Revised: November 4, 2011
Accepted: March 17, 2012
Published online: March 27, 2012
Liver disease in human immunodeficiency virus (HIV)-infected individuals encompasses the spectrum from abnormal liver function tests, liver decompensation, with and without evidence of cirrhosis on biopsy, to non-alcoholic liver disease and its more severe form, non-alcoholic steatohepatitis and hepatocellular cancer. HIV can infect multiple cells in the liver, leading to enhanced intrahepatic apoptosis, activation and fibrosis. HIV can also alter gastro-intestinal tract permeability, leading to increased levels of circulating lipopolysaccharide that may have an impact on liver function. This review focuses on recent changes in the epidemiology, pathogenesis and clinical presentation of liver disease in HIV-infected patients, in the absence of co-infection with hepatitis B virus or hepatitis C virus, with a specific focus on issues relevant to low and middle income countries.
- Citation: Crane M, Iser D, Lewin SR. Human immunodeficiency virus infection and the liver. World J Hepatol 2012; 4(3): 91-98
- URL: https://www.wjgnet.com/1948-5182/full/v4/i3/91.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i3.91
There are 33 million people infected with human immunodeficiency virus (HIV) globally with the greatest burden of disease in low and middle income countries. With the increased availability of antiretroviral therapy (ART), the number of people surviving with HIV and presenting with liver disease is increasing[1]. Most clinical trials and cohort studies have studied liver disease in HIV-infected individuals living in high income countries but liver disease is likely to emerge as an important co-morbidity in HIV-infected patients in low and middle income countries.
In the absence of co-infection with either hepatitis B virus (HBV) or hepatitis C virus (HCV), liver disease in HIV-infected individuals encompasses the spectrum from abnormal liver function tests, liver decompensation, with and without evidence of cirrhosis on biopsy, to non-alcoholic liver disease (NAFLD) and its more severe form, non-alcoholic steatohepatitis (NASH) and hepatocellular cancer (HCC)[2-6]. This review focuses on recent changes in the epidemiology, pathogenesis and clinical presentation of liver disease in HIV-infected patients with a specific focus on issues relevant to low and middle income countries.
Liver disease, including liver-related mortality secondary to chronic liver disease and HCC, is an emerging management problem in HIV-infected individuals in high income countries, where people are surviving longer on ART[1]. In the most recent data collection on adverse events of anti-HIV drugs (D:A:D) study of over 33 000 individuals, liver related deaths were the commonest cause of non-acquired immunodeficiency syndrome (AIDS) mortality amongst HIV-infected individuals[1]. While liver related deaths were strongly associated with viral hepatitis (84% of liver related deaths, 11% of total deaths), 16% of the liver related deaths (or 2.3% of the total deaths) in this population occurred in the absence of viral hepatitis[1].
Liver-related mortality in HIV-infected patients not co-infected with HBV or HCV has not been widely studied in low and middle income countries. In a retrospective South American study of serious non-AIDS events in the LATINA cohort (comprising over 6000 HIV-infected individuals on ART from Brazil, Mexico, Peru and Argentina), terminal liver failure or cirrhosis was the leading cause of death with 54/130 (42%) confirmed or probable cases based on clinical, laboratory and histological findings[7]. In this study, co-infection with HBV or HCV and low CD4 count were the major risk factors[7]. Similarly, a post-mortem study of 86 HIV-infected individuals undergoing autopsy in rural South Africa demonstrated that 10% had liver related conditions at the time of death[8]; however, it is likely that co-infection with viral hepatitis was a contributing factor.
Chronic liver disease, as measured by raised alanine aminotransferase (ALT), has been widely studied in HIV-infected individuals, particularly in high income settings. In a Swiss cohort of 2365 HIV-infected individuals not co-infected with either HBV or HCV, 385 (16%) had chronically elevated ALT (defined as > 2 × upper limit of normal)[9]. Risk factors associated with elevated ALT were high HIV RNA and prolonged ART exposure as well as high body mass index (BMI), alcohol abuse and increasing age[9]. A number of studies have now shown a link between BMI, high cholesterol levels, diabetes mellitus and liver disease in HIV mono-infection in high income countries[9-11], indicating lifestyle may play a significant role in the development of liver disease amongst HIV-infected individuals. Of concern, high BMI and diabetes mellitus are increasingly reported in low and middle income countries[12]. Ocama et al[13] recently reported that following 36 mo of ART in a cohort of 546 individuals in Kampala, only 1.5% had grade 3 aspartate aminotransferase (AST) elevations. In this study, a subset (n = 470) of patients were tested for HBV surface antigen and 9% were positive. A study of 59 individuals in Mexico showed a moderately strong positive correlation between elevated transaminases and HIV RNA in individuals not receiving ART and without viral hepatitis co-infection[14], consistent with reports from North America[11].
Data on HCC in HIV mono-infection in both low and high income countries is limited. In a study of over 3500 HIV-infected Chinese individuals, HCC was one of the leading causes of morbidity and mortality; however, this was primarily due to co-infection with viral hepatitis[15]. In a French prospective database study, a low CD4 count was a major risk factor for HCC with the rate ratio, or risk, increasing from 2 to 7.6 when comparing patients with a high CD4 count (> 500 cells/mL) to those with a low CD4 count (< 50 cells/mL) respectively[16]. Importantly for low and middle income countries, it has been demonstrated that the use of ART in individuals with low CD4 count (< 350 cells/mL) was associated with a reduced risk of hospitalization for liver related complications[17].
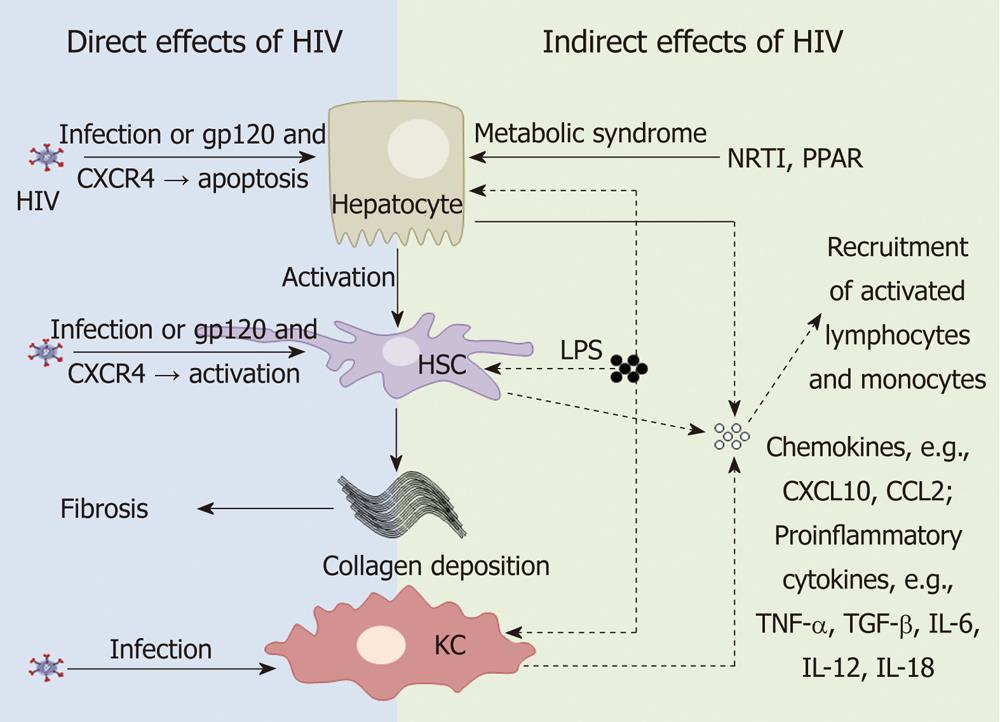
HIV may alter liver function by either direct or indirect mechanisms. HIV predominantly infects CD4+ T-cells, monocyte/macrophages and dendritic cells. However, there are multiple studies now showing HIV infection of a wide range of non hemapoietic cells, including cells in the liver. In addition, changes in gastrointestinal (GI) tract permeability via massive depletion of GI tract associated CD4+ T-cells by HIV may have indirect consequences on immune activation and liver disease (Figure 1).
There are numerous studies demonstrating HIV infection of hepatic cells. Kupffer cells, differentiated tissue macrophages that reside in the liver, can be infected by HIV in vivo[18-20]. In vitro studies suggest that HIV infection of primary Kupffer cells leads to productive infection[21,22].
HIV RNA has also been detected in sinusoidal cells and hepatocytes in vivo[18,19]. Primary human sinusoidal cells have also been shown to be permissive to HIV infection in vitro[23]. A number of studies have demonstrated HIV infection of hepatocyte cell lines[24]. Infection of hepatocyte cell lines is thought to be CD4-independent as most hepatocyte cell lines, as well as primary hepatocytes, do not express CD4[24]. HIV infection of hepatocytes cells may therefore occur via receptor-mediated endocytosis or alternative co-receptors[25]. Hepatocytes may act as a transient HIV reservoir and promote CD4+ T cell infection by cell-cell contact[26].
HIV can also induce hepatocyte apoptosis in vitro via gp120 signalling through CXCR4 in the absence of infection[27]. Hepatocyte apoptosis can trigger pro-fibrotic activity of hepatic stellate cells (HSC) activity, as has been demonstrated in both HIV-HBV co-infection and HIV-HCV co-infection[28,29]. Further work is needed to determine the precise role of HIV-induced hepatic cell apoptosis and liver disease in HIV mono-infection.
HSCs are lipid storing cells and the main cells responsible for fibrogenesis in the liver. HIV infection of HSCs, including primary HSCs and the LX-2 stellate cell line, has recently been reported[30]. While HSCs express the HIV co-receptors CCR5 and CXCR4, HIV infection of HSCs appeared to be CD4-independent[30]. However, gp120 has also recently been shown to activate HSC via ligation of CXCR4[31]. HSCs infected with HIV or exposed to gp120 showed increased activation and fibrogenesis, as measured by alpha-smooth muscle actin and collagen production and increased levels of monocyte chemotactic protein-1 (MCP-1 or CCL-2). CCL-2 binds to CCR2 which is primarily expressed on activated pro-inflammatory monocytes and migration of these cells into the liver could potentially contribute to hepatic fibrosis and the accelerated progression to liver disease observed in HIV-HCV co-infected individuals[30,31].
HIV infection of GI tract associated CD4+ T-cells leads to increased permeability to bacterial endotoxins such as lipopolysaccharide (LPS). Increased systemic levels of LPS are hypothesised to contribute to chronic immune activation in HIV-infected patients via activation of monocytes[32]. Kupffer cells are the main cell type in the liver that responds to LPS. When stimulated through ligation of the LPS receptor, toll like receptor (TLR)-4, Kupffer cells produce pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interleukin-6 (IL-6), IL-12 and IL-18[33]. Under normal physiological conditions, Kupffer cells remain tolerant, or refractory, to repeated LPS stimulation[33]. Elevated LPS has been shown to contribute to liver disease progression in alcoholic liver disease[34], as well as in NAFLD and NASH[35-37], chronic HCV[38] and HIV-HCV co-infection[39]. In addition to activation of Kupffer cells, LPS also directly activates HSC to produce CCL-2[40], and in vitro following co-culture with monocytes, induces hepatocytes to produce chemokines CXCL9, 10 and 11[41]. These chemokines will induce chemotaxis of both T-cells and monocytes to the liver. In HIV-infected individuals, an increase in systemic LPS levels may therefore potentially contribute to liver disease progression, although to date this has not been demonstrated in the setting of HIV alone (Figure 1).
Finally, HIV may also contribute to the metabolic syndrome associated with NAFLD or NASH via several mechanisms. ART and chronic inflammation can promote insulin resistance[42], where free fatty acids are released from adipose tissue, leading to increased intrahepatic triglyceride droplets (macrosteatosis) or via mitochondrial toxicity (microsteatosis) and oxidative stress[43,44]. HIV has also been shown to modify the activity of peroxisome proliferator-activated receptors (PPAR)[45]. PPARs are a family of nuclear receptor transcription factors that regulate insulin sensitivity, glucose and lipid metabolism, as well as inflammation, tissue repair, carcinogenesis and fibrosis, and are expressed by hepatocytes, HSCs and Kupffer cells[46-48]. Two HIV accessory proteins, Vpr and Nef, have been shown to suppress PPAR-γ subtype activity in vitro[49,50]. One small study in HIV-infected individuals also showed a reduction in the mRNA levels of PPAR-γ and a correlation with increased liver fibrosis[51]. Suppression of PPAR-γ activity by HIV may therefore represent another mechanism by which HIV contributes to liver disease progression.
Portal hypertension has been described recently in HIV mono-infected individuals without other known risk factors for liver disease. Individuals may present with decompensated portal hypertension, such as ascites or bleeding esophageal varices, without histological cirrhosis on liver biopsy[2-5]. This clinical scenario has been variably termed “noncirrhotic portal hypertension”(NCPH)[52], idiopathic portal hypertension[3] or “cryptogenic pseudocirrhosis”[4] to differentiate it from cirrhosis from other etiologies.
Numerous case reports and series of individuals with NCPH in HIV have been described[2,52-60], including those undergoing liver transplantation[61]. NCPH has not yet been described in low and middle income countries. It is possible that NCPH has not been recognized due to limited availability of liver biopsy. Alternatively, the impact of NCPH may be overshadowed by more common diseases such as co-infection with viral hepatitis, pyogenic infections or tuberculosis[8,62,63]. NCPH has been described in adolescence[64] but the lead time prior to clinical presentation of NCPH may mean it remains a future problem for low and middle income countries.
Liver histology from individuals with HIV mono-infection and NCPH is variable and includes periportal or perisinusoidal fibrosis, low grade inflammation and steatosis[65]. However, a common pattern appears to be portal vein occlusion and focal fibrous obliteration of small portal veins, so-called “hepatic venopathy”, often in the setting of nodular regenerative hyperplasia (NRH)[52,57].
A hypercoagulable state may contribute to the hepatic venopathy and NRH, as described in the antiphospholipid syndrome associated with rheumatoid arthritis[66]. Hypercoagulable states were identified in 8 out of 10 individuals with NCPH in one case series[57]. In a recent case-control study, NRH was seen in all 5 out of 11 individuals with NCPH where histology was available, and protein C and S activity was lower in cases than controls[67]. In another small case-control study including 15 individuals with confirmed esophageal varices and absence of cirrhosis on liver biopsy, periportal fibrosis was the most common hepatic lesion described and NRH was only seen in 1 patient[65]. A strong association between prolonged exposure to didanosine (ddI) and the development of NCPH was found in this study. Two cross sectional studies looking at associations between multiple clinical factors and NCPH have also demonstrated an association with prior and current use of ddI[2], as well as NASH[5]. ddI is now almost never used in high income countries but still frequently used in low and middle income countries, and therefore NCPH may soon be seen more frequently in these settings[68].
Hepatotoxicity due to ART may be related to agents from a number of classes, including nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors[43] (Table 1). The severity of hepatotoxicity may range from transient elevations in transaminase levels to hepatic failure and death, via a variety of mechanisms. NNRTI such as nevirapine and efavirenz may cause hypersensitivity[69-71]. NRTI, primarily ddI, may cause direct mitochondrial toxicity leading to abnormal liver function[72]. Other mechanisms by which ART causes liver-related toxicity include direct cell stress and disturbances in lipid/sugar metabolism and steatosis, as seen with protease inhibitors[43]. The protease inhibitors ritonavir, tipranavir and darunavir have all been associated with elevations in ALT[43].
| Viral hepatitis | HBV, HCV, (HDV) | Co-infection common (up to 10%) |
| HAV, HEV | Self-limited acute increase in ALT | |
| Drug hepatotoxicity | Alcohol | Limited data in low and middle income countries |
| ART1 | Nevirapine | Hypersensitivity, usually early (< 12 wk) |
| Efavirenz | Direct liver cell stress or hypersensitivity | |
| Abacavir | Hypersensitivity, (predominantly in HLA B57 carriers) | |
| ddI, d4T | Mitochondrial toxicity with long-term use | |
| Ritonavir | Steatosis, metabolic disturbance | |
| Darunavir | Hypersensitivity | |
| Tipranavir | Hepatic failure reported with ritonavir 200 mg | |
| Maraviroc | Hypersensitivity with liver involvement | |
| Anti-TB therapy2 | Rifampicin | Drug interactions with ART and direct hepatotoxicity |
| Isoniazid | Hepatotoxicity may be increased in HIV | |
| Pyrazinamide | Dose-related hepatotoxicity | |
| Hepatotropic infections | Schistosomiasis | Leads to portal hypertension |
| Leishmaniasis | Fever +/- hepatosplenomegaly | |
| Herpes viruses inc EBV CMV HHV6 HSV | Often cause raised transaminases, occasionally symptomatic hepatitis | |
| Liver abscess | Unlikely to cause chronic liver disease | |
| HIV cholangiopathy | Usually when CD4 < 200 cells/μL | |
| NAFLD | ART-related, prevalence unknown |
NAFLD is now commonly reported in many high income countries in the presence and absence of HIV infection. The spectrum of NAFLD ranges from mild steatosis to NASH, which may progress to severe fibrosis and cirrhosis. NAFLD may be associated with other features of the metabolic syndrome, including central obesity, insulin resistance, diabetes mellitus and dyslipidemia, such as raised triglycerides, low high-density lipoproteins and raised low-density lipoproteins. NAFLD has been identified in up to 30% of HIV mono-infected Americans, although this study was unable to demonstrate whether the prevalence of NAFLD was different to HIV-negative individuals[6]. While risk factors for NAFLD may be similar in people with or without HIV[73], a small study has suggested that people with HIV and NAFLD may be more physically active and less obese than HIV-negative individuals with NAFLD, and that other factors such as HIV itself or ART may contribute to NAFLD[74]. NAFLD is frequently described in the setting of HIV-HCV co-infection[75-77] where ART including ddI or stavudine may also be implicated[75]. Insulin resistance and NASH have been described in individuals with liver disease in HIV mono-infection[5,52]. Insulin resistance and exposure to ddI and/or stavudine were factors associated with advanced fibrosis in 681 HIV-HCV co-infected individuals in Spain[78]. Few studies of NAFLD have been published from low and middle income countries.
Co-infection with Mycobacterium tuberculosis and its treatment are also important causes of liver disease in low and middle income countries and are summarised in Table 1. Hepatitis related to isoniazid therapy is common[79], and HIV or ART may increase the risk of isoniazid-related hepatotoxicity, as demonstrated in a small Ethiopian study[80]. Concomitant anti-tuberculosis therapy was associated with a 5-fold increased risk of abnormal aminotransferase levels in Ugandan individuals commencing NNRTI-based ART[81].
Schistosomiasis may also cause liver disease but was an uncommon cause of liver disease in HIV-infected individuals in Uganda in one study of 77 patients[62]. In a recent study using transient elastography (TE, FibroScan®) in 1000 individuals in Uganda, 14% of the 500 HIV-infected individuals had positive schistosomiasis serology[82]. Significant fibrosis (≥ F2, liver stiffness measurement ≥ 9.3 kPa) was detected in 17% of HIV-infected individuals. Positive schistosomiasis serology was not a significant predictor of cirrhosis [odds ratio 1.7, (0.9-3.3); P = 0.10] in this study but the authors concluded that schistosomiasis may play a role in the burden of liver disease in HIV-infected individuals in Uganda[82]. Further work is required to better understand the true relationship between HIV, schistosomiasis and liver disease.
The impact of other infections, including visceral leishmaniasis (also known as Kala-Azar), on liver disease in HIV infection is unclear but has been reported[83] and may cause chronic disease[84]. Amebic liver abscesses are frequently described in HIV infection[85] but are usually treatable and are unlikely to contribute significantly to ongoing liver disease[62]. HIV cholangiopathy in individuals with CD4 T-cell counts < 200 cells/mL may be due to infections with cytomegalovirus, cryptosporidium or microsporidium[63], although prevalence in low and middle income countries has not been reported.
Alcohol is a common cause of liver disease world-wide and is likely to be as important in low and middle income countries, as it is in HIV-infected individuals in high income countries[86]. Alcohol is responsible for significant morbidity and mortality in South Africa[87] and may also contribute to significant fibrosis detected by TE in Uganda[62]. However, data on alcohol consumption and its impact on liver disease in the setting of HIV in low and middle income countries are limited.
The availability of liver biopsy is limited in low and middle income countries and non-invasive measures of liver fibrosis, such as TE, have been studied in many liver conditions but largely in high income countries[88]. Algorithms to diagnose liver disease severity based on a range of biochemical and hematological indices, such as FibroTest®, Hepascore or AST to platelet ratio index, have also been used[89]. Most data regarding TE come from studies of individuals with HCV[88]. TE is most reliable at detecting cirrhosis (F4) or severe fibrosis (F3/4) but is less reliable at differentiating absent or mild fibrosis (F0/1) from significant fibrosis or greater (F2/3/4)[88]. In many studies considered in a recent meta-analysis, the sensitivity for detecting at least significant fibrosis was less than 70%[88]. The mean area under the receiver operator characteristic curve (AUROC) was 0.84 (95% CI: 0.82-0.86), where a diagnostic tool is considered good if the AUROC is greater than 80% and excellent if the AUROC is greater than 90%[88]. The AUROC for the detection of cirrhosis was 0.94 (95% CI: 0.93-0.95).
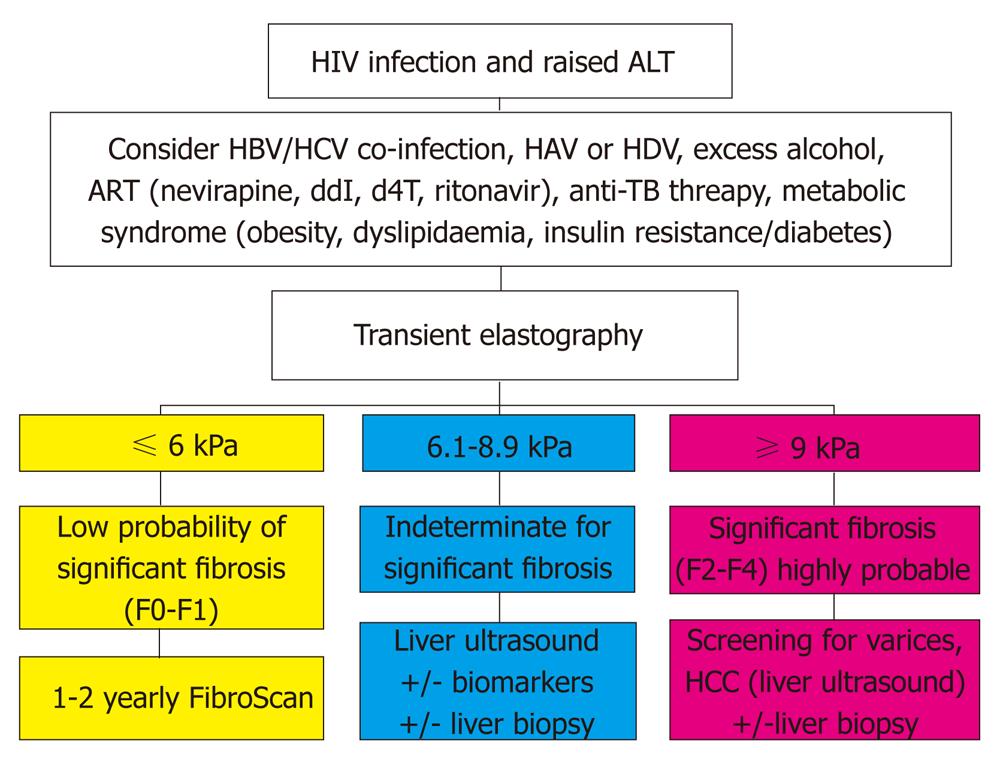
Non-invasive measures like TE have been used to detect severe fibrosis in HIV-infected individuals with persistently elevated transaminase levels[2,5,90]. Few studies in low and middle income countries have been reported to date[82]. However, the convenience of TE and or biochemical or hematological indices offers great potential for screening and monitoring liver disease in large cohorts of HIV-infected individuals in low and middle income countries and there is a great need for further work in this area. A proposed algorithm for the use of TE in evaluating HIV-infected individuals is presented in Figure 2[91].
Liver disease in HIV-infected individuals, in the absence of co-infection with HBV or HCV, is an emerging issue in all settings. While ART related toxicities are an obvious cause, there is emerging evidence that HIV infection may have a direct impact on the pathogenesis of liver fibrosis, NAFLD and NASH and subsequent progression to liver disease. Further research is needed to determine the causal link between HIV infection and liver disease progression. Two potentially important risk factors for low and middle income countries in the development of liver disease are prolonged exposure to high HIV RNA and low CD4 count, providing further support for earlier initiation of ART. One of the major obstacles to research into the epidemiology of the true incidence of liver disease in both high income and low and middle income countries is the lack of suitable non-invasive methods of determining liver disease progression. However, with the advent of newer convenient technologies, such as TE and non invasive plasma markers, we may see this change in the near future.
Peer reviewers: Dr. Andrea Mancuso, Epatologia e Gastro-enterologia, AO Ospedale Niguarda Cà Granda, Piazza Ospedale Maggiore 3, 20162 Milano, Italy; Dr. Lang Zhuo, Department of Cell and Tissue Engineering, Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos 04-16, Singapore 138669, Singapore
S- Editor Wu X L- Editor Roemmele A E- Editor Li JY
| 1. | Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, Monforte A, Kirk O, Friis-Moller N, Phillips A. Factors associated with specific causes of death amongst HIV-positive individuals in the D: A: D Study. AIDS. 2010;24:1537-1548. [PubMed] |
| 2. | Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103-107. [PubMed] |
| 3. | Chang PE, Miquel R, Blanco JL, Laguno M, Bruguera M, Abraldes JG, Bosch J, Garcia-Pagan JC. Idiopathic portal hypertension in patients with HIV infection treated with highly active antiretroviral therapy. Am J Gastroenterol. 2009;104:1707-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Panos G, Farouk L, Stebbing J, Holmes P, Valero S, Randell P, Bower M, Gazzard B, Anderson M, Nelson M. Cryptogenic pseudocirrhosis: a new clinical syndrome of noncirrhotic portal hypertension (unassociated with advanced fibrosis) that can be detected by transient elastography in patients with HIV. J Acquir Immune Defic Syndr. 2009;52:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Ingiliz P, Valantin MA, Duvivier C, Medja F, Dominguez S, Charlotte F, Tubiana R, Poynard T, Katlama C, Lombès A. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, Parker R, Lifson A, Capozza T. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Belloso WH, Orellana LC, Grinsztejn B, Madero JS, La Rosa A, Veloso VG, Sanchez J, Ismerio Moreira R, Crabtree-Ramirez B, Garcia Messina O. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med. 2010;11:554-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Garcia-Jardon M, Bhat VG, Blanco-Blanco E, Stepian A. Postmortem findings in HIV/AIDS patients in a tertiary care hospital in rural South Africa. Trop Doct. 2010;40:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Kovari H, Ledergerber B, Battegay M, Rauch A, Hirschel B, Foguena AK, Vernazza P, Bernasconi E, Mueller NJ, Weber R. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis. 2010;50:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, Hale B, Hames C. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. [PubMed] |
| 12. | Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 624] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 13. | Ocama P, Castelnuovo B, Kamya MR, Kirk GD, Reynolds SJ, Kiragga A, Colebunders R, Thomas DL. Low frequency of liver enzyme elevation in HIV-infected patients attending a large urban treatment centre in Uganda. Int J STD AIDS. 2010;21:553-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Mata-Marín JA, Gaytán-Martínez J, Grados-Chavarría BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejía A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virol J. 2009;6:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zhang YX, Gui XE, Zhong YH, Rong YP, Yan YJ. Cancer in cohort of HIV-infected population: prevalence and clinical characteristics. J Cancer Res Clin Oncol. 2011;137:609-614. [PubMed] |
| 16. | Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152-1159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 429] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 17. | Crum-Cianflone NF, Grandits G, Echols S, Ganesan A, Landrum M, Weintrob A, Barthel R, Agan B. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr. 2010;54:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Bréchot C, Marche C. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol. 1990;21:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Hufert FT, Schmitz J, Schreiber M, Schmitz H, Rácz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr. 1993;6:772-777. [PubMed] |
| 21. | Gendrault JL, Steffan AM, Schmitt MP, Jaeck D, Aubertin AM, Kirn A. Interaction of cultured human Kupffer cells with HIV-infected CEM cells: an electron microscopic study. Pathobiology. 1991;59:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Schmitt MP, Gendrault JL, Schweitzer C, Steffan AM, Beyer C, Royer C, Jaeck D, Pasquali JL, Kirn A, Aubertin AM. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retroviruses. 1990;6:987-991. [PubMed] |
| 23. | Steffan AM, Lafon ME, Gendrault JL, Schweitzer C, Royer C, Jaeck D, Arnaud JP, Schmitt MP, Aubertin AM, Kirn A. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Cao YZ, Friedman-Kien AE, Huang YX, Li XL, Mirabile M, Moudgil T, Zucker-Franklin D, Ho DD. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553-2559. [PubMed] |
| 25. | Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1658] [Article Influence: 61.4] [Reference Citation Analysis (16)] |
| 26. | Fromentin R, Tardif MR, Tremblay MJ. Human hepatoma cells transmit surface bound HIV-1 to CD4+ T cells through an ICAM-1/LFA-1-dependent mechanism. Virology. 2010;398:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Iser DM, Avihingsanon A, Wisedopas N, Thompson AJ, Boyd A, Matthews GV, Locarnini SA, Slavin J, Desmond PV, Lewin SR. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS. 2011;25:197-205. [PubMed] |
| 29. | Macias J, Japón MA, Sáez C, Palacios RB, Mira JA, García-García JA, Merchante N, Vergara S, Lozano F, Gómez-Mateos J. Increased hepatocyte fas expression and apoptosis in HIV and hepatitis C virus coinfection. J Infect Dis. 2005;192:1566-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, Milani S, Gessani S, Fantuzzi L, Liotta F. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2823] [Cited by in RCA: 2773] [Article Influence: 138.7] [Reference Citation Analysis (4)] |
| 33. | Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 343] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691-701. |
| 36. | Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G107-G116. [PubMed] |
| 37. | Zhan YT, An W. Roles of liver innate immune cells in nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:4652-4660. [PubMed] |
| 38. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 40. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 513] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 41. | Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, Bertoletti A. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Núñez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Núñez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology. 2010;52:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 742] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 45. | Lemoine M, Capeau J, Serfaty L. PPAR and Liver Injury in HIV-Infected Patients. PPAR Res. 2009;2009:906167. [PubMed] |
| 46. | Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol. 1995;2:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715-35722. [PubMed] |
| 48. | Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319-1327. [PubMed] [DOI] [Full Text] |
| 49. | Otake K, Omoto S, Yamamoto T, Okuyama H, Okada H, Okada N, Kawai M, Saksena NK, Fujii YR. HIV-1 Nef protein in the nucleus influences adipogenesis as well as viral transcription through the peroxisome proliferator-activated receptors. AIDS. 2004;18:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Shrivastav S, Kino T, Cunningham T, Ichijo T, Schubert U, Heinklein P, Chrousos GP, Kopp JB. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor {gamma} and inhibits adipocyte differentiation: implications for HIV-associated lipodystrophy. Mol Endocrinol. 2008;22:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Lemoine M, Barbu V, Girard PM, Kim M, Bastard JP, Wendum D, Paye F, Housset C, Capeau J, Serfaty L. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS. 2006;20:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Vispo E, Moreno A, Maida I, Barreiro P, Cuevas A, Albertos S, Soriano V. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS. 2010;24:1171-1176. [PubMed] |
| 53. | Arey B, Markov M, Ravi J, Prevette E, Batts K, Nadir A. Nodular regenerative hyperplasia of liver as a consequence of ART. AIDS. 2007;21:1066-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Bihl F, Janssens F, Boehlen F, Rubbia-Brandt L, Hadengue A, Spahr L. Anticoagulant therapy for nodular regenerative hyperplasia in a HIV-infected patient. BMC Gastroenterol. 2010;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, Galambos MR, Rubin RA. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol. 2008;6:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Vispo E, Maida I, Barreiro P, Moreno V, Soriano V. Upper gastrointestinal bleeding may unmask didanosine-associated portal hepatopathy in HIV/HCV co-infected patients. HIV Clin Trials. 2008;9:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Mendizabal M, Craviotto S, Chen T, Silva MO, Reddy KR. Noncirrhotic portal hypertension: another cause of liver disease in HIV patients. Ann Hepatol. 2009;8:390-395. [PubMed] |
| 60. | Dinh MH, Stosor V, Rao SM, Miller FH, Green RM. Cryptogenic liver disease in HIV-seropositive men. HIV Med. 2009;10:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Tateo M, Sebagh M, Bralet MP, Teicher E, Azoulay D, Mallet V, Pol S, Castaing D, Samuel D, Duclos-Vallée JC. A new indication for liver transplantation: nodular regenerative hyperplasia in human immunodeficiency virus-infected patients. Liver Transpl. 2008;14:1194-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Ocama P, Katwere M, Piloya T, Feld J, Opio KC, Kambugu A, Katabira E, Thomas D, Colebunders R, Ronald A. The spectrum of liver diseases in HIV infected individuals at an HIV treatment clinic in Kampala, Uganda. Afr Health Sci. 2008;8:8-12. [PubMed] |
| 63. | Feld JJ, Ocama P, Ronald A. The liver in HIV in Africa. Antivir Ther. 2005;10:953-965. [PubMed] |
| 64. | Kochin I, Magid M, Arnon R, Glasscock A, Kerkar N, Miloh T. Variceal bleeding in an adolescent with HIV diagnosed with hepatoportal sclerosis and nodular regenerative hyperplasia. J Pediatr Gastroenterol Nutr. 2010;50:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Kovari H, Ledergerber B, Peter U, Flepp M, Jost J, Schmid P, Calmy A, Mueller NJ, Muellhaupt B, Weber R. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis. 2009;49:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Cesari M, Schiavini M, Marchetti G, Caramma I, Ortu M, Franzetti F, Galli M, Antinori S, Milazzo L. Noncirrhotic portal hypertension in HIV-infected patients: a case control evaluation and review of the literature. AIDS Patient Care STDS. 2010;24:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Yarchoan R, Mitsuya H, Thomas RV, Pluda JM, Hartman NR, Perno CF, Marczyk KS, Allain JP, Johns DG, Broder S. In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 1989;245:412-415. [PubMed] |
| 69. | Chu KM, Boulle AM, Ford N, Goemaere E, Asselman V, Van Cutsem G. Nevirapine-associated early hepatotoxicity: incidence, risk factors, and associated mortality in a primary care ART programme in South Africa. PLoS One. 2010;5:e9183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Coffie PA, Tonwe-Gold B, Tanon AK, Amani-Bosse C, Bédikou G, Abrams EJ, Dabis F, Ekouevi DK. Incidence and risk factors of severe adverse events with nevirapine-based antiretroviral therapy in HIV-infected women. MTCT-Plus program, Abidjan, Côte d'Ivoire. BMC Infect Dis. 2010;10:188. [PubMed] |
| 71. | Mbougua JB, Laurent C, Kouanfack C, Bourgeois A, Ciaffi L, Calmy A, Gwet H, Koulla-Shiro S, Ducos J, Mpoudi-Ngolé E. Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon. BMC Public Health. 2010;10:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Murphy RA, Sunpath H, Kuritzkes DR, Venter F, Gandhi RT. Antiretroviral therapy-associated toxicities in the resource-poor world: the challenge of a limited formulary. J Infect Dis. 2007;196 Suppl 3:S449-S456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Guaraldi G, Squillace N, Stentarelli C, Orlando G, D'Amico R, Ligabue G, Fiocchi F, Zona S, Loria P, Esposito R. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 74. | Mohammed SS, Aghdassi E, Salit IE, Avand G, Sherman M, Guindi M, Heathcote JE, Allard JP. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr. 2007;45:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | McGovern BH, Ditelberg JS, Taylor LE, Gandhi RT, Christopoulos KA, Chapman S, Schwartzapfel B, Rindler E, Fiorino AM, Zaman MT. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Rodríguez-Torres M, Govindarajan S, Solá R, Clumeck N, Lissen E, Pessôa M, Buggisch P, Main J, Depamphilis J, Dieterich DT. Hepatic steatosis in HIV/HCV co-infected patients: correlates, efficacy and outcomes of anti-HCV therapy: a paired liver biopsy study. J Hepatol. 2008;48:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, Thomas DL. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585-592. [PubMed] |
| 78. | Blanco F, Barreiro P, Ryan P, Vispo E, Martín-Carbonero L, Tuma P, Labarga P, Medrano J, González-Lahoz J, Soriano V. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18:11-16. [PubMed] |
| 79. | Tedla Z, Nyirenda S, Peeler C, Agizew T, Sibanda T, Motsamai O, Vernon A, Wells CD, Samandari T. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in hiv-infected adults in Botswana. Am J Respir Crit Care Med. 2010;182:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Yimer G, Aderaye G, Amogne W, Makonnen E, Aklillu E, Lindquist L, Yamuah L, Feleke B, Aseffa A. Anti-tuberculosis therapy-induced hepatotoxicity among Ethiopian HIV-positive and negative patients. PLoS One. 2008;3:e1809. [PubMed] |
| 81. | Weidle PJ, Moore D, Mermin J, Buchacz K, Were W, Downing R, Kigozi A, Ndazima V, Peters P, Brooks JT. Liver enzymes improve over twenty-four months of first-line non-nucleoside reverse transcriptase inhibitor-based therapy in rural Uganda. AIDS Patient Care STDS. 2008;22:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Stabinski L, Reynolds S, Ocama P, Newell K, Ndyanabo A, Thomas D, Gray R, Wawer M, Quinn T, Kirk G. High prevalence of unexplained liver fibrosis associated with HIV in rural southwestern Uganda. Proceedings of the 17th Conference on Retroviruses and Opportunistic Infections (CROI). 2010;689. |
| 83. | Alexandrino-de-Oliveira P, Santos-Oliveira JR, Dorval ME, Da-Costa FC, Pereira GR, da Cunha RV, Paniago AM, Da-Cruz AM. HIV/AIDS-associated visceral leishmaniasis in patients from an endemic area in Central-west Brazil. Mem Inst Oswaldo Cruz. 2010;105:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Bourgeois N, Bastien P, Reynes J, Makinson A, Rouanet I, Lachaud L. 'Active chronic visceral leishmaniasis' in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med. 2010;11:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Hung CC, Ji DD, Sun HY, Lee YT, Hsu SY, Chang SY, Wu CH, Chan YH, Hsiao CF, Liu WC. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Negl Trop Dis. 2008;2:e175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 87. | Schneider M, Norman R, Parry C, Bradshaw D, Plüddemann A. Estimating the burden of disease attributable to alcohol use in South Africa in 2000. S Afr Med J. 2007;97:664-672. [PubMed] |
| 88. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1090] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 89. | Calès P, Halfon P, Batisse D, Carrat F, Perré P, Penaranda G, Guyader D, d'Alteroche L, Fouchard-Hubert I, Michelet C. Comparison of liver fibrosis blood tests developed for HCV with new specific tests in HIV/HCV co-infection. J Hepatol. 2010;53:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Merchante N, Pérez-Camacho I, Mira JA, Rivero A, Macías J, Camacho A, Gómez-Mateos J, García-Lázaro M, Torre-Cisneros J, Pineda JA. Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther. 2010;15:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Moreno S, García-Samaniego J, Moreno A, Ortega E, Pineda JA, del Romero J, Tural C, von Wichmann MA, Berenguer J, Castro A. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat. 2009;16:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |