Published online Aug 27, 2013. doi: 10.4254/wjh.v5.i8.425
Revised: July 24, 2013
Accepted: August 4, 2013
Published online: August 27, 2013
Processing time: 165 Days and 3.8 Hours
AIM: To investigate the roles of peribiliary glands around the bile ducts in the pathophysiology of the biliary tract.
METHODS: The expression of fetal pancreatic markers, pancreatic duodenal homeobox factor 1 (PDX1) and hairy and enhancer of split 1 (HES1) and endodermal stem/progenitor (S/P) cell markers [CD44s, chemokine receptor type 4 (CXCR4), SOX9 and epithelial cell adhesion molecule (EpCAM)] were examined immunohistochemically in 32 normal adult livers (autopsy livers) and 22 hepatolithiatic livers (surgically resected livers). The latter was characterized by the proliferation of the peribiliary glands. Immunohistochemistry was performed using formalin-fixed, paraffin-embedded tissue sections after deparaffinization. Although PDX1 and HES1 were expressed in both the nucleus and cytoplasm of epithelial cells, only nuclear staining was evaluated. SOX9 was expressed in the nucleus, while CD44s, CXCR4 and EpCAM were expressed in the cell membranes. The frequency and extent of the expression of these molecules in the lining epithelia and peribiliary glands were evaluated semi-quantitatively based on the percentage of positive cells: 0, 1+ (focal), 2+ (moderate) and 3+ (extensive).
RESULTS: In normal livers, PDX1 was infrequently expressed in the lining epithelia, but was frequently expressed in the peribiliary glands. In contrast, HES1 was frequently expressed in the lining epithelia, but its expression in the peribiliary glands was focal, suggesting that the peribiliary glands retain the potential of differentiation toward the pancreas and the lining epithelia exhibit properties to inhibit such differentiation. This unique combination was also seen in hepatolithiatic livers. The expression of endodermal S/P cell markers varied in the peribiliary glands in normal livers: SOX9 and EpCAM were frequently expressed, CD44s infrequently, and CXCR4 almost not at all. The expression of these markers, particularly CD44s and CXCR4, increased in the peribiliary glands and lining epithelia in hepatolithiatic livers. This increased expression of endodermal S/P cell markers may be related to the increased production of intestinal and gastric mucin and also to the biliary neoplasia associated with the gastric and intestinal phenotypes reported in hepatolithiasis.
CONCLUSION: The unique expression pattern of PDX1 and HES1 and increased expression of endodermal S/P cell markers in the peribiliary glands may be involved in biliary pathophysiologies.
Core tip: Immunohistochemical analysis showed that pancreatic duodenal homeobox factor 1 was more frequently expressed in the peribiliary glands than epithelia lining the bile duct and was accompanied by the reciprocal expression pattern of hairy and enhancer of split 1. These results may reflect maintenance of the biliary tract and the increased expression of endodermal stem/progenitor cell markers may be involved in the unique pathophysiologies of the peribiliary glands.
- Citation: Igarashi S, Sato Y, Ren XS, Harada K, Sasaki M, Nakanuma Y. Participation of peribiliary glands in biliary tract pathophysiologies. World J Hepatol 2013; 5(8): 425-432
- URL: https://www.wjgnet.com/1948-5182/full/v5/i8/425.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i8.425
The biliary tract and pancreas are embryologically derived from the foregut and several factors, such as pancreatic duodenal homeobox factor 1 (PDX1) and hairy and enhancer of split 1 (HES1), are reportedly involved in their development and differentiation[1-3]. For example, PDX1, a transcription factor crucial for the development of the pancreas and a marker of pancreatic progenitor cells, was shown to be expressed in the fetal biliary tract[4,5]. In contrast, HES1, which is also a fetal transcription factor that represses pancreatic exocrine and endocrine differentiation, is also important for the development and differentiation of the biliary tract[4,5]. In HES1-/HES1- mice, the biliary tract was shown to continuously express PDX1 and the pancreatic acini appeared and replaced biliary epithelial cells in the biliary tract[5]. Our previous study showed that PDX1 was expressed extensively in epithelia lining the fetal bile ducts, but not at all in adult bile ducts, whereas HES1 was expressed in the adult bile ducts[4]. These findings supported reciprocal roles for PDX1 and HES1 in the development of bile ducts. However, the expression and significance of these proteins in the peribiliary glands remain to be clarified.
Peribiliary glands composed of branched tubuloalveolar seromucinous glands are found around the extrahepatic and intrahepatic large bile ducts of humans at all ages[1,6-10]. These glands communicate with bile duct lumens through their own conduits[6-8] and are relatively dense in the hilar bile ducts, cystic duct and periampullary region[7-10]. They secrete several substances such as lactoferrin and lysozyme[7]. Recently, the peribiliary glands were reported to be stem cell niches of the biliary tree[11] and these stem cells were shown to be capable of differentiating into hepatobiliary and pancreatic cells[11-13]. According to Carpino et al[12], these peribiliary glands harbor stem/progenitor (S/P) cells of the liver, bile duct and pancreas, which express endodermal S/P cell markers, such as C-X-C chemokine receptor type 4 (CXCR4), PDX1, HES1, SOX9/17, epithelial cell adhesion molecule (EpCAM) and CD44s, and these cells also weakly express adult liver, bile duct and pancreatic markers, such as albumin and cystic fibrosis transmembrane conductance regulator (CFTR)[11-13].
These S/P cells in the peribiliary glands are likely to be central to normal tissue turnover and injury repair, and may play key roles in the pathophysiology of several biliary tract diseases[12]. In hepatolithiasis, the peribiliary glands proliferate markedly, secrete large amounts of mucin into the bile duct lumen[14,15] and may be involved in stone formation and even cholangiocarcinogenesis[16]. However, the roles of S/P cells in the peribiliary glands in hepatolithiasis have not been examined.
In this study, we examined the expression of fetal pancreatic markers (PDX1 and HES1) and endodermal S/P cell markers (CD44s, CXCR4, SOX9 and EpCAM) in the lining epithelia and peribiliary glands immunohistochemically, using 32 normal livers and 22 hepatolithiatic livers, and then tried to evaluate the roles and significance of these markers in the biliary tract pathophysiologies in hepatolithiasis.
Case selection: Thirty-two histologically normal livers from 32 patients (range of age: 45-81 years old with an average age of 63 years; 20 males and 12 females) were obtained from our recent autopsy series with minimal autolytic changes and at least one tissue section was obtained from the hepatic hilus containing hilar bile duct(s) with peribiliary glands in each case. In addition, 22 hepatolithiatic livers were obtained from our surgical cases and the age and sex of these cases were similar to those of normal livers. All stone-containing bile ducts exhibited the marked proliferation of peribiliary glands and failed to show neoplastic biliary epithelial lesions[6,7,14] and at least two tissue sections were obtained from these stone-containing bile ducts in each case.
Tissue preparation: All tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. More than 20 consecutive 4-μm-thick sections were cut from each paraffin block and one section was stained with hematoxylin and eosin (HE) for histological observations. The remaining sections were used for immunohistochemistry.
Immunostaining was performed using formalin-fixed, paraffin-embedded tissue sections of normal and hepatolithiatic livers. The antibodies and their sources, optimal dilution and antigen retrieval are shown in Table 1. After deparaffinization and the blocking of endogenous peroxidase, the sections were incubated first in a protein block solution (DakoCytomation), then overnight at 4 °C with the primary antibodies against PDX1 and HES1 and the markers for endodermal S/P cells (CD44s, CXCR4, SOX9 and EpCAM). Sections were then treated with secondary antibodies conjugated to a peroxidase-labeled polymer (EnVision system, Dako Cytomation). Color development was performed using DAB and the sections were counterstained with hematoxylin or methyl green. As positive controls, islet cells in normal pancreatic tissue (one case) for PDX1, fibroblasts in cirrhotic liver tissue (one case) for HES1, neural cells in a normal human brain (one case) for CXCR4, fibroblasts in cirrhotic liver tissue (one case) for CD44s, normal human embryonic tissue (one case) for SOX9, and bile ducts in a normal liver (one case) for EpCAM were used as shown in Table 1. Negative controls were carried out with non-immunized serum substituted for the primary antibodies, resulting in no signal detection.
| Primary antibody | Clone (product code) | Company | Optional dilution | Antigen retrieval method | Positive control (N/C/M) |
| PDX1 | goat poly. (sc-14664) | Santa Cruz | 1:100 | Citrate buf. AC | Islet cells in the human pancreas |
| HES1 | rabbit poly. (H2034-35) | US Biological | 1:500 | Citrate buf. MW | Human fibroblasts (N) |
| CXCR4 | mouse mono. (35-8800) | ZYMED | 1:100 | PK | Neural cells in the human brain (M) |
| CD44s | mouse mono. (M 7082) | Dako | 1:100 | Dako Target Retrieval Solution No.S1700 | Human fibroblasts (M/C) |
| SOX9 | rabbit poly. (AB5535) | MILLIPORE | 1:1000 | Boro buf. PC | Embryonic tissue (N) |
| EpCAM | mouse mono. (ab46714) | Abcam | 1:5 | Dako Target Retrieval Solution No.S1700 | Bile ducts in the human liver (M) |
Although PDX1 and HES1 were expressed in both the nucleus and cytoplasm of epithelial cells, only nuclear staining was evaluated. Sox9 was expressed in the nucleus, while CD44s, CXCR4 and EpCAM were expressed in the cell membranes. The immunoreactivity of epithelial cells in the lining epithelia and peribiliary glands was semi-quantitatively graded based on the percentage of positive cells, as follows: 0, no expression of each marker in the lining epithelia or peribiliary glands; 1+ (focal), the expression of each marker in less than one third of the lining epithelia and peribiliary glands, respectively; 3+ (extensive), the expression of each marker in more than two thirds of the lining epithelia and peribiliary glands, respectively; and 2+ (moderate), the expression of each marker in the lining epithelia and peribiliary glands between 1+ and 3+, respectively. The staining intensity was rather stronger in the cases of extensive expression and was rather weaker in the cases of focal expression.
Intergroup comparisons were made using Mann-Whitney’s U test. The results were considered significant if the P value < 0.05.
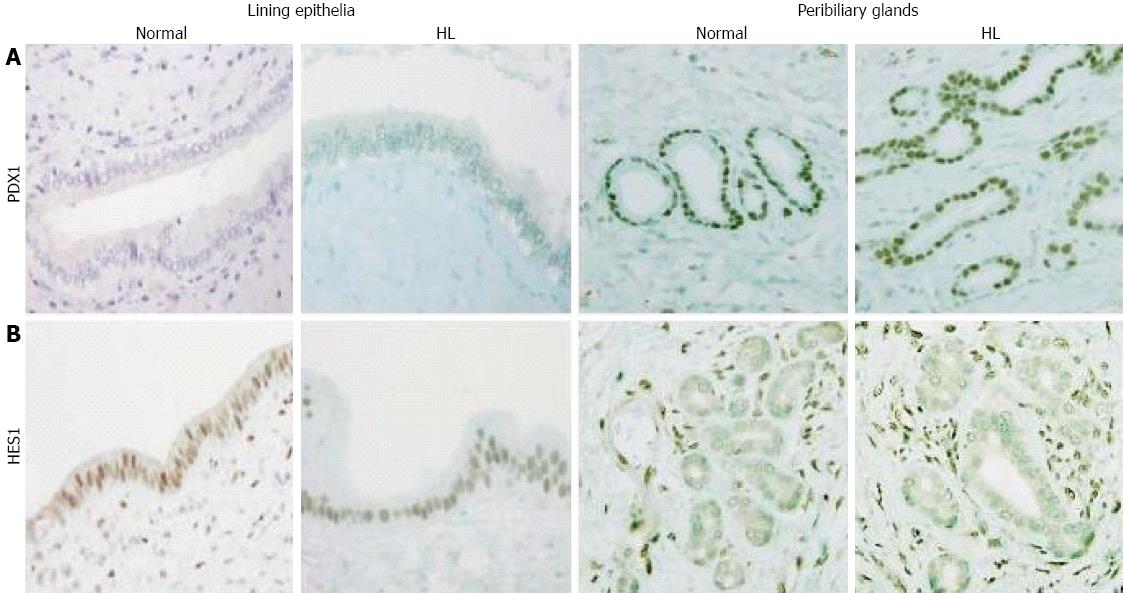
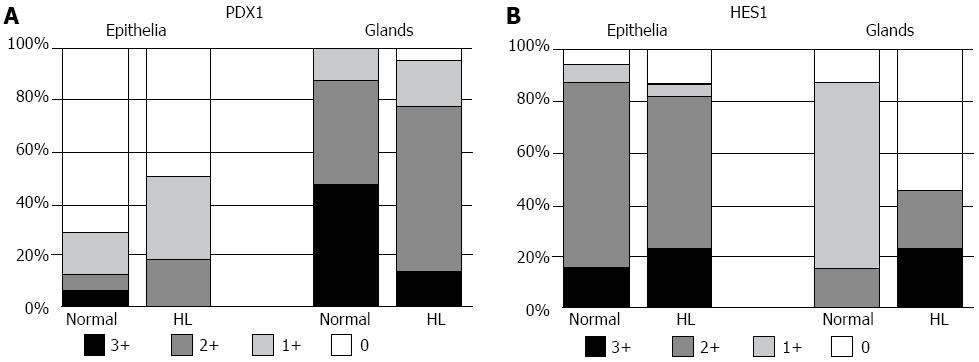
In normal livers, PDX1 was infrequently and focally expressed in the lining epithelia but was extensively expressed in the peribiliary glands, with 28 of 32 cases showing moderate to extensive expression (Figures 1A and 2A). In contrast, HES1 was extensively expressed in the lining epithelia, with 28 of 32 cases showing moderate to extensive expression (Figures 1B and 2B), while its expression was infrequent and focal in the peribiliary glands. The patterns for the expression of PDX1 and HES1 in the peribiliary glands and lining epithelia in hepatolithiatic livers were similar to those in normal livers.
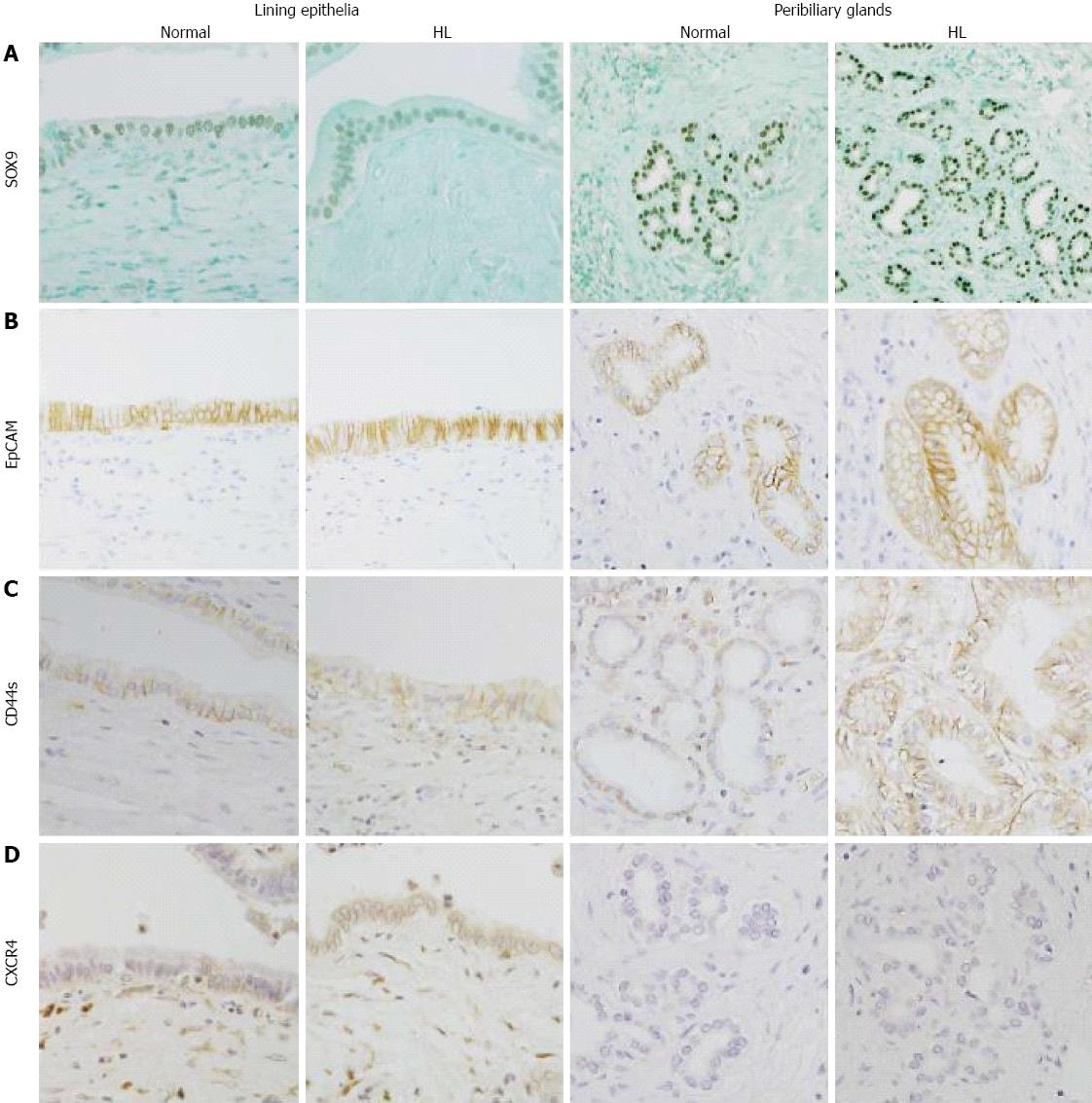
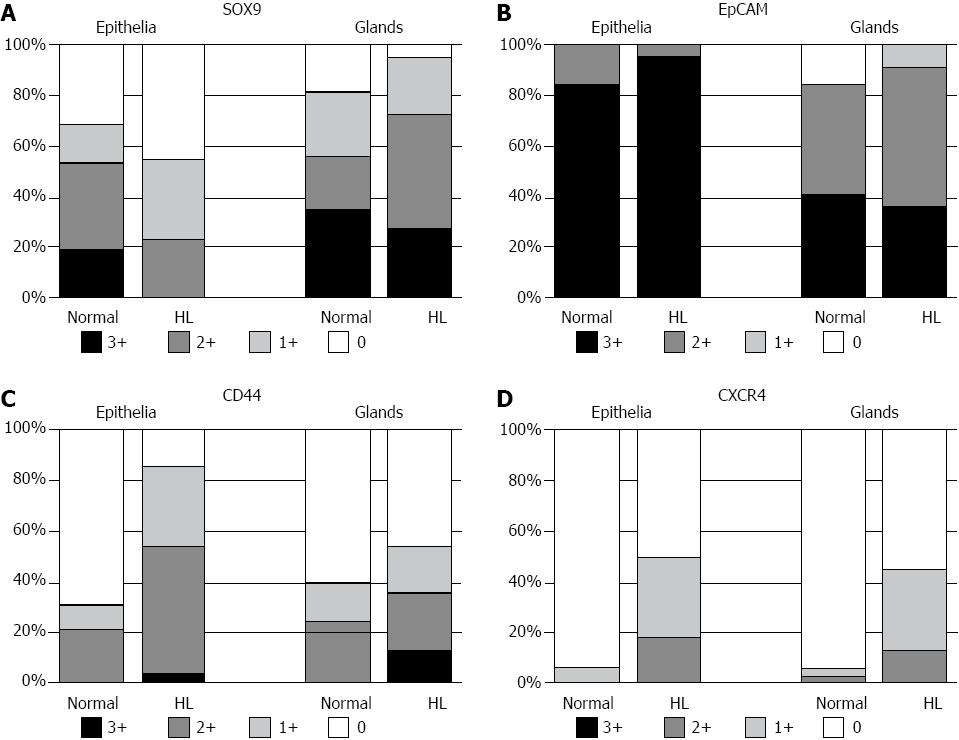
In normal livers, SOX9 was frequently expressed in the lining epithelia and also in the peribiliary glands, with 17 and 18 cases showing moderate to extensive expression, respectively (Figure 3A). EpCAM was frequently expressed in the lining epithelia and peribiliary glands (Figure 3B), with the level of moderate to extensive expression. In hepatolithiatic livers, the degree of SOX9 expression was relatively low in the lining epithelia and relatively high in the peribiliary glands in comparison with normal livers, whereas the frequency and degree of EpCAM expression in both the lining epithelia and peribiliary glands were similar to those in normal livers (Figure 4A and Figure B).
CD44s was moderately to extensively expressed in the lining epithelia and peribiliary glands in 7 and 8 of 32 normal livers, respectively (Figure 3C), and its expression in the lining epithelia and peribiliary glands was increased in the frequency and degree in the hepatolithiatic livers, with this increase being significant in the lining epithelia (P < 0.01) (Figure 4C). CXCR4 was expressed in the lining epithelia and peribiliary glands of two and one normal livers, respectively. Its expression in the lining epithelia and peribiliary glands was increased in the frequency and degree in the hepatolithiatic livers (Figure 3D), with this increase being significant in the lining epithelia (P < 0.01) (Figure 4D).
The number of peribiliary glands in hepatolithiatic livers was markedly higher than that in normal livers[6,7,14]. Therefore, the actual number of glandular cells expressing each marker of these fetal pancreatic markers and endodermal S/P cell markers in the peribiliary glands could be regarded as more in hepatolithiatic livers than expected by the above-mentioned semi-quantitative approach.
The results of this study can be summarized as follows: (1) In normal livers, PDX1 was extensively expressed in the peribiliary glands and was infrequently expressed in the lining epithelia of the bile ducts, and while the opposite was true for HES1, this unique expression pattern was retained in hepatolithiatic livers; (2) Endodermal S/P cell markers were variably expressed in the peribiliary glands and EpCAM and SOX9 were frequently detected in the peribiliary glands of normal livers, whereas CXCR4 was rarely detected; and (3) In hepatolithiatic livers, the expression of the S/P markers, particularly CD44s and CXCR4, in the lining epithelia and peribiliary glands was increased. Taken together, the reciprocal expression of PDX1 and HES1 in the lining epithelia and peribiliary glands may be important for physiological maintenance of the biliary tract. The expression of endodermal S/P cell markers was variable in the peribiliary glands and tended to be higher in hepatolithiatic livers than in normal livers, implying the pathogenetic roles of S/P cells in the biliary pathophysiologies of hepatolithiasis.
HES1 is known to inhibit or repress the differentiation of pancreas, whereas PDX1 is involved in its promotion[2-5]. Our recent study supported their roles in the biliary tract differentiation and maturation in humans[1,4]. The present study showed that PDX1 was infrequently and focally expressed in the lining epithelia of normal livers, but was extensively expressed in the peribiliary glands. In contrast, HES1 was extensively expressed in the lining epithelia, although infrequently and usually focally expressed in the peribiliary glands. Taken together, it seems possible that the peribiliary glands expressing PDX1 in adults retain potential properties for the promotion of pancreatic development as seen in the biliary epithelia of the fetal bile duct, while the lining epithelia of the bile duct expressing HES1 keep properties for the inhibition of pancreatic development.
Our previous studies showed that the pancreatic exocrine acini were occasionally present in the peribiliary glands of adults and expressed enzymes such as amylase, lipase and chymotrypsin[17,18]. Carpino et al[12] also reported that pancreatic genes such as CFTR were weakly expressed in the peribiliary glands[11,13]. Thus, it seems possible that the peribiliary glands may have the potential to become pancreatic cells and the biliary tract can be regarded as an incomplete pancreas[1]. Gerber et al[19] proposed one hypothesis in which glandular cells within the peribiliary glands could migrate via their conduits and renew or replace the lining epithelia of the bile ducts. Sutton et al[20] also showed that peribiliary glands renewed the biliary lining epithelia as a repair process. Taken together, glandular cells in the peribiliary glands may lose PDX1 expression but gain HES1 expression during their migration toward the lining layer. Interestingly, this unique expression pattern of PDX1 and HES1 in the lining epithelia and peribiliary glands was generally retained in hepatolithiatic livers, suggesting that the above-mentioned renewal process may be maintained in hepatolithiasis.
Recent studies have shown multipotent, endoderm S/P cells to be present in human peribiliary glands at all ages[11-13]. In the studies using cell cultures and tissue explants, isolated cells of the peribiliary glands expanded in vitro and these cells readily and efficiently showed cell lineages differentiating into liver, the biliary tree or pancreatic cells[11-13]. It was found in this study that the markers of endodermal S/P cells such as EpCAM, CD44s, CXCR4 and SOX9[13,21-23] were variably expressed in the peribiliary glands and also in the lining epithelium of hilar bile ducts, supporting that peribiliary glands are a niche for endodermal S/P cells[11-13]. The different expression patterns of endoderm S/P cell markers may reflect heterogeneous S/P cell components within the peribiliary glands.
Mucin hypersecretion is a frequent finding in cases of hepatolithiasis[15,16]. Mucin secreted from the bile ducts is central to calcium bilirubinate stones. According to our previous studies, gel-forming mucins such as MUC2 and MUC5AC were shown to be important for the development of calcium bilirubinate stones and these mucins were detected in the glands showing the intestinal and gastric metaplasia in the bile ducts and peribiliary glands with hepatolithiasis[14,15]. The increased expression of endodermal S/P cell markers in the lining epithelia and peribiliary glands in hepatolithiatic livers may be involved in these metaplastic processes[24].
Interestingly, the peribiliary glands were shown to be dense at the hepatic hilar regions, the cystic duct of gallbladder, and the periampullary region, where cholangiocarcinomas (CCs) are likely to occur. Furthermore, CCs and precursor lesions, such as biliary intraepithelial neoplasm and intraductal papillary neoplasm of bile duct (IPNB), are known to develop in the stone-containing bile ducts in hepatolithiasis[25]. This study showed that the expression of these S/P cell markers tended to be higher in the peribiliary glands and also lining epithelia of hepatolithiatic livers than in those of normal livers. CD44s-positive cells were also increased in the lining epithelia and peribiliary glands in hepatolithiasis, while CD44s was not typically expressed in the normal biliary tract. It was reported that CD44s was expressed in CCs[26]. CXCR4 is a chemokine receptor, its interaction with its ligand was reportedly involved in cholangiocarcinogenesis[27] and the frequent expression of CXCR4 in the lining epithelia and peribiliary glands in hepatolithiasis was shown in this study. Taken together, this study suggested that CD44s and CXCR4 may also be related to the neoplastic changes in bile ducts with hepatolithiasis. Our previous study showed that PDX1 expressed in preneoplastic and neoplastic biliary epithelia was related to their proliferative activities; therefore, it also seems likely that PDX1 expression in the peribiliary glands of hepatolithiasis may be related to their proliferation and neoplastic process[6,7,14].
The increased expression of pancreatic and endodermal S/P cell markers in the lining epithelium and peribiliary glands may be also related to the unique features of the neoplastic processes of the biliary tract[28]. Our recent study showed that hilar cholangiocarcinomas shared features with pancreatic duct adenocarcinomas (PDAC)[29]. Furthermore, IPNB, which is known to develop in hepatolithiasis, often shows intestinal or gastric phenotypes[24]. Therefore, it is possible that the lining epithelia and peribiliary glands expressing pancreatic and endodermal S/P cell markers may be related to the similarities of hilar cholangiocarcinoma to PDAC and may be also involved in the intestinal or gastric metaplasia of IPNB.
In conclusion, the peribiliary glands frequently and extensively expressed PDX1 but focally expressed HES1 and this unique expression pattern may be involved in the biliary tract maintenance. The expression of endoderm S/P cell markers in the peribiliary glands and also lining epithelia may be involved in the intestinal and gastric metaplasia occurring in these glands in hepatolithiasis. The lining epithelia and peribiliary glands expressing pancreatic and endodermal S/P cell markers in hepatolithiasis may be also involved in cholangiocarcinogenesis with pancreatic and gastrointestinal phenotypes. Further studies are needed to clarify the exact roles of the peribiliary glands expressing endodermal S/P cell markers in the pathophysiologies of biliary diseases in hepatolithiasis.
Pancreatic duodenal homeobox factor 1 (PDX1), a transcription factor crucial for the development of the pancreas and a marker of pancreatic progenitor cells, was shown to be expressed in the fetal biliary tract. In contrast, hairy and enhancer of split 1 (HES1), which is also a fetal transcription factor that represses pancreatic exocrine and endocrine differentiation, is also important for the development and differentiation of the biliary tract.
PDX1 was infrequently and focally expressed in the lining epithelia of normal livers, but was extensively expressed in the peribiliary glands. In contrast, HES1 was extensively expressed in the lining epithelia, although infrequently and usually focally expressed in the peribiliary glands. Taken together, it seems possible that the peribiliary glands expressing PDX1 in adults retain potential properties for the promotion of pancreatic development as seen in the biliary epithelia of the fetal bile duct, while the lining epithelia of the bile duct expressing HES1 keep properties for the inhibition of pancreatic development.
In this study, the authors examined the expression of fetal pancreatic markers (PDX1 and HES1) and endodermal stem/progenitor (S/P) cell markers in the lining epithelia and peribiliary glands immunohistochemically, using 32 normal livers and 22 hepatolithiatic livers, and then tried to evaluate the roles and significance of these markers in the biliary tract pathophysiologies in hepatolithiasis.
The study’s unique combination was also seen in hepatolithiatic livers. The expression of these markers, particularly CD44s and chemokine receptor type 4, increased in the peribiliary glands and lining epithelia in hepatolithiatic livers. This increased expression of endodermal S/P cell markers may be related to the increased production of intestinal and gastric mucin and also to the biliary neoplasia associated with the gastric and intestinal phenotypes reported in hepatolithiasis.
The authors have investigated the pathophysiologies of peribiliary glands (currently known to be stem cell niches of pancreas and the biliary tree) in hepatolithiasis by performing immunohistochemistry using a set of fetal pancreatic markers and endoderm S/P cell markers. The present work clearly demonstrated the roles of peribiliary glands as biliary epithelial renewal and metaplasia and supported the previous findings as stem cell niches. It also provides interesting insights into the roles of peribiliary glands in inflammation-associated pancreatic duct cancer and cholangiocarcinoma.
| 1. | Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas. Pathol Int. 2010;60:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 2. | Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Gerrish K, Gannon M, Shih D, Henderson E, Stoffel M, Wright CV, Stein R. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J Biol Chem. 2000;275:3485-3492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Igarashi S, Matsubara T, Harada K, Ikeda H, Sato Y, Sasaki M, Matsui O, Nakanuma Y. Bile duct expression of pancreatic and duodenal homeobox 1 in perihilar cholangiocarcinogenesis. Histopathology. 2012;61:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552-570. [PubMed] |
| 7. | Nakanuma Y, Zen Y, Portman BC. Diseases of the bile ducts. MacSween’s Pathology of the Liver. Edinburg: Churchill Livingstone 2011; 491-562. |
| 8. | Terada T, Nakanuma Y, Ohta G. Glandular elements around the intrahepatic bile ducts in man; their morphology and distribution in normal livers. Liver. 1987;7:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Tsuneyama K, Kono N, Yamashiro M, Kouda W, Sabit A, Sasaki M, Nakanuma Y. Aberrant expression of stem cell factor on biliary epithelial cells and peribiliary infiltration of c-kit-expressing mast cells in hepatolithiasis and primary sclerosing cholangitis: a possible contribution to bile duct fibrosis. J Pathol. 1999;189:609-614. [PubMed] |
| 10. | Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 11. | Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Semeraro R, Carpino G, Cardinale V, Onori P, Gentile R, Cantafora A, Franchitto A, Napoli C, Anceschi M, Brunelli R. Multipotent stem/progenitor cells in the human foetal biliary tree. J Hepatol. 2012;57:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Nakanuma Y, Yamaguchi K, Ohta G, Terada T. Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1988;19:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Sasaki M, Ikeda H, Nakanuma Y. Expression profiles of MUC mucins and trefoil factor family (TFF) peptides in the intrahepatic biliary system: physiological distribution and pathological significance. Prog Histochem Cytochem. 2007;42:61-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers. II. A possible source of cholangiocarcinoma. Hepatology. 1990;12:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express alpha-amylase isozymes, trypsin and pancreatic lipase: an immunohistochemical analysis. Hepatology. 1993;18:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers. Heterotopic pancreas in the liver. Gastroenterology. 1990;98:1333-1337. [PubMed] |
| 19. | Gerber MA, Thung SN. Liver stem cells and development. Lab Invest. 1993;68:253-254. [PubMed] |
| 20. | Sutton ME, op den Dries S, Koster MH, Lisman T, Gouw AS, Porte RJ. Regeneration of human extrahepatic biliary epithelium: the peribiliary glands as progenitor cell compartment. Liver Int. 2012;32:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 658] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 22. | Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 24. | Ikeda H, Sasaki M, Ishikawa A, Sato Y, Harada K, Zen Y, Kazumori H, Nakanuma Y. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Ashida K, Terada T, Kitamura Y, Kaibara N. Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology. 1998;27:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 28. | Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Gandou C, Harada K, Sato Y, Igarashi S, Sasaki M, Ikeda H, Nakanuma Y. Hilar cholangiocarcinoma and pancreatic ductal adenocarcinoma share similar histopathologies, immunophenotypes, and development-related molecules. Hum Pathol. 2013;44:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
P- Reviewers Pinlaor S, Tanaka T S- Editor Zhai HH L- Editor Roemmele A E- Editor Ma S