Published online Jul 27, 2012. doi: 10.4254/wjh.v4.i7.231
Revised: September 2, 2011
Accepted: November 8, 2011
Published online: July 27, 2012
The use of herbal supplements has increased considerably over the last decade. We report a case of an elderly woman who began taking Move Free Advanced for arthritis, which in addition to glucosamine and chondroitin, contained two herbal ingredients, Chinese skullcap and Black Catechu. Our patient presented with significant cholestasis and hepatitis which significantly improved after discontinuation of the supplement. Since neither the patient nor the treating physician recognized this supplement as a potential hepatotoxin, she resumed taking the supplement and again suffered from considerable hepatotoxicity. Liver biopsy at that time was consistent with acute drug induced liver injury. She, once again, recovered after discontinuation of the supplement. Review of the literature confirms that Chinese skullcap has been implicated as a possible hepatotoxic agent which was demonstrated in this case.
- Citation: Yang L, Aronsohn A, Hart J, Jensen D. Herbal hepatoxicity from Chinese skullcap: A case report. World J Hepatol 2012; 4(7): 231-233
- URL: https://www.wjgnet.com/1948-5182/full/v4/i7/231.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i7.231
The use of complementary and alternative medicine has increased fivefold over the past 10 years, with approximately 42% of Americans taking some form of herbal medication[1]. Herbal supplements causing drug induced liver injury is also increasingly being recognized. Chinese skullcap (Scutellaria baicalensis) belongs to the mint family and has been studied as a possible anti-inflammatory agent[2]. This herbal supplement is currently available in the United States as Move Free Advanced® which is an over the counter arthritis remedy comprised of glucosamine, chondroitin, Chinese skullcap and black catechu. Recently, reports of hepatotoxicity have been described after taking this supplement[3]. We report a case of an elderly woman who experienced biopsy proven Chinese skullcap induced hepatotoxicity that reoccurred with re-challenge.
A 78-year old caucasian woman initially presented to her primary care physician with acute painless jaundice. She was otherwise asymptomatic. Past medical history was significant for osteoarthritis and hyperlipidemia. Past surgical history included a cholecystectomy. She was not on any prescription medications, but took a multivitamin daily and three weeks prior had started Move Free Advanced®, a glucosamine/chondroitin supplement with a recommended dose of one tablet twice a day. She denied alcohol use and had no risk factors for viral hepatitis. Initial studies revealed a serum bilirubin of 7.2 mg/dL (normal value 0.1-1.2), aspartate aminotransferase (AST) 1053 U/L(normal value 15-56), alanine aminotransferase (ALT) 1626 U/L(normal value 15-59), alkaline phosphatase (ALP) 354 U/L (normal value 33-131) and Gamma-glutamyl transpeptidase (GGT) 599 U/L (normal value 11-63) . Hepatitis A, B and C serologic studies were negative. She was hospitalized at an outside hospital briefly for two days for observation during which time she was not given any medications. Upon discharge home, she remained off all medications and two weeks later her bilirubin had decreased to 2.3 mg/dL, AST 415, U/L ALT 678 U/L and ALP 279 U/L.
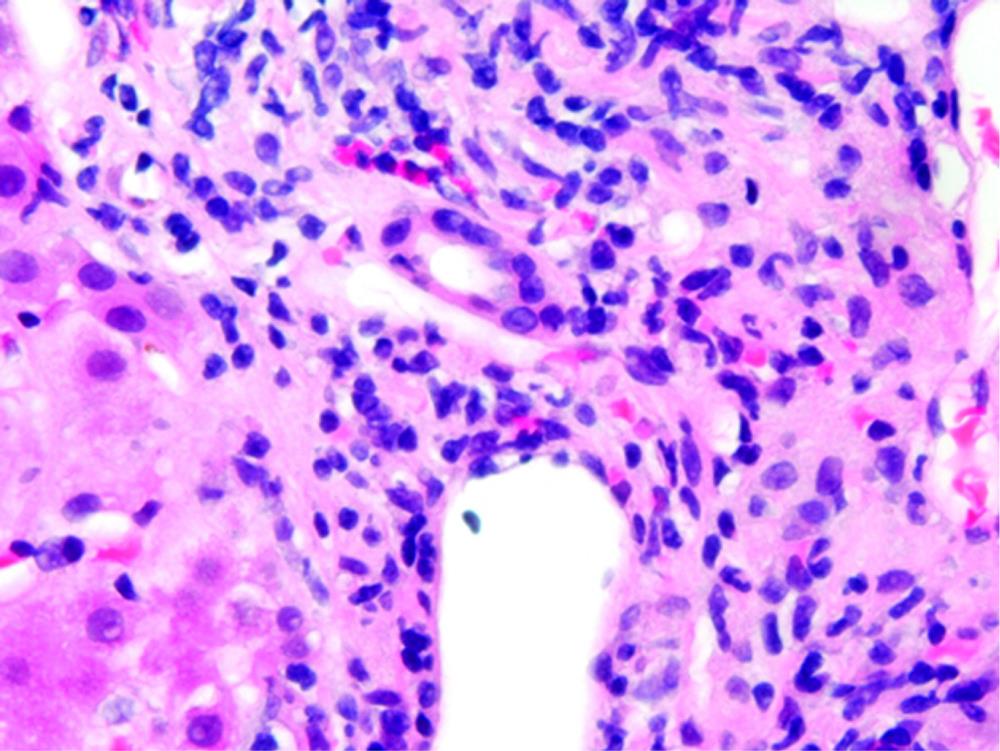
Her osteoarthritis worsened and after consulting her physician she was told that glucosamine and chondroitin were not hepatotoxic and thus probably not the cause of her hepatitis, so she restarted the Move Free Advanced supplement. Within 2 wk, she noted jaundice again and her laboratory studies revealed: bilirubin 4.7 mg/dL, AST 1177 U/L, ALT 1206 U/L, and Alkaline phosphatase 286 U/L. An abdominal/pelvis computed tomography (CT) was performed and was unrevealing. A percutaneous liver biopsy was performed. Upon referral to the University of Chicago Medical Center the following laboratory tests were all normal: ANA, p-ANCA, smooth muscle antibody, serum electrophoresis and iron studies. The bottle of Move Free Advanced revealed that in addition to glucosamine and chondroitin, it contained two herbal ingredients, Chinese skullcap and Black Catechu. The relative concentrations of Chinese skullcap and Black Catechu are not known since the herbal component is listed on the package as “Uniflex Proprietary Extract - 250 mg”. Review of the liver biopsy showed portal tracts containing mild, predominately mononuclear cell infiltrates, with many eosinophils. There was also significant lobular inflammatory cell infiltrates, including eosinophils. Numerous acidophil bodies were seen and scattered ballooned hepatocytes were also present. No fibrosis was seen and iron staining was negative (Figure 1). This was consistent with acute drug induced hepatitis. She was instructed to discontinue her Move Free Advanced supplement. Four weeks after stopping, her bilirubin normalized to 0.9 mg/dL, AST 80 U/L, ALT 120 U/L, ALP126 and GGT 142.
The diagnosis of herbal drug induced hepatotoxicity is one of exclusion and requires taking a detailed medication history. Several scoring systems exist to standardize the evaluation of drug induced toxicity, such as the Council for International Organizations of Medical Sciences scale, and can be useful in diagnosing herbal medication induced hepatotoxicity[4]. Rechallenging remains the gold standard to determine causality but is not clinically recommended.
In this case, the patient developed an acute hepatitis clinical picture that improved following discontinuation, and then returned when she re-challenged herself with the glucosamine/condroitin supplement. Also, the liver biopsy supports the impression of drug-induced hepatitis. Although there are no documented cases of hepatotoxicity for gluocosamine, chondroitin or black catechu, Chinese skullcap has been implicated as a hepatotoxin in the past[3].
Chinese skullcap (Scutellaria baicalensis) is a plant that belongs to the Mint family and is also known as huang qin, baikal and scutellaria. It is related to American Skullcap (Scutellaria lateirflora) and even though they are not the same, both are often referred to as Skullcap or scutellaria making differentiation difficult. They are both commonly used as a relaxant. Recently the combination of Chinese skullcap and Black Catechu has been studied for possible anti-inflammatory properties[2].
One of the early documented cases of Chinese skullcap hepatotoxicity described a 49-year old woman who developed acute hepatitis that resolved but returned after she rechallenged herself to the an herbal preparation that contained mistletoe, skullcap, kelp and motherwort[5]. At that time, mistletoe was the only herb known to contain a potential hepatotoxin, so it was suggested as the causative agent. However, later evaluation of the mixture suggested mistletoe was probably not an ingredient, and therefore raised the possibility that one of the other ingredients was the hepatotoxin[6]. Four cases of acute hepatitis have been reported in women taking the herbal preparations Kalms and Neurelax, both of which contained skullcap and Valerian[7]. Fulminant hepatic failure occurred in a 28 year old man with multiple sclerosis who was taking zinc, skullcap and pau d’ arco[8]. Finally, in a recently published case report, 2 elderly women were found to have hepatotoxicity after taking the same product as our patient, Move Free Advanced[3]. Both of these patients had improvement in liver tests after discontinuation of the supplement. A limitation of this case report was that neither of the patients had a re-challenge of the supplement and no liver biopsy specimens were available, as was demonstrated in this case.
Treatment of herbal induced hepatoxicity involves withdrawal of the offending agent. More advanced cases of liver failure require supportive care and can be fatal or lead to liver transplantation.
In our case, herbal hepatotoxicity from Skullcap was most likely the causative agent of liver injury based upon liver histology and re-challenge. This case highlights the importance of considering herbal supplement induced hepatotoxicity in the differential of any patient who presents with acute hepatitis. This case also highlights the importance of checking manufacturers’ labels when drug induced hepatitis is suspected because herbal additives are not always evident on first glance.
| 1. | Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, Kaptchuk TJ, Eisenberg DM. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262-268. [PubMed] |
| 2. | Burnett BP, Silva S, Mesches MH, Wilson S, Jia , Q . Safety evaluation of a combination, defined extract of Scutellaria Baiscalensis and Acacia Catechu. J Food Biochem. 2007;31:797-825. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy. 2010;30:750, 258e-262e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Harvey J, Colin-Jones DG. Mistletoe hepatitis. Br Med J (Clin Res Ed). 1981;282:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Fletcher HF. Mistletoe hepatitis. Br Med J. 1981;282:186-187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 7. | MacGregor FB, Abernethy VE, Dahabra S, Cobden I, Hayes PC. Hepatotoxicity of herbal remedies. BMJ. 1989;299:1156-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Peer reviewers: Sonia Ramos, PhD, Department of Metabolism and Nutrition, Instituto del Frio (CSIC), Jose Antonio Novais, 10, 28040 Madrid, Spain; Byoung-Joon Song, PhD, Senior Investigator, Laboratory of Membrane Biochemistry and Biophysics, National Institute on Alcohol Abuse and Alcoholism, NIH 5625 Fishers Lane, Room 2S-30, Bethesda, MD 20892, United States; Agustin Castiella, MD, Gastroenterology and Hepatology Unit, Hospital Mendaro, Mendaro, Spain
S- Editor Wu X L- Editor A E- Editor Wu X