubated in the dark at 37 °C for 30 min. The stained cell suspensions were processed for flow cytometry analysis to determine the amount of DNA at different phases of the cell cycle using a FACScanto apparatus (BD Pharmingen, San Diego, CA, United States) with the loaded software. A total of 30000 cells from each sample were collected for evaluation of each data file.
All experiments were performed in triplicate (n = 3). Data are presented as the mean ± SE for each group, and these were compared for significant differences using a one-way analysis of variance test, followed by a post-hoc analysis (Tukey’s multiple comparison test) using Prism 5 (GraphPad Software Inc; San Diego, CA, United States).
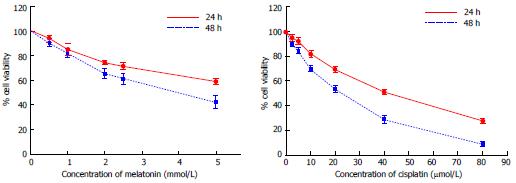
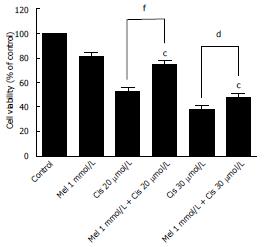
As shown in Figure 1, melatonin (concentration 0.5-5 mmol/L) and cisplatin (concentration 2.5-80 μmol/L) reduced the viability of HepG2 cells in a time-concentration dependent manner. The 50% CC50 of melatonin were 6.25 mmol/L and 3.74 mmol/L and of cisplatin were 38.53 μmol/L and 17.53 μmol/L, at 24 h and 48 h, respectively. The concentrations of melatonin and cisplatin used in the combination treatment were selected from the minimal anti-proliferative effect of melatonin, which significantly decreased percent cell viability, and the most tolerable cytotoxic effect of cisplatin, which induced cell death at a rate below 50%. Therefore, 1 mmol/L melatonin was used in combination with 20 and 30 μmol/L cisplatin for 48 h. Melatonin increased the viability of HepG2 cells compared with cisplatin treatment alone. The combined treatment significantly reduced cell viability compared with the control and significantly increased cell viability compared with the cisplatin treatment alone (Figure 2).
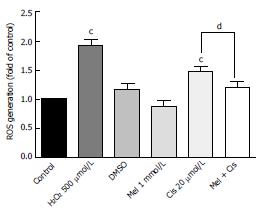
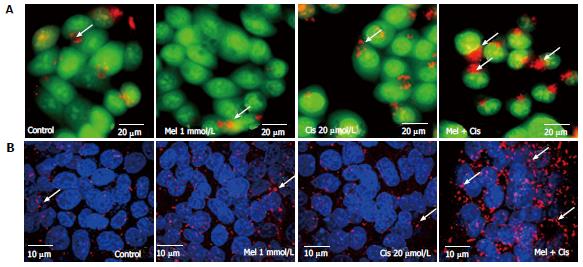
Anti-ROS production: To determine whether melatonin reduced the ROS production due to cisplatin, the biochemical basis of intracellular ROS was explored using a fluorescein-labeled dye, DCFH-DA. The results showed significantly increased intracellular ROS in cisplatin-treated cells. However, the combined treatment with melatonin (1 mmol/L) reduced the intracellular ROS level (Figure 3).
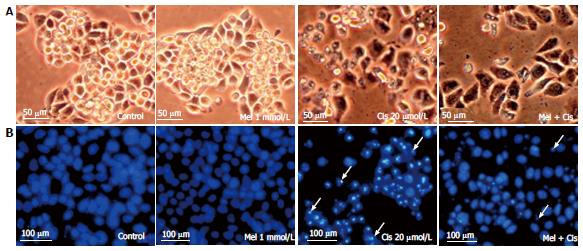
Reduction of cellular damage and apoptosis formation: The combined effect of melatonin and cisplatin was evaluated in unstained in HepG2 cells under an inverted light microscope (Figure 4A) and in HepG2 cells stained with DAPI under a fluorescence microscope (Figure 4B). Melatonin treatment resulted in no morphological changes and the absence of apoptotic nuclei. Cisplatin treatment induced intense morphological changes, with cell shrinkage and typical apoptotic nuclear features, such as nuclear condensation and apoptotic bodies. The combined melatonin and cisplatin treatment revealed unchanged cell morphology and a reduction of apoptotic bodies.
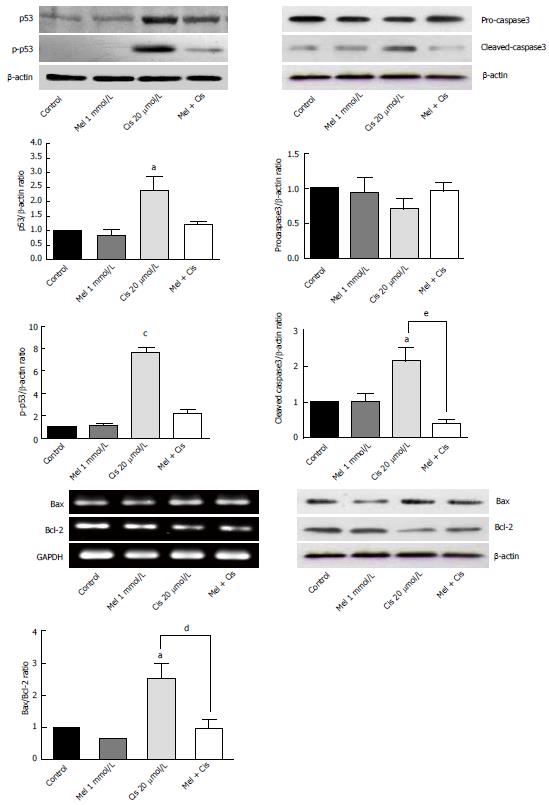
Regulation of apoptosis vs anti-apoptosis genes and protein expression: To determine apoptotic events in cells, the expression of the genes and proteins involved in apoptosis were analyzed. As shown in Figure 5, Western blotting results indicated that melatonin (1 mmol/L) had no significant effect on proteins associated with apoptosis (p53, p-p53, pro-caspase3 and cleaved-caspase3), whereas cisplatin significantly increased p53 and p-p53 levels. Gene and protein expression analyses of pro-apoptosis Bax and anti-apoptosis Bcl-2 levels revealed slight decreases in the Bax/Bcl-2 ratio with melatonin treatment while resulting in a significantly increased Bax/Bcl-2 ratio with cisplatin treatment. In the combined treatment, melatonin significantly ameliorated the pro-apoptotic effects of cisplatin.
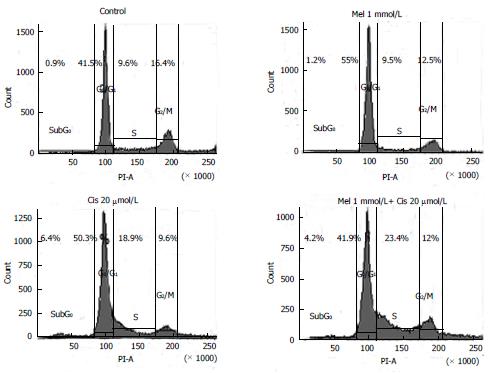
Cell cycle regulation: To determine the effects of the treatments on HepG2 cell cycle control, DNA accumulation was measured by flow cytometry at different phases of the cell cycle. The phases of cell cycle arrest were determined at G0/G1 by melatonin and at subG0, G0/G1, and S by cisplatin. In the combined treatment, melatonin reduced cisplatin cell cycle arrest at the subG0 through G0/G1 phases but significantly increased cell cycle arrest at the S phase (Figure 6).
Autophagy regulation: The combined effect of melatonin and cisplatin in HepG2-treated cells was evaluated for lysosomal staining with acridine orange dye and observation under an inverted microscope to measure fluorescence. Both melatonin and cisplatin caused slight increases in the acidic lysosomal compartments; an increase in acridine orange intensity was highly apparent in the combined treatment (Figure 7A). In the immunofluorescence assay with the anti-LC3 antibody, melatonin and cisplatin treatment alone induced minor autophagy events in HepG2 cells compared with the control. However, the combination of melatonin and cisplatin treatment significantly enhanced autophagy, as indicated by the fluorescence intensity of LC3 scattered throughout the cytoplasm (Figure 7B).
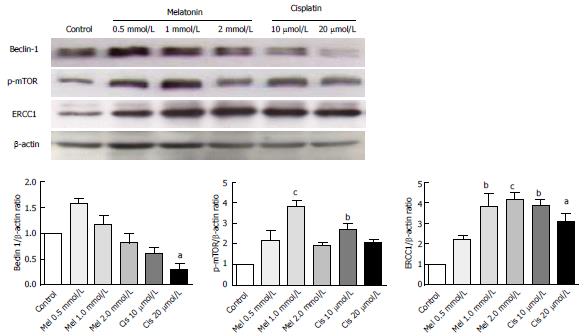
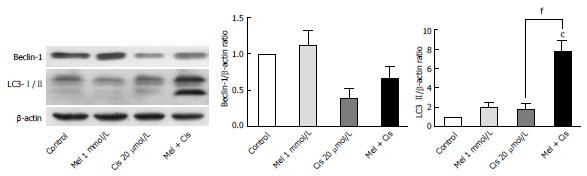
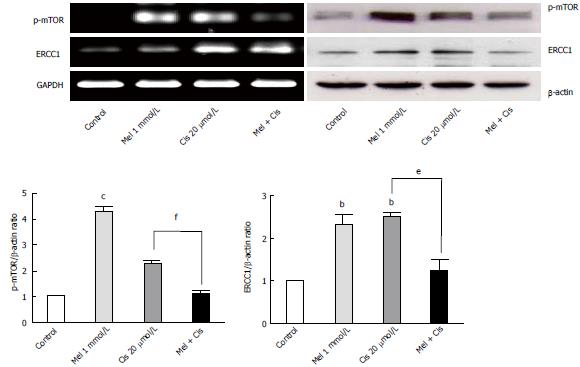
Alteration of autophagy regulators and the DNA repair system: To explore the potential pathways of melatonin and cisplatin effects on autophagy regulation and DNA repair process in HepG2-treated cells, the relationship between the proteins Beclin-1, mTOR and ERCC1 were analyzed. Melatonin (0.5 and 1 mmol/L) increased Beclin-1, p-mTOR, and ERCC1 in a concentration-dependent manner (Figure 8). Conversely, cisplatin (10 and 20 μmol/L) decreased Beclin-1, p-mTOR, and ERCC1 in a concentration-dependent manner. At the selected concentrations (1 mmol/L melatonin and 20 μmol/L cisplatin), melatonin significantly increased p-mTOR and ERCC1 from the basal levels, whereas cisplatin decreased Beclin-1 and increased p-mTOR and ERCC1 from the basal levels.
Evaluation of the autophagy process in melatonin- and cisplatin-treated HepG2 cells showed changes in Beclin-1 (an initiator of autophagy) and LC3-II (a mature marker of autophagy). Melatonin (1 mmol/L) treatment alone slightly increased both Beclin-1 and LC3-II, but cisplatin (20 μmol/L) treatment alone decreased Beclin-1 and slightly increased LC3-II (Figure 9). The combined treatment showed that melatonin prevented cisplatin from affecting the level of Beclin-1 but significantly increased the effect of cisplatin on the LC3-II level.
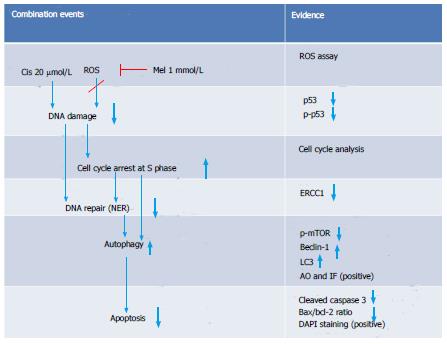
The analysis of mTOR and ERCC1 mRNA and protein expression in the treated HepG2 cells revealed that melatonin (1 mmol/L) or cisplatin (20 μmol/L) treatment alone significantly increased p-mTOR and ERCC1 from the basal levels. However, the combined treatment resulted in the suppression of both p-mTOR and ERCC1 compared with the basal cellular levels (Figure 10). (See a schematic overview of the results in Figure 11)
Cisplatin chemotherapy has been used to kill several solid cancer cells with satisfactory results. However, due to its efficient cytotoxic effects, cisplatin produces adverse drug effects and chemo-resistance. Therefore, adjuvant therapy with melatonin has been proposed, and extensive studies were performed, with variable outcomes[22]. In the present study, we set out to determine whether melatonin could mediate a protective effect against cisplatin-induced apoptosis using HepG2 cells and attempted to identify the potential molecular pathways of melatonin action. The results demonstrated that the overall effect of this adjuvant in chemotherapy depends on a number of melatonin effects on cells. When melatonin was combined with cisplatin in the HepG2 cell line, the expression of the apoptosis mediators phosphorylated p53, cleaved caspase 3, and Bax decreased, whereas anti-apoptotic Bcl-2 increased. These biochemical results correlated well with decreases in the intracellular ROS level and the recovery of cell morphology. The results indicated the anti-oxidative stress property of melatonin, which directly scavenges reactive oxygen species induced by cisplatin anticancer drugs[31,32], thus reducing adverse cisplatin drug effects in hepatocytes.
In addition to the extended results from the alteration of the anti-apoptotic Bcl-2 level, we found that melatonin decreased the expression ratio of Bax/Bcl-2, which was affected by cisplatin. Thus, an apoptosis event in the combined treatment was switched to cell survival. The expression of p53 protein was also subjected to a switch by the melatonin and cisplatin combination, suggesting a direct effect of both on the cell cycle. The pro-death effect of cisplatin, which was mostly mediated by p53, indicated that the cell cycle specificity of cisplatin in HepG2 cells affected G0/G1 phase. In the combined treatment, the cell cycle arrest was changed to the S and G2/M phases. Therefore, melatonin must have increased the cisplatin effect on cell cycle arrest through inhibition at the S (DNA synthesis) and M (mitosis) phases. These are considered the most anti-proliferative (oncostatic) effects of melatonin. However, the cell cycle arrest by melatonin in the treated cells could also be mediated by other cell cycle control proteins, such as the calcium/calmodulin pathway[33] and autophagy regulation pathway. Both pathways are related to the anti-proliferation of cells and pro-survival.
In our study, DNA damage may have been induced in HepG2 cells by the cisplatin genotoxic chemical and ROS by-products of cell metabolism[34]. The subsequent response reactions include the activation of a DNA damage checkpoint, which arrests cell cycle progression and leads to apoptosis[35]. We demonstrated that there was a survival switch from cisplatin-induced apoptosis to autophagy in the combination treatment with melatonin. The indicators included acridine orange staining, an immunohistochemistry assay for autophagy, and the measurement of mRNA and proteins associated with autophagy [i.e., mTOR, Beclin-1, and chain3-II (LC3-II)]. Compared with cisplatin treatment alone, the combination treatment showed a high intensity of positive fluorescence staining in the lysosomal compartment of the cell, suggesting increased autophagy activity. Cisplatin acted on p53 and Bcl-2 to induce apoptosis, but in the combined treatment, Bcl-2 was decreased to an extent lower than the basal level. This could occur through reduced binding between the Bcl-2/Bcl-xL complex and Beclin-1, followed by a release of Beclin-1 to initiate autophagy formation. Thus, the combined treatment could change apoptosis to autophagy.
The basal levels of Beclin-1 and mTOR, major regulators of autophagy, were evaluated in HepG2 cells. Cisplatin treatment alone up-regulated mTOR and down-regulated Beclin-1 expression compared with the basal levels, thus decreasing autophagy and promoting the apoptosis pathway. Melatonin treatment alone up-regulated both mTOR and Beclin-1 expression compared with the basal levels, and this up-regulation by melatonin played a significant role in the survival of HepG2 cells. In the combined treatment, melatonin caused autophagy through the final formation of the autophagosome and was shown by an increased microtubule-associated protein-light LC3-II level via the up-regulation of p-mTOR and Beclin-1. This changed the apoptotic signal caused by cisplatin. Overall, melatonin attenuated cisplatin-induced HepG2 cell death by inducing an autophagy survival signal[36].
A novel autophagy regulation mediated by melatonin was identified in this study. Both melatonin and cisplatin exhibited concentration-dependent effects on mTOR, Beclin-1, and DNA ERCC1. ERCC1 is a marker of DNA repair, whereby ERCC1 is the rate-limiting enzyme in the NER system, a pathway known to remove cisplatin lesions from DNA[37]. The analysis of protein expression in this study revealed that increases in ERCC1 from the basal levels in HepG2 cells by each treatment were associated with an increase in mTOR expression, suggesting a tight relationship between DNA repair and autophagy. We also found that both mTOR and ERCC1 were significantly decreased by melatonin induction in the co-treatment group compared with the cisplatin-treated group. However, the only slight reduction in ERCC1 in our assay using rapamycin as an inhibitor of mTOR demonstrated that the melatonin effect on ERCC1 was not directly to the mTOR activation pathway (data not included). Therefore, melatonin reduced cisplatin-induced DNA damage, resulting in decreased activation of the DNA repair capacity and might be involved in transcriptional regulation or an epigenetic mechanism. As previously shown, p-p53 is a transcription factor for apoptotic gene expression and also plays a role in DNA repair via the subsequent up-regulation of genes in the NER pathway[38-40]. AP-1 is a transcription factor for autophagy and ERCC1 regulation in DNA damage pathways[41]. In the combined treatment, melatonin and cisplatin may encounter transcription inactivation. That is, after cisplatin treatment, DNA damage occurs, and ERCC1 is induced. An increased level and activation of mTOR induced by melatonin counter-balances the role of p-p53 in the DNA damage and repair process induced by cisplatin. This finding is the first report that melatonin can activate ERCC1 in a concentration-dependent manner.
Interestingly, significant decreases in both mTOR and ERCC1 compared with the basal levels were identified in the combined treatment, suggesting a positive response of the cells. The expression level of ERCC1 has been previously suggested to be a prognostic marker of cancers, and ERCC1 over-expression has been associated with to cisplatin resistance[42]. In our research, ERCC1 decreased compared with the cisplatin-treated group, indicating an improvement in cancer prognosis.
In conclusion, melatonin exerted an oncostatic effect on cisplatin-treated hepatocellular carcinoma cells via a counter-balance between the roles of apoptosis-related p53 and Bcl-2 and autophagy proteins. The alteration of Beclin-1, mTOR, and ERCC1 levels determined the pro-death and pro-survival effects of treatment in this cancer cell line. Therefore, during cisplatin treatment of hepatocellular carcinoma, the administration of a high dose of melatonin as an adjuvant in cancer therapy should ameliorate the adverse effects of cisplatin.
We thank the Core Instrument Facilities, Faculty of Science, Mahidol University for providing access to the laboratory instruments and facilities. Special thanks are extended to Dr. Peter Hanna for his help in the English correction of the manuscript.
Liver cancer is one of the major causes of death in humans worldwide due to the limitation of drugs for treatment. Frequently, chemotherapy with platinum-based cisplatin is provided during the late stage of the disease. This drug causes excessive side effects and sometimes induces cancer drug resistance.
Melatonin is a hormone produced in our body to maintain several normal physiological functions, which may be altered during the course of cancer. To reduce cisplatin side effects and still maintain the drug’s efficacy, we treated human hepatocellular carcinoma (HepG2) cells with cisplatin and a high concentration of melatonin. The results showed that melatonin could reduce the oxidative stress effects of cisplatin by altering the stages of cell cycle arrest and apoptosis mediators such as p53. Melatonin switched cisplatin-induced apoptosis in HepG2 cell to autophagy-induced survival via Bcl-2, Beclin-1 and mTOR. In addition, melatonin reduced cisplatin-induced DNA damage by decreasing the activation of excision repair cross complementary-1 (ERCC1) in the DNA repair system.
Melatonin can activate DNA ERCC1 in a concentration-dependent manner. An analysis of protein expression in this study revealed that increases in ERCC1 from the basal levels in HepG2 cells by each treatment were associated with an increase in mTOR expression, suggesting a tight relationship between DNA repair and autophagy. In the combined treatment, melatonin and cisplatin may result in transcriptional inactivation between mTOR and ERCC1.
During cisplatin treatment of hepatocellular carcinoma, the administration of melatonin as an adjuvant in cancer therapy should ameliorate the adverse cisplatin effects while maintaining the drug’s efficacy.
ERCC1 is the rate-limiting endonuclease in the nucleotide excision repair (NER) pathway, which is the major pathway for removing cisplatin from the DNA strand. ERCC1 is a good predictive biomarker for cancer treatment with cisplatin. ERCC1-negative tumor patients were associated with a higher survival rate than ERCC1-positive tumor patients treated with adjuvant platinum-based regimens.
This study has a good structure and is easy to read despite many abbreviations, even for inexperienced readers in this field. It is a very important and up-to-date subject. The work is interesting, technically correct, and well written and describes an important role for melatonin as an adjuvant to cisplatin, a therapy known to cause severe toxicity in the body. The studies were well-designed, clearly presented, highly mechanistic, and demonstrated novel pathways regulated by melatonin to improve health outcomes associated with hepatic cancer and cisplatin-induced hepatotoxicity. This study will open up new research areas in this field. The authors used myriad techniques and approaches to tackle this deadly disease.
| 1. | Arslan C, Ozdemir E, Dogan E, Ozisik Y, Altundag K. Secondary hematological malignancies after treatment of non-metastatic breast cancer. J BUON. 2011;16:744-750. [PubMed] |
| 2. | Gerets HH, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28:69-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 540] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 3. | Varan A, Batu A, Cila A, Soylemezoğlu F, Balcı S, Akalan N, Zorlu F, Akyüz C, Kutluk T, Büyükpamukçu M. Optic glioma in children: a retrospective analysis of 101 cases. Am J Clin Oncol. 2013;36:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Noguchi K, Yokoo H, Nakanishi K, Kakisaka T, Tsuruga Y, Kamachi H, Matsushita M, Kamiyama T. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol. 2012;10:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000;57:1229-1235. [PubMed] |
| 6. | Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2990] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 7. | Cassone VM. Effects of melatonin on vertebrate circadian systems. Trends Neurosci. 1990;13:457-464. [PubMed] |
| 8. | Shochat T, Haimov I, Lavie P. Melatonin--the key to the gate of sleep. Ann Med. 1998;30:109-114. [PubMed] |
| 9. | Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189-200. [PubMed] |
| 10. | Cheung RT. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res. 2003;34:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Park KH, Kang JW, Lee EM, Kim JS, Rhee YH, Kim M, Jeong SJ, Park YG, Kim SH. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J Pineal Res. 2011;51:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ. Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J Pineal Res. 2008;44:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Sánchez-Hidalgo M, Lee M, de la Lastra CA, Guerrero JM, Packham G. Melatonin inhibits cell proliferation and induces caspase activation and apoptosis in human malignant lymphoid cell lines. J Pineal Res. 2012;53:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Shiu SY, Leung WY, Tam CW, Liu VW, Yao KM. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(Kip1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): potential implications on prostate cancer chemoprevention and therapy. J Pineal Res. 2013;54:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res. 2010;49:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Liu L, Xu Y, Reiter RJ. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone. 2013;55:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Reiter RJ. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin Investig Drugs. 2012;21:819-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ, Dong TF, Fu QL. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, Mills E. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther. 2012;11:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2886] [Cited by in RCA: 2825] [Article Influence: 176.6] [Reference Citation Analysis (0)] |
| 24. | Hubbard VM, Valdor R, Macian F, Cuervo AM. Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology. 2012;13:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1471] [Cited by in RCA: 1718] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 26. | Ramírez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol. 2008;181:293-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 28. | Lorin S, Pierron G, Ryan KM, Codogno P, Djavaheri-Mergny M. Evidence for the interplay between JNK and p53-DRAM signalling pathways in the regulation of autophagy. Autophagy. 2010;6:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Hoeijmakers JH. Genome maintenance mechanisms are critical for preventing cancer as well as other aging-associated diseases. Mech Ageing Dev. 2007;128:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 512] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1481] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 33. | Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Rodriguez-Rocha H, Garcia-Garcia A, Panayiotidis MI, Franco R. DNA damage and autophagy. Mutat Res. 2011;711:158-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 2443] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 36. | Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 37. | Bowden NA, Ashton KA, Avery-Kiejda KA, Zhang XD, Hersey P, Scott RJ. Nucleotide excision repair gene expression after Cisplatin treatment in melanoma. Cancer Res. 2010;70:7918-7926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Slee EA, O’Connor DJ, Lu X. To die or not to die: how does p53 decide? Oncogene. 2004;23:2809-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Seo YR, Jung HJ. The potential roles of p53 tumor suppressor in nucleotide excision repair (NER) and base excision repair (BER). Exp Mol Med. 2004;36:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Andrieux LO, Fautrel A, Bessard A, Guillouzo A, Baffet G, Langouët S. GATA-1 is essential in EGF-mediated induction of nucleotide excision repair activity and ERCC1 expression through ERK2 in human hepatoma cells. Cancer Res. 2007;67:2114-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Steffensen KD, Waldstrøm M, Jakobsen A. The relationship of platinum resistance and ERCC1 protein expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
P- Reviewers: Guglielmo R, Sanchez-Barcelo EJ, Witt-Enderby PA S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL