Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.188
Revised: January 22, 2014
Accepted: February 20, 2014
Published online: April 27, 2014
Processing time: 201 Days and 17.4 Hours
The prevalence of obesity and related conditions like non-alcoholic fatty liver disease (NAFLD) is increasing worldwide and therapeutic options are limited. Alternative treatment options are therefore intensively sought after. An interesting candidate is the natural polyphenol resveratrol (RSV) that activates adenosinmonophosphate-activated protein kinase (AMPK) and silent information regulation-2 homolog 1 (SIRT1). In addition, RSV has known anti-oxidant and anti-inflammatory effects. Here, we review the current evidence for RSV-mediated effects on NAFLD and address the different aspects of NAFLD and non-alcoholic steatohepatitis (NASH) pathogenesis with respect to free fatty acid (FFA) flux from adipose tissue, hepatic de novo lipogenesis, inadequate FFA β-oxidation and additional intra- and extrahepatic inflammatory and oxidant hits. We review the in vivo evidence from animal studies and clinical trials. The abundance of animal studies reports a decrease in hepatic triglyceride accumulation, liver weight and a general improvement in histological fatty liver changes, along with a reduction in circulating insulin, glucose and lipid levels. Some studies document AMPK or SIRT1 activation, and modulation of relevant markers of hepatic lipogenesis, inflammation and oxidation status. However, AMPK/SIRT1-independent actions are also likely. Clinical trials are scarce and have primarily been performed with a focus on overweight/obese participants without a focus on NAFLD/NASH and histological liver changes. Future clinical studies with appropriate design are needed to clarify the true impact of RSV treatment in NAFLD/NASH patients.
Core tip: The prevalence of obesity and related conditions like non-alcoholic fatty liver disease (NAFLD) is increasing. Therapeutic options are limited and alternative treatment options are sought after. An interesting candidate is resveratrol (RSV), a known AMP-activated protein kinase and silent information regulation-2 homolog 1 activator with anti-oxidant and anti-inflammatory properties. Here, we review the current evidence for RSV-mediated effects and address the different aspects of NAFLD and non-alcoholic steatohepatitis pathogenesis. We review the in vivo evidence from animal studies and clinical trials. Uniformly, animal studies report a decrease in hepatic triglyceride accumulation and improvements in histological fatty liver changes, whereas results from the few clinical trials are equivocal.
- Citation: Heebøll S, Thomsen KL, Pedersen SB, Vilstrup H, George J, Grønbæk H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J Hepatol 2014; 6(4): 188-198
- URL: https://www.wjgnet.com/1948-5182/full/v6/i4/188.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i4.188
The prevalence of obesity is increasing worldwide and consequently related conditions like non-alcoholic fatty liver disease (NAFLD) have increased. NAFLD now affects up to one-third of adults and a growing number of children in developed countries[1-3]. Early stages of NAFLD involve pathological accumulation of triglyceride (TG) in the liver, a fairly benign condition. However, some individuals elicit an inflammatory response that can progress to cirrhosis, cirrhosis complications and an increased risk of liver cancer[4]. NAFLD is now the third leading cause of liver transplantation in the United States[5]. Thus, NAFLD and especially the subtype non-alcoholic steatohepatitis (NASH) are thought to become a major health issue in the United States and throughout the world[6].
Therapeutic options are limited and include weight loss, which is hard to obtain and sustain[7], bariatric surgery, Vitamin E and glitazone treatment, especially the latter with a risk of significant side effects[8-11]. Alternative treatment options are therefore warranted and intensively sought after[12-15].
A potential new therapeutic option is the polyphenol resveratrol (RSV). RSV is found in a number of plants, although in low concentrations. It is known as an activator of AMP-activated protein kinase (AMPK) and silent information regulation 2 homolog 1 (SIRT1), thereby mimicking a condition of caloric restriction in vivo. In addition, RSV has anti-oxidant and anti-inflammatory properties. All of these effects could in theory be beneficial for the treatment of NAFLD and a number of experimental and clinical studies have been performed.
The aim of the present review is to provide a comprehensive description of the rationale for RSV treatment for NAFLD and to review the present evidence from RSV intervention in experimental and clinical NAFLD studies.
RSV is primarily recognized as an AMPK and SIRT1 activator[16-18]. AMPK and SIRT1 are both central in the metabolism of many different cell types, rendering RSV with pleiotropic effects in various tissues. To date, studies have been unable to determine if RSV activates AMPK, SIRT1 or both, directly or indirectly[19], a matter of ongoing debate[20]. Regardless, the effects of the enzymes are closely interdependent. Recently, Park et al[21] proposed a mechanism involving a direct RSV-mediated inhibition of cAMP-specific phosphodiesterases and identified the cAMP effector protein Epac1 as a key mediator, which may lead to activation of first AMPK[21] and then SIRT1 through the up-regulation of NAD+[20]. Furthermore, RSV may also act independently of AMPK/SIRT1; however, the mechanisms are not clarified.
Through the activation of AMPK, SIRT1 and alternative routes including anti-inflammatory and anti-oxidant actions, RSV may inhibit the development or progression of steatosis and steatohepatitis. Former attempts to use the AMPK activator metformin in the treatment of NAFLD have largely been abandoned because clinical studies showed no effect on histological NASH changes, despite a general decrease in hepatic steatosis and transaminase levels[22-24].
The AMPK pathway regulates energy homeostasis, both intracellularly and at the whole-body level. Through the action of upstream kinases, AMPK responds to changes in the AMP/ATP ratio and thus serves as an intracellular sensor of energy levels, e.g., in the situation of fasting, calorie restriction or accelerated ATP consumption[25].
AMPK activation in the liver shuts down anabolic processes like cholesterol and TG biosynthesis by reducing the activities of, e.g., sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS). AMPK activation also promotes catabolic processes, e.g., fatty acid (FA) β-oxidation by inactivation of acetyl-CoA carboxylase (ACC) and promotion of carnitine palmitoyltransferase-1 (CPT-1) activity[26-28]. In vivo, it has been shown that chronic AMPK activation limits TG accumulation in both high-fat and control diet fed rats[29]. These AMPK-mediated effects have been shown in in vitro and in vivo studies, using RSV as an AMPK activator[28]. As an example, RSV treatment of HepG2 cells in high glucose media dose-dependently attenuated enhanced FAS expression, increased ACC activity and elevated TG accumulation[30].
In adipose tissue, the AMPK effects are similar, impairing lipolysis and promoting mitochondrial β-oxidation, thereby decreasing the level of circulating FFAs and the FFA load on the liver[26]. Both hepatic and peripheral insulin sensitivity is augmented.
SIRT1 is a member of the sirtuin family and a NAD+-dependent deacetylase that acts as a master metabolic sensor of NAD+. Thus, it adapts gene expression and metabolic activity in response to the intracellular energy state. SIRT1 is mainly found in the nucleus, where it functions as a transcriptional repressor through histone, transcription factor, co-factor and enzyme deacetylation[31]. Following the SIRT1 metabolic effects, the molecule is thought to link calorie restriction and healthy aging and/or longevity. A number of studies have confirmed this in vivo and in vitro[32-34], while few have disputed it[35].
In the liver, SIRT1 is implicated in the control of energy metabolism through deacetylation and activation of especially peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and the lipid-sensing transcription factor peroxisome proliferator-activated receptor (PPARα), resulting in increased FA β-oxidation[36]. PGC-1α stimulates mitochondrial biogenesis, thereby increasing the mitochondrial content in hepatocytes[37]. In unison with AMPK, SIRT1 deacetylates and regulates SREBP-1c and liver X receptor (LXR), which govern lipid and cholesterol metabolism[38-41]. Adenovirus-mediated overexpression of SIRT1 specifically in mouse liver has been shown to reduce liver fat by down-regulation of SREBP-1c and FAS and up-regulation of expression of genes that control FA β-oxidation[42].
SIRT1 is an inhibitor of inflammation, repressing especially NF-κB transcription and activation as shown in liver and adipose tissue[31,36,43]. Anti-inflammatory effects of RSV have also been demonstrated in in vitro and in vivo studies, however, better documented in adipose tissue[44-48] than in hepatic cells or tissue[18,49-52].
Targeting SIRT1 activation for treatment of NAFLD has been suggested[53] as SIRT1 expression is decreased in dietary NAFLD models and NAFLD patients[54-56] and moderate SIRT1 overexpression protects mice from developing NAFLD[57].
The pathogenesis of NAFLD and NASH is far from clarified and especially the factors that drive disease progression towards a more progressive, inflammatory phenotype are not fully characterized. Recently, a “multiple parallel hit hypothesis” has been proposed by Tilg and Moschen. Here, TG accumulation is viewed as an “innocent bystander”, while a number of different parallel hits lead to NASH development[58]. Thus, it appears that there are 3 types of NAFLD patients: the “Good Fat Storer” (the NAFLD patient with a benign course); “the Bad Fat Storer” (the patient who develops immediate NASH); and the “Unfortunate Good Fat Storer” (the NAFLD patient that experiences additional hits and becomes a NASH patient)[59]. Only the latter two may require pharmacological treatment however, bearing in mind that NAFLD may be an independent risk factor for type 2 diabetes[60,61].
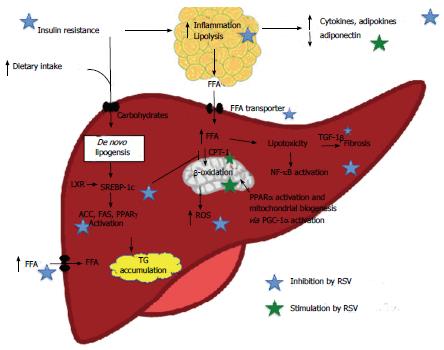
Hepatic steatosis occurs most often in the setting of obesity and metabolic syndrome and is the result of lipid overload, primarily with increased free fatty acid (FFA) flux and TG accumulation. Several mechanisms are involved[58,62] and some may be targeted directly or indirectly by RSV treatment. An illustration of the proposed RVS effects on NAFLD pathogenesis is shown in Figure 1.
Insulin resistance (IR) results in increased lipolysis of TG in adipose tissue, resulting in elevated levels of circulating FFAs. Hepatic uptake of both diet- and lipolysis-derived FFAs is unregulated with limitless hepatocyte uptake via fatty acid transporters (e.g., CD36). The bulk of hepatic TG (two-thirds) is derived from circulating FFA from lipolysis[62]. RSV may decrease lipolysis in adipose tissue through improvement of peripheral insulin sensitivity, as documented in several studies[63,64]. Furthermore, RSV may favorably modulate the expression of fatty acid transporters[65-67].
Dietary fat contributes approximately 15% to the overall FFA load on the liver[62]. Increased fat intake increases circulating FFA, whereas elevated carbohydrate intake, especially in the form of fructose, increases de novo lipogenesis[68].
Normally, de novo synthesis accounts for 5% of hepatic fat content. However, in subjects with NASH, up to 25% of the hepatic fat content may be caused by de novo lipogenesis[62]. The process is regulated independently by insulin and glucose. In the postprandial and in the IR state, insulin stimulates the transcription factor SREBP-1c that promotes transcription of all genes involved in lipogenesis, among them ACC, FAS and peroxisome proliferator-activated receptor (PPARγ)[69,70]. Glucose stimulates lipogenesis through stimulation of the transcription factor carbohydrate response element binding protein (ChREBP)[71]. An upstream activator of both SREBP-1c and ChREBP is LXR, which is a transcription factor governing lipid and cholesterol metabolism[72,73].
Results from in vitro and in vivo studies show that RSV inhibits hepatic lipogenesis by AMPK/SIRT1-mediated inhibition of SREBP-1c, ACC and FAS activity[27,28,37,44,74].
Under normal physiological conditions, the mitochondria handle FFA by β-oxidation. Inadequate β-oxidation may be involved in NAFLD development due to the increased FFA flux through the liver[62,68] and both de- and increased β-oxidation rates are reported[75]. Regardless, SREBP-1c inhibits FA oxidation by indirect inhibition of CPT-1.
RSV may increase FA β-oxidation by stimulating mitochondrial biogenesis through PGC-1α activation[37], increasing mitochondrial number[37,76], increasing uncoupling protein 2 expression[76] and by increasing CPT-1 expression and activity[27,77].
Hepatic inflammation is the hallmark of NASH and the inflammation is driven by several inflammatory hits that may include both intra- and extrahepatic factors[58].
Among the intrahepatic factors are the excess cholesterol, FFA and lipotoxic intermediates, which elicit a number of damaging effects, collectively named “lipotoxicity”[78]. This is recently reviewed in comprehensive reviews[78,79]. Converging the harmful factors is the NF-κB pathway in immune cells, hepatocytes and hepatic stellate cells (HSC), resulting in an inflammatory, pro-fibrogenetic and pro-apoptotic hepatic environment. NF-κB activation enhances transcription of pro-inflammatory cytokines[80,81], with increased hepatic transcription of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and the TNFα receptor, as shown in NASH patients[82]. Kupffer cells and damaged hepatocytes release, e.g., transforming growth factor 1β (TGF-1β) that acts on quiescent HSC, inducing an activated state and thereby fibrosis. In addition, extracellular molecules, endotoxins, such as lipopolysaccharide (LPS) (via toll-like receptor 4), TNFα, IL-1, IL-6 and reactive oxygen species, can induce NF-κB-mediated activation of the HSCs.
The anti-inflammatory effects of RSV are also documented in the liver. Animal studies have shown reduced hepatic macrophage infiltration[51] and TNFα levels[49,52,83], as well as inhibition of the NF-κB pathway[50,52,83]. Only one study has focused on NASH-like fibrosis[52], probably due to the lack of appropriate animal models. However, other hepatic fibrosis models have shown RSV-mediated mitigating effects on markers of hepatic fibrosis[84-87] and HSC activation[86,88,89].
A number of studies report endoplasmatic reticulum stress, lipid peroxidation and oxidative stress as causative or early events in NASH pathogenesis[90-93]. RSV is a known anti-oxidant compound and has been shown to lower hepatic oxidative stress in rodent diabetes and NAFLD models[49,51,94-98]. One potential mechanism is interference in the Keap1/Nrf2 pathway, leading to up-regulation of anti-oxidant enzymes[99,100].
The extrahepatic inflammatory factors include dietary factors (e.g., trans-fatty acids and fructose), gut-derived factors (e.g., microbiota composition and bacterial byproducts) and adipose tissue-derived factors (e.g., hypoadiponectinemia, adipo and cytokines, namely leptin, resistin, IL-6, TNF-α and monocyte chemotactic protein-1 (MCP-1)), that induce a state of whole-body low-grade inflammation in obesity[101]. Several lines of evidence document the anti-inflammatory effects of RSV in adipose tissue, especially by inhibiting NF-κB activation[46,102,103], lowering IL-6 levels[47,104] and macrophage infiltration[103], and modulating circulating cytokines and adipokines[45,50,103,105]. RSV-mediated reduction of LPS-induced liver pathology and oxidative stress has also been reported[106,107].
In summary, RSV has AMPK/SIRT1 activating, anti-inflammatory and anti-oxidant effects that may act in unison, combating the different hits in the pathogenesis of NAFLD and NASH development.
Animal studies of RSV effects on NAFLD are numerous and span a wide variety of models, intervention periods and RSV doses. The studies can be divided into low-dose [RSV doses 7-45 mg/kg bodyweight (BW) daily] and high-dose studies (RSV 45-300 mg/kg BW daily). Some of the studies have hepatic steatosis as the primary endpoint, others as secondary. The RSV treatment is generally started from the beginning of the study and therefore most of the studies concentrate on the preventive effect of RSV and not the therapeutic effect. Almost uniformly, the studies report beneficial effects of RSV treatment on NAFLD pathology. In addition, RSV treatment in experimental models generally reduce circulating insulin and glucose levels[18,37,45,46,50-52,63,105,108-110] and, in some instances, weight[50,52,110-113], circulating transaminases[44,52,77,110] and lipids[45,50,65,96,108,111,112].
In Table 1 we present a list of published RSV animal studies with hepatic histological NAFLD/NASH data.
| Ref. | Focus | Model | RSV dose | RSV exposure(wk) | Histology/LW | TG/CH content | LiverAMPK activation | Suggested RSV actions/mechanisms |
| Baur et al[37] (2006) | Longevity | Middle-aged DIO mice | 22.4 mg/kg BW = 0.04% in diet | 27 | +/+ | ND | + | Elevated PGC-1α deacetylation as marker of enzymatic SIRT1 activity. Phosphorylation of ACC |
| Ahn et al[65] (2008) | NAFLD/NASH | DIO mice (1% CH in diet) | 1.25 g/kg diet | 8 | +/+ Decrease in NASH | +/- | ND | Reduced hepatic expression of FAS, PPARγ, CD36. Increased PPARα expression |
| Kang et al[46] (2010) | Insulin signaling | DIO mice | 30 mg/kg BW | 2 | +/ND | ND | - | Increased Akt phosphorylation, improving insulin signaling |
| Labbé et al[109] (2011) | NAFLD, metabolic profile, longevity | Werner syndrome mice | 0.04% RSV in diet | Life-long ( ≤ 22.5 mo) | +/ND | ND | - | Decreased FAS expression Decreased HCC prevalence |
| Tauriainen et al[115] (2011) | Obesity, NAFLD | DIO mice | 2 or 4 g/kg diet | 15 | +/ND | ND | ND | Increased SIRT1 expression in liver tissue. High-dose most effective |
| Cho et al[111] (2012) | NAFLD | DIO mice (1% CH in diet) | 7 mg/kg BW or 30 mg/kg BW | 10 | +/ND | +/+ | ND | Suppressed FAS activity. Activation of FA β-oxidation in liver. The lower dose is more efficient than the higher dose |
| Zhou et al[108] (2012) | Overall transcriptomic and metabolic profiling | DIO mice | 0.04% or 0.02% RSV + quercetin 0.02% | 26 | +/ND | -/+ | ND | Modulation of inflammation and FA β-oxidation. Combination with quercetin was more effective than RSV alone |
| Jeon et al[51] (2012) | Cognitive deficit | DIO mice | 200 mg/kg BW | 20 | +/ND | ND | ND | Attenuation of hepatic lipid peroxidation and macrophage infiltration |
| Shiozaki et al[97] (2012) | Longevity | SAMP10 mice | 0.04% RSV | 20 | +/ND | ND | ND | Inhibition of ACC. Improved mitochon-drial number, redox status and activity |
| Gao et al[114] (2013) | NAFLD | T0901317-treated mice | 200 mg/kg BW | < 1 | +/+ | +/ND | + | Inhibition of ACC. Unchanged expression SREBP-1c and related genes |
| Li et al[110] (2013) | NAFLD | HFS mice | 50 mg/kg BW | 3 | +/ND Decrease in NASH | ND | ND | Inhibition of NF-κB-induced inflamma-tion and MDA-induced oxidative stress. Protection against NASH fibrosis |
| Bujanda et al[49] (2008) | NAFLD | Fasting/feeding special diet, rats | 10 mg daily | 4 | +/- | ND | ND | Hepatic TNF-α decrease. Improved oxidant/antioxidant markers (MDA, NOS, SOD, e.g., catalase) |
| Shang et al[74] (2008) | NAFLD | HFD rats | 100 mg/kg BW | 10 | +/+ | +/- | + | Suppressed SREBP-1c and FAS gene expression |
| Poulsen et al[76] (2012) | NAFLD | HFD rats | 100 mg daily | 8 | +/- | +/ND | - | Increased UCP2 expression Increased mitochondrial number |
| Gomez-Zorita et al[103] (2012) | NAFLD | Obese Zucker rats | 15 mg/kg BW or 45 mg/kg BW | 6 | +/+ | +/? | ND | Increased CPT-1α and ACO No effect on activity of lipogenic enzymes |
| Bagul et al[94] (2012) | Oxidative stress | High fructose fed rats | 10 mg/kg BW | 8 | +/ND | ND | ND | Attenuation of hepatic oxidative stress, e.g., with increased level of NRF2 |
| Franco et al[96] (2013) | Oxidative stress and NAFLD | Obese ‘early weaned’ rats | 30 mg/kg BW | 4 | +/ND | +/ND | ND | Decreased markers of oxidative stress |
| Xin et al[66] (2013) | NAFLD | HFS rats | 50 or 100 mg/kg BW | 13 | +/ND | +/+ | ND | Decreased hepatic LDLr and SRB1 mRNA and protein express |
| Cho et al[67] (2008) | Hyperlipidemia | HFD hamsters | 0.25 g/kg diet | 8 | +/+ | +/+ | ND | Decreased HMG-CoA expression and modulated lipoprotein expression |
| Burgess et al[110] (2011) | Metabolic syndrome | HFD mini-swine | 100 mg/kg daily | 11 | (+)/ND | ND | ND | Improved insulin sensitivity |
In 2006, Baur et al[37] published a much-cited study on the effect of RSV on the health and survival of mice on a high-fat diet (HFD). HFD and low-dose RSV (10 mg/d) were fed to the mice from senescence to death. Besides increased survival and a number of beneficial metabolic effects, the study showed RSV-mediated hepatic AMPK activation, ACC inhibition, decreased FAS transcription and increased mitochondrial number. RSV also decreased liver weight and the degree of steatosis. This study triggered a number of other mouse studies, often using C57BL/6J diet-induced obese (DIO) mice.
Regarding NAFLD data, the studies report a decrease in hepatic TG [45,50,65,96,111,114] and/or cholesterol accumulation[45,108,111-113] and liver weight[37,65,112], along with improvement in histological fatty liver changes[37,46,51,52,65,96,108,111,113-115]. Only a few studies report NASH changes in the histological specimens. Ahn et al[65] and Li et al[52] find that RSV supplementation represses development of histological steatosis and steatohepatitis and also fibrosis in the latter study. Tauriainen et al[115] find that high-dose RSV represses steatosis and hepatocyte ballooning in a model with minimal hepatic inflammation.
Although tested in a few studies, only two mouse studies are able to verify RSV-mediated AMPK activation in liver tissue[105,114]. Also, no subsequent mouse studies have investigated hepatic PGC-1α deacetylation as a marker of SIRT1 activation. However, other markers of AMPK/SIRT1 activation have been documented in several mouse studies, among them inhibition of FAS expression and activation[65,109,111], inhibition of ACC activation[97,114], augmentation of FA β-oxidation[111], inhibition of PPARγ and SREBP-1c expression and stimulation of PPARα expression[52,65]. In mouse models, RSV treatment inhibits NF-κB activation[50,52] and lowers hepatic expression of inflammation markers[52]. Furthermore, oxidative stress is alleviated by RSV treatment in a number of mouse models[51,52,97].
In a long-term study of high-dose RSV treatment alone and in combination with another polyphenolic compound, namely quercetin, transcriptomic and metabonomic data demonstrated that combination therapy results in a significant restoration of gene sets in functional pathways of glucose and lipid metabolism (glycolysis and FA β-oxidation), inflammation/immunity, liver function and the cardiovascular system, which were altered by HFD feeding[108].
Also, in mutant Werner syndrome mice (showing premature signs of aging, e.g., fatty liver), RSV treatment reversed liver steatosis and lipid peroxidation[109]. Microarray and biological enrichment analyses on liver tissues suggested that RSV mainly decreases lipogenesis and increases genes involved in the insulin signaling pathway and glutathione metabolism. The authors also observed a lower prevalence of hepatocellular carcinoma, however, an increase in lymphomas and other solid tumors was observed.
Numerous different rat models of NAFLD report on RSV effects on NAFLD relevant endpoints, along with a single hamster study. Similar to the mouse studies, the conclusions are positive overall. The studies show a decrease in liver weight[67,74,77], hepatic TG[27,44,66,67,74,76,77] and/or cholesterol accumulation[66,67], and histological fatty liver[49,66,67,74,76,77].
The first to describe RSV effects on NAFLD in rats was Shang et al[74], using a HFD rat model in which the HFD was started 6 wk prior to the high-dose RSV treatment (100 mg/kg BW daily). This study therefore focused on the therapeutic effects of RSV. Besides alleviating NAFLD changes, it demonstrated that high-dose RSV treatment promotes phosphorylation and activation of AMPK and suppresses expression of FAS and SREBP-1c. This is backed by a recent study in which the HFD was added to a high amount of sucrose. Alberdi et al[27] found that low-dose RSV treatment for 6 wk activates AMPK and PGC-1α, increases CPT-1 and decreases ACC activities with no change in the mRNA expression of SREBP-1c, PPARα, SIRT1 and PGC-1α. Yet, not all rat studies find AMPK activation either. At variance, our group found no increase in AMPK phosphorylation or expression of related genes in spite of improvement in fatty liver changes, along with an increase in the hepatic mitochondrial content[76].
Obese Zucker rats have been used in low-dose RSV studies[44,77], with a reduction in hepatic lipid content and alanine aminotransferase levels, along with activation of AMPK and increased CPT-1 activity, which is important for the rate of FA β-oxidation. Resveratrol treatment also improved the inflammatory status of visceral adipose tissue[44] and reduced liver oxidative stress[77]. Here, the effect on lipogenetic enzyme activity was equivocal.
Similar results on inflammatory and oxidative status was found by Bujanda et al[49] in a model in which cycles of fasting and feeding with a high carbohydrate-fat free diet induced steatosis. Low dose RSV for 4 wk lowered hepatic TNFα levels and reduced markers of lipid peroxidation and hepatic oxidative stress.
To our knowledge, only one study reports no RSV effect on hepatic lipid levels. Andersen et al[116] used a dietary rat model and high-dose RSV treatment for 8 wk, yet found no decrease in liver TG, FFA or cholesterol content. Also, they found no effect on liver SIRT1 protein expression.
Taken together, the current evidence shows that RSV prevents NAFLD-like hepatic steatosis in rodent NAFLD models. This may be caused by inhibition of adipose tissue lipolysis, inhibition of hepatic de novo lipogenesis and an increase in FA β-oxidation. A graphic illustration of the proposed RVS effects on NAFLD pathology is shown in Figure 1. Development of steatohepatitis may be attenuated by an inhibition of adipose tissue and hepatic inflammation and reduction of oxidative stress. However, few studies have used appropriate animal NASH models and there is only one study documenting an alleviating effect on NASH fibrosis. RSV could exert some of these effects through AMPK activation but AMPK activation is not found in all studies. Also, hepatic SIRT1 activation has not been verified in an experimental NAFLD model. Studies focusing on the therapeutic effect as opposed to the preventive effect of RSV on NAFLD and especially NASH are few but warranted.
In a porcine model of metabolic syndrome, Burgess et al[110] found that high-dose RSV treatment had mitigating effects on insulin resistance and transaminase levels. Oil red O staining showed a decrease in hepatic lipid accumulation. However, a HE stain found no difference in histology between the control, HFD and HFD with RSV groups, signifying that steatosis was not sufficiently induced in this model.
So far, only a few clinical RSV trials on efficacy outcomes have been concluded and none of these studies have focused on fatty liver disease per se. Two studies on obese but otherwise healthy male participants report liver data. In the study by Timmers et al[117], 11 participants received a daily dose of 150 mg RSV or placebo for 30 days in a double-blind, cross-over design. Results suggest a number of beneficial metabolic effects, among these a reduction of liver transaminases and liver fat by magnetic resonance (MR) spectroscopy. In another study, 24 participants received a dose of 1.5 g RSV or placebo daily for 4 wk[118] and there was no effect on liver fat content (MR spectroscopy) or transaminase levels. This is consistent with another study in 45 non-obese postmenopausal women receiving a dose of 75 mg for 12 wk where no effect on liver fat (MR spectroscopy) or any other physiological parameter could be demonstrated[119].
Future clinical studies should focus on patients with biopsy verified NAFLD and NASH to determine any efficacy of RSV treatment in this setting.
So far, clinical studies of RSV effects on steatosis are scarce and the overall positive effects seen in rodent studies are still missing. None of the studies have included verified NAFLD patients or histological data and the studies differ significantly in the RSV dose used. New clinical studies should focus on the RSV effects in patients rather than healthy or near-healthy individuals[120], in this case, histologically verified NAFLD/NASH patients. In this setting, the focus should be on the therapeutic effects of RSV and not its preventive effects, as reported in the majority of animal studies.
| 1. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 2. | Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496-500.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 3. | Bloomgarden ZT. Nonalcoholic fatty liver disease and insulin resistance in youth. Diabetes Care. 2007;30:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kopec KL, Burns D. Nonalcoholic fatty liver disease: a review of the spectrum of disease, diagnosis, and therapy. Nutr Clin Pract. 2011;26:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 6. | Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S-225S. [PubMed] |
| 8. | Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75:103-112. [PubMed] |
| 9. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1255] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 10. | Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;3:CD007176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Kostapanos MS, Kei A, Elisaf MS. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol. 2013;5:470-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 13. | Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S, Filis K, Papalampros A. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J Hepatol. 2013;5:160-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Kelishadi R, Farajian S, Mirlohi M. Probiotics as a novel treatment for non-alcoholic Fatty liver disease; a systematic review on the current evidences. Hepat Mon. 2013;13:e7233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tomeno W, Yoneda M, Imajo K, Ogawa Y, Kessoku T, Saito S, Eguchi Y, Nakajima A. Emerging drugs for non-alcoholic steatohepatitis. Expert Opin Emerg Drugs. 2013;18:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2852] [Cited by in RCA: 2815] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 17. | Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 18. | Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1284] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 20. | Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 522] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 21. | Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1080] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 22. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 25. | Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol. 2010;16:3731-3742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C. AMPK: Lessons from transgenic and knockout animals. Front Biosci (Landmark Ed). 2009;14:19-44. [PubMed] |
| 27. | Alberdi G, Rodríguez VM, Macarulla MT, Miranda J, Churruca I, Portillo MP. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition. 2013;29:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1426] [Cited by in RCA: 1427] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 29. | Henriksen BS, Curtis ME, Fillmore N, Cardon BR, Thomson DM, Hancock CR. The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding. Diabetol Metab Syndr. 2013;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015-20026. [PubMed] |
| 31. | Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570-2580. [PubMed] |
| 33. | Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1455] [Article Influence: 58.2] [Reference Citation Analysis (14)] |
| 34. | Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998-16003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 996] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 35. | Herranz D, Serrano M. Impact of Sirt1 on mammalian aging. Aging (Albany NY). 2010;2:315-316. [PubMed] |
| 36. | Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 897] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 37. | Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3166] [Cited by in RCA: 3276] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 38. | Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 501] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 39. | Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959-33970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (8)] |
| 41. | Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 42. | Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104:12861-12866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 438] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 43. | Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol Res. 2013;67:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 45. | Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 46. | Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Cullberg KB, Olholm J, Paulsen SK, Foldager CB, Lind M, Richelsen B, Pedersen SB. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur J Pharm Sci. 2013;49:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond). 2008;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Do GM, Jung UJ, Park HJ, Kwon EY, Jeon SM, McGregor RA, Choi MS. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444-1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 52. | Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, Weng X. Resveratrol modulates autophagy and NF-κB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Colak Y, Ozturk O, Senates E, Tuncer I, Yorulmaz E, Adali G, Doganay L, Enc FY. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:HY5-HY9. [PubMed] |
| 54. | Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Chen LL, Deng XQ, Li NX. [Effects of calorie restriction on SIRT1 expression in liver of nonalcoholic fatty liver disease: experiment with rats]. Zhonghua Yixue Zazhi. 2007;87:1434-1437. [PubMed] |
| 56. | Costa Cdos S, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, Mottin CC, Guaragna RM. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 58. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1915] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 59. | Fujii H, Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 2012;47:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 60. | Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Fatty liver is an independent risk factor for the development of Type 2 diabetes in Korean adults. Diabet Med. 2008;25:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2676] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 63. | Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2983] [Cited by in RCA: 3250] [Article Influence: 162.5] [Reference Citation Analysis (1)] |
| 64. | Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Ahn J, Cho I, Kim S, Kwon D, Ha T. Dietary resveratrol alters lipid metabolism-related gene expression of mice on an atherogenic diet. J Hepatol. 2008;49:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Xin P, Han H, Gao D, Cui W, Yang X, Ying C, Sun X, Hao L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem Toxicol. 2013;52:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 869] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 69. | Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737-12742. [PubMed] |
| 70. | Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:13656-13661. [PubMed] |
| 71. | Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116-9121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 521] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 72. | Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019-11025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 595] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 73. | Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1373] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 74. | Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 75. | Degli Esposti D, Hamelin J, Bosselut N, Saffroy R, Sebagh M, Pommier A, Martel C, Lemoine A. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem Res Int. 2012;2012:387626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Poulsen MM, Larsen JØ, Hamilton-Dutoit S, Clasen BF, Jessen N, Paulsen SK, Kjær TN, Richelsen B, Pedersen SB. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res. 2012;32:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Gómez-Zorita S, Fernández-Quintela A, Macarulla MT, Aguirre L, Hijona E, Bujanda L, Milagro F, Martínez JA, Portillo MP. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 79. | Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 80. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4403] [Cited by in RCA: 4658] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 81. | Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 82. | Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 83. | Chang CC, Chang CY, Huang JP, Hung LM. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin J Physiol. 2012;55:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010;33:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008;28:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Di Pascoli M, Diví M, Rodríguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, Bosch J, García-Pagán JC. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2013;58:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 87. | Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010;30:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 88. | Godichaud S, Krisa S, Couronné B, Dubuisson L, Mérillon JM, Desmoulière A, Rosenbaum J. Deactivation of cultured human liver myofibroblasts by trans-resveratrol, a grapevine-derived polyphenol. Hepatology. 2000;31:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Souza IC, Martins LA, Coelho BP, Grivicich I, Guaragna RM, Gottfried C, Borojevic R, Guma FC. Resveratrol inhibits cell growth by inducing cell cycle arrest in activated hepatic stellate cells. Mol Cell Biochem. 2008;315:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:595-617, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Serviddio G, Bellanti F, Vendemiale G, Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 93. | Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr. 2012;32:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 94. | Bagul PK, Middela H, Matapally S, Padiya R, Bastia T, Madhusudana K, Reddy BR, Chakravarty S, Banerjee SK. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol Res. 2012;66:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 95. | Rocha KK, Souza GA, Ebaid GX, Seiva FR, Cataneo AC, Novelli EL. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009;47:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Franco JG, Lisboa PC, Lima NS, Amaral TA, Peixoto-Silva N, Resende AC, Oliveira E, Passos MC, Moura EG. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J Nutr Biochem. 2013;24:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 97. | Shiozaki M, Hayakawa N, Shibata M, Koike M, Uchiyama Y, Gotow T. Closer association of mitochondria with lipid droplets in hepatocytes and activation of Kupffer cells in resveratrol-treated senescence-accelerated mice. Histochem Cell Biol. 2011;136:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812:719-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 100. | Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18-H24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 448] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 101. | Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 102. | Zagotta I, Dimova EY, Funcke JB, Wabitsch M, Kietzmann T, Fischer-Posovszky P. Resveratrol suppresses PAI-1 gene expression in a human in vitro model of inflamed adipose tissue. Oxid Med Cell Longev. 2013;2013:793525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Gómez-Zorita S, Fernández-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013;29:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond). 2010;34:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Chen S, Li J, Zhang Z, Li W, Sun Y, Zhang Q, Feng X, Zhu W. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can J Physiol Pharmacol. 2012;90:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 106. | Sebai H, Sani M, Yacoubi MT, Aouani E, Ghanem-Boughanmi N, Ben-Attia M. Resveratrol, a red wine polyphenol, attenuates lipopolysaccharide-induced oxidative stress in rat liver. Ecotoxicol Environ Saf. 2010;73:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Farghali H, Cerný D, Kameníková L, Martínek J, Horínek A, Kmonícková E, Zídek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Zhou M, Wang S, Zhao A, Wang K, Fan Z, Yang H, Liao W, Bao S, Zhao L, Zhang Y. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J Proteome Res. 2012;11:4961-4971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Labbé A, Garand C, Cogger VC, Paquet ER, Desbiens M, Le Couteur DG, Lebel M. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Burgess TA, Robich MP, Chu LM, Bianchi C, Sellke FW. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg. 2011;146:556-564. [PubMed] |
| 111. | Cho SJ, Jung UJ, Choi MS. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br J Nutr. 2012;108:2166-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 112. | Chen Q, Wang E, Ma L, Zhai P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, Xie LP. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 114. | Gao M, Liu D. Resveratrol suppresses T0901317-induced hepatic fat accumulation in mice. AAPS J. 2013;15:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari A, Herzig KH, Lecklin A, Mervaala E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation. J Nutr Metab. 2011;2011:525094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Andersen G, Burkon A, Sulzmaier FJ, Walker JM, Leckband G, Fuhst R, Erbersdobler HF, Somoza V. High dose of dietary resveratrol enhances insulin sensitivity in healthy rats but does not lead to metabolite concentrations effective for SIRT1 expression. Mol Nutr Food Res. 2011;55:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 1001] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 118. | Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 376] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 119. | Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 120. | Smoliga JM, Colombo ES, Campen MJ. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging (Albany NY). 2013;5:495-506. [PubMed] |
P- Reviewer: Fink MP S- Editor: Song XX L- Editor: Roemmele A E- Editor: Wu HL