Published online May 27, 2013. doi: 10.4254/wjh.v5.i5.264
Revised: March 1, 2013
Accepted: March 6, 2013
Published online: May 27, 2013
Processing time: 116 Days and 0.6 Hours
Assessment of liver fibrosis and steatosis is crucial in chronic liver diseases in order to determine the prognosis, the need of treatment, as well as monitor disease progression and response to treatment. Liver biopsy is limited by its invasiveness and patient acceptability. Transient elastography (TE, Fibroscan®) is a non-invasive tool with satisfactory accuracy and reproducibility to estimate liver fibrosis and steatosis. TE has been well validated in major liver diseases including chronic hepatitis B and C, non-alcoholic fatty liver disease, alcoholic liver disease, primary biliary cirrhosis, and primary sclerosing cholangitis. As alanine aminotransferase (ALT) is one of the major confounding factors of liver stiffness in chronic hepatitis B, an ALT-based algorithm has been developed and higher liver stiffness measurements (LSM) cutoff values for different stages of liver fibrosis should be used in patients with elevated ALT levels up to 5 times of the upper limit of normal. Otherwise falsely-high LSM results up to cirrhotic range may occur during ALT flare. TE is also useful in predicting patient prognosis such as development of hepatocellular carcinoma (HCC), portal hypertension, post-operative complications in HCC patients, and also survival. Unfortunately, failed acquisition of TE is common in obese patients. Furthermore, obese patients may have higher LSM results even in the same stage of liver fibrosis. The new XL probe, a larger probe with lower ultrasound frequency and deeper penetration, increases the success rate of TE in obese patients. The median LSM value with XL probe was found to be lower than that by the conventional M probe, hence cutoff values approximately 1.2 to 1.3 kPa lower than those of M probe should be adopted. Recent studies revealed a novel ultrasonic controlled attenuation parameter (CAP) of the machine is a useful parameter to detect even low-grade steatosis noninvasively. CAP may also be used to quantify liver steatosis by applying different cutoff values. As both LSM and CAP results are instantly available at same measurement, this makes TE a very convenient tool to assess any patients who are suspected or confirmed to suffer from chronic liver diseases.
Core tip: Transient elastography (TE, Fibroscan®) is a non-invasive tool with satisfactory accuracy to estimate liver fibrosis and steatosis. Liver stiffness measurement (LSM) with TE has been well validated to detect advanced fibrosis in most liver diseases. LSM is useful in predicting hepatocellular carcinoma (HCC), portal hypertension, post-operative complications in HCC patients, and survival. The new XL probe increases the success rate of TE in obese patients. A novel ultrasonic controlled attenuation parameter (CAP) of the machine is useful to detect steatosis noninvasively. Simultaneous LSM and CAP results make TE very convenient to assess any patients with suspected or confirmed liver diseases.
- Citation: Wong GLH. Transient elastography: Kill two birds with one stone? World J Hepatol 2013; 5(5): 264-274
- URL: https://www.wjgnet.com/1948-5182/full/v5/i5/264.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i5.264
Liver fibrosis is the natural wound-healing response to parenchymal injury in chronic liver diseases. Simple steatosis is a usually reversible and benign condition, but steatosis may be part of the more sinister condition in the setting of steatohepatitis where inflammation and hepatocyte changes co-exist[1-3]. Both liver fibrosis and steatohepatitis may eventually result in liver cirrhosis and its various complications. Sensitive detection and accurate staging of liver fibrosis and steatosis is now essentially indispensable in the decision process of treatment in chronic viral hepatitis as well as predicting disease prognosis[4,5]. It is also vital to monitor disease progression and response to treatment.
Liver biopsy has been the “gold standard” for assessing liver fibrosis and steatosis in the last few decades[6,7]. However, it has numerous limitations namely its invasive nature, risk of complications, patient discomfort, and sampling errors[8,9]. Complications associated with liver biopsy are rare but can be severe and even life threatening. Pain and hypotension are the predominant complications for which patients are hospitalized[10]. Clinically significant intraperitoneal hemorrhage is the rarest but most serious bleeding complication of percutaneous liver biopsy, which may happen more often in older age patients with cirrhosis or liver cancer[11]. Inadvertent puncture of gallbladder may lead to choleperitoneum[12]. The mortality rate among patients after percutaneous liver biopsy is approximately 1 in 10000 to 1 in 12000[13]. All these problems make it impractical to perform serial biopsies to assess disease progression in routine clinical practice[5]. The cost of liver biopsy is generally high as an in-patient bed, at least as day admission to hospital, is required. Including the charges of specialist doctor, nursing care and histologic examinations, it usually costs at the range of USD $800 to USD $1200 in Hong Kong.
The diagnostic accuracy of liver biopsy is limited by the sampling variability[14-16]. The average size of biopsy is 15 mm in length, which represents 1/50000 the size of the entire liver. There is significant variability in the histologic assessment of two readings of the same biopsy by the same pathologist, and between two pathologists, even among those who are highly specialized[8]. This variability is low for the diagnosis of cirrhosis (kappa coefficient of concordance ≥ 0.80), moderate for earlier fibrosis stages (kappa 0.70-0.80), but high for the activity grades (kappa 0.40-0.50)[8]. Fortunately, the variability is usually low for the diagnosis of steatosis (kappa coefficient of concordance ≥ 0.80)[4]. Given the above limitations to the practice of liver biopsy, a noninvasive transient elastography has been proposed as an alternative tool.
Transient elastography (TE; Fibroscan®; Echosens, Paris, France) measures liver stiffness[17] in patients suffering from different chronic liver diseases[18]. An ultrasound transducer probe is mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency (50 Hz) are transmitted by the transducer, inducing a plastic shear wave that propagates through the underlying tissues. Pulse-echo ultrasound acquisition is used to follow the propagation of the shear wave and to measure its velocity, which is directly related to tissue stiffness. The stiffer the tissue, the faster the shear wave propagates. TE measures liver stiffness in a volume that approximates a cylinder 1 cm in diameter and 4 cm in length, between 25 to 65 mm underneath the skin surface. This volume is at least 100 times bigger than a biopsy sample, and therefore should be more representative of the liver parenchyma[17]. Results of liver stiffness measurement (LSM) are expressed in kPa and correspond to the median of 10 validated measurements according to Sandrin et al[17]. According to the manufacturer, the examination is considered reliable if ≥ 10 valid measurements are acquired, the success rate (number of valid acquisitions divided by the number of attempts) is over 60%, and the ratio of the interquartile range to the median of 10 measurements (IQR/M) is ≤ 0.3[17].
It is important to assess liver steatosis, not only because non-alcoholic fatty liver disease (NAFLD) is the commonest liver disease[19], but also that steatosis often coexists in other chronic liver diseases likely chronic hepatitis C (CHC)[20]. A new physical parameter based on the properties of ultrasonic signals acquired by the machine has been recently developed to assess liver steatosis by applying the property that liver steatosis affects ultrasound propagation[21]. This controlled attenuation parameter (CAP), is measuring ultrasound attenuation (go and return path) at 3.5 MHz using signals acquired by the M probe of TE machine. Ultrasound attenuation is a physical property of the medium of propagation which corresponds to the loss of energy as ultrasound travels through the medium. Due to attenuation, the intensity of the emitted ultrasound decreases exponentially with depth[21]. At a given frequency, the ultrasound-attenuation coefficient (α) can be expressed in dB/m. The CAP is measured only on validated measurements according to the same criteria used for LSM, and on the same signals. This ensures that the operator obtains a liver ultrasonic attenuation simultaneously and in the same volume of liver parenchyma as the LSM. The final CAP value was the median of individual CAP values using the same valid measurements[22].
TE has the advantages of being painless, rapid (usually less than 5 min) and easy to perform at the bedside or in the outpatient clinic. The examination is performed on a non-fasting patient lying supine with the right arm placed behind the head to facilitate access to the right upper quadrant of the abdomen. The tip of the probe transducer is placed on the skin between the rib bones at the level of the right lobe of the liver where liver biopsy would be performed. Once the measurement area has been located, the operator presses the button on the probe to start an acquisition. The software determines whether each measurement is successful or not. The cost of a TE examination ranges from USD $100 to USD $150 in Hong Kong, which is much lower than a liver biopsy examination. It is obvious that TE is a user- and patient-friendly, but it would be even more important to be an accurate tool to assess liver fibrosis and steatosis.
Reproducibility of TE is an important feature for its widespread clinical application. The reproducibility of LSM was excellent for both inter-observer and intra-observer agreement, with intraclass correlation coefficients (ICC) of 0.98[23]. However, interobserver agreement was significantly reduced in patients with lower degrees of liver fibrosis (ICC for F0-1 and F2 were 0.60 and 0.99 respectively), with liver steatosis (ICC for steatosis < 25% and 25% of hepatocytes 0.98 and 0.90 respectively), and with increased body mass index (ICC for body mass index ≥ 25 kg/m2 and < 25 kg/m2 were 0.98 and 0.94 respectively).
Using TE to assess liver fibrosis has been widely validated in different liver diseases, including CHC[4,24,25], chronic hepatitis B (CHB)[26-28], co-infection with HIV[29], NAFLD[30,31], alcoholic liver disease[32], primary biliary cirrhosis, primary sclerosing cholangitis (PSC)[33], post-liver transplantation setting[34], and in cystic fibrosis[35,36]. In these studies, TE was valid with liver histology being the gold standard. In general, all these studies confirm that TE has good overall accuracy to diagnose advanced fibrosis and cirrhosis (though some uncommon diseases like PSC and cystic fibrosis are under-represented by small numbers of patients), independent of the underlying etiology[37,38]. The remaining controversy is the optimal cutoff values to diagnose advanced fibrosis and cirrhosis, which differ according to particular etiologies. This has significant implication when a clinician interprets TE results. The suggested diagnostic performance and cutoff values for histologic cirrhosis (F4) based on published studies are summarized in Table 1.
| Ref. | Biopsies (n) | Prevalence of cirrhosis (F4) | Etiologies | Proposed cutoff values (kPa) | Sensitivity | Specificity | NPV | PPV | Positive LR | Negative LR | AUROC |
| Castéra et al[4], 2005 | 183 | 25% | HCV | 12.5 | 87% | 91% | 95% | 77% | 9.7 | 0.1 | 0.95 |
| Fraquelli et al[23], 2007 | 200 | 12% | All | 11.9 | 91% | 89% | 98% | 53% | 8.3 | 0.1 | 0.9 |
| Arena et al[24], 2008 | 150 | 19.3% | HCV | 14.8 | 94% | 92% | 98% | 73% | 11.3 | 0.07 | 0.99 |
| Ziol et al[25], 2005 | 251 | 19% | HCV | 14.6 | 86% | 96% | 97% | 78% | 23.1 | 0.1 | 0.97 |
| Chan et al[26], 2009 | 161 | 25% | HBV | 13.4 | 60% | 93% | 88% | 75% | 85 | 0.43 | 0.93 |
| Marcellin et al[27], 2009 | 173 | 8% | HBV | 11 | 93% | 87% | 99% | 38% | 7 | 0.08 | 0.93 |
| Wong et al[28], 20101 | 238 | 23.5% | HBV | 9.0 (normal ALT) 12.0 (elevated ALT) | 54% | 99% | 67% | 98% | 3.3 | 0.7 | 0.88 |
| de Lédinghen et al[29], 2006 | 72 | 23.6% | HCV-HIV | 11.8 | 100% | 92.7% | 82% | 100% | 13.7 | 0 | 0.97 |
| Nobili et al[30], 20081 | 52 | 5.8% | NAFLD | 10.2 | 100% | 100% | 100% | 100% | ∞ | 0 | 1 |
| Wong et al[31], 2010 | 246 | 10.1% | NAFLD | 10.3 | 92% | 88% | 99% | 46% | 7.5 | 0.09 | 0.95 |
| Nahon et al[32], 2008 | 174 | 53.7% | ALD | 22.7 | 84% | 83% | 82% | 85% | 5.24 | 0.19 | 0.87 |
| Corpechot et al[33], 2006 | 95 (66 PBC, 29 PSC) | 16% | PBC/PSC | 17.3 | 93% | 95% | 99% | 78% | 18.6 | 0.1 | 0.96 |
| Carrión et al[34], 2006 | 124 | 11% | HCV-LT | 12.5 | 100% | 87% | 100% | 50% | 7.7 | 0 | 0.98 |
| Witters et al[36], 2009 | 66 | NA | Cystic fibrosis | 6.5 | 100% | 81% | NA | NA | NA | NA | 0.92 |
| Coco et al[75], 2007 | 228 | 20.2% | HCV/HBV | 14 | 78% | 98% | 82% | 98% | 39 | 0.2 | 0.96 |
| Ganne-Carrié et al[106], 2006 | 775 | 15.5% | All | 14.6 | 79% | 95% | 96% | 74% | 15.8 | 0.1 | 0.95 |
| Foucher et al[107], 2006 | 354 | 13.3% | All | 17.6 | 77% | 97% | 92% | 91% | 25.7 | 0.2 | 0.96 |
| Gómez-Domínguez et al[108], 2006 | 94 | 17% | All | 16 | 89% | 96% | 98% | 80% | 22.3 | 0.1 | 0.94 |
| Vergara et al[109], 2007 | 169 | 38.5% | HCV-HIV | 14.6 | 93% | 88% | 94% | 86% | 7.8 | 0.1 | 0.95 |
| Rigamonti et al[110], 2008 | 95 | 17% | HCV-LT | 12 | 93% | 93% | 99% | 74% | 14 | 0.1 | 0.9 |
| Yoneda et al[111], 2007 | 67 | 7.5% | NAFLD | 17 | 100% | 98% | 95% | 64% | 50 | 0 | 0.99 |
In a retrospective study of 115 patients of mixed etiologies of chronic liver diseases, CAP was found efficient to detect low grade steatosis (> 10%), with a sensitivity of 91% and specificity of 81% at a cutoff value of 238 dB/m[21]. The accuracy of CAP was confirmed in two prospective studies of mixed etiologies[39,40], as well as in individual etiology, including CHB, CHC, NAFLD and alcoholic liver disease[41-44]. The suggested diagnostic performance and cutoff values for different degrees of steatosis are summarized in Table 2. As a well-validated tool, TE is also well-investigated in different aspects of clinical applications.
| Ref. | Biopsies (n) | Study design | Etiologies | AUROC≥S1 (11%) | AUROC≥S2 (34%) | AUROC≥S3 (67%) | Cutoff values for≥S1 (dB/m) | Sen | Spe | NPV | PPV | Cutoff values for≥S2 (dB/m) | Sen | Spe | NPV | PPV | Cutoff values for≥S3 (dB/m) | Sen | Spe | NPV | PPV |
| Sasso et al[21], 2010 | 115 | Retrospective | All | 0.91 | 0.95 | 0.89 | 238 | 91% | 81% | 87% | 87% | 259 | 89% | 86% | 92% | 80% | 292 | 100% | 78% | 100% | 28% |
| de Lédinghen et al[39], 2012 | 112 | Prospective | All | 0.84 | 0.86 | 0.93 | 215 | 252 | 296 | ||||||||||||
| Myers et al[40], 2012 | 153 | Prospective | All | 0.81 | 283 | 76% | 79% | 64% | 87% | ||||||||||||
| Beaugrand et al[41], 2010 | 74 | Retrospective | ALD | 0.81 | 0.87 | 0.82 | |||||||||||||||
| Beaugrand et al[42], 2010 | 96 | Retrospective | ALD/NAFLD | 0.86 | 0.87 | 0.77 | |||||||||||||||
| Cardoso et al[43], 2010 | 133 | Retrospective | CHB | 0.82 | 0.81 | - | |||||||||||||||
| Sasso et al[44], 2012 | 615 | Retrospective | CHC | 0.8 | 0.86 | 0.88 |
The severity of liver fibrosis is the key factor of timing and choice of therapy. This is particularly relevant in chronic viral hepatitis. Current international guidelines recommend antiviral therapy for CHB patients with significant liver fibrosis[45-47]. As TE has been repeatedly shown to have satisfactory accuracy to exclude and diagnose advanced fibrosis and cirrhosis as mentioned above, more than half of the patients might reach treatment decision without the need for confirmatory liver biopsies[26]. TE is also found to be more cost-effective than liver biopsy[48]. TE has been incorporated in the international guidelines of CHB and CHC[45,46]. TE, together with other non-invasive parameters, can also be used as the screening tool for cirrhosis in asymptomatic people[49-51], as well as the diagnostic and/or prognostic tool of NAFLD, such that the need of liver biopsy can be reduced[52,53].
A few longitudinal studies have been reported that patients responding to treatment had low or decreased liver stiffness[54]. In fact, both reduction in fibrosis and necroinflammation might contribute to the decrease in liver stiffness[55]. In a prospective study of 71 CHB patients on antiviral therapy, paired liver biopsy and TE were both performed at baseline and at 1 year of treatment[56]. Although TE remained accurate in distinguishing patients with insignificant disease from those with advanced fibrosis or cirrhosis at both time points, the absolute change in liver stiffness correlated poorly with the change in histological fibrosis stage, and resolution of advanced fibrosis could only be assumed with significantly decreased liver stiffness to 5.0 kPa or less after antiviral treatment[56].
TE is found useful to identify cirrhotic patients with higher risk of portal hypertension, and cutoff values of 17.6 kPa and 21.0 kPa having sensitivity ≥ 90% in order to detect patients with hepatic venous pressure gradient (HVPG) above 10-12 mmHg[57,58]. Presence of varices could be excluded with a liver stiffness below 12.5-19.8 kPa[59,60]. Unfortunately, these suggested cutoff values overlap with those for detecting histologic cirrhosis in most chronic liver diseases. Hence there seems no significant new information provided by TE regarding screening endoscopy for varices among cirrhotic patients.
TE is also useful to predict the risk other liver-related complications and death. A dose-response relationship between LSM and risk of hepatocellular carcinoma (HCC) was found in both CHB and CHC patients (Table 3). Taking patients with LSM ≤ 10.0 kPa as reference, the hazard ratios of developing HCC were 17, 21, 26, and 46 in patients with LSM at 10.1-15.0, 15.1-20.0, 20.1-25.0 and above 25.0 kPa respectively, in a prospective cohort of 866 CHC patients[61]. Patients with LSM ≤ 8.0 kPa acted as the control group, the hazard ratios of developing HCC were 3.1, 4.7, 5.6 and 6.6 in patients with LSM at 8.1-13.0, 13.1-18.0, 18.1-23.0 and above 23.0 kPa respectively in another cohort of 1130 CHB patients[62]. LSM, as well as FibroTest, can also predict 5-year survival of patients with CHC; the prognostic values of LSM remained even after adjustments for treatment response, patient age, and degree of necroinflammation[63].
| Chronic hepatitis B patients | Chronic hepatitis C patients | ||
| LSM (kPa) | HR of HCC | LSM (kPa) | HR of HCC |
| ≤ 10.0 | Referent | ≤ 8.0 | Referent |
| 10.1-15.0 | 17 | 8.1-13.0 | 3.1 |
| 15.1-20.0 | 21 | 13.1-18.0 | 4.7 |
| 20.1-25.0 | 26 | 18.1-23.0 | 5.6 |
| > 25.0 | 46 | > 23.0 | 6.6 |
LSM is also an important prognostic tool in patients confirmed to have HCC. A prospective study of 105 HCC patients demonstrated that a LSM cutoff of 12.0 kPa had the sensitivity of 86% and specificity of 72% in predication of major post-operative complications[64]. This cutoff might also identify patients with more severe operative blood loss and higher transfusion rate[64]. Another study of 133 HCC patients revealed that patients of LSM ≥ 13.4 kPa had a nearly 2-fold increase in the risk of HCC recurrence compared to those with LSM < 13.4 kPa[65].
Liver steatosis is a common histological feature in the general population and in patients with chronic liver disease. Its prevalence is high: almost 30% in the general population[66,67], 50% in patients with CHC[68], above 80% in severely obese patients[69]. Liver steatosis plays a pivotal role in CHC, as metabolically (instead of virally) induced steatosis is associated with a lower response rate to antiviral treatment[70] and liver fibrosis progression[71]. Steatosis may also increase the risk of HCC[72]. Detection of liver steatosis is also important to the potential donors for liver transplantation, as their extent of steatosis is directly related to primary non-functioning of the graft, which may result in mortality or the need for re-transplantation[73]. Liver steatosis is also a risk factor for post-operative complications and mortality after liver resection[74].
There have been enough data to prove TE is accurate and applicable in different clinical settings. How this tool also has a few shortcomings that any user should keep in mind.
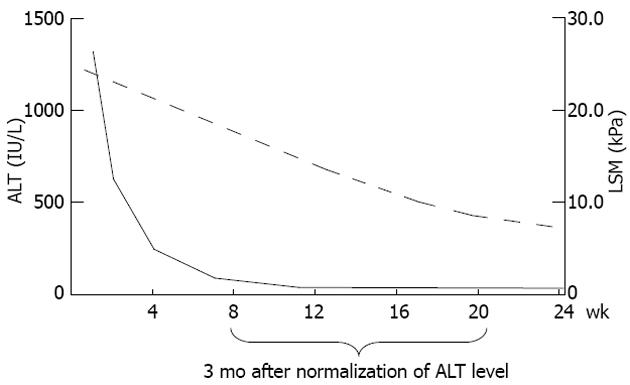
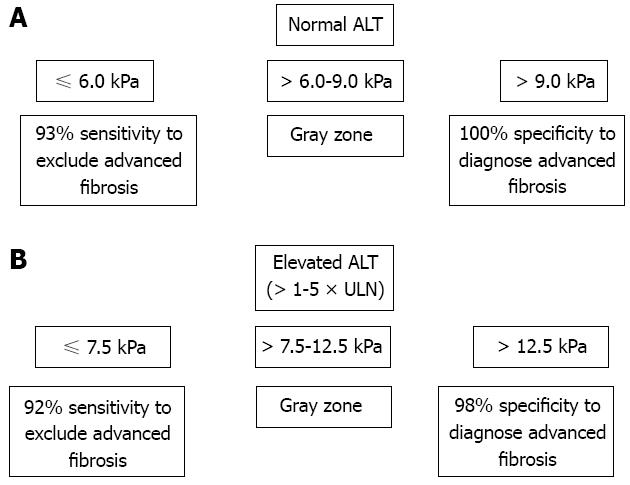
Not only liver fibrosis but also other factors contribute to the liver stiffness. LSM has been consistently found to be falsely elevated in acute hepatitis manifested as alanine aminotransferase (ALT) flares[75,76]. Severe hepatic necroinflammation may lead to LSM values well within the cirrhotic range even in the absence of fibrosis on histology[55,77,78]. In this setting, LSM tends to decrease considerably after the resolution of acute hepatitis. Therefore, applying TE in this scenario can be misleading and not recommended until at least 3 mo after normalization or at least stabilization of ALT levels below 5 times the upper limit of normal[26,76] (Figure 1). An ALT-based algorithm has been developed and higher LSM cutoff values for different stages of liver fibrosis should be used in patients with elevated ALT levels (Figure 2). This also leads to another advantage of liver biopsy over TE that at this stage the necroinflammatory score is only available in histologic assessment.
Extrahepatic cholestasis[79], hepatic congestion[80,81], hepatic amyloidosis[82] and recent food intake (within 60 min)[83] were also found associated with a falsely high LSM values. Fortunately, the degree of liver steatosis does not appear to affect LSM results, therefore TE remains an accurate tool for fibrosis assessment in CHC and NAFLD[24,31]. A recent study found that the correlation between LSM and fibrosis stage was less strong in CHB and NAFLD than in CHC patients[84]. Our recent study showed that NAFLD patients with BMI 30 kg/m2, the lowest limit of an abnormal BMI in NAFLD, would have higher LSM values by M probe even in the same fibrosis stage[85]. This provocative finding may lead to concern about the M probe accuracy in obese patients. The emergence of XL probe is a possible solution to this issue.
It has been noted that unreliable and failed LSM occur at about 3% and 11.6% to 18.4% in all TE examinations, respectively, and they are independently associated body mass index (BMI) > 30 kg/m² in both Caucasians and Chinese[86,87]. The success rate of LSM with M probe would be as low as 75%[31] in NAFLD patients with BMI > 30 kg/m2. The low LSM success rate among obese patients is likely related to the thick subcutaneous fat, which hinders the transmission of shear waves and ultrasound waves through the liver parenchyma[87]. Patients with extreme very high and very low BMI were recently found to have higher LSM values in an Indian population[88]. Subjects with narrow intercostal space, high riding liver, hyperinflated lungs, ascites or free peritoneal fluid[17] may also have lower success rate or failed acquisition of LSM.
A recent study challenged the validity of the reliability criteria suggested by the manufacturer of 1165 patients with chronic liver diseases who underwent LSM within 3 mo of liver biopsy. The investigators found the number of successful acquisitions, and its success rate having no influence on the diagnostic accuracy[89]. Furthermore, LSM remained reliable even if the ratio of the interquartile range to the median of 10 measurements (IQR/M) > 0.30, provided that the median LSM < 7.1 kPa. These new findings implied that LSM results were more reliable than what was previously described.
Only a few studies on CAP have been published so far, and a few of them were only in abstract form. Hence CAP needs further validation in larger populations. Furthermore, CAP is not yet available in the measurements with the XL probe, which is designed for overweight and obese patients who are particularly at risk of liver steatosis[69]. Therefore, further development and calibration of CAP in XL probe is warranted.
In general, serum markers have modest accuracy to diagnose advanced liver fibrosis[90,91]. TE has certain advantages over serum markers, as TE provides a more direct measurement of fibrosis, is less affected by inter-current health disorders, and is theoretically applicable to all chronic liver diseases. On the other hand, the diagnostic performance was particularly affected in patients with elevated serum ALT levels[55]. Hence a second non-invasive test independent of the serum ALT or AST levels may be a good supplementary test for LSM. Among various serum test formulae, Forns index[92] and Hui index[90] are composed of clinical parameters other than ALT or AST levels. We demonstrated that a combined LSM-Forns algorithm improved the accuracy to predict advanced liver fibrosis in 238 CHB patients[28]. In this combined algorithm, low LSM or low Forns index could be used to exclude advanced fibrosis with a high sensitivity of 95%. To confirm advanced fibrosis, agreement between high LSM and high Forns index could improve the specificity up to 99% to 100%[28].
The combination of TE and FibroTest was found to have the best diagnostic performance compared to either test alone in patients with CHC[4]. When TE and FibroTest matched (present in 70%-80% of cases), results were also concordant, respectively in 84%, 95% and 94% of patients with liver fibrosis ≥ F2, ≥ F3 and F = 4[4]. The combination of LSM and FibroTest allowed exclusion of significant fibrosis (≥ F2) in nearly 80% of 100 CHB patients in inactive carrier stage.
The development of S and XL probes aim to cater for different population groups of various body-build types (Table 4). S probe contains a higher frequency ultrasonic transducer and shallower measurements below the skin surface, which suit pediatric subjects and those with small body build[93]. XL probe contains a lower frequency and a more sensitive transducer, a deeper focal length, larger vibration amplitude and a higher depth of measurements below the skin surface[94]. This probe serves obese subjects with “XL” body builds. Data concerning the validations of these new probes are emerging.
| Probe | Frequency of ultrasound (MHz) | Depth (mm) |
| S | 5 | 15-40 |
| M | 3.5 | 25-65 |
| XL | 2.5 | 35-75 |
With the XL probe, LSM could be successfully performed in more obese patients compared to the M probe[95]. In our validation study involving 286 patients, LSM using XL probe documented reliable results in 92% of patients, compared to 80% using M probe (64). In another study of 193 NAFLD patients, a cutoff value had reasonable sensitivity (78%), specificity (78%), positive predictive value (60%), and good negative predictive value (89%) for F3 or greater disease[96]. However, the median LSM by the XL probe was consistently found to be approximately 1.0 to 1.2 kPa lower than that of the M probe at the same stage of liver fibrosis in all of the histologic reports[95,96]. A recent exploratory study of 517 overweight patients having different etiologies, XL cutoff values of 4.8 kPa and 10.7 kPa, 6.0 kPa and 12.0 kPa with the M probe[85], for patients with BMI > 25-30 kg/m2. Patients with BMI > 30 kg/m2 might use M probe cut-offs for the XL probe. More studies are warranted to delineate the proper cutoff values of LSM using the XL probe in various etiologies.
Recent enthusiasm on spleen stiffness measurement (SSM) leads us to the non-invasive evaluation of portal hypertension, which is conventionally assessed by HVPG via hepatic angiogram[97]. SSM was recently found accurate to predict portal hypertension and esophageal varices[98,99]. The clinical role of SSM will be explored more in the near future.
Acoustic radiation force impulse (ARFI) is another new imaging technology based on the shear acoustic waves remotely induced by the radiation force of a focused ultrasonic beam[100]. ARFI may be even more accurate than TE for both significant and severe classes of liver fibrosis in CHC patients[101]. ARFI was also used for SSM in CHB and CHC patients[102]. Another technique called real-time tissue elastography (RTE) is incoporated into B-mode ultrasography machine[103]. RTE is also found accurate to diagnose liver fibrosis and portal hypertension[104,105]. All these new technologies are also promising and should be further validated in the near future.
TE is a non-invasive, accurate and reproducible test of advanced liver fibrosis, cirrhosis and steatosis. This tool has been validated in a wide spectrum of liver diseases. TE is also useful to predict patient outcomes. Further studies should explore the appropriate cutoff values of newer XL and S probes, and exploring the prognostic role of CAP.
| 1. | Lonardo A, Bellentani S, Ratziu V, Loria P. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient - death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 934] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 3. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 4. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 5. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 6. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 7. | Campbell MS, Reddy KR. Review article: the evolving role of liver biopsy. Aliment Pharmacol Ther. 2004;20:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 9. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1635] [Article Influence: 96.2] [Reference Citation Analysis (2)] |
| 10. | Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118:96-98. [PubMed] |
| 11. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 12. | Di Virgilio D, Colella F, Lancia A, Nicolella U, Ravallese F, Lucherini M, Cavacece A. Intracholecystic hemorrhage: an atypical complication after liver needle biopsy. Ann Ital Med Int. 1992;7:179-181. [PubMed] |
| 13. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 14. | Poynard T, Benhamou Y, Thabut D, Ratziu V. Liver biopsy: the best standard...when everything else fails. J Hepatol. 2009;50:1267-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Poynard T, Lenaour G, Vaillant JC, Capron F, Munteanu M, Eyraud D, Ngo Y, M’Kada H, Ratziu V, Hannoun L. Liver biopsy analysis has a low level of performance for diagnosis of intermediate stages of fibrosis. Clin Gastroenterol Hepatol. 2012;10:657-663.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Ratziu V, Bugianesi E, Dixon J, Fassio E, Ekstedt M, Charlotte F, Kechagias S, Poynard T, Olsson R. Histological progression of non-alcoholic fatty liver disease: a critical reassessment based on liver sampling variability. Aliment Pharmacol Ther. 2007;26:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 18. | Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol. 2010;25:1726-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Clouston AD, Jonsson JR, Powell EE. Steatosis as a cofactor in other liver diseases: hepatitis C virus, alcohol, hemochromatosis, and others. Clin Liver Dis. 2007;11:173-189, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 654] [Article Influence: 40.9] [Reference Citation Analysis (3)] |
| 22. | Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 660] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 24. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1097] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 26. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 27. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 28. | Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2010;31:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175-179. [PubMed] |
| 30. | Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 31. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 32. | Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Lédinghen V, Douvin C, Marcellin P, Ganne-Carrié N, Trinchet JC, Beaugrand M. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 34. | Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 35. | Karlas T, Neuschulz M, Oltmanns A, Güttler A, Petroff D, Wirtz H, Mainz JG, Mössner J, Berg T, Tröltzsch M. Non-invasive evaluation of cystic fibrosis related liver disease in adults with ARFI, transient elastography and different fibrosis scores. PLoS One. 2012;7:e42139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Witters P, De Boeck K, Dupont L, Proesmans M, Vermeulen F, Servaes R, Verslype C, Laleman W, Nevens F, Hoffman I. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros. 2009;8:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1091] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 38. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 39. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 40. | Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 41. | Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Fournier C, Sandrin L, Miette V, Sasso M. Validation of controlled attenuation parameter (CAP) as a non-invasive marker of steatosis in 228 patients with chronic liver disease from various causes. J Hepatol. 2010;52:S35-S36. |
| 42. | Beaugrand M, Ziol M, de Ledinghen V, Douvin C, Fournier C, Sandrin L, Miette V, Sasso M. Controlled attenuation parameter: A novel FibroScan®-based tool to detect and quantify steatosis. Preliminary study in patient with alcoholoc and non alcoholic fatty liver disease. J Hepatol. 2010;52:S158-S159. |
| 43. | Cardoso AF, Sasso MC, Miette V, Fournier C, Sandrin L, Beaugrand M, Douvin C, de Ledinghen V, Poupon R, Ziol M. Controlled attenuation parameter: A novel FibroScan-based tool to detect and quantify steatosis in chronic hepatitis B. Controlled Attenuation Parameter: A Novel FibroScan-based Tool to Detect and Quantify Steatosis in Chronic Hepatitis B. Available from: http://onlinelibrary.wiley.com.easyaccess1.lib.cuhk.edu.hk/doi/10.1002/hep.23974/pdf. |
| 44. | Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 45. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 46. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 47. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (2)] |
| 48. | Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol. 2009;24:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Castera L. Screening the general population for cirrhosis using transient elastography: finding a needle in a haystack? Gut. 2011;60:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 51. | Stepanova M, Aquino R, Alsheddi A, Gupta R, Fang Y, Younossi Z. Clinical predictors of fibrosis in patients with chronic liver disease. Aliment Pharmacol Ther. 2010;31:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, Loria P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 53. | Festi D, Schiumerini R, Marzi L, Di Biase AR, Mandolesi D, Montrone L, Scaioli E, Bonato G, Marchesini-Reggiani G, Colecchia A. Review article: the diagnosis of non-alcoholic fatty liver disease -- availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Arima Y, Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Kobayashi K, Ichino N. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res. 2010;40:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Chan HL. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chu SH, Chan FK, Sung JJ, Chan HL. On-treatment monitoring of liver fibrosis with transient elastography in chronic hepatitis B patients. Antivir Ther. 2011;16:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 58. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 59. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 60. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrié N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 61. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 62. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (2)] |
| 63. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-1979, 1979.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 64. | Wong JS, Wong GL, Chan AW, Wong VW, Cheung YS, Chong CN, Wong J, Lee KF, Chan HL, Lai PB. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg. 2013;257:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Jung KS, Kim SU, Choi GH, Park JY, Park YN, Kim do Y, Ahn SH, Chon CY, Kim KS, Choi EH. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan®). Ann Surg Oncol. 2012;19:4278-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2721] [Article Influence: 123.7] [Reference Citation Analysis (3)] |
| 67. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 68. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 69. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [PubMed] |
| 70. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 71. | Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM, Dhumeaux D. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003;52:288-292. [PubMed] |
| 72. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 73. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1830] [Article Influence: 91.5] [Reference Citation Analysis (15)] |
| 74. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 75. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 76. | Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 581] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 78. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [PubMed] |
| 79. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 80. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 81. | Lebray P, Varnous S, Charlotte F, Varaut A, Poynard T, Ratziu V. Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology. 2008;48:2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Janssens F, Spahr L, Rubbia-Brandt L, Giostra E, Bihl F. Hepatic amyloidosis increases liver stiffness measured by transient elastography. Acta Gastroenterol Belg. 2010;73:52-54. [PubMed] |
| 83. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 84. | Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, Marzano A, Rizzetto M. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 85. | Wong GL, Chan HL, Choi PC, Chan AW, Lo AO, Chim AM, Wong VW. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11:295-302.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 87. | Wong GL, Wong VW, Chim AM, Yiu KK, Chu SH, Li MK, Chan HL. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol. 2011;26:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 88. | Das K, Sarkar R, Ahmed SM, Mridha AR, Mukherjee PS, Das K, Dhali GK, Santra A, Chowdhury A. “Normal” liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology. 2012;55:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 90. | Hui AY, Chan HL, Wong VW, Liew CT, Chim AM, Chan FK, Sung JJ. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol. 2005;100:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 92. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 726] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 93. | Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, Grulich-Henn J, Schenk JP, Teufel U. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 2012;171:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 95. | de Lédinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, Le Bail B, Choi PC, Chermak F, Yiu KK. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 96. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 97. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 98. | Abraldes JG, Reverter E, Berzigotti A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology. 2013;57:1278-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 100. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 443] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 101. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 102. | Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Noninvasive assessment of liver fibrosis via spleen stiffness measurement using acoustic radiation force impulse sonoelastography in patients with chronic hepatitis B or C. J Viral Hepat. 2012;19:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Koizumi Y, Hirooka M, Kisaka Y, Konishi I, Abe M, Murakami H, Matsuura B, Hiasa Y, Onji M. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography--establishment of the method for measurement. Radiology. 2011;258:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Colombo S, Buonocore M, Del Poggio A, Jamoletti C, Elia S, Mattiello M, Zabbialini D, Del Poggio P. Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012;47:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 105. | Ochi H, Hirooka M, Koizumi Y, Miyake T, Tokumoto Y, Soga Y, Tada F, Abe M, Hiasa Y, Onji M. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology. 2012;56:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 107. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 966] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 108. | Gómez-Domínguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, Ríos MJ, García-García JA, Camacho A, López-Cortés L. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Rigamonti C, Donato MF, Fraquelli M, Agnelli F, Ronchi G, Casazza G, Rossi G, Colombo M. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut. 2008;57:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 111. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
P- Reviewers Al-Shamma S, Guerrero-Romero F, Lonard A, Roeb E, Singh V S- Editor Gou SX L- Editor A E- Editor Li JY