Published online Jun 27, 2012. doi: 10.4254/wjh.v4.i6.191
Revised: September 1, 2011
Accepted: June 23, 2012
Published online: June 27, 2012
Glycogen storage disease type Ia (GSD-Ia; also called von Gierke disease) is an autosomal recessive disorder of carbohydrate metabolism caused by glucose-6-phosphatase deficiency. There have been many reports describing hepatic tumors in GSD patients; however, most of these reports were of hepatocellular adenomas, whereas there are only few reports describing focal nodular hyperplasia (FNH) or hepatocellular carcinoma (HCC). We report a case with GSD-Ia who had undergone a partial resection of the liver for FNH at 18 years of age and in whom moderately differentiated HCC had developed. Preoperative imaging studies, including ultrasonography, dynamic computer tomography (CT) and magnetic resonance imaging, revealed benign and malignant features. In particular, fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT revealed the atypical findings that FDG accumulated at high levels in the non-tumorous hepatic parenchyma and low levels in the tumor. Right hemihepatectomy was performed. During the perioperative period, high-dose glucose and sodium bicarbonate were administered to control metabolic acidosis. He had multiple recurrences of HCC at 10 mo after surgery and was followed-up with transcatheter arterial chemoembolization. The tumor was already highly advanced when it was found by chance; therefore, a careful follow-up should be mandatory for GSD-I patients as they are at a high risk for HCC, similar to hepatitis patients.
- Citation: Mikuriya Y, Oshita A, Tashiro H, Amano H, Kobayashi T, Arihiro K, Ohdan H. Hepatocellular carcinoma and focal nodular hyperplasia of the liver in a glycogen storage disease patient. World J Hepatol 2012; 4(6): 191-195
- URL: https://www.wjgnet.com/1948-5182/full/v4/i6/191.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i6.191
Glycogen storage disease type Ia (GSD-Ia; also called von Gierke disease) is an autosomal recessive disorder of carbohydrate metabolism caused by glucose-6-phosphatase deficiency[1]. The incidence of GSD-I is 1 in 100 000 to 1 in 300 000 live births[2]. Clinical manifestations of this disease include abdominal distension due to hepatomegaly, rounded doll-like face, growth retardation, hypoglycemia during fasting leading to lactic acidosis, and a bleeding tendency due to impaired platelet agglutinability[2]. While it has been reported that hepatic tumors develop in GSD-Ia patients, the majority of these tumors are not malignant but are usually benign, e.g., hepatocellular adenoma (HCA)[3]. Although focal nodular hyperplasia (FNH) or hepatocellular carcinoma (HCC) can develop in GSD patients, it is a rare event, occurring at a much lower rate than HCA[4]. We report herein a case with GSD-Ia in whom FNH and HCC developed metachronously.
A 39 year old man with GSD-Ia, diagnosed with a liver biopsy by laparotomy at 1 year of age, was referred to our institute. He had developed metabolic acidosis after he had caught a cold and was transferred to hospital by ambulance, where a hepatic tumor was found on computer tomography (CT). He previously underwent a partial resection of the liver for a tumor in segment 4, resulting in FNH at 18 years of age (Figure 1). The patient had never smoked and seldom drank. His height was 153 cm and his weight was 36.6 kg due to the growth retardation of GSD-Ia. He had a typical doll-like face.
On physical examination, the bulbar conjunctiva showed no icterus. The liver was palpable 8 cm below the costal arch, while the spleen was not palpable. Laboratory workup revealed elevated levels of serum aspartate aminotransferase (65 IU/L; normal, 13-33 IU/L), alanine aminotransferase (88 IU/L; normal, 8-42 IU/L) and gamma-glutamyl transferase (127 IU/L; normal, 0-75 IU/L). Serum total bilirubin, total protein, albumin and prothrombin time were within normal limits. The levels of total cholesterol (279 mg/dL; normal, 128-219 mg/dL), triglyceride (830 mg/dL; normal, 30-149 mg/dL) and uric acid (8.1 mg/dL; normal, 3.6-7.0 mg/dL) were elevated. Blood urea nitrogen (28.8 mg/dL; normal, 8.0-22.0 mg/dL) and creatinine (1.83 mg/dL; normal, 0.6-1.1 mg/dL) levels were also elevated. Fasting blood glucose was 64 mg/dL (normal, 70-109 mg/dL). Serum alpha-fetoprotein was undetectable but protein induced by vitamin K absence or antagonist-II was elevated at 139 mAU/mL (normal, 0-30 mAU/mL). The patient had lactic acidosis on blood gas analysis (pH: 7.229; PaCO2: 25.2 mmHg; HCO3-: 10.2 mmol/L; Base excess: -15.9 mmol/L; Lactate: 7.3 mmol/L).
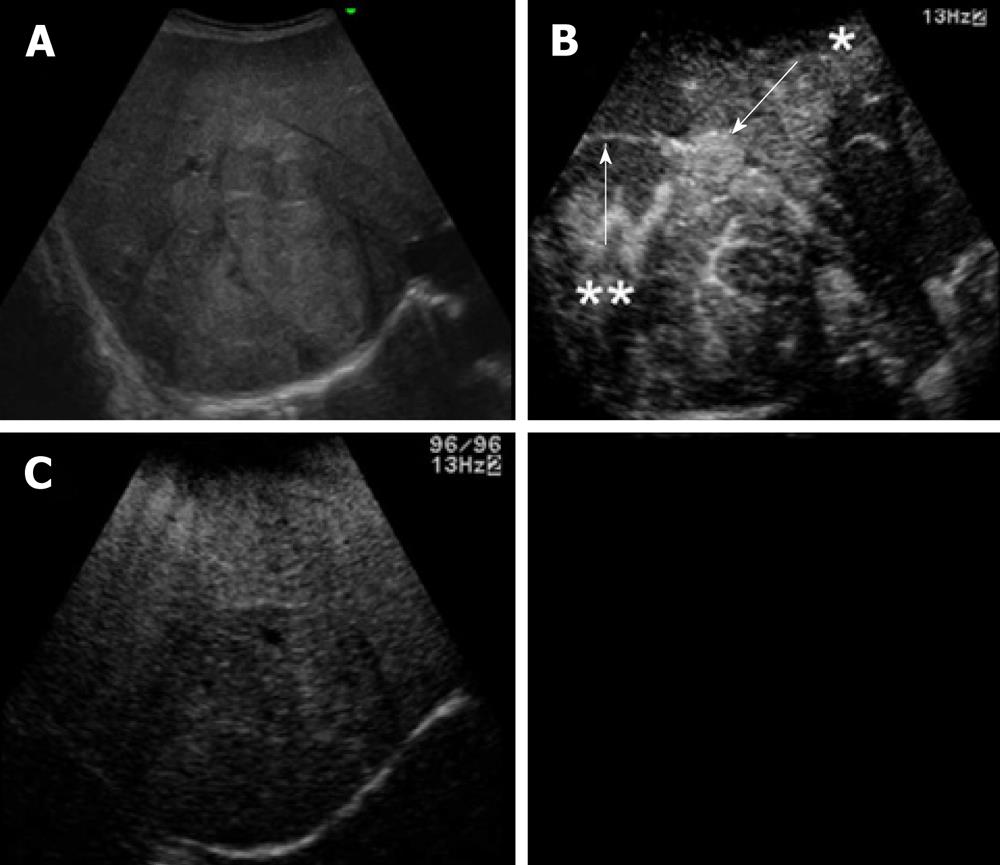
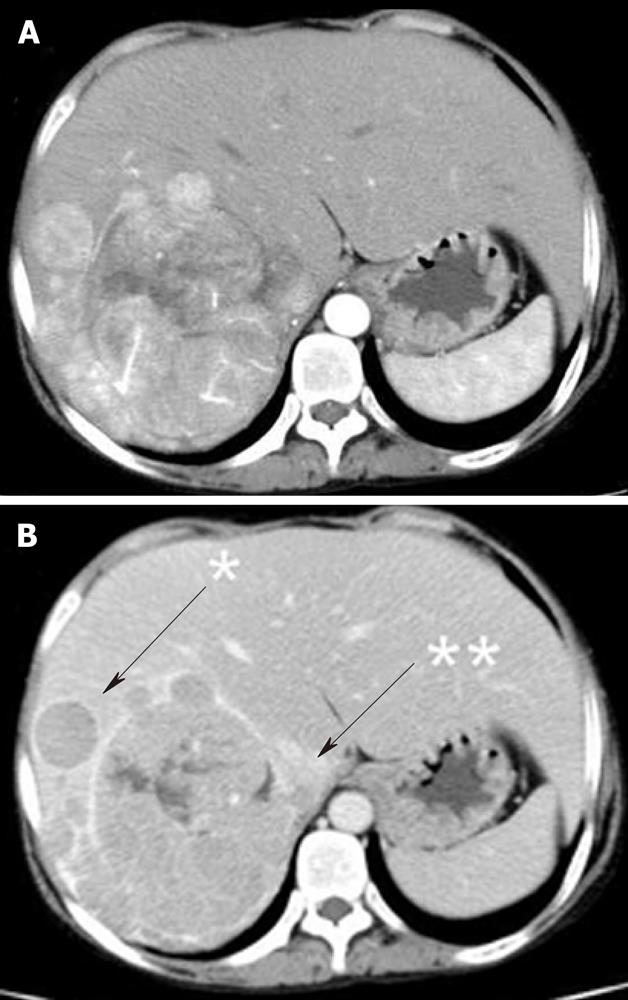
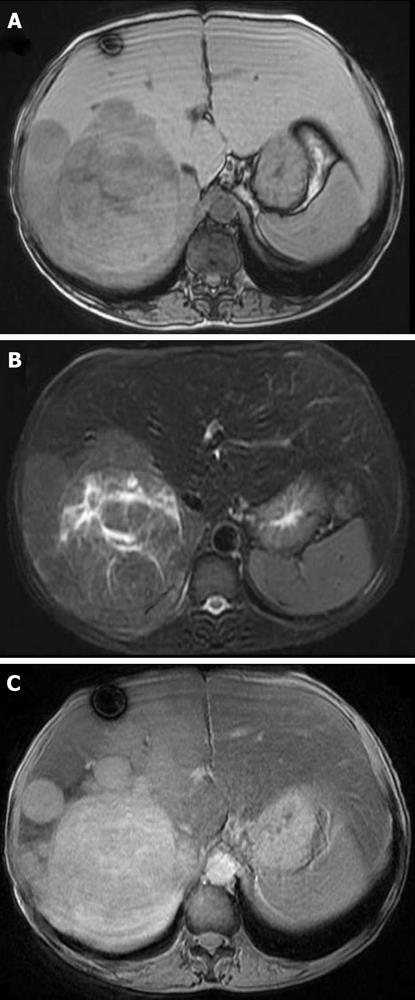
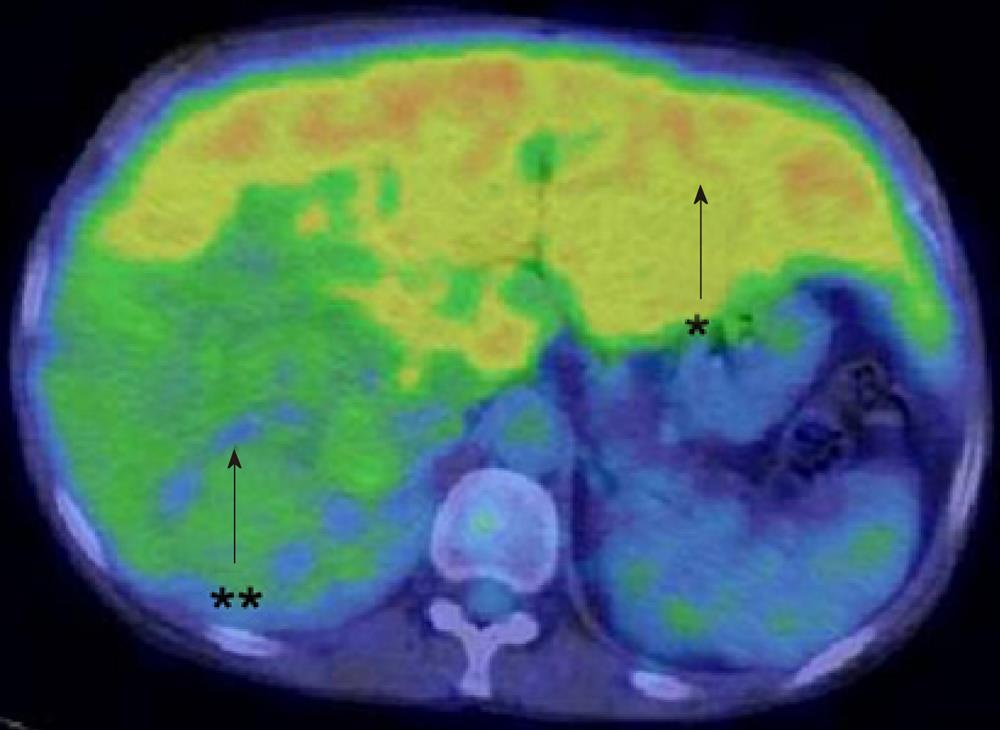
Ultrasonography (US) showed a heterogeneous echoic mass with a capsule of 10 cm in diameter in the right lobe. The tumor was accompanied with many daughter nodules around the capsule. Contrast-enhanced US with perfluorobutane microbubbles showed a highly enhanced mass in the early phase, whereas a washout effect was not obvious in the Kupffer phase (Figure 2). A dynamic CT scan showed a high-density tumor measuring 10 cm in diameter in the early phase and an iso-low-density tumor in the late phase. It had a capsule and septums, leading to the preoperative diagnosis of HCA. On the other hand, it had daughter nodules and the capsule was partially torn, suggestive of malignancy (Figure 3). Magnetic resonance imaging (MRI) showed a low-intensity tumor in T1-weighted images (WI) and a slightly high-intensity tumor in T2WI. Superparamagnetic iron oxide-enhanced MRI revealed the low uptake of Resovist, which was suggestive of HCC (Figure 4). Fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT showed a high accumulation of FDG with a maximum standardized uptake value (SUVmax) of 4.2 in the non-tumorous hepatic parenchyma and a relatively low uptake of FDG with a SUVmax of 2.7 in the tumor (Figure 5).
The patient underwent a right hemihepatectomy without diagnostic confirmation of the giant hepatic tumor. During the perioperative period, high-dose glucose and sodium bicarbonate were administered to control the metabolic acidosis, as described previously[5]. The postoperative course was uneventful and he left the hospital on postoperative day 14.
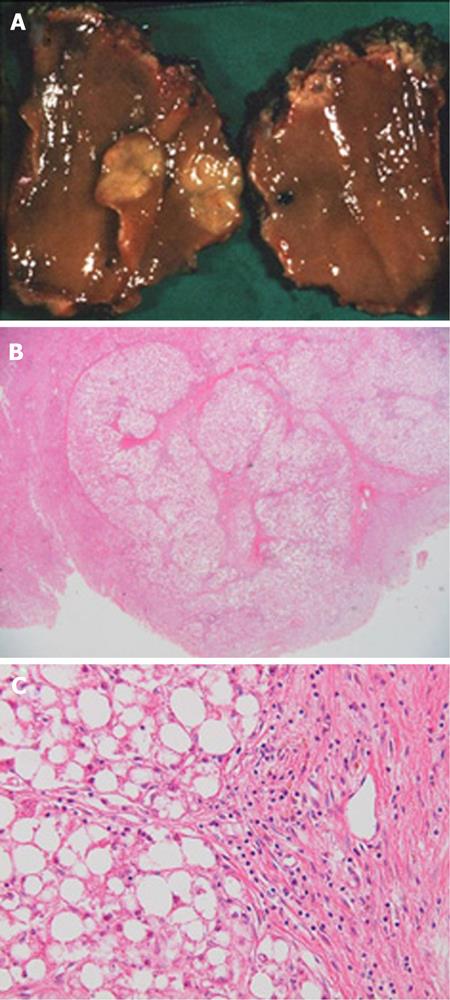
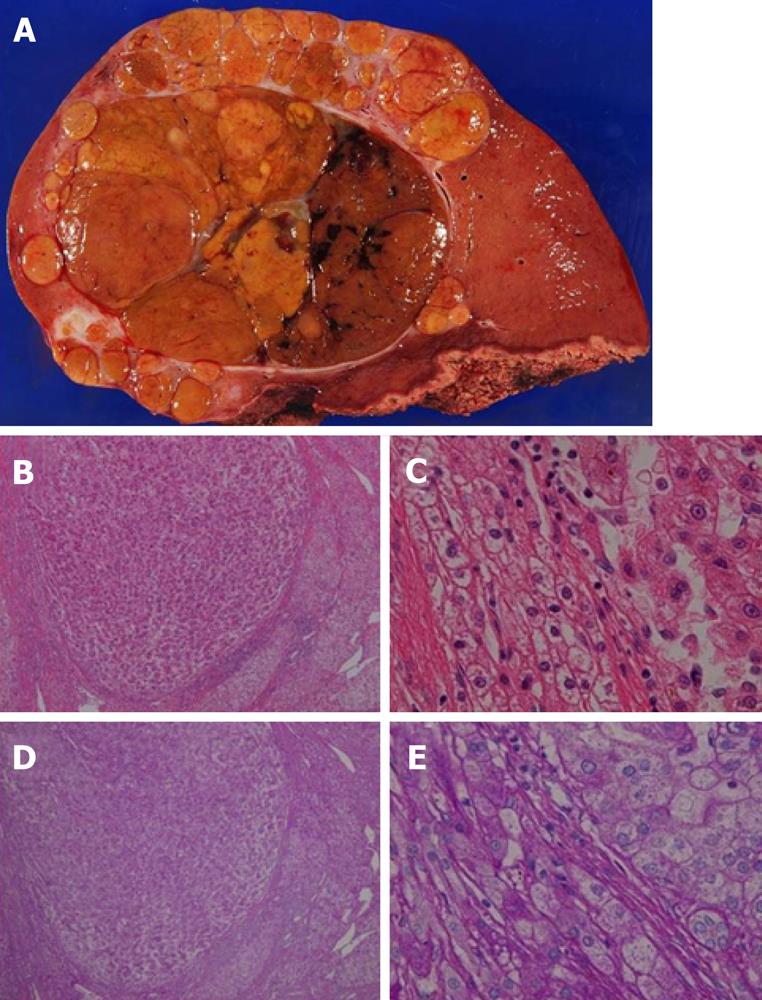
The weight of the resected specimen was 1914 g. The tumor was 100 mm in diameter with multiple satellite nodules. The main tumor contained septums and hemorrhagic regions. It also contained a capsule with some extracapsular invasion. Microscopic examination revealed that the tumor cells were swollen and polygonal with large round or irregular nuclei with rough chromatin aggregations, obvious nucleoli and mitoses. Multinucleated tumor cells were also found and many blood lakes had formed in the tumor. These findings resulted in the diagnosis of moderately differentiated HCC with intrahepatic metastases, whereas no adenomatous components were observed in the tumor. In the non-tumorous liver parenchyma, cells with a clear cytoplasm were positive for periodic acid-Schiff (PAS) staining; this finding was consistent with that of GSD (Figure 6).
The present case had multiple recurrences of HCC that were beyond the Milan criteria at approximately 10 mo after surgery. He was followed-up with transcatheter arterial chemoembolization.
GSD-Ia is an autosomal recessive disorder caused by glucose-6-phosphatase deficiency in the liver, kidneys and intestinal mucosa. The disease is characterized by the impaired conversion of glucose from glucose-6-phosphate in the liver, resulting in fasting hypoglycemia and lactic acidosis[1,2]. In the present case, the development of hypoglycemia and lactic acidosis led to the opportunity to use imaging studies for the diagnosis of a hepatic tumor.
Although US, CT, MRI and CT combined with arterial portography and hepatic arteriography are generally considered to be effective for the diagnosis of HCC, preoperative diagnosis was difficult in this case. Each imaging study revealed benign and malignant features. Moreover, FDG-PET/CT revealed the very interesting findings that FDG accumulated at high levels in the non-tumorous hepatic parenchyma and at low levels in the tumor. As GSD-I is characterized by glucose-6-phosphatase deficiency, it was hypothesized that the FDG ingested by hepatic cells was phosphorylated by hexokinase to FDG-6-phosphate, which accumulated in non-tumorous cells without dephosphorylation by glucose-6-phosphatase.
Liver tumors are common in GSD-I, the majority of which are HCAs[3]. Talente et al[3] described that 27 of 37 (73%) GSD-Ia patients had HCAs detected by US. Meanwhile, the concomitant occurrence of other tumor types is rare. Sumimoto et al[4] described 22 GSD-I cases with liver tumors and reported HCA, HCC, FNH and hepatoblastoma in 16, 3, 2 and 1 patients, respectively. To the best of our knowledge, there are no reports describing a case of GSD-I with FNH and HCC.
Some authors have reported malignant formations from adenomas in GSD-I patients[6,7]. Bianchi summarized 10 cases of HCC in GSD-I patients reported in the literature and observed that the transition from HCA into HCC occurred in 50% of these cases[6]. The pathogenesis of HCC formation in GSD-I is not well understood. Some authors have described hypothetical causes, including the accumulation of abnormal metabolites in hepatic cells acting as carcinogens[8] and proliferative or neoplastic changes in hepatic cells caused by long-term hypoglycemia-induced chronic hormonal stimulation (e.g., low insulin and high glucagon levels)[7]. For these reasons, once GSD-I is diagnosed, it is necessary to maintain normal blood glucose levels to prevent HCC. In fact, in some cases of GSD-I with adenoma, regression of adenoma after nutrition therapy has been reported[6,9]. In our case, it was not obvious whether HCC developed from an adenoma since the patient was not followed-up after his initial surgery for FNH and there were no components of HCA in the resected specimen upon microscopic evaluation.
With regard to liver tumors in GSD-I patients, surgery might be indicated for HCC or even adenoma if it is likely to cause bleeding or complaints of compression[10]. When it is difficult to control metabolic acidosis in GSD-I patients, a liver transplantation could be performed[11,12]. As the present case had only 1 episode of acidosis and the tumor was so large that it did not meet the Milan criteria, hepatectomy and not liver transplantation was indicated.
Concerning perioperative management, hypoglycemia and lactic acidosis are common problems for GSD patients. Although glycogenolysis and glycogenesis are normal in these patients, the release of free glucose from the liver into the blood is greatly impaired. Since glycolysis of glucose 6-phosphate is intact or even intensified under hormonal counter regulation, similar to a fasting state, the production of pyruvate and lactate is increased[13]. Therefore, high-dose glucose and sodium bicarbonate were administered, as reported previously[5], resulting in the prevention of severe perioperative hypoglycemia and acidosis (data not shown).
Unfortunately, this patient was not followed-up well and over 20 years had passed since his initial operation for FNH. The tumor was already highly advanced when it was found by chance; therefore, careful follow-up should be mandatory for GSD patients as they are at a high risk of developing HCC, similar to hepatitis patients[14].
In conclusion, we report a very rare case of FNH and HCC that developed metachronously in a patient with GSD-Ia.
| 1. | Chen YT. Glycogen storage diseases. The metabolic and molecular bases of inherited disease, 8th ed., vol. 1. New York: McGraw-Hill 2001; 1521-1552. |
| 2. | Greene HL. Glycogen storage diseases. Cecil textbook of medicine, 22nd ed. London: WB Saunders 2004; 1270-1272. |
| 3. | Talente GM, Coleman RA, Alter C, Baker L, Brown BI, Cannon RA, Chen YT, Crigler JF, Ferreira P, Haworth JC. Glycogen storage disease in adults. Ann Intern Med. 1994;120:218-226. [PubMed] |
| 4. | Sumimoto S, Motoi T, Mikawa H, Honde H, Kobayashi N, Tanaka K, Ozawa K, Sudo M, Yamamura H. Type Ia glycogen storage disease with multiple hepatic adenomas and a highly differentiated hepatic carcinoma: a case report and review of the literature. Ann Paediatr Jap. 1988;34:47-55. |
| 5. | Oshita A, Itamoto T, Amano H, Ohdan H, Tashiro H, Asahara T. Perioperative management of benign hepatic tumors in patients with glycogen storage disease type Ia. J Hepatobiliary Pancreat Surg. 2008;15:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr. 1993;152:Suppl 1: S63-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Miller JH, Gates GF, Landing BH, Kogut MD, Roe TF. Scintigraphic abnormalities in glycogen storage disease. J Nucl Med. 1978;19:354-358. [PubMed] |
| 8. | Zangeneh F, Limlbeck GA, Brown BI, Emch JR, Arcasoy MM, Goldenberg VE, Kelley VC. Hepatorenal glycogenosis (type I glycogenosis) and carcinoma of the liver. J Pediatr. 1969;74:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Parker P, Burr I, Slonim A, Ghishan FK, Greene H. Regression of hepatic adenomas in type Ia glycogen storage disease with dietary therapy. Gastroenterology. 1981;81:534-536. [PubMed] |
| 10. | Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002;161 Suppl 1:S20-S34. [PubMed] |
| 11. | Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008;12:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Nuoffer JM, Mullis PE, Wiesmann UN. Treatment with low-dose diazoxide in two growth-retarded prepubertal girls with glycogen storage disease type Ia resulted in catch-up growth. J Inherit Metab Dis. 1997;20:790-798. [PubMed] [DOI] [Full Text] |
| 14. | Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Peer reviewer: Dr. Stefan Rose-John, Department of Biochemistry, University of Kiel, Olshausenstrasse 40, Kiel D-24098, Germany
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X