INTRODUCTION
Hepatic encephalopathy (HE) is a potentially reversible syndrome manifested by a wide spectrum of changes in consciousness, ranging from subtle behavioral abnormalities to deep coma and death. These neuropsychiatric conditions emerge as the major complication of acute or chronic liver disease.
The earliest known document to date on HE is the one in the Ancient Library of the University of Padova, Italy, authored by Giovanni Battista Morgagni in 1765. Morgagni’s book of medicine described a case of liver cirrhosis associated with a possible HE complication[1]. Then and onwards, a long road has been traveled and much information has been compiled. There are many factors involved in HE, as a neurotransmission disorder in the central nervous system and in the neuromuscular system, that could lead to impairment of fine motor coordination, alterations in sleep patterns and cognition. Besides, in most cases only minor morphological changes were found in the brain; these are astrocyte swelling and Alzheimer type II astrocyte[2]. Ammonium was initially indicated as the molecule responsible for the HE but may be incorrectly judged in advanced stages and may be only a scapegoat. Ammonia plasma levels are increased two- to three-fold in patients with mild to moderate cirrhotic HE and up to ten-fold in patients with acute liver failure (ALF)[3]. Ammonia is a neurotoxic molecule and its role has been widely studied. Hepatic and inter-organ trafficking of ammonia and its metabolite, glutamine (GLN), lead to hyperammonemic conditions. GLN metabolism via glutaminase is located in the intestinal epithelial cells and in the colonic epithelial cells[4]. Removal of hepatic ammonia is a differentiated work that includes the periportal hepatocyte, through the urea cycle and the perivenous hepatocytes converting ammonia into GLN via glutamine synthetase. When the liver is under pathological conditions, one or both types of hepatocytes could be damaged and the ammonia plasma level starts to rise. Minimal HE (formerly subclinical HE) is an initial clinical state with subtle abnormalities that can only be assessed by specific neuropsychometric and/or neurophysiological tests[5,6].
In 2002, a new classification of HE was proposed by the Working Party (World Congress of Gastroenterology)[7,8]. Basically, 3 types were proposed: type A, HE associated with acute liver failure; type B or HE associated with portal-systemic hepatocellular by-pass without intrinsic disease; and type C, or HE associated with cirrhosis and portal hypertension and portal systemic shunts. In this classification, minimal hepatic encephalopathy (MHE) was recognized as a subtype of C type HE. In 2009, the International Society for the Study of Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) completed this classification and added the experimental models associated with each type[9].
Although significant progress has been made in understanding the knowledge of molecular aspects involved in the development of HE, many questions remain unanswered and controversial issues need to be clarified. This editorial will focus on issues where, to the best of our knowledge, more research is needed in order to clarify, at least partially, controversial topics.
TOXIC SUBSTANCES
There are many neurotoxic substances and among them, related to HE, ammonia and manganese.
Ammonia in the central nervous system, muscle and kidney
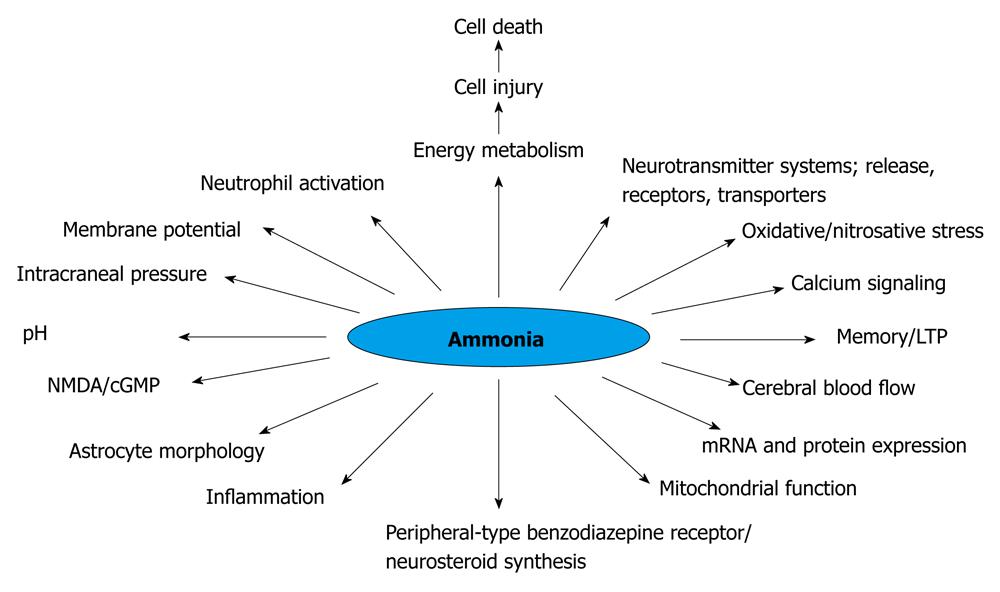
It is well known that hyperammonemia (Figure 1), astrocyte changes and impairment in neurotransmission could lead to HE. Patients with liver injury concomitantly decrease the capability of detoxification of ammonia to urea, shifting this metabolic pathway to the muscular system and to the astrocyte in the central nervous system (CNS). In ALF, the progression of HE is associated with an increased risk of brain edema that could lead to brain herniation, a major cause of death. Therefore it is clear that when the ALF is complicated by HE, the risk to life is increased. The severity of HE is also associated with difference in survival[10]. The development of brain edema and ultimate death is a unique complication of ALF. There are factors that can be viewed with the ability to identify clinical situations with a higher possibility of developing brain edema, such as deep encephalopathy, hyperacute liver failure (acetaminophen-induced ALF), severe hyperammonemia, younger age and infection[11-13].
Figure 1 Changes induced by hyperammonemia (modified)[183].
NMDA: N-methyl-D-aspartate; LTP: Long term potentials.
Currently, an area of controversy is to establish conclusively whether the percentages of deaths could be attributable to cerebral edema or to multiorgan failure. So, brain edema is a key for understanding the pathophysiology of ALF[14]. Chronic liver failure (CLF) spreads minimal to mild edema located surrounding the blood-brain barrier. The edema in CLF has very different consequences than those seen in acute liver failure[15].
In normal circumstances, the liver metabolizes all the ammonia coming from the small and large intestine. In the small intestine, the main source of energy in the enterocytes is glutamine, liberating ammonia[16].
Ammonia is sent to the liver through the portal vein. The liver metabolic stage of ammonia takes place in two major places, the periportal hepatocyte through the urea cycle takes care of the major part of the ammonia and the hepatocytes near the central vein transform the small quantities left into glutamine. Ammonia is also produced in healthy individuals by muscle and the kidney. These last two tissues have the ability to shift to ammonia detoxifying organs in the case of liver failure. Skeletal muscular tissue, due to its large size, becomes the main ammonia detoxifying organ in the case of chronic liver failure[17]. Muscular glutamine-synthase becomes important due to the failing liver and brain metabolic activity[18]. Hypoproteic diet is a very common procedure in treating patients with liver failure, although a normal proteic diet may be metabolically more adequate and can be safely administered to the cirrhotic patient[19]. Hypoproteic diet may decrease the muscular mass and therefore the ammonia detoxifying ability. Recently, diet supplementation with branched chain amino acids has been shown to decrease minimal hepatic encephalopathy and to increase muscle mass[20].
The kidney is an organ capable of synthesizing and degrading ammonia. In normal conditions, the kidneys and liver interact closely to maintain the ammonia homeostasis. The kidneys are ammonia producers and only 30% of the ammonia produced is excreted with the urine. In liver failure and metabolic acidosis, the kidney has the ability to increase ammonia elimination to 70% of the produced ammonia[21,22]. Aquaporin 2 plays an important role in the regulation of water. Its expression is increased in the urine of cirrhotic patients, with a significant increase in patients with ascites, and is higher in compensated cirrhotic patients[23]. The Rh B and C glycoproteins group participates in the elimination of ammonia in the kidney[24,25]. Plasma ammonia concentration has been shown to be related to serum creatinine and the glomerular filtration rate. Renal dysfunction seems to increase cognitive impairment in patients with liver cirrhosis and might be implicated in the pathogenesis of hepatic encephalopathy[26]. Extra-hepatic ammonia metabolism appears to be the target of novel ways of treatment in chronic liver failure.
Manganese
Manganese is an essential trace metal that is involved in the metabolism of carbohydrates, lipids and proteins and has an important function as a cofactor for a number of enzymes[27]. It exists as divalent (Mn+2) and trivalent forms in the plasma[28]. Both, divalent via an undefined transporter and trivalent Mn via the receptor-mediated endocytosis, may be transported into the brain, across the blood-brain and the blood-cerebrospinal fluid barriers and accumulate in the brain[29]. Manganese is neurotoxic, particularly affecting the actions of certain proteins (i.e., receptors) that interact with the neurotransmitter dopamine, probably via striatal dopamine depletion, N-methyl-D-aspartate (NMDA) excitotoxicity or oxidative/nitrosative stress. Moreover, three basic Mn cellular neurotoxicity mechanisms can be described: (1) mitochondrial dysfunction and disruption of energy metabolism; (2) the inflammatory activation of the glia; and (3) disruption of the synaptic transmission and neuronal-glial communication[30]. Furthermore, manganese has prooxidant activity and direct toxic effects have been observed in dopaminergic neurons. Manganese induces a decrease in the content of peroxidase and catalase in the substantia nigra. This metal produces active oxygen species, i.e., superoxide hydrogen peroxide and hydroxy radical, and also produces 6-hydroxydopamine or other toxic catecholamines. Manganese induces the autooxidation of dopamine followed by the formation of toxic (semi)quinones and dopamine depletion[30,31]. Besides this, apoptosis may play a role in the dopaminergic neurotoxicity associated with manganese, the first metal to be reported to induce this form of cell death[31,32]. The early biochemical events show the impairment of energy metabolism and the process may require new synthesis of proteins such as c-Fos and c-Jun. In addition, manganese induces phosphorylation of c-Jun and SEK1/MKK4 (c-Jun N-terminal kinase) and tyrosine phosphorylation of several proteins. Manganese activates specific signal cascades including the c-Jun N-terminal kinase pathway[32].
In chronic exposure with Mn+2, a decrease of GABA concentration in discrete regions of the SNC as the globus pallidus, but not in substance nigra or hippocampus, was observed[33,34]. This effect on GABA levels could be due to the direct action of the Mn+2 on the expression of glutamic decarboxylase, an enzyme that regulates GABA synthesis[35].
Many authors suggest the participation of Mn+2 in the pathogenesis of the HE, in addition to ammonia[36-38]. The increased Mn+2 in plasma and as a deposit in the CNS is believed to be due to decreased elimination of Mn+2via biliary excretion[39], and to increased systemic availability due to portal-systemic shunting associated with chronic liver disease[39,40]. Manganese (Mn+2) brain deposits have also been demonstrated in a rat model of cirrhosis[41] and recently high plasma levels of Mn+2 and deposits of Mn+2 in the hippocampus of rats with MHE and altered integrity of the blood-brain barrier (BBB) (unpublished results) were demonstrated. These results also show that the sharp effects that Mn+2 produces on the aminoacidergic neurotransmitter can be opposite to the chronic effects. In this way, when the Mn+2 accumulates in the synapsis[34], it produces a consistent neuropathy with an excitotoxic effect, suggesting that the mechanism of glutamate is involved in the development of the pathology described by the Mn+2. Manganese may operate at the same time on the hypothalamus GABAergic and glutamatergic neurons that integrate the self-regulation neuronal network.
Moreover, in patients it has been found that there is correlation between plasma levels of Mn+2 with deposits of Mn+2 in basal ganglia registered by MRI[37,38,41,42]. As Krieger et al[36] stated, in end-stage liver disease, the question is whether the increased hyperintensity of Mn+2 in the globus pallidus indicates chronic Mn+2 intoxication or is an adaptive process, leading to improved efficacy of astrocyte ammonia detoxification. Patients with chronic liver failure have shown increased plasma and brain levels of manganese, displaying many of the clinical and pathological features associated with manganese toxicity[37,38,43,44]. The divalent manganese and magnesium have some comparable important overlapping functions[45]. Therefore, the work of Bjerring et al[46] is an interesting approach that shows that hypermagnesemia does not prevent intracranial hypertension or affect the brain content of glutamate, glutamine, or aquaporin-4 expression. Interestingly, divalent manganese also offers fields of study to explore the pathogenesis of the HE more deeply.
ROLE OF ASTROCYTES
Cerebral edema is a response to injuries such as stroke and HE and it is well known that the initial step involves the swelling of astrocytes[47,48]. Mechanisms mediating the astrocyte swelling and the subsequent brain edema remain poorly understood[49].
Glutamine
In HE due to ALF, most frequently caused by drug toxicity and viral hepatitis, marked astrocyte swelling has been demonstrated by electron transmission microscopy in patients[50]. The main determinant molecule involved in astrocyte swelling, at least triggering this pathological condition, is ammonia. Astrocyte’s glutamine synthetase (GS) plays a detoxifying role of ammonia, by amidation of glutamate (GLU) to GLN. In hyperammonemic conditions, GLN is increased in the astrocyte and astrocyte swelling occurs. In rats with induced hyperammonemia, astrocyte swelling was reduced when an inhibitor of GS, methionine sulphoximine, was administered[51]. This could explain why the edema is focused primarily on the astrocyte, because the neurons and capillaries and other membranes in general of the CNS have unusually low water permeability[52]. Glutamime could have a relevant role in oxidative stress/nitrosative as a critical factor in ammonia-induced cell injury[53-55].
Albrecht et al[56] proposed the theory of the trojan horse to explain the glutamine pathway that leads to astrocyte damage. In summary, glutamine is a “stealth” carrier of ammonia. Glutamine enters mitochondria via a histidine-sensitive glutamine carrier, which is potentiated by ammonia. Glutamine is then hydrolyzed by phosphate-activated glutaminase, located in the inner mitochondrial membrane, yielding glutamate and ammonia. Significant amounts of glutamine have been shown to be metabolized in astrocytes. Thus, the generation of mitochondrial ammonia may reach high levels, inducing the mitochondrial potential membrane, reactive oxygen species (ROS), with the potential consequence of morpho-functional oxidative damage of the mitochondria[57,58] and its respiratory chain[58].
Blood brain barrier
Astrocytes are important components of the BBB. Any change in astrocytes is also a potential change in the integrity of the BBB. Besides this, three major causes of astrocyte swelling are considered: (1) cellular edema; (2) vasogenic edema; and (3) aquaporins (AQP). Of these three, the latest are aquaporins which were described in 1992 as water channels and since then many isoforms have been identified[59]. So far seven AQPs: AQP1, AQP3, AQP4, AQP5, AQP8, AQP9 and AQP11 have been identified in various animal tissues in the CNS. Via real-time studies, including the above mentioned AQPs, we also observed the expression of AQP6 and AQP7 in mouse cortical neurons[60].
In CNS AQP1, 9 and 4 are mainly described and associated with brain edema. CNS AQP-4 and AQP-9 are mainly located in astrocytes, while AQP 1 is in the choroid plexus. AQP4 is the most important of the three mentioned in the development of cerebral edema observed in ALF or CLF. Two different isoforms of AQP4 have been shown to exist. Both isoforms are found in the brain[60]. AQP4 is highly expressed in the plasma membrane of astrocytes. It is plentiful in astrocyte cells bordering the subarachnoidal space, ventricles and blood vessels. High levels of AQP4 were noted in areas where astrocytes come into direct contact with capillaries, ependymal layer and pia. In addition, the basolateral membrane of the ependymal cells that line the subfornical organ is positive for AQP4. The sites of AQP expression in the brain suggest a role in the movement of water across the blood brain barrier and thus in cerebrospinal fluid dynamics and the formation of brain edema[60,61]. Importantly, the AQPs are also associated with apoptosis in the CNS. Considering the AQP localization in the astrocyte, the rectifying potassium channel Kir4.1 and the slow K+ channel (Kslo), a dynamic view of Ast trafficking of fluid, can be constructed, likening it to ontogenetically modified epithelial cells[60-62].
Related to vasogenic edema, the ion channels, exchangers and transporters are important factors in cell volume regulation[59,60]. Changes in these systems may result in the loss of ion homeostasis[62] and the subsequent accumulation of intracellular water. These ion transporters and exchangers include the Na-K-Cl cotransporter-1 (NKCC1) that plays an important role in cell swelling/brain edema. Jayakumar et al[63] suggest that activation of NKCC1 is important in astrocyte swelling by ammonia and such activation is mediated by NKCC1 as well as by its oxidation/nitration and phosphorylation.
N-methyl-D-aspartate
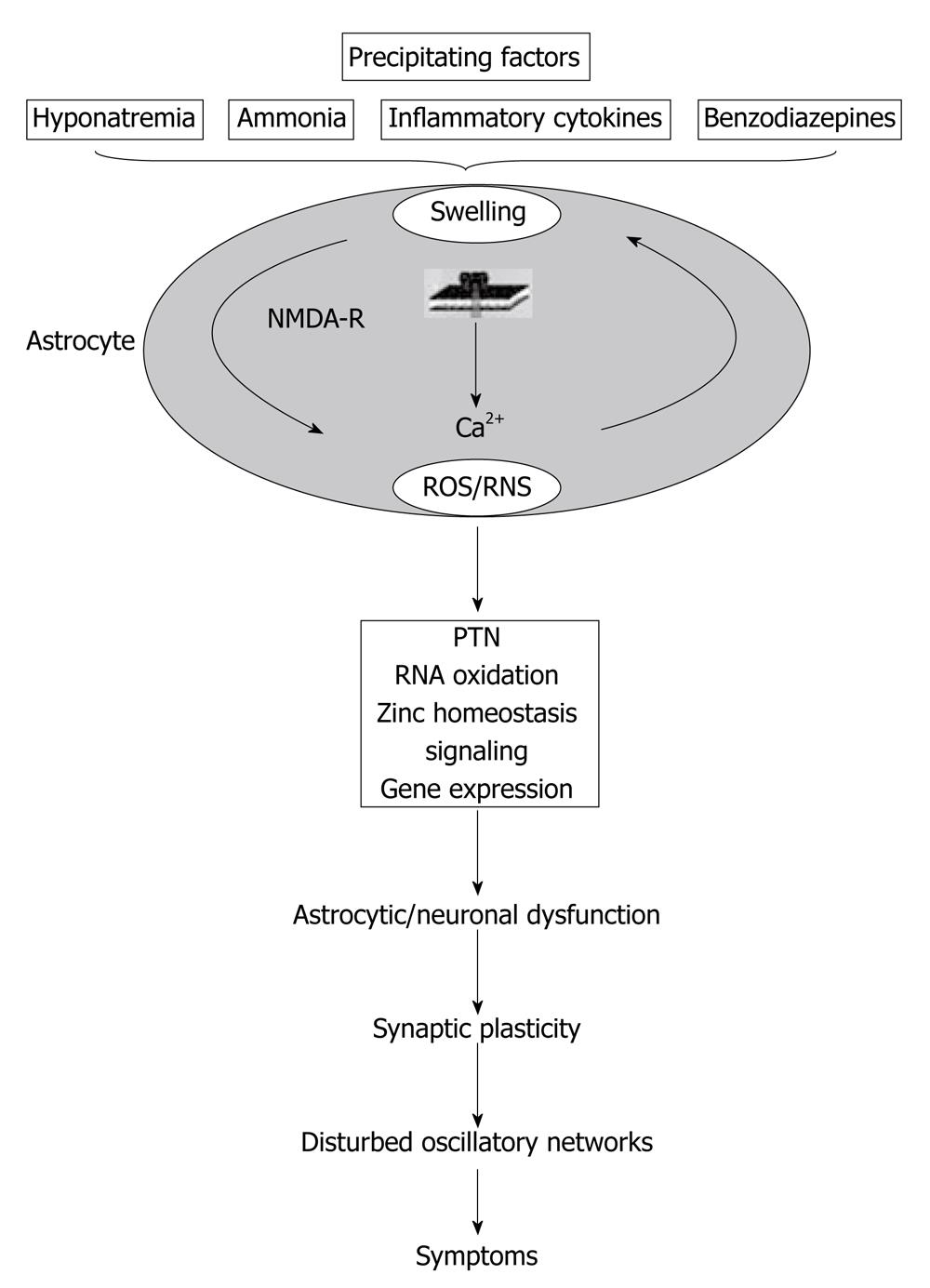
Another point of view that should be included in the subject of swelling of astrocytes is the NMDA receptor pathway. As Häussinger et al[64] summarized, ammonia induces astrocyte swelling which is, in part, counteracted by volume-regulatory osmolyte depletion but leaves the astrocyte vulnerable towards swelling by a heterogeneous set of precipitating factors[64]. Astrocyte swelling involves NMDA receptor activation and the generation of ROS/RNS, which again favors astrocyte swelling. In this way, an autoamplificatory-signaling loop is generated. Consequences are PTN, oxidation of RNA, zinc mobilization and effects on gene transcription. This may impair glioneuronal communication and synaptic plasticity, resulting in disturbance of oscillatory networks, which finally accounts for the HE symptoms (Figure 2)[64].
Figure 2 Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity.
PTN: Protein tyrosine nitration (reproduced with permission)[64]. NMDA: N-methyl-D-aspartate; ROS: Reactive oxygen species.
Extracellular space
Another key component and little considered in HE is the extracellular space (ECS) diffusion parameters that may significantly affect communication between neurons as well as between neurons and glia. The diffusion of transmitters and other neuroactive substances through the ECS is also the underlying mechanism of extrasynaptic or so-called ‘‘volume transmission” in the brain[65,66]. Any decrease in the ability of neuroactive substances, ions or metabolites to diffuse through nervous tissue may represent a serious clinical problem due to the potential for disrupting brain function[67].
So far, it is not known whether astrocyte swelling, proliferation and hypertrophy during physiological and pathological states lead to persistent and functionally significant changes in ECS diffusion parameters. Astrocyte swelling is an early event in numerous pathological states, such as ischemia, hyponatremia and hepatic encephalopathy, and likely results from reduced extracellular osmolarity, elevated extracellular K1 concentration and/or glutamate[68,69]. When rapid cellular, particularly astrocyte, swelling occurs, water moves from the extra to the intracellular compartment. This causes a decrease in ECS volume and changes in the geometry of the intercellular spaces, e.g., during repetitive neuronal activity, ischemia, X-irradiation and experimental autoimmune encephalomyelitis[70-72]. Several experimental conditions have been shown to result in ECS volume changes due to the movement of water from the extra to the intracellular space. In particular, these include a decrease in the extracellular osmotic pressure[73] and the accumulation of excitatory amino acids[74]. After the initial phase of swelling, cells, particularly astrocytes, actively down-regulate their volume by regulatory volume decrease (RVD) and by a net release of KCl, taurine and other amino acids[74,75]; in this way, ECS volume can return to normal values. Even later, when glia become reactive, astrogliosis may result in the formation of additional and persistent diffusion barriers formed, for example, by the hypertrophy of fine glial processes or by an accumulation of macromolecules in the ECS (e.g., extracellular matrix proteins and cytokines) produced by neurons and glia[76]. Syková[76] found that astrogliosis results in the formation of persistent diffusion barriers. By this mechanism, glial cells could significantly affect neuronal excitability synaptic as well as extrasynaptic transmission and be involved in plastic changes. Finally, the upstream sensors and transducers of cell volume changes, a number of integral membrane proteins, including integrins, growth factor receptors and cytokine receptors, have been assigned roles as sensors of cell volume perturbations. Direct evidence for roles in osmo-/volume sensing is so far limited with respect to cytokine receptors and calcium-sensing receptors; hence, only the possible roles of integrins and growth factor receptors need further studies[77].
BLOOD BRAIN BARRIER
BBB is a diffusion barrier, a specialized system of brain microvasculature, essential for the normal function of the CNS. BBB is composed by the endothelial capillary cells, the capillary basement membrane, astrocyte end-feet ensheathing the vessels and pericytes embedded within the basement membrane[78]. The most relevant features of BBB endothelial cells which differentiate them from other endothelial cells are tight junctions and sparse pinocytic vesicular transport. BBB breakdown or alterations in transport systems play an important role in the pathogenesis of many CNS diseases, such as HIV-1 encephalitis, Alzheimer's disease, ischemia, tumors, multiple sclerosis and Parkinson's disease[79]. The pathophysiology of HE is closely linked to changes in the BBB. HE and the BBB is a field in which many controversies and many aspects not yet understood remain even today. It is well known that ammonia plasma levels correlate with CNS damage in liver failure[80]. Ammonia blood concentration is of major importance in the evaluation of patients with known or suspected HE[81]. Since Loockwood et al[82] published the first relevant data in this topic, many answers and new questions arose. Is arterial or venous ammonia concentration by itself an early marker and/or predictor in HE initiation or progression? Is BBB ammonia permeability increased in hyperammonemic states and/or in HE? Different views based on different data from different experiments or clinical work place this issue at a dynamic controversy. Ong et al[83] and Kramer et al[84] conclude that arterial, venous and partial pressure of blood ammonia in patients correlated with HE severity. On the other hand, Goldbecker et al[85] in a study with patients with liver fibrosis conclude that no increased BBB ammonia permeability was demonstrated or related to the development of HE. In a historical view, not many years ago, it was considered that ammonium did not pass from blood to brain tissue. Later, Stahl’s work[86] demonstrates increased ammonia blood brain extraction in HE. Then, BBB permeability and integrity changes were demonstrated with PET, showing that brain ammonia concentration was significantly higher (80%) in HE, measured with the isotope 13N by Lookwood et al[87]. A key data for this asseveration could be the permeability surface area product (PS), still not completely elucidated, in the framework of BBB. It is of interest that under their working conditions, Goldbecker et al[85] found no changes on PS. Maybe we could see a little further with a little help from some experimental data. In a model of MHE[57] that displays moderate hyperammonemia and portal hypertension, it was clearly demonstrated that BBB has morphofunctional changes. Transmission electron microscopy revealed tight junction disruption in BBB capillaries in the hippocampal area, with mild edema of astrocytes and ECS. Moreover, a hippocampal increased number of capillaries and capillaries area per field was also documented. That means that in an experimental MHE, increased permeability and angiogenesis in the hippocampal area was seen. Furthermore, energy failing, pathological changes in the endothelial mitochondria and decreased nitric oxide synthase (NOS) activity were documented[57]. Jensen et al[88] suggest that BBB leakage induce microglial reactions. These involve modulation of immunomolecules, cytokine and growth factor gene expression. In this sense, Rodrigo et al[89] conclude that chronic hyperammonemia is sufficient to induce microglial activation and neuroinflammation during HE. It may be important to start paying attention to other components of the BBB in HE, components that remain largely unknown as pericytes and the blood-cerebrospinal fluid barrier[90]. Pericytes are involved in angiogenesis and its recruitment to the developing blood vessels and attachment of pericytes to the abluminal surface require heparin sulphate proteoglycan N-sulfation to retain platelet derived growth factor-beta (PDGFRb) homodimers and to activate the receptor PDGFRb signaling[91]. Recently, new data helped to introduce this new player in HE development. Armulik et al[92] describes a novel and critical role for pericytes in the integration of endothelial and astrocyte functions at the neurovascular unit and in the regulation of the BBB. The increased permeability occurs by endothelial transcytosis, a process that is rapidly arrested by the drug imatinib. Besides, by employing a metabolic profiling of serum samples by high-field 1H-nuclear magnetic resonance spectroscopy based on metabonomics approach, Jimenez et al[93] explored a methodology that could enhance diagnosis and monitor disease progression and patient response to treatment during MHE. It is also important to keep in mind that BBB is a very dynamic structure and could be damaged in different stages of HE. Our laboratory has shown[94] that the altered integrity of BBB gets back to normal in a HE-PVL induced model after portal pressure returns to a normal value. Moreover, in a group of animals with portal hypertension (PVL-induced) plus acetaminophen, behavioral changes and increased the BBB alterations were observed[95]. Therefore it can be concluded that the BBB is an area where further research is needed to integrate all components and generate a view of the totality in HE. To understand the pathophysiology of BBB, the data contributed to its components conducted in isolation, e.g., culture of astrocytes, are important but do not provide a comprehensive vision of BBB as a network. BBB, as a key access to the CNS is, without any doubt, a special issue that needs further exploration in order to know the specific role of the carrier or receptors in HE.
CENTRAL NERVOUS SYSTEM CELL DEATH
Perhaps one of the areas that may present greater potential for conflict is the cell death in the CNS in HE. Beyond what can be considered “physiological replacement”, there is a need to determine whether there is a mechanism of lethal cell injury as part of the pathophysiology of HE. Mitochondria are a major target in hyperammonemic conditions and could trigger different pathways. Many of them (a-f) have an important overlap.
Mitochondria, NMDA and energy metabolism
Acute exposure of brain preparations to pathophysiologically relevant concentrations of ammonia has numerous metabolic and neurophysiological effects, including alterations of synaptic inhibition and excitation[96] effects on cerebral energy metabolism and modifications of neurotransmitter-related processes. The effects on cerebral energy metabolism are associated with alterations of mitochondrial function[97], such as in reductions of brain ATP concentrations[98]. Two possible mechanisms have been proposed to explain ammonia-induced reductions in brain ATP concentration: a mechanism involving NMDA receptors and inhibition of the tricarboxylic acid cycle[99]. NMDA receptor is a mechanism identified with the potential to cause neuronal cell death in liver failure mediated by excitotoxicity, lactic acidosis, oxidative/nitrosative stress and the presence of pro-inflammatory cytokines[100]. The role of NMDA receptor in the reduction of ATP levels can be explained by two mechanisms: increased consumption of ATP due to activation of Na+-K+-ATPase and decreased synthesis of ATP in mitochondria due to impairment of calcium homeostasis[99]. NMDA receptor activation also results in mitochondrial swelling[101]. Ammonia-induced depletion of ATP is prevented by the administration of antagonists of NMDA receptor[102].
Acute ammonia intoxication leads to a rapid increase in intramitochondrial calcium content in the brain, followed by a reduction in the calcium capacity and calcium uptake rate. Injection of ammonia results in increased spontaneous calcium efflux from rat brain mitochondria and in potent inhibition of Na+-induced calcium efflux. NMDA receptors in rat brain in vivo alters mitochondrial calcium homeostasis at several distinct steps and independent of the mitochondrial permeability transition (PTP)[103]. Proliferation of astrocytic mitochondria has been reported in conditions of chronic hyperammonemia, attributed to increased energy requirements[50]. In addition, chronic hyperammonemia similar in magnitude to that observed in end-stage chronic liver failure leads to down-regulation of functional NMDA receptor and prevents loss of ATP[99].
Calcium
The calcium ion has important roles in cell growth, signal transduction[104-106] and HE[107]. Most cell calcium is sequestered in the endoplasmic/sarcoplasmic-reticulum (ER/SR) membrane system and this sequestration produces an ER-to-cytoplasm gradient difference of greater than three orders of magnitude. Up to 50% of the calcium so sequestered is mobilizable by inositol triphosphate (IP3), which is formed through the hydrolysis of inositol 4, 5-biphosphate by phosphoinosital-dependent phospholipase C[108]. Calcium stored in the ER is not inert; however, a draining of calcium ions from the ER can cause secretion of resident proteins[109]. The transduction of the ER/SR calcium filling/depleting signal may be mediated in part by the phenomenon of “capacitative calcium entry”[110,111] in which a sustained trans-sarcolemmal calcium entry is coupled to a depletion of intracellular Ca2+ storage that is itself initiated by IP3. In HE, glutamate neurotoxicity has been well documented and glutamate is mainly mediated by excessive activation of the NMDA type of glutamate receptors[112].
Mitochondria are implicated at multiple stages of glutamate neurotoxicity, including the sequestration of Ca entering via the NMDA receptor, the bioenergetic collapse that triggers the ischemic release of glutamate, the generation of reactive oxygen species and the triggering of an apoptotic cascade under certain circumstances[112,113]. Excessive activation of NMDA receptor leads to the opening of its ion channel, allowing the entry of Ca from the extracellular space into the cytoplasm of the post synaptic neuron[103]. Much of this Ca entering the cell is sequestered by mitochondria[113]. The uptake of calcium by the mitochondria is driven by the mitochondrial membrane potential and will compete with the mitochondrial ATP synthase for protons. The abnormal cell calcium regulation has been implicated in cell-cycle progression[114,115] and may participate or precipitate cell death[116,117]. Calcium ATPase located on the ER/SR membrane (SERCA)[117,118] and, to a lesser extent, both plasma-membrane calcium ATPase and the mitochondria contribute to cell-calcium sequestration[118].
In neurons, intracellular calcium levels are controlled by ion channels in the plasma membrane such as NMDA receptors, voltage-gated calcium channels and certain α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, as well as by calcium exchange pathways between the cytosol and internal calcium stores, including the SERCA and mitochondria[118]. Synaptic activity and the subsequent opening of ligand and/or voltage-gated calcium channels can initiate cytosolic calcium transients which propagate towards the cell soma and enter the nucleus via its nuclear pore complexes embedded in the nuclear envelope. Recently, it was described that in hippocampal neurons the morphology of the nucleus affects the calcium dynamics within the nucleus[119-121].
The action of the calcium ion in the nucleus opens up new research possibilities in the interplay of calcium in HE with cellular structures, receptors, SERCA, mitochondria and nucleus[122]. The SERCA isogene group belongs to a family of ion-motive ATPase (“P” ATPase) whose reaction cycle involves the generation of an aspartyl-phosphate intermediate and is represented by three isogenes SERCA overexpression, produced through the use of an adenovirus vector or via a transgenic mouse model[123,124], which has suggested the existence of a phenotype consistent with facilitated intracellular calcium sequestration[123]. This finding supports the functional role of SERCA previously attributed to it through in vitro experimentation. The calcium compartmentalized in the SR may participate in cell-cycle progression. Moreover, the experimental SERCA blockade leads to a quiescent G0-like state[115].
Altered bioenergetics and oxidative stress are also thought to play a major role in mitochondrial disorder inducing the PTP, involving the mechanism of ammonia neurotoxicity. The PTP is a Ca2+-dependent, cyclosporin A sensitive process due to the opening of a pore in the inner mitochondrial membrane that leads to a collapse of ionic gradients and ultimately to mitochondrial dysfunction. Many of the factors that facilitate the induction of the MPT are also known to be implicated in the mechanism of HE, including free radicals, Ca2+, nitric oxide, alkaline pH and glutamine[124]. Our laboratory has shown that oxidative stress was involved in hippocampal damage and curcumin protects against this oxidative stress in experimental MHE. These protective effects may be attributed to its antioxidant properties[125].
Peripheral-type benzodiazepine receptors
Another mitochondrial membrane receptor associated with alterations of cerebral energy metabolites and astrocytes swelling is the mitochondrial "peripheral-type" benzodiazepine receptors (PTBRs)[126]. PTBRs are 18 kDa mitochondrial membrane proteins and with a still elusive function in cell death. PTBRs are located in the mitochondrial membrane cells in the periphery and in astrocytes and they are not allosterically coupled to GABAA receptors[127]. Itzhak el al[128] described an increased PTBRs site in brain in mice with acute hyperammonemia resulting from toxic liver injury. Exposure of glioma cells in culture to PTBRs agonists results in proliferation and swelling of mitochondrial[129], phenomena which have been described following administration of ammonia resins to animals with chronic liver failure. PTBRs cause increases in state IV and decreases in state III mitochondrial respiration rates, resulting in a significant decrease in the respiration control[130].
The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced PTP opening in rat brain mitochondria. PTP opening is important in mitochondrial events leading to programmed cell death[113,131]. In addition to a specific anti-PBR antibody, delayed Ca2+-induced dissipation of membrane potential (ψm) and diminished cyclosporine A-sensitive Ca2+ efflux, which are both indicative for the suppression of PTP opening[132]. Moreover, anti-PBR antibody caused partial retention of Ca2+ in the mitochondrial matrix in spite of ψm dissipation and reduced activation of respiratory rate at Ca2+-induced PTP opening. A release of pro-apoptotic factors, apoptotic inducing factor (AIF) and cytochrome c, from rat brain mitochondria was shown at threshold Ca2+ load. Anti-PBR antibody blocks the release of AIF but does not affect the cytochrome c release. The endogenous PBR ligand, protoporphyrin IX, facilitated PTP opening and phosphorylation of the mitochondrial proteins, thus inducing effects opposite to anti-PBR antibody[132]. It is also interesting that in experimental ALF, moderate hypothermia prevents cerebral edema and reduces the up-regulation of astrocytic PTBRs[133].
Central nervous system cell death
A recent study from our laboratory has shown astrocyte death associated with mitochondrial dysfunction in hippocampal CNS in an experimental model of MHE[58]. The presence of DNA fragmentation with a higher ratio of the Bcl-2 family members Bax/Bcl-xL in the outer mitochondrial membrane and cytochrome c release indicate the presence of apoptosis. A marked decrease of cytochrome oxidase (complex IV of the electron transport chain) was also observed; mitochondria from these animals showed less ability to maintain the membrane potential (ΔΨm) stabilized[58].
Ammonia induced neuronal and oligodendroglial death, triggered apoptosis and activated caspases and calpain. Probably due to calpain activation, ammonia caused the cleavage of the cyclin-dependent kinase 5 activator, p35, to p25, the cdk5/p25 complex known to lead to neurodegeneration[134]. It is important to note that hyperammonemia induces different degrees of cellular damage and it mainly depends on the stage of development and whether the exposure is acute or chronic. Hyperammonemia during development is associated with neuronal cell loss and cerebral atrophy that leads to mental retardation and cerebral palsy in pediatric patients. Among the various pathogenic mechanisms involved, alterations in axonal and dendritic growth and cerebral energy have been demonstrated[135]. Acute hyperammonemia also results in decreased activities of free radical scavenging enzymes and again, free radical formation due to ammonia exposure is prevented by either NMDA receptor antagonists or NOS inhibitors[53,136].
Autophagy of mitochondria and/or selective mitophagy most likely play an important role in removing damaged organelles. Although cell death is often accompanied by autophagy, it is still controversial whether autophagy promotes or prevents cell death. When damaged mitochondria are removed by autophagy, this will prevent cytochrome c release and activation of caspases, whereas a block in autophagy would promote caspase-dependent cell death[137].
Studies containing opposite views have been reported. On one hand, Kosenko et al[138] did not observe apoptosis in an experimental model of acute intoxication with large ammonia doses. The animals (rats) were sacrificed 11 min after injection of ammonium acetate. This acute ammonia intoxication study did not affect caspase-9 or caspase-3 activities, the mitochondrial membrane potential remained unaltered in non-synaptic brain mitochondria, indicating that ammonia did not induce PTP formation in brain in vivo. Also, the nuclear level of p53 did not change, whereas its cytoplasmic level increased approximately two-fold. In agreement with the theory that translocation of the p53 from cytosol to nuclei is an essential step for induction of apoptosis, the authors did not find apoptotic nuclei in the brain. They conclude that this data supports the idea that ammonia neurotoxicity does not involve apoptosis and points to impaired p53 transfer from cytoplasm to nuclei as a possible preventer of apoptosis. They also reported disturbances in the mitochondrial electron transport chain in brain mitochondria from rats injected with ammonia. On the other hand, in our laboratory, an opposite view in a chronic model of MHE PVL-induced cellular death was registered[58]. We found a 5 times higher expression of the proapoptotic member of the Bcl-2 family, Bax. Moreover, an increase of 2.3-times in the number of TUNEL-positive, simultaneously marked with GFAP was also observed in the hippocampal area.
A significant decrease in the mitochondrial respiratory control of MHE animals and a significant decrease (46%) of cytochrome oxidase (complex IV) were recorded. In addition, these mitochondria showed less ability to maintain membrane potential (∆ψm) (28% lower). The swelling experiments showed that mitochondria from MHE animals spontaneously tend to swell. This and the decreased ∆ψm could indicate an MPT mechanism. We conclude that the cellular death could be regarded as one of the earliest steps in the development of experimental MHE. Even although they are different experiments, e.g., an acute with animal sacrifice at 11 min after administration of ammonium and the other, chronic with sacrifice at 10 d of the PVL, the results, seemingly opposite, can be complementary with a broader view.
Programmed cell death
Cell swelling was shown to activate the release of excitatory amino acids, glutamate and aspartate, in astrocyte cultures[139-141]. In the brain, excitatory amino acids can promote neuronal cell damage via over-activation of glutamate receptors and could lead to cell death. One hypothetical route for astrocyte swelling-activated release of organic osmolytes is the ubiquitously expressed volume-regulated anion channel(s) (VRACs), which is activated by cell swelling and is permeable to a variety of inorganic and small organic anions, including the amino acids taurine, glutamate and aspartate. Cell shrinkage is a morphological feature of apoptosis, known as apoptotic volume decrease (AVD). AVD is an isosmotic cell shrinkage which is seen early after apoptotic stimuli and seems to be a prerequisite for apoptosis[142].
Under physiological conditions, brain cells, when subjected to osmotic fluctuations, will undergo regulatory volume increase/decrease (RVI/RVD) to achieve homeostatic balance with neurons in the brain being additionally protected by the BBB. However, during AVD following an apoptotic trigger, the cell undergoes anisotonic shrinkage that involves the loss of water and ions, particularly monovalent ions e.g., K+, Na+ and Cl-. AVD results from a loss of KCl via K+and Cl- channels and concomitant loss of water[143]. VRAC seems to be the anion channel involved in AVD in several human and animal cells. Various K+ channels, including inner membrane mitochondrial K+, appear to be involved in AVD, depending on the cell type or stimulus used[144]. Among these are the two-pore K+ channels that have been implicated in RVD in multiple cell types[145]. Inhibitors of swelling-activated K+ and Cl- channels attenuate AVD and several groups have suggested that the same channels are involved in RVD and in AVD[146]. Moreover, apoptotic cells exhibit an augmented RVD response that could reflect that volume-sensitive channels are more sensitive to cell swelling[147]. Whether the sensor and trigger mechanism for RVD and AVD have identical components with a different set point is still under discussion. In most cells, Cl- conductance (gCl) is significantly lower than K+ conductance (gK) under steady-state. Consequently, an increase in gK+ alone results in K+ loss and Na+ uptake and not in KCl loss and cell shrinkage. This means that activation of VRAC is a necessity for initiation of AVD and in congruence with this, depolarization of the membrane potential during AVD has been demonstrated in several cell types[148-150]. Therefore, VRAC is a principal pathway for mediating organic osmolyte release in astrocyte swelling that could lead to cellular death[151].
Sharing pathways: Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes
Although stroke is a pathological entity by itself, it shares some pathways or an important part of a pathway with HE. Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS) are a pathology that share many altered pathways with HE, such as cerebral edema, BBB abnormalities and alterations in brain mitochondria respiratory chain. MELAS is one of the most common and widely studied maternally inherited mitochondrial diseases that is frequently associated with the m.3243A > G point mutation in the mitochondrial tRNA LeuUUR gene[152]. The clinical phenotype is multisystemic but the triad of lactic acidosis, seizures and stroke-like episodes remains crucial to the diagnosis and reflects the complex and unique pathogenesis of this syndrome[153].
The levels of ventricular CSF lactate correlate with the severity of neurological impairment[154]. Besides, the increase in brain ROS activity was demonstrated in MELAS[155], as it was also clearly documented in HE[53]. MELAS experimental studies in cybrids showed that severe defects in protein synthesis and respiratory chain function segregate with the mutation, although the pathogenic threshold is high; more than 90% mutant mtDNAs are required to cause dysfunction[153]. However, most MELAS patients have well below 95% mutant mtDNA, suggesting that the data from cybrid studies may not be directly extrapolated to the clinical status. As in HE, the pathogenic mechanism of strokes and vasogenic edema cannot be explained by the available data. The same challenge for MELAS and HE is that without a better understanding of the pathogenesis, rational therapeutic intervention has not been possible.
The strokes, non-ischemic in origin and therefore called “stroke-like episodes”, are at least partially reversible and do not conform to distribution of large cerebral arteries, but rather affect small arterioles and capillaries of the cortex while sparing the adjacent white matter[155]. The recurrent strokes are associated with vasogenic edema, as demonstrated by MR diffusion weighted imaging studies, suggesting that they may be due to increased permeability in the BBB, perhaps caused by mitochondrial dysfunction in the endothelium of cerebral small vessels[156]. Furthermore, the accumulation of ventricular lactate indicates severe energy failure in the brain due to mitochondrial dysfunction and acute hypoxia during stroke-like episodes. Translational defects of the mitochondrial respiratory chain subunits and pathological alterations in the microvasculature and in BBB components have been documented in patients with MELAS, thus supporting the notion that BBB permeability may be increased due to mitochondrial respiratory failure in the cortical microvasculature[157], as it has also been demonstrated in MHE[57]. In MELAS, severe defects of respiratory chain complexes in immortalized endothelial cells and astrocytes as well as in primary astrocytes harboring the m.3243A > G mutation were associated[158,159]. Furthermore, the defects in EC cells with the MELAS mutation correlate with lower transendothelial electrical resistance, indicating increased permeability of the BBB endothelial cells[160] as it was also documented in experimental MHE[94].
NEUROENDOCRINE AXIS
Little is known about neuroendocrine changes that occur in portal-systemic hepatic encephalopathy and/or MHE. Scorticati et al[161] studied plasma prolactin (PRL) levels in our laboratory and the involvement of hyperammonemia, nitric oxide (NO) and dopaminergic and adrenergic systems in the control of this hormone secretion in a male rat model of mHE. The authors conducted in vivo studies to determine plasma ammonia and PRL levels, dopamine (DA), dihydroxyphenylacetic acid (DOPAC), epinephrine and norepinephrine content in medial basal hypothalamus (MBH) and anterior pituitary (APs). In addition, NOS activity and protein expression were evaluated in APs. In in vitro studies, the APs from intact rats were incubated with different doses of ammonia and PRL secretion was determined. In ex vivo studies, the APs from normal and PH rats were incubated in the presence of ammonia and/or a NOS inhibitor, NG-nitro-L-arginine-methyl ester (L-NAME), and PRL secretion was determined. It is well known that this model, PVL, has moderate hyperammonemia but the rest of the data obtained was a bit surprising, like a decrease in plasma PRL levels, a significant increase in norepinephrine content in both MBH and AP and an increase in NOS activity and NOS protein expression in APs. Also, in vitro the authors found reduced PRL secretion from APs and the presence of L-NAME, an inhibitor of NOS, abrogated the inhibitory effect of ammonia on PRL secretion from APs from control and MHE rats. Authors conclude that plasma PRL levels were decreased in MHE rats probably due to the high ammonia levels. The central noradrenergic system could also mediate this decrease. Also, the increase in NOS activity and/or content in AP induced NO production that directly inhibited PRL secretion from the AP, without the participation of the dopaminergic system. These results demonstrate the alteration of the neuroendocrine axis in a model of MHE and opens an interesting area of study that remains largely unexplored[161].
NEURONAL ENVIRONMENT, NEUROPLASTICITY, BEHAVIOR AND MEMORY
Pioneer investigations about memory processes began in 1957. Brenda Milner stated that certain forms of memory were stored in the hippocampus and the medial temporal lobe[162]. Since then, many works have described the relationship between the neuron and its environment in normal and pathological states. Investigations led by Kandel[163] have shown how the process of neuroplasticity modifies and adapts neuronal behavior in response to learning stimuli and transforms short term memory into long term memory. In the case of HE, although one of most important roles has been given to ammonia metabolism in astrocytes, many other mechanisms have been proposed such as gabaergic theory, manganese, receptor changes and modifications in permeability of BBB, among others. The NMDA receptor is the predominant molecular device for controlling synaptic plasticity and memory function[164]. Nonetheless, little is known about how these processes can modify neuronal behavior or how such behavior might be expressed by the neuron. In the early nineties, our workgroup found changes in norepinephrine uptake in CNS in portal vein of rats with experimental prehepatic portal hypertension induced by portal vein ligation (PVL)[165] and baro-reflex alteration[166]. These results triggered several other questions. Would changes in the peripheral nervous system be the expression of changes at the central nervous system level?
We found changes in norepinephrine´s metabolism in discrete diencephalic regions in PVL rats[167]. Moreover, ascending along the brain stem, we arrived at the hippocampus where we found changes in capillaries and astrocytes[168]. As described above, many molecule concentrations (in plasma, cerebrospinal fluid, etc) are modified or differentially expressed in portal hypertension acting on neurons, the glia, or both. It remains unclear whether BBB modifications selectively act on all those molecules or if these might modify the BBB integrity. Besides, inflammation and immunity have also been given general consideration regarding the pathophysiology of HE. In the words of Yirmiya[169], “in addition, immune-like processes are involved in tissue remodeling, which is a continuous process of dynamic alterations in a specific tissue or a whole organ that facilitates morphological and functional adaptations to the ever changing environmental demands”. Therefore, immunomodulation of learning, memory, neural plasticity and neurogenesis are chapters worth considering in HE[169].
Reichenberg et al[170] found that experimental immune activation by endotoxin produced alterations in emotional states and decreased performance in memory tests. Moreover, endotoxin-induced changes in emotional parameters were found to have a complex time course, characterized by an early elevation in anxiety levels followed by an increase in depressed mood[170]. Evidence suggests that glia and the soluble form of tumor necrosis factor-α (TNF-α) may be involved in a specific form of synaptic scaling[171,172]. Accumulating evidence suggests that inflammatory cytokines, such as TNF-alpha, interleukin (IL)-1 and IL-6, meditate these disturbances. In animals, sickness behavior can be induced by administration of cytokines. Antagonists or synthesis blockers of cytokines abolish sickness behavior in response to various immune challenges and a similar response was also demonstrated in humans[170].
Learning impairment is present in cirrhotic patients with neuropsychological abnormalities consistent with attention deficit secondary to MHE[173]. Patients with MHE score lower than controls in memory tasks, predominantly due to deficits in attention and visual perception[174]. Hilsabeck et al[175] found that cognitive impairment on a visuoconstruction task ranged from 9% to 38% of patients on a measure of complex attention, visual scanning and tracking, and psychomotor speed. The greater viral C hepatitis (HCV) disease severity indicated by liver fibrosis was associated with greater cognitive dysfunction. Objective cognitive impairment was not related to subjective cognitive complaints or psychiatric symptoms. These findings suggest that a significant number of patients with chronic HCV have experienced cognitive difficulties that may interfere with activities of daily living and quality of life[175]. Hepatocyte growth factor/scattered factor (HGF/SF) possess motogenic activity, which is essential for normal development of other organ systems and is a conserved mechanism that regulates trans-telencephalic migration of interneurons in embryonic state[176]. Porto-systemic encephalopathy (PSE) is often preceded by ascites and this condition is characterized by gastro-intestinal capillary increased permeability, endotoxin translocation, activation of cytokines and failure to endotoxin removal. Lipopolysaccharide is elevated in portal blood and activates Kupffer cells through the CD14/toll-like receptor-4 complex to produce ROS via NADPH oxidase[177,178].
Thus, it might be possible that the chain of events triggered by endotoxin could lead to a derangement of neuronal functioning. Some evidence supports the idea that both direct and indirect effects of lipopolysaccharides/endotokinemia might be related to neuron plasticity and memory changes, as well as mood alterations, as seen in PSE. Currently, there is no evidence that directly supports the relationship between HE, lipopolysaccharides and neuron derangements. HE is a neurological complication that affects attention and memory. Experimental animal models have been used to study HE, the most frequent being the porto-caval shunt (PCS). In order to investigate learning impairment and brain functional alterations in this model, Méndez et al[179] assessed reversal learning and neural metabolic activity in a PCS rat model.
PCS and sham-operated rats were tested for reversal learning in the Morris water maze. Brains were then processed for cytochrome oxidase histochemistry. The PCS group had reversal learning impairment and a reduction in cytochrome oxidase activity in the prefrontal cortex, ventral tegmental area and accumbens shell nucleus. These results suggest that this model of portosystemic HE shows learning impairments that could be linked to dysfunction in neural activity in the prefrontal cortex and regions involved in motivated behavior[179].
The memory consolidation at the cellular level is based on the facilitation or attenuation of transmission at specific synapses. Besides, antibodies against L1 glycoprotein and neural cell adhesion molecules (NCAM) can impair memory consolidation. Moreover, learning is followed by altered expression and glycosylation of L1 and in NCAM[180].
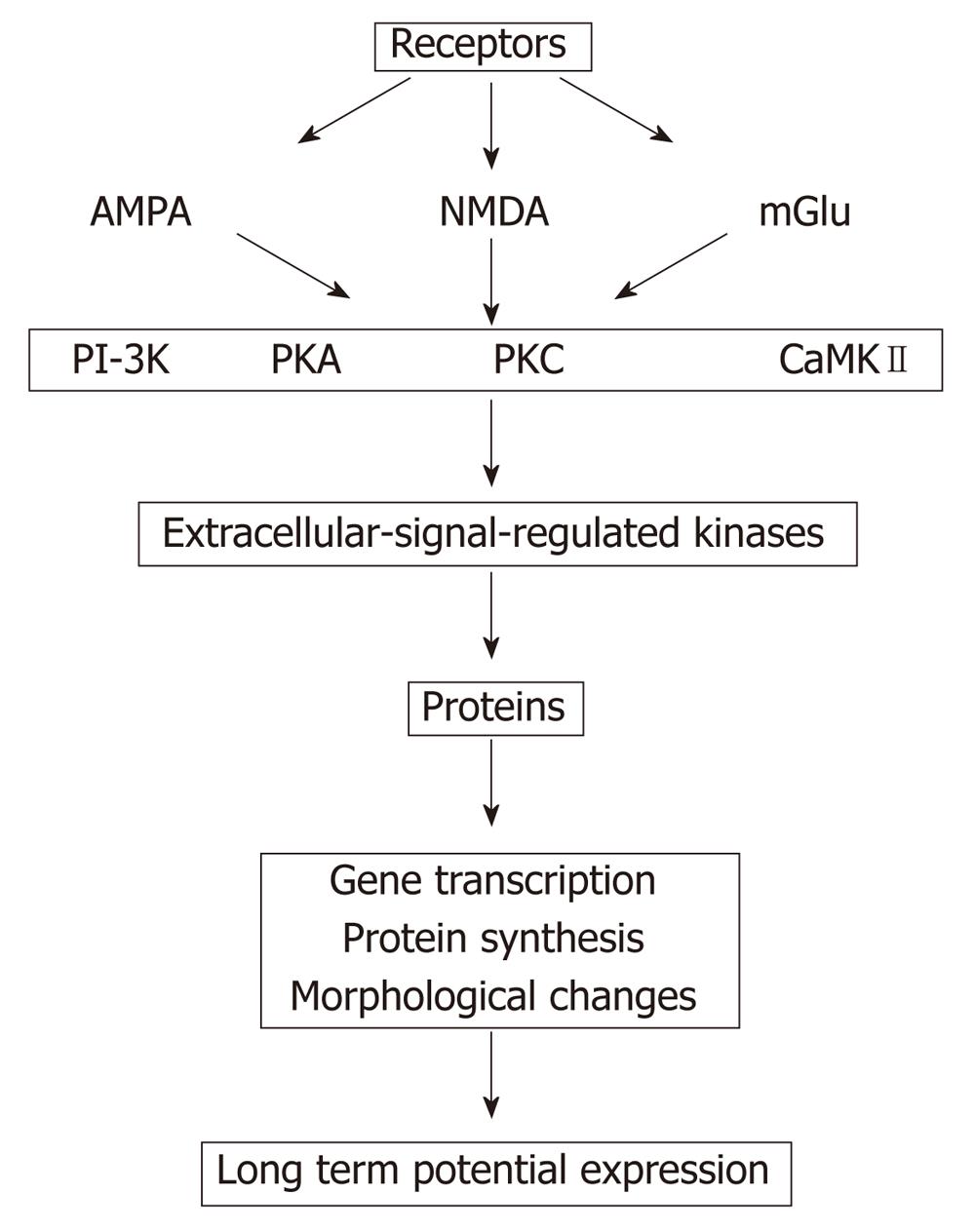
Addition of 8 Br-cGMP to slices treated with ammonia restores both phosphodiesterase activation and maintenance of long term potentials (LTP)[181]. Impairment of LTP in hyperammonemia may be involved in the impairment of the cognitive function in patients with HE (Figure 3)[182]. Although many theories have been proposed, the intimate mechanism whereby a liver pathology triggered HE remains to be known. There is insufficient evidence to determine if moderate hyperammonemia and/or portal hypertension, major complications in liver disease, or if one or more mediators (for instance, manganese, ammonia, HGF, TGFB, interleukins, reactive oxygen species, neurotransmitters, aquaporins, etc) are responsible or co-responsible for triggering MHE.
Figure 3 Proposed sequences that finally induce changes in the long term potentials, which is down regulated in hyperammonemia (modified from Lynch[182]).
AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NMDA: N-metil D-aspartato; mGlu: Metabotropic glutamate receptors; PI-3K: Phosphatidylinositol 3-kinases; PKA and PKC: Protein kinase A and C; CaMKII: Ca2+/calmodulin-dependent protein kinases II.