Published online Mar 27, 2013. doi: 10.4254/wjh.v5.i3.133
Revised: January 6, 2013
Accepted: January 29, 2013
Published online: March 27, 2013
A 26-year-old male presented with three weeks of jaundice after the self-initiation of the injectable anabolic steroid, Mastabol [Dromastanolone Di-Propionate (17 beta-Hydroxy-2alpha-methyl-5alpha-androstan-3-one propionate)]. He reported dark urine, light stools, and pruritus. He denied abdominal pain, intravenous drug use, intranasal cocaine, blood transfusions, newly placed tattoos, or sexually transmitted diseases. He used alcohol sparingly. Physical exam revealed jaundice with deep scleral icterus. The liver was palpable 2 cm below the right costal margin with no ascites. The peak bilirubin was 23.6 mg/dL, alkaline phosphatase was 441 units/L, and aspartate aminotransferase/alanine aminotransferase were 70 units/L and 117 units/L respectively. A working diagnosis of acute intrahepatic cholestasis was made. Liver biopsy revealed a centrilobular insult with neutrophilic infiltrates and Ito cell hyperplasia consistent with acute drug induced cholestasis. The patient’s clinical symptoms resolved and his liver enzymes, bilirubin, and alkaline phosphatase normalized. Anabolic steroids with 17 alpha carbon substitutions have been associated with a bland variety of cholestatic injury with little hepatocellular injury. Cholestasis, under these circumstances, may be secondary to the binding of drugs to canalicular membrane transporters, accumulation of toxic bile acids from canalicular pump failure, or genetic defects in canalicular transport proteins. Mastabol is an injectable, 17 beta hydroxyl compound with no alpha alkyl groups at the 17 carbon position. As such, it has been reported to have little potential toxic effects on the liver. This is the first known reported case of Mastabol-induced cholestatic liver injury. It highlights the need for physicians to consider such widely available substances when faced with hepatic injury of unclear etiology.
- Citation: Hymel BM, Victor DW, Alvarez L, Shores NJ, Balart LA. Mastabol induced acute cholestasis: A case report. World J Hepatol 2013; 5(3): 133-136
- URL: https://www.wjgnet.com/1948-5182/full/v5/i3/133.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i3.133
Mastabol is a readily available, injectable, synthetic dihydrotestosterone with no aromatization potential which limits its estrogen effects. This is a particularly attractive feature to the bodybuilding community. Mastabol is not regulated, in any way, by the Food and Drug Administration (FDA). There are very few medical indications for the use of anabolic steroids; one of which is the treatment of certain rare forms of aplastic anemia[1].
The adverse hepatic effects of anabolic steroids have largely focused on cholestatic effects, but there are several other types of hepatic injury which have been linked to these compounds. Case reports have suggested that anabolic steroids may play a role in the so called toxicant-associated steatohepatitis[2]. In 2005, Socas et al[3] reported anabolic-androgenic induced hepatocellular adenoma occurance in two bodybuilders. Typically, anabolic androgens result in cholestatic liver injury, but in 2002, Stimac et al[4] reported a case of anabolic steroid induced hepatocellular necrosis. A case report was published by Patil et al[5] describing a spontaneous hepatic rupture with hemorrhage and shock in a male with a history of anabolic steroid use.
Anabolic steroids with substitution groups at the 17 carbon position can have particularly undesirable effects on the liver. In particular, 17 alpha alkyl substitutions result in a decreased first-pass hepatic metabolism and, therefore, can allow for a greater oral bioavailability. These compounds are known to provoke a highly characteristic intrahepatic cholestasis via their direct toxic effects[6]. In 2007, Kafrouni et al[7] reported on a case of cholestatasis in two male bodybuilders, ages 40 and 31, who were utilizing the anabolic steroids superdrol and superdrol/holodrol, respectively, as part of their training regimens. Within weeks of ingestion, they both presented with symptoms of weight loss, jaundice, and pruritus. The symptoms resolved and liver panels normalized upon discontinuation of the substances. Ursodeoxycholic acid for pruritus, and in one of the cases, a short course of prednisone were also utilized. The use of methandienone by mouth daily and stanozolol by intramuscular injection resulted in severe cholestatic liver injury in a 28 year-old-male, as reported by Habscheid et al[8] in 1999. In this case, the patient’s condition deteriorated over a seven week course after the steroids were discontinued and improvement in jaundice and liver chemistries corresponded with the initiation of ursodiol[8].
Like other anabolic steroids, the mass building effects of Mastabol are thought to be secondary to enhanced protein synthesis and positive nitrogen balance. Other possible suggested uses for anabolic steroids include the stimulation of red blood cells, enhancement of male sexual characteristics, and palliation of androgen responsive, recurrent breast cancers in postmenopausal females. The 17 alpha substituted anabolic steroid, oxymetholone, has been FDA approved for treatment of anemia in patients with deficient red blood cell production[9]. Contraindications, as per the manufacturer, include drug hypersensitivity, development of male breast cancer, prostate cancer, and serious cardiac, liver, and renal impairment. The vast majority of anabolic steroids are not regulated or endosed by the FDA, and as they are available over-the-counter, are often assumed to be generally safe. Further, searching the term, Mastabol, in any internet search engine can produce up to 14 000 results detailing how it can be accessed. However, a pubmed search of “Mastabol” revealed no results, which further highlights the significance of this case report.
Mastabol (17 beta-Hydroxy-2alpha-methyl-5alpha-androstan-3-one propionate) is a common anabolic steroid used by bodybuilders as an over-the-counter supplement to augment their training efforts. Its chemical structure does not include a 17 alpha alkyl group, and as such, has been marketed on the internet as having little to no potential hepatotoxic affects. Here, we present the case of a 26-year-old male with biopsy proven drug induced cholestasis seven to ten days following the initiation of the injectable body building supplement, Mastabol. To our knowledge, this is the first reported case of adverse hepatobiliary effects associated with Mastabol.
A 26-year-old male presented with three weeks of jaundice beginning ten days after the self- initiation of the injectable bodybuilding supplement, Mastabol. He had previously been healthy and was working full time as an offshore oil field wire operator. Upon the onset of jaundice, he also reported associated dark colored urine and light colored stools. Days later, he developed generalized pruritus that progressively worsened such that he was unable to sleep at night. He reported losing 40 pounds over a three month period, roughly two thirds of which was through diet and exercise prior to the onset of illness. He denied nausea, emesis, abdominal pain, diarrhea, constipation, fever, chills, or night sweats. He reportedly followed the dosing instructions on the label. Further, he denied any exposure to known hepatitis carriers, exposure to intravenous drugs, intranasal cocaine, blood transfusions, newly placed tattoos, and sexually transmitted diseases, such as herpes. He reported a monogamous relationship with his wife.
Further social history was significant for drinking alcohol sparingly, one to two drinks per month, and smoked one pack of cigarettes daily for approximately ten years. He denied known toxic exposure at work. Past medical history included asthma and bronchitis as a child with no adult episodes and reported peptic ulcer disease four years ago. The patient’s family history was negative for liver disease, including hepatitis, jaundice, cirrhosis, or malignancy. He was taking no prescribed medications and no herbal or over-the-counter medications except the previously mentioned Mastabol.
Physical exam revealed a jaundiced, oriented male with normal vital signs. Deep scleral icterus was noted. There was no stigmata of cirrhosis or portal hypertension. Several excoriated lesions were noted on the lower extremities. The liver was palpable 2 cm below the right costal margin in the midaxillary line on inspiration, the spleen was not palpable, and there was no evidence of ascites. No joint tenderness or effusion was noted. Neurological exam was non-focal with no asterixis.
On data evaluation, peak bilirubin was 23.6 mg/dL with a direct fraction of 20.5 mg/dL, alkaline phosphatase was 441 units/L, and aspartate aminotransferase/alanine aminotransferase were 70 units/L and 117 units/L respectively. The albumin was 3.1 and international normalized ratio 0.98 with all other chemistries and blood counts unremarkable. All other causes of hepatitis, including viral, autoimmune, and genetic, were ruled out by appropriate testing.
A working diagnosis of acute, nonobstructive, intrahepatic cholestatic hepatitis secondary to Mastabol was made. The patient was placed on phenobarbitol for management of pruritus, and a liver biopsy was scheduled. He was also urged to avoid any other medications in the interim.
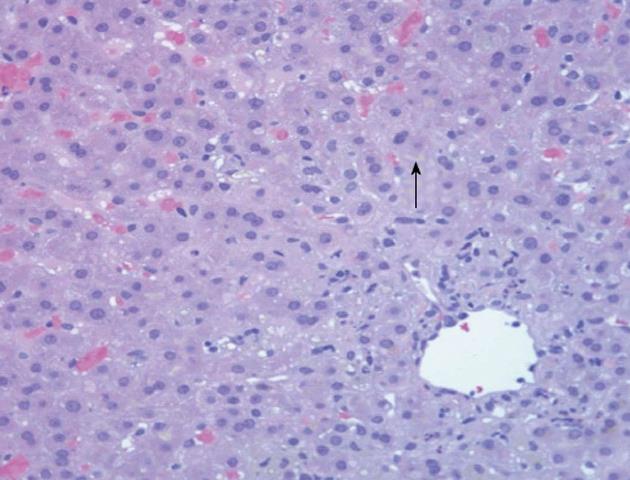
The biopsy revealed a centrilobular insult with neutrophilic infiltrates and mild Ito (stellate) cell hyperplasia consistent with severe acute cholestatic hepatitis consistent with a drug induced liver injury (Figure 1). The hepatic stellate cell is normally a quiescent cell situated in the perisinusoidal space. However, with liver injury, they are activated resulting in hyperplasia and can contribute to hepatic fibrosis over time by secreting collagen.
Eventually, the patient’s clinical symptoms resolved and his liver enzymes, bilirubin, and alkaline phosphatase trended toward normal.
This patient’s presentation, physical examination, laboratory data, histology, and recovery were all consistent with severe cholestasis secondary to the anabolic steroid, Mastabol. In particular, this case was illustrative of the, so called, bland type of cholestatic injury with significant bilirubin and alkaline phosphatase elevation and only mild aminotransferase elevation, thus indicating minimal hepatocellular injury.
Drug-induced adverse reactions on the liver can occur through direct effects on the hepatocytes, effects on bilirubin metabolism/binding, cholestatic mechanisms, viral mechanisms, or nonspecific/mixed effects[10]. The addition of alkyl groups to the 17 alpha position ties up the bonds needed to change the molecule to the less active keto form, and therefore, results in a greater half life and risk of toxicity. Here, we will focus our discussion on the proposed cholestatic mechanisms of liver injury.
Drug-induced cholestasis can be of several varieties: bland, meaning that there is limited injury to hepatocytes, inflammatory, sclerosing, or ductopenic (disappearance of bile ducts)[6]. Seventeen alpha substituted anabolic steroids appear to lead to the bland cholestatic variety with little hepatocellular injury and aminotransferases often elevated less than five-fold[11]. Although the exact mechanism of cholestasis under these circumstances is not known, it may be secondary to the binding of the drugs to canalicular membrane transporters or accumulation of toxic bile acids due to canalicular pump failure. Genetic defects in canalicular transport proteins may also play a role[6]. Other possible mechanisms include decreased permeability of the biliary epithelium to water, decreases in the bile salt-independent fraction of bile secretion, and/or the interference with the hepatic disposal of bile salts[12].
In support of the theory of injury at the level of the canaliculi, electron microscopy of rat livers a following 17 carbon substituted anabolic steroid administration confirmed canalicular changes of dilation and loss of microvilli[10]. Further, cholestatic effects have been attributed to interference with bile flow with potential sites of anabolic injury at the canalicular, pericanalicular microfibrillar network, and the basolateral plasma membrane all resulting in canalicular contraction[13]. The steroid-like agent, icterogenin, also leads to cholestasis and canalicular distortion, lending further support to this theory[13].
In addition, biliary excretion has been found to be significantly decreased in the presence of 17 alkylated anabolic steroids[13]. The lesion produced by the androgens to result in poor excretion has been compared to that causing Dubin-Johnson Syndrome, whereby there is a mutation in the canalicular multidrug resistance protein resulting in poor conjugated bilirubin excretion[13].
Whatever the mechanism of injury, these patients often present with mild jaundice which is usually reversible on drug discontinuation. The reaction appears to be dose related and predictable. Hepatic dysfunction often resolves quickly with the discontinuation of the anabolic steroid in anicteric cases and within months in patients presenting with icterus[11]. In patients without jaundice, continuation of the offending agent has been noted to induce a tolerance to the adverse effects of anabolic steroids with amelioration of hepatic enzymes levels[11].
In conclusion, supplemental anabolic steroids are often poorly regulated but are commonly used by resistance trainers to augment their training programs. Seventeen alpha alkylated anabolic steroids have been historically associated with hepatic cholestasis. This is the first described case of the highly available steroid, Mastabol, causing severe cholestasis in the absence of alkyl groups at the 17 alpha position.
| 1. | Sanchez-Medal L, Gomez-Leal A, Duarte L, Guadalupe Rico M. Anabolic androgenic steroids in the treatment of acquired aplastic anemia. Blood. 1969;34:283-300. [PubMed] |
| 2. | Schwingel PA, Cotrim HP, Salles BR, Almeida CE, dos Santos CR, Nachef B, Andrade AR, Zoppi CC. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011;31:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Socas L, Zumbado M, Pérez-Luzardo O, Ramos A, Pérez C, Hernández JR, Boada LD. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Stimac D, Milić S, Dintinjana RD, Kovac D, Ristić S. Androgenic/Anabolic steroid-induced toxic hepatitis. J Clin Gastroenterol. 2002;35:350-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Patil JJ, O’Donohoe B, Loyden CF, Shanahan D. Near-fatal spontaneous hepatic rupture associated with anabolic androgenic steroid use: a case report. Br J Sports Med. 2007;41:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Toxic and Drug Induced Hepatitis. Harrisons’s Practice. McGraw Hill Medical. 2008;. |
| 7. | Kafrouni MI, Anders RA, Verma S. Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol. 2007;5:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | Habscheid W, Abele U, Dahm HH. Severe cholestasis with kidney failure from anabolic steroids in a body builder. Dtsch Med Wochenschr. 1999;124:1029-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Pavlatos AM, Fultz O, Monberg MJ, Vootkur A. Review of oxymetholone: a 17alpha-alkylated anabolic-androgenic steroid. Clin Ther. 2001;23:789-801; discussion 771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Rao R. Mechanism of drug induced heptotoxicity. Ind J Pharmacol. 1973;5:313-318. |
| 11. | Hepatic Effects of 17 Alpha Alkylated Anabolic Androgenic Steroids. The Foundation for Care Management. HIV Hotline. 1998;8:2-5. |
| 12. | Berthelot P. Mechanisms and prediction of drug-induced liver disease. Gut. 1973;14:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Hormonal Derivatives and Related Drugs. Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. Philadelphia: Lippincott Williams and Wilkins 1999; 557-560. |
P- Reviewers Stefanovic B, Lonardo A S- Editor Song XX L- Editor A E- Editor Li JY