Published online Dec 27, 2012. doi: 10.4254/wjh.v4.i12.342
Revised: November 17, 2012
Accepted: November 24, 2012
Published online: December 27, 2012
Hepatitis C virus (HCV) infects more than 170 million people worldwide, and thereby becomes a series global health challenge. Chronic infection with HCV is considered one of the major causes of end-stage liver disease including cirrhosis and hepatocellular carcinoma. Although the multiple functions of the HCV proteins and their impacts on the modulation of the intracellular signaling transduction processes, the drive of carcinogenesis during the infection with HCV, is thought to result from the interactions of viral proteins with host cell proteins. Thus, the induction of mutator phenotype, in liver, by the expression of HCV proteins provides a key mechanism for the development of HCV-associated hepatocellular carcinoma (HCC). HCC is considered one of the most common malignancies worldwide with increasing incidence during the past decades. In many countries, the trend of HCC is attributed to several liver diseases including HCV infection. However, the development of HCC is very complicated and results mainly from the imbalance between tumor suppressor genes and oncogenes, as well as from the alteration of cellular factors leading to a genomic instability. Besides the poor prognosis of HCC patients, this type of tumor is quite resistance to the available therapies. Thus, understanding the molecular mechanisms, which are implicated in the development of HCC during the course of HCV infection, may help to design a general therapeutic protocol for the treatment and/or the prevention of this malignancy. This review summarizes the current knowledge of the molecular mechanisms, which are involved in the development of HCV-associated HCC and the possible therapeutic strategies.
- Citation: Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O, Hassan M. Hepatitis C virus-related hepatocellular carcinoma: An insight into molecular mechanisms and therapeutic strategies. World J Hepatol 2012; 4(12): 342-355
- URL: https://www.wjgnet.com/1948-5182/full/v4/i12/342.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i12.342
Chronic infection with hepatitis C virus (HCV) is considered one of the major causes of end-stage liver disease including cirrhosis and hepatocellular carcinoma. HCV infects more than 170 million people worldwide[1], and thereby becomes a series global health challenge. In the last decades, understanding the molecular pathogenesis of HCV infection was hampered by the lack of a suitable infection model, however, the establishment of both HCV replicons[2,3] and small animal models[3], helped to a better understanding the molecular mechanisms of both life cycle and the etiopathogenesis of the virus.
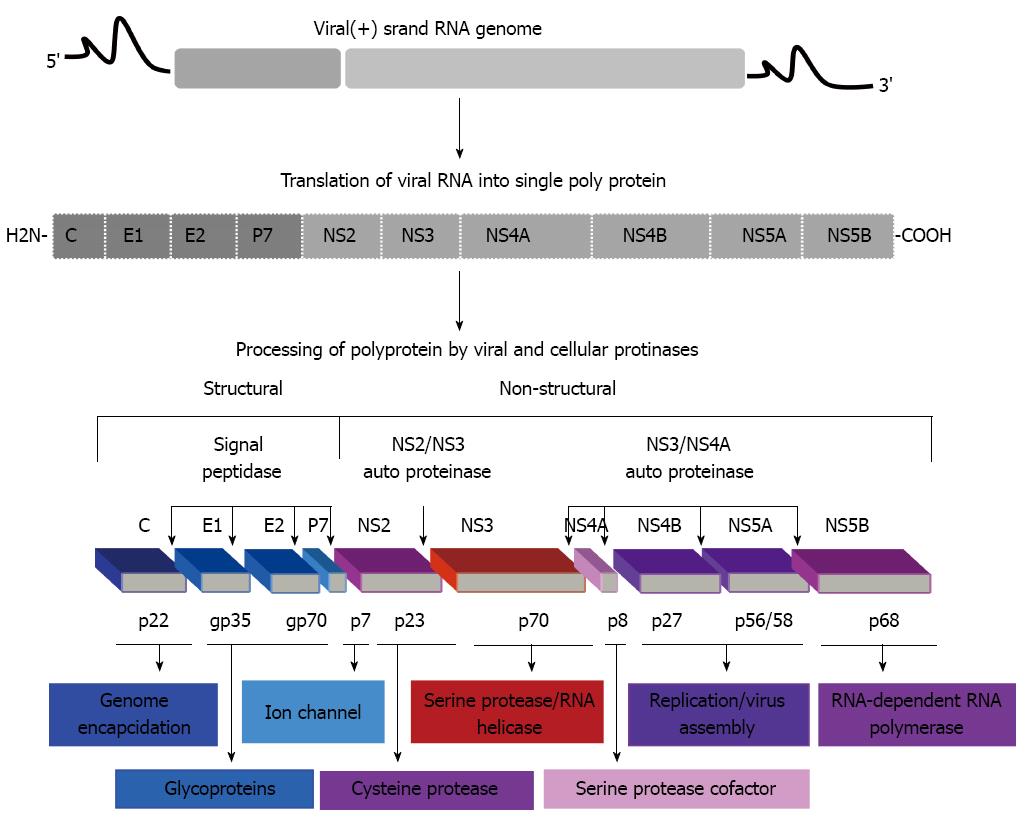
HCV is an enveloped virus with positive-sense RNA genome of 9.6 kb that encodes for a single polyprotein[4]. This single polyprotein can be cleaved by both viral and cellular proteases into 10 mature proteins including, structural (Core, E1, E2/p7) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins (Figure 1).
The natural course of HCV infection is the progression to fibrosis and cirrhosis, and subsequently to hepatocellular carcinoma (HCC) in a significant proportion of HCV infected patients[5-7]. Thus, beside its role in the cause of chronic infection that is mostly associated with the development of fibrosis and cirrhosis, HCV may play an integral role in the development of HCC via mechanisms mediated by viral proteins-host cell interaction[8,9].
Because of the close association of cirrhosis with HCV-related HCC, the molecular mechanisms of HCV-mediated carcinogenesis are intensively discussed in the context of liver diseases, such as chronic inflammation, steatosis and fibrosis. However, these liver diseases seem to be the main cause for the development of cirrhosis[10-12]. Although the multiple functions of the HCV proteins and their impacts on the modulation of the intracellular signaling transduction processes, the drive of carcinogenesis during HCV infection, is thought to result from the interactions of viral proteins with host cell proteins[13-16]. Thus, the induction of mutator phenotype, in liver, by the expression of HCV proteins provides a key mechanism for the development of HCV-associated HCC.
Based on their proliferative potential that is widely documented in vitro and in vivo, some of HCV viral proteins including core, NS3, NS5A and NS5B have been shown to possess an oncogenic potential[17-19]. These documented oncogenic potenial results mainly from the interference of HCV viral proteins with cellular proteins, which are responsible for the regulation of cell cycle control[20]. Under normal physiological conditions, the cell cycle progression is regulated by consecutive activation of cyclin and cyclin-dependent kinase (CDK) complexes[21]. For example, active cyclin-CDK complexes in G1 results in the phosphorylation of the retinoblastoma family of proteins (pRb, p130 and p107), leading the activation of the members of transcription factor family E2F, and subsequently the upregulation of cellular genes, which are characteristic for the progression of G1 phase of the cell cycle[22]. In addition to cyclin-CDK complexes, the regulation of these checkpoints are p53 and rb pathways-dependent activation[23,24].
Up on the transcriptional activation of the cycline-dependent kinase inhibitor p21, during G1/S transition, by p53, p21 becomes available to bind and to inhibit CDK2, leading to cell cycle arrest[21]. Thus, anti-growth signals, such as checkpoint activation can play an essential role by limiting the replication of oncogenic viruses, in response to viral infection including HCV.
The interference of cellular proteins and HCV core protein is considered a major risk factor for the progression of HCC. As widely reported, the expression of HCV core protein in a transgenic mouse model was found to be sufficient to induce tumor formation in liver[25]. In addition, HCV core can trigger the activation of peroxisome proliferator-activated receptor alpha that, in turn, may contribute to HCC[26-28]. Also the expression of HCV core protein was found to promote the immortalization of primary human hepatocytes as well as to reverse replicative senescence[29]. In addition to the activation of telomerase in the immortalized hepatocytes, the HCV core protein was found to increase the expression of interleukin (IL)-6, gp130, leptin receptor, and signal transducer and activator of transcription 3[29]. However, the upregulation of these genes, in response to the expression of HCV core protein, thought to be involved in the regulation of c-myc and cyclin D1, and subsequently leading to the promotion of cellular transformation[30].
The role of NS3 in the neoplastic transformation of hepatocytes in vivo and in vitro[17,31-33]. Also, the enhancement of transformation and tumorigenicity upon transfection with HCV NS3 DNA has been reported in the non-tumorigenic mouse fibroblast cell line NIH 3T3 into nude mice[34]. Moreover, the HCV NS3 C-terminal-deleted protein showed both transforming and oncogenic potential[35]. The expression of the NS3 protein in human hepatocytes was found to induce transformed characters with reduced population doubling time as well as anchorage-independent growth and tumor development that is associated with the increased phosphorylation of extracellular regulated protein kinases and p38 proteins[36]. Also, the NS3 protein has been shown to form complexes with p53[19], and to inhibit p21 promoter activity[37].
The oncogenic potential of HCV NS5A protein has been shown to be mediated by suppression of the cell cycle regulatory gene p21 in response to its interaction to p53[38-40]. In addition, NS5A protein has been reported to suppress the expression of the mitotic spindle protein ASPM through the PKR-p38 signaling pathway, as well as the induction of aberrant mitoses, chromosome instability and HCC[41].
In addition to its ability to form a cytoplasmic complex with Rb in infected cells[42], the HCV NS5B-dependent downregulation of Rb results in the enhancement of E2F-dependent transcription as well as the promotion cellular proliferation[21]. Also, the cell-cycle checkpoint, the mitotic spindle checkpoint, is a target for HCV NS5B. Since the interaction of the HCV polymerase NS5B with Rb results in the degradation of Rb and activates the MAD2 promoter[43]. Thus, the loss of host-cell genomic stability due to deregulation of Rb pathway may result from viral infection.
Chronic inflammation, which generally associated with the increased proliferation of tissue cells, and an increased rate of random mutations, leads mostly to chromosomal instability[44-48], and ultimately to both tumor progression and invasion[8,49,50]. Also, the correlation between chronic inflammation and the promotion of carcinogenesis has been reported in several clinical studies dealing with HCV-associated liver cirrhosis and HCC[51-53]. Accordingly, the interaction of viral proteins with cellular factors in host cells[21,54], and the augmentation of chronic liver disease during the course of HCV infection, suggests a central role for viral proteins in the regulation of chronic inflammation leading to the initiation and subsequently progression of HCC[55-59]. Thus, HCV-associated chronic inflammation seems to result from the dysregulation of cell cycle control[60] and the loss of tumor-suppressor gene functions[61], together with the induction of the proinflammatory cytokines, such as tumor necrosis factor (TNF)-α. Since the significant increase of proinflammatory cytokines was noted in HCV-expressing cells[31,62], and in clinical samples including liver biopsies and sera of HCV-infected patients[54,63]. Therefore, the association between chronic inflammation and the development of HCC, during the infection with HCV is considered. Indeed, the chronic inflammatory process of HCV infection that is thought to be responsible for the promotion of the increased mutation rate in the regenerating hepatocytes, and thereby contributes to the development of HCC[45-48]. In contrast, the rare occurrence of HCC in auto-immune hepatitis[64,65], indicates that the inflammation alone cannot be the reason for a high incidence of HCC in HCV-infected patients[66-68]. Although the role of HCV-associated chronic inflammation is considered to be the primary inducer of liver fibrosis and cancer, the molecular mechanisms whereby the chronic inflammation mediates the progression of liver fibrosis and subsequently HCC are not fully understood. As widely reported, chemokines produced in the liver during HCV infection are involved mainly in the regulation of migration of activated T cells from the periphery to infected parenchyma[69]. More important, these chemokines and their receptors are associated not only with viral control, but also with immune-mediated liver inflammation[70]. Accordingly, in a hepatotropic viral infection in humans, a marked intrahepatic non-specific mononuclear infiltrate during viral persistence was reported[71], suggesting an essential role for the intrahepatic chemoattraction of non-specific T cells in the modulation of liver damage[69]. Thus, besides their functional role in viral clearance, chemokines and their receptors are implicated in the development of chronic tissue inflammation. In fact, the modulation of these pathways seems to be essential for generating an efficient immune response, as well as for the regulation of the inflammatory process during the course of the chronic infection with HCV, a viral strategy to escape from immune control[72].
Generally, chemokines and their receptors are the main actor in the regulation of multistep pathway of inflammatory processes, which are responsible for the migration of lymphocytes to the liver[73,74]. In chronic hepatitis C, the expression of different chemokines in the liver has been documented in several studies[75-78]. The most reported chemokines include CXCL10 that is produced by hepatocytes and sinusoidal endothelial cells[76,77], CXCL9 and CXCL11, which are increased in the serum and liver of patients with chronic hepatitis C[76,79]; CCL5 that is elevated in chronic hepatitis C and it is produced by hepatocytes, sinusoidal endothelial cells and biliary epithelium[80]. However, the expression of all these chemokines in the liver can be induced directly by HCV. Since the induction of CXCL10, CXCL9 and CCL5, in hepatocytes, by HCV proteins, including NS5A and core has been reported[81]. Although the dominant role of chemokines in the modulation of HCV-associated inflammation, the precise mechanisms, which are involved in the regulation of HCV-associated chronic inflammation still remain to be discussed in detail. The mechanisms that thought to be involved in the regulation of HCV-associated chronic inflammation are induction of IL-1β, IL-6 and TNF-α by HCV core and NS3 proteins, indirect induction of CXCL10 and CXCL9 by HCV core and NS5A proteins.
Generally, a variety of adverse stimuli including viruses such as HCV can trigger fibrogenesis. However, the ability of HCV and its proteins to induce fibrosis is mediated either direct by the interference of HCV proteins with various cellular pathways[82-84], or indirect via steatosis[26,85], or type 2 diabetes[86-88] - dependent mechanisms, which finally lead to the deregulation of released cytokines[89-91].
As widely recognized, the excess synthesis and deposition of extracellular matrix (ECM) that is mainly directed by the induction of cytokine release, is mostly associated with the increased severity of liver disease[92,93]. As a result, the matrix metalloproteinase (MMPs) including, MMP-1, -2, -3, -8, -9, -12, -13 and -14, become inactive and fail to remove excess ECM[94-96], and subsequently disturb the balance between fibrogenesis and fibrolysis in the liver[97-100], an evidence for the development of liver fibrosis. Therefore, the inhibition of MMPs in response to repeated liver injury can lead to the dysfunction of ECM, and subsequently to undesired tissue remodeling, architectural disruption and a fibrogenic response.
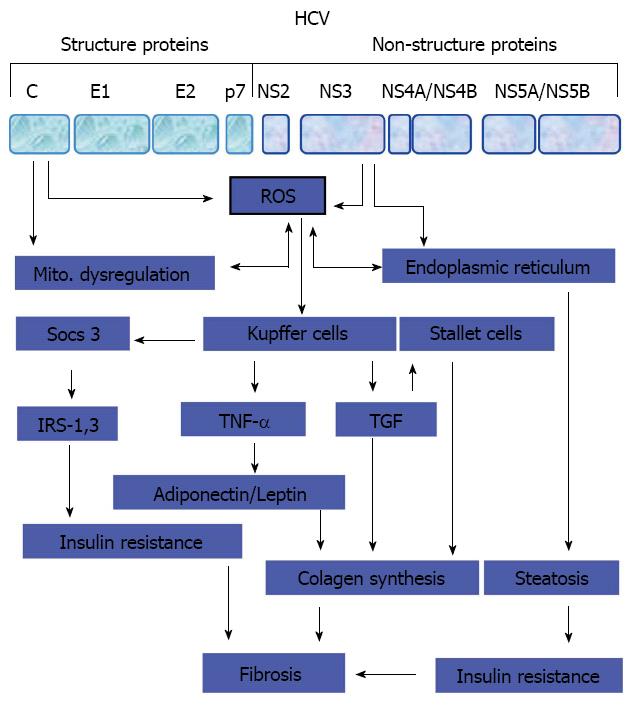
As known, the source of fibrogenic cytokines and growth factors in liver is activated liver macrophages, such as Kupffer cells, proliferating bile ductular epithelia, endothelia, mononuclear cells, and myofibroblasts[101-103]. Therefore, the stimulation of hepatic stellate cells and provascular fibroblasts by fibrogenic cytokines and growth factors mediates their transformation into myofibroblasts, the main source of collagens, MMPs and tissue inhibitor of MMPs, resulting in the accumulation of ECM that is responsible for the balance between fibrogenesis and fibrolysis in the liver[104-106]. However, a proposed model for the development of liver fibrosis during the course of HCV infection is outlined in Figure 2.
Transforming growth factor β1 (TGF-β1) is the most prominent profibrogenic cytokine that can be released from any cell type during inflammation, tissue regeneration and fibrogenesis[107-111]. Thus, besides its multiple functions, the TGF-β1 is strongly involved in the regulation of the production and deposition of the major ECM proteins[112,113].
As known, fibrosis results from the deposition of ECM material around the liver parenchyma. This deposition is mediated by the promotion of liver fibrogenesis that results mainly from either the inflammation of liver stellate cells or from hepatocyte damage in response to the generated reactive oxygen species (ROS) by Kupffer cells[114,115].
Besides the role of epithelial-mesenchymal transition paradigm in the development of fibrosis and HCC, epithelial cells are considered important mediators for progressive fibrosis and HCC[116]. During the progression of chronic liver diseases, such as HCV infection, hepatocytes undergo transition from tumor-suppressive pSmad3C pathway, a characteristic pathway of epithelial cells, to JNK/pSmad3L pathway that is, in turn, mediates the activation of myofibroblasts leading to the promotion of liver fibrosis and subsequently increasing the risk of cancer[117-119]. The loss of both epithelial homeostasis and acquisition of migratory mesenchymal phenotype are known to be essential for tumor invasion[120-122]. In this context, the role of HCV-induced JNK pathway[17,36,54], is thought to be essential for the regulation of Smad3L-depndent signaling[36,123]. Thus, the possible interaction of Smad3L-depndent signaling with oncogenic pathways including, the activator protein 1 may be responsible for the augmentation of the mesenchymal phenotype of hepatocytes[124]. Thus, the interaction of HCV core with Smad3[125,126], and the subsequent inhibition of TGF-β-induced Smad3 transcriptional activity provides evidence for the contribution of HCV core protein in the regulation of TGF-β signaling and its downstream biological responses seem to be a possible mechanism for the development of HCV-associated HCC[54,127].
The elevation of TGF-β2 production in the sera of HCV-infected patients or in core-expressing liver cells[54], in HCC biopsies[128], besides the significant cell proliferation in HCV core-expressing cells[36,54,123], suggest a central role for TGF-β signaling pathway in the regulation of HCV-associated HCC. Although the functional role of TGF-β in liver tumorigenesis as well as the implication of EMT in HCC development is not well elucidated, the contribution of HCV oncogenic potential in the course of hepatocarcinoma is widely documented[17,36,54,129]. However, the possible factors and mediators, which are thought to be involved in the regulation of HCV-associated fibrosis are ROS, TGF-β1, TNFα, epidermal growth factor (EGF), insulin-like growth factor, micro integral membrane protein (TiMP)-1, TiMP-3, MMP-1, MMP3, MMP-8.
HCC is the only malignancy whose occurrence in patients is associated with the appearance of risk factors, such as chronic liver inflammation and cirrhosis[130-132]. However, the extensive epidemiological studies performed in the last decades led to the identification of major risk factors of HCC and thereby helped to understand the pathogenesis of HCC[133-135]. Although the advances that made in the understanding of HCC pathogenesis, little is known about the molecular mechanisms of this malignancy. The most changes that occur in liver tissues are thought to result from either viral infection or the exposure to hepatotoxic agents leading to significant changes in the cellular signaling pathways and their target genes that are responsible in the regulation of tumor formation. These pathways include Wnt/β-catenin[8,136], p53[137,138], pRb[139-141], mitogen-activated protein (MAP) kinases[142,143], stress signaling[144-147], Ras[148-150], epidermal growth factor receptor[151-153], TGF-β[54,154], and JAK/STAT[155].
Wnt/β-catenin pathway has been reported to be involved in the regulation of HCC development in response to viral infection including HCV[156]. Also, the up-regulation of frizzled-7 and dephosphorylation of β-catenin is frequently observed in HCC[157-160]. Therefore, the targeted inactivation of Wnt pathway is considered a potential therapeutic target for the prevention or the ablation of HCV-associated HCV. Moreover, the increase of the mutation in β-catenin in HCC patients in response to either HCV infection[161] or the exposure to aflatoxin[162,163], provides evidence for the involvement of Wnt pathway in the regulation of HCV-associated HCC.
The tumor suppressor P53 gene, which can be inactivated by single point mutation[164,165], is one of the most studied proteins in the context of tumor development. Although the expression of this protein at normal levels in most tumors, under normal physiological conditions, the level of cellular p53 is low. The alteration of the expression level of p53 in response to either intracellular or extracellular stress signals can lead to significant changes that mostly vary from down regulation to up-regulation[164-166]. However, the loss of p53 function as tumor suppressor protein is controlled by defects in p53 signaling.
Retinoblastoma, pRb1 is a major cellular barrier to cancer development that controls cell cycle progression through a mechanism including, the repression of the E2F transcription factor family of proteins[167,168]. The phosphorylation of pRb and subsequently G1/S cell cycle transition is mainly correlated with activation of CDKs in different tumor types including, HCC[20,169-173]. In according, HCV core protein-induced acceleration of liver cells was found to be associated with activation of CDKs, inhibition of pRb and the up regulation of E2F1[20,54]. In addition, there is strong correlation between the loss of pRB and the inhibition of functional p53 in HCV core expressing cells[21], as well as in different tumor types including HCC[169-171]. The inhibition of CDK inhibitors p16INK4A, p21(WAF1/CIP1), and p27Kip1 in response to frequent mutation, or HCV infection was found to be associated with carcinogenesis of most HCC cases[172,173]. Also, the disruption of pRb pathway in various tumor types including, HCC has been reported in several studies[20,174], an evidence for the critical role of pRB in carcinogenesis.
Although HCV is a single-stranded RNA virus, and its genome is never integrated into the genome of hepatocytes[175], and no known oncogenic properties have been reported for its genes, a significant portion of HCV-infected patients with induced cirrhosis has been shown to develop HCC[20]. Thus, HCV-induced oncogenesis seems to result from the interference of HCV proteins with the intracellular signal transduction processes via mechanism includes dysregulation of cell cycle control.
Core protein, the most viral protein that is widely reported to interact with several intracellular signal transduction pathways, and thereby orchestrate their function, as oncogenic mediator, by indirect activation of TNF-α receptor[176,177], Raf-1 kinase[173,178], MAP kinase[36,179], E2F1/Rb[21] and nuclear factor kappa B[180,181] pathways. Also, the inhibition of TNF-α-induced apoptosis and the modulation of other cytokines activities during the course of HCV infection may prolong survival of infected hepatocytes, and subsequently leads to the accumulation of genetic damages that mediate the processes of the malignancy[182-184].
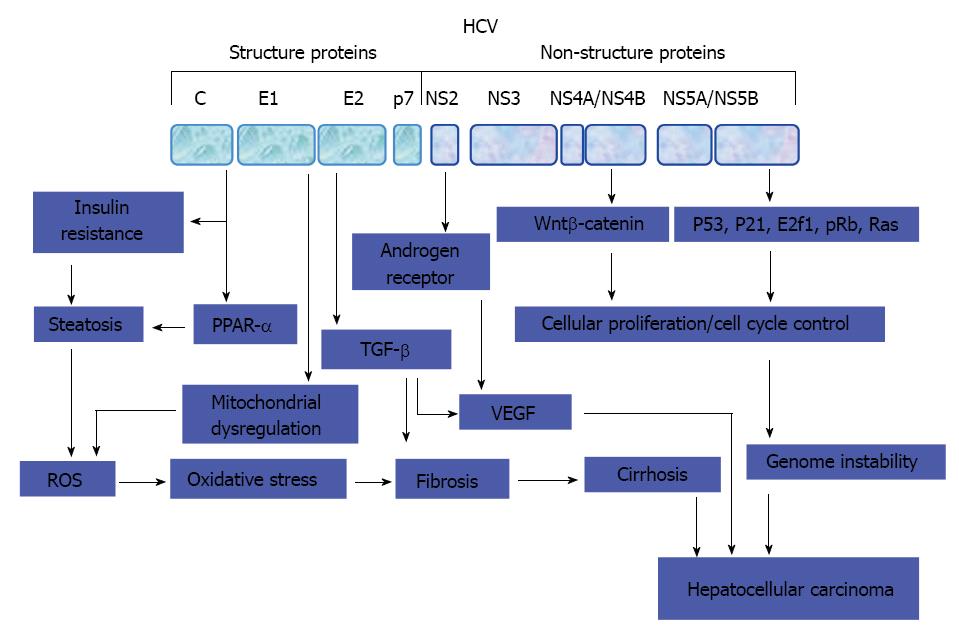
Although the direct involvement of HCV core protein in the development of HCC has been demonstrated in transgenic mice[25,185], the mechanism(s) whereby HCV core triggers HCC is not completely addressed in human. Apart from HCV core protein, the role of other viral proteins such as, nonstructural proteins NS5A and NS3 in the development of hepatocarcinogenesis is less clear[17-19,38-41]. A suggested model for the development of HCV-associated HCC is outlined in Figure 3. Also, the possible mechanisms of HCV-associated HCC are: (1) activation of cellular oncogenes such as, Ras, c-Myc, E2F1 by HCV proteins; (2) inactivation of tumor suppressor genes such as p21, p53, Rb by HCV proteins; and (3) HCV proteins-induced dysregulation of Wnt/β-catenin, MAPK, JAK/STAT, PI3K/Akt, EGF-β, TGF-β pathways.
Currently, the available HCC therapy is limited and usually with no clinical benefit for patients with advanced disease[186-188]. Although surgery or liver transplantation can successfully cure small or slow-growing tumors, the success is hampered because of donor organ shortage as well as the rapid and frequent recurrence of HCC in the transplanted liver[189-191].
Despite the potentially curative and palliative approaches are available for the treatment of HCC[192,193], there is no effective systemic chemotherapy for HCC treatment. Apart from limited benefit of the available therapies, the choice of the HCC treatment depends on several factors including, cancer stage, resources, and practitioner expertise.
Several anticancer agents including, sorafenib have shown promise in the treatment of patients with HCC[194]. Sorafenib is a small molecule multikinase inhibitor with antiproliferative, antiangiogenic and pro-apoptotic properties. Although its limited benefit for patients with advanced HCC and compensated cirrhosis, the treatment with sorafenib is associated with the increase in overall survival[195,196]. However, the relative success with sorafenib, despite the commonly reported side effects, has prompted its clinical utilization as a relevant therapeutic either alone or in combination with other treatments[197,198]. In addition, the reliability of sorafenib as relevant therapeutic approaches for HCC encouraged to test other small molecules, such as brivanib and erlotinib[199-202], and monoclonal antibodies, such as bevacizumab,and cetuximab[203,204] for their therapeutic potential in patients with hepatocellular carcinoma. Based on the successful clinical development of sorafenib in HCC treatment, the era of the molecularly targeted agents undergoes active clinical development.
Although the efficacy of antiviral therapy on HCV viral status and underlying liver function in patients is still unclear, antiviral treatment may render patients with HCV-related HCC to tolerate HCC treatments and thereby may improve prognosis[205,206]. However, based on its success, the clinical management of chronic HCV can improve the prevention of the late recurrence of HCC. Whereas, the high viral load has been shown to be an HCC recurrence risk that can be common to all HCV carriers-independent from their HCcAg status, alanine aminotransferase (ALT) levels, and stage of chronic infection[207,208]. Interferon-α therapy was found to reduce significantly the risk for hepatocellular carcinoma, especially among virologic or biochemical responders[209]. For example, patients with sustained biochemical response, independent from viral load, were at reduced risk for HCC, when compared with patients with sustained virologic response[210,211]. However, the degree of these reduced HCC risk is thought to be variable related to ALT levels[212,213], suggesting that the reduced risk of HCC recurrence is not associated only with disappearance of viremia, but also with amelioration of hepatic inflammation.
The key signal transduction pathways, which are involved in the regulation of the pathogenesis of HCV-associated HCC are considered a roadmap for the development of clinical relevant approach for the treatment or the prevention of HCV-associated HCC. Currently, the targeted therapies, which are developed for the pathways that are mentioned in the context of HCV-mediated HCC development are either in clinical development or already proved for clinical use. These include therapies that target endothelial growth factor receptor, insulin growth factor 1[214,215], vascular endothelial growth factor receptor 1-3[216], in addition to those target c-MET[217], Ras/Raf and MEK[169,173], Akt/mTOR[218], pathways. Other signaling pathways such as, Jak-STAT, and TGF-β[54], need more attention to investigate their clinical relevance and therapeutic potential in the treatment of HCC or HCV-associated HCC.
Chronic infection with HCV can lead to cirrhosis and hepatocellular carcinoma. Although the allegation of clinicians and researchers that the presence of cirrhosis is the main output for the development of HCC in individuals with chronic HCV infection, the direct role of HCV infection in the development of HCC in non-cirrhotic individuals has been suggested.
Generally, the induction of cancer is a multistep-dependent mechanism. In HCV infection, however, some of these steps might be bypassed during the development of HCV-associated HCC. Therefore, the overall effects that can be achieved by the expression of viral proteins including core protein, even in the absence of a complete set of genetic aberrations, are essential for carcinogenesis. Apart from conventional process of the induction of HCC, a plausible explanation might be given for many unusual events that take place in HCV-infected patients. The incidence of HCC in patients with HCV is known to correlate with the progression of liver fibrosis. However, the degree of liver fibrosis and the status of the infection may influence the risk of HCC occurrence in HCV-infected patients. Although HCC without cirrhosis in HCV-infected patients is rare, the direct implication of viral proteins in the development of HCC has been recently reported. Thus, the contribution of the viral infection to the development of HCC is thought to result from chronic hepatitis and/or cirrhosis.
Although there is no evidence that HCV by itself is oncogenic, the development of HCC in non-cirrhotic HCV-infected individuals is less frequent, so that a direct oncogenic effect of viral proteins is considered.
Commonly, in patients with HCV-related HCC, the tumors seem to be solitary, smaller sized, and encapsulated, when compared to those of hepatitis B virus (HBV)-related HCC. Because of the most of HCC that occurs in patients with chronic hepatitis and liver cirrhosis, is associated with infection with HBV or HCV, the treatment of these hepatitis viruses with anti-viral agents and chemoprevention approaches may decrease the risk of HCC.
However, the key signal transduction pathways, which are involved in the regulation of the pathogenesis of HCV-associated HCC, are considered a roadmap for the development of clinical relevant approach for the treatment or the prevention of HCV-associated HCC.
Thus, the development of novel targeted therapies based on the inhibition of the signaling pathways, which are directly involved in the regulation HCV-mediated initiation, progression and invasion of HCC may provide a better picture of the clinical utility and treatment options for patients with HCV-associated HCC.
| 1. | Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 985] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 2. | Kambara H, Fukuhara T, Shiokawa M, Ono C, Ohara Y, Kamitani W, Matsuura Y. Establishment of a novel permissive cell line for the propagation of hepatitis C virus by expression of microRNA miR122. J Virol. 2012;86:1382-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Uprichard SL, Chung J, Chisari FV, Wakita T. Replication of a hepatitis C virus replicon clone in mouse cells. Virol J. 2006;3:89. [PubMed] |
| 4. | Suzuki T, Aizaki H, Murakami K, Shoji I, Wakita T. Molecular biology of hepatitis C virus. J Gastroenterol. 2007;42:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Miura M, Maekawa S, Kadokura M, Sueki R, Komase K, Shindo H, Ohmori T, Kanayama A, Shindo K, Amemiya F. Analysis of viral amino acids sequences and the IL28B SNP influencing the development of hepatocellular carcinoma in chronic hepatitis C. Hepatol Int. 2011;Aug 17; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Yamashita T, Honda M, Kaneko S. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2011;26:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Banerjee A, Ray RB, Ray R. Oncogenic potential of hepatitis C virus proteins. Viruses. 2010;2:2108-2133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Chen L, Zhang Q, Chang W, Du Y, Zhang H, Cao G. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer. 2012;48:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Delpuech O, Trabut JB, Carnot F, Feuillard J, Brechot C, Kremsdorf D. Identification, using cDNA macroarray analysis, of distinct gene expression profiles associated with pathological and virological features of hepatocellular carcinoma. Oncogene. 2002;21:2926-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Verna EC, Valadao R, Farrand E, Pichardo EM, Lai JC, Terrault NA, Brown RS. Effects of ethnicity and socioeconomic status on survival and severity of fibrosis in liver transplant recipients with hepatitis C virus. Liver Transpl. 2012;18:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Neuman MG, Schmilovitz-Weiss H, Hilzenrat N, Bourliere M, Marcellin P, Trepo C, Mazulli T, Moussa G, Patel A, Baig AA. Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int J Hepatol. 2012;2012:231210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Sumie S, Kawaguchi T, Kuromatsu R, Takata A, Nakano M, Satani M, Yamada S, Niizeki T, Torimura T, Sata M. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One. 2011;6:e26840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kannan RP, Hensley LL, Evers LE, Lemon SM, McGivern DR. Hepatitis C virus infection causes cell cycle arrest at the level of initiation of mitosis. J Virol. 2011;85:7989-8001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Soliman HH, Nagy H, Kotb N, Alm El-Din MA. The role of chemokine CC ligand 20 in patients with liver cirrhosis and hepatocellular carcinoma. Int J Biol Markers. 2012;27:e125-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Gurtsevitch VE. Human oncogenic viruses: hepatitis B and hepatitis C viruses and their role in hepatocarcinogenesis. Biochemistry (Mosc). 2008;73:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Hassan M, Ghozlan H, Abdel-Kader O. Activation of c-Jun NH2-terminal kinase (JNK) signaling pathway is essential for the stimulation of hepatitis C virus (HCV) non-structural protein 3 (NS3)-mediated cell growth. Virology. 2005;333:324-336. [PubMed] |
| 18. | Kasprzak A, Adamek A, Przybyszewska W, Olejniczak K, Biczysko W, Mozer-Lisewska I, Zabel M. p21/Wafl/Cipl cellular expression in chronic long-lasting hepatitis C: correlation with HCV proteins (C, NS3, NS5A), other cell-cycle related proteins and selected clinical data. Folia Histochem Cytobiol. 2009;47:385-394. [PubMed] |
| 19. | He QQ, Cheng RX, Sun Y, Feng DY, Chen ZC, Zheng H. Hepatocyte transformation and tumor development induced by hepatitis C virus NS3 c-terminal deleted protein. World J Gastroenterol. 2003;9:474-478. [PubMed] |
| 20. | Nguyen H, Mudryj M, Guadalupe M, Dandekar S. Hepatitis C virus core protein expression leads to biphasic regulation of the p21 cdk inhibitor and modulation of hepatocyte cell cycle. Virology. 2003;312:245-253. [PubMed] |
| 21. | Hassan M, Ghozlan H, Abdel-Kader O. Activation of RB/E2F signaling pathway is required for the modulation of hepatitis C virus core protein-induced cell growth in liver and non-liver cells. Cell Signal. 2004;16:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Harrington EA, Bruce JL, Harlow E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci USA. 1998;95:11945-11950. [PubMed] |
| 24. | Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493-8497. [PubMed] |
| 25. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] |
| 26. | Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008;118:683-694. [PubMed] |
| 27. | Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Mayhew CN, Bosco EE, Fox SR, Okaya T, Tarapore P, Schwemberger SJ, Babcock GF, Lentsch AB, Fukasawa K, Knudsen ES. Liver-specific pRB loss results in ectopic cell cycle entry and aberrant ploidy. Cancer Res. 2005;65:4568-4577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Katoh M, Katoh M. STAT3-induced WNT5A signaling loop in embryonic stem cells, adult normal tissues, chronic persistent inflammation, rheumatoid arthritis and cancer (Review). Int J Mol Med. 2007;19:273-278. [PubMed] |
| 31. | Hassan M, Selimovic D, Ghozlan H, Abdel-Kader O. Induction of high-molecular-weight (HMW) tumor necrosis factor(TNF) alpha by hepatitis C virus (HCV) non-structural protein 3 (NS3) in liver cells is AP-1 and NF-kappaB-dependent activation. Cell Signal. 2007;19:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Basu A, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein modulates the interferon-induced transacting factors of Jak/Stat signaling pathway but does not affect the activation of downstream IRF-1 or 561 gene. Virology. 2001;288:379-390. [PubMed] |
| 33. | Sakamuro D, Furukawa T, Takegami T. Hepatitis C virus nonstructural protein NS3 transforms NIH 3T3 cells. J Virol. 1995;69:3893-3896. [PubMed] |
| 34. | Smirnova IS, Aksenov ND, Vonsky MS, Isaguliants MG. Different transformation pathways of murine fibroblast NIH 3T3 cells by hepatitis C virus core and NS3 proteins. Cell Biol Int. 2006;30:915-919. [PubMed] |
| 35. | Zemel R, Gerechet S, Greif H, Bachmatove L, Birk Y, Golan-Goldhirsh A, Kunin M, Berdichevsky Y, Benhar I, Tur-Kaspa R. Cell transformation induced by hepatitis C virus NS3 serine protease. J Viral Hepat. 2001;8:96-102. [PubMed] |
| 36. | Erhardt A, Hassan M, Heintges T, Häussinger D. Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP-1 in a HepG2 Tet-Off cell line. Virology. 2002;292:272-284. [PubMed] |
| 37. | Ray RB, Steele R, Meyer K, Ray R. Hepatitis C virus core protein represses p21WAF1/Cip1/Sid1 promoter activity. Gene. 1998;208:331-336. [PubMed] |
| 38. | Qadri I, Iwahashi M, Simon F. Hepatitis C virus NS5A protein binds TBP and p53, inhibiting their DNA binding and p53 interactions with TBP and ERCC3. Biochim Biophys Acta. 2002;1592:193-204. [PubMed] |
| 39. | Lan KH, Sheu ML, Hwang SJ, Yen SH, Chen SY, Wu JC, Wang YJ, Kato N, Omata M, Chang FY. HCV NS5A interacts with p53 and inhibits p53-mediated apoptosis. Oncogene. 2002;21:4801-4811. [PubMed] |
| 40. | Majumder M, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol. 2001;75:1401-1407. [PubMed] |
| 41. | Wu SC, Chang SC, Wu HY, Liao PJ, Chang MF. Hepatitis C virus NS5A protein down-regulates the expression of spindle gene Aspm through PKR-p38 signaling pathway. J Biol Chem. 2008;283:29396-29404. [PubMed] |
| 42. | Munakata T, Liang Y, Kim S, McGivern DR, Huibregtse J, Nomoto A, Lemon SM. Hepatitis C virus induces E6AP-dependent degradation of the retinoblastoma protein. PLoS Pathog. 2007;3:1335-1347. [PubMed] |
| 43. | Munakata T, Nakamura M, Liang Y, Li K, Lemon SM. Down-regulation of the retinoblastoma tumor suppressor by the hepatitis C virus NS5B RNA-dependent RNA polymerase. Proc Natl Acad Sci USA. 2005;102:18159-18164. [PubMed] |
| 44. | Slaga TJ, Scribner JD. Inhibition of tumor initiation and promotion by anti-inflammatory agents. J Natl Cancer Inst. 1973;51:1723-1725. [PubMed] |
| 45. | Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 194] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 46. | Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Marks F, Fürstenberger G, Müller-Decker K. Tumor promotion as a target of cancer prevention. Recent Results Cancer Res. 2007;174:37-47. [PubMed] |
| 48. | Argyris TS. Tumor promotion by abrasion induced epidermal hyperplasia in the skin of mice. J Invest Dermatol. 1980;75:360-362. [PubMed] |
| 49. | Jin X, Moskophidis D, Mivechi NF. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 2011;14:91-103. [PubMed] |
| 50. | Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R. Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol. 2010;2010:272170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800-806. [PubMed] |
| 52. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 53. | Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med. 2010;49:633-636. [PubMed] |
| 54. | Hassan M, Selimovic D, Ghozlan H, Abdel-kader O. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology. 2009;49:1469-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S, Viguier M, Perret C. Oncogenic β-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122:586-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 56. | Takata A, Kuromatsu R, Ando E, Iwamoto H, Fukushima N, Sumie S, Torimura T, Sata M. HCC develops even in the early stage of chronic liver disease in elderly patients with HCV infection. Int J Mol Med. 2010;26:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Klopstock N, Katzenellenbogen M, Pappo O, Sklair-Levy M, Olam D, Mizrahi L, Potikha T, Galun E, Goldenberg D. HCV tumor promoting effect is dependent on host genetic background. PLoS One. 2009;4:e5025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Sarfraz S, Hamid S, Ali S, Jafri W, Siddiqui AA. Modulations of cell cycle checkpoints during HCV associated disease. BMC Infect Dis. 2009;9:125. [PubMed] |
| 59. | Huang XX, McCaughan GW, Shackel NA, Gorrell MD. Up-regulation of proproliferative genes and the ligand/receptor pair placental growth factor and vascular endothelial growth factor receptor 1 in hepatitis C cirrhosis. Liver Int. 2007;27:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Yamashita T, Kaneko S, Hashimoto S, Sato T, Nagai S, Toyoda N, Suzuki T, Kobayashi K, Matsushima K. Serial analysis of gene expression in chronic hepatitis C and hepatocellular carcinoma. Biochem Biophys Res Commun. 2001;282:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, Matsuda M, Fujii H, Scoazec JY, Ohgaki H. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Asakawa M, Kono H, Amemiya H, Matsuda M, Suzuki T, Maki A, Fujii H. Role of interleukin-18 and its receptor in hepatocellular carcinoma associated with hepatitis C virus infection. Int J Cancer. 2006;118:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Tanaka Y, Furuta T, Suzuki S, Orito E, Yeo AE, Hirashima N, Sugauchi F, Ueda R, Mizokami M. Impact of interleukin-1beta genetic polymorphisms on the development of hepatitis C virus-related hepatocellular carcinoma in Japan. J Infect Dis. 2003;187:1822-1825. [PubMed] |
| 64. | Delhem N, Cottrez F, Carpentier A, Miroux C, Moralès O, François V, Groux H, Auriault C, Pancré V. [Role of the Regulatory T lymphocytes in hepatitis C fibrosis progression]. Bull Cancer. 2008;95:1029-1038. [PubMed] |
| 65. | Yousuf M, Kiyosawa K, Sodeyama T, Yoda H, Nakano Y, Furuta S. Development of hepatocellular carcinoma in a man with auto-immune chronic active hepatitis. J Gastroenterol Hepatol. 1992;7:66-69. [PubMed] |
| 66. | Stauffer JK, Scarzello AJ, Jiang Q, Wiltrout RH. Chronic inflammation, immune escape, and oncogenesis in the liver: a unique neighborhood for novel intersections. Hepatology. 2012;56:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 68. | Wang BB, Cheng JY, Gao HH, Zhang Y, Chen ZN, Bian H. Hepatic stellate cells in inflammation-fibrosis-carcinoma axis. Anat Rec (Hoboken). 2010;293:1492-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Riezu-Boj JI, Larrea E, Aldabe R, Guembe L, Casares N, Galeano E, Echeverria I, Sarobe P, Herrero I, Sangro B. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422-431. [PubMed] |
| 70. | Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011;52:871-878. [PubMed] |
| 71. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. [PubMed] |
| 72. | Price DA, Klenerman P, Booth BL, Phillips RE, Sewell AK. Cytotoxic T lymphocytes, chemokines and antiviral immunity. Immunol Today. 1999;20:212-216. [PubMed] |
| 73. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. [PubMed] |
| 75. | Patzwahl R, Meier V, Ramadori G, Mihm S. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. J Virol. 2001;75:1332-1338. [PubMed] |
| 76. | Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236-6243. [PubMed] |
| 77. | Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536-5544. [PubMed] |
| 78. | Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Pérez-Hornedo J, González-Mateos F, García-Garzón S, Bienvenido A, Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632-641. [PubMed] |
| 79. | Bièche I, Asselah T, Laurendeau I, Vidaud D, Degot C, Paradis V, Bedossa P, Valla DC, Marcellin P, Vidaud M. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology. 2005;332:130-144. [PubMed] |
| 80. | Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, García-Monzón C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861-2870. [PubMed] |
| 81. | Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27-37. [PubMed] |
| 82. | Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Teixeira R, Marcos LA, Friedman SL. Immunopathogenesis of hepatitis C virus infection and hepatic fibrosis: New insights into antifibrotic therapy in chronic hepatitis C. Hepatol Res. 2007;37:579-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol. 2002;160:641-654. [PubMed] |
| 85. | Tanaka A, Uegaki S, Kurihara H, Aida K, Mikami M, Nagashima I, Shiga J, Takikawa H. Hepatic steatosis as a possible risk factor for the development of hepatocellular carcinoma after eradication of hepatitis C virus with antiviral therapy in patients with chronic hepatitis C. World J Gastroenterol. 2007;13:5180-5187. [PubMed] |
| 86. | Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver Int. 2011;31 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Livingston SE, Deubner H, Bruden DL, McMahon BJ, Homan CE, Townshend-Bulson LJ, Bruce MG, Hennessy TW, Williams JL, Gretch DR. Factors associated with the progression of fibrosis on liver biopsy in Alaska Native and American Indian persons with chronic hepatitis C. Can J Gastroenterol. 2010;24:445-451. [PubMed] |
| 88. | Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Sayed-Ahmed L, Kotb N, El-Serogy H, El-Shazly S, Eid M. TNF-alpha and CXCL-10 correlation with insulin resistance in patients with chronic hepatitis C virus infection. Egypt J Immunol. 2010;17:101-111. [PubMed] |
| 90. | Hung CH, Lee CM, Chen CH, Hu TH, Jiang SR, Wang JH, Lu SN, Wang PW. Association of inflammatory and anti-inflammatory cytokines with insulin resistance in chronic hepatitis C. Liver Int. 2009;29:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Peterson MC. Elevated circulating transforming growth factor beta-1 may explain poorer renal survival in type II diabetics with chronic hepatitis C. Med Sci Monit. 2007;13:RA81-RA85. [PubMed] |
| 92. | Kasahara A, Hayashi N, Mochizuki K, Oshita M, Katayama K, Kato M, Masuzawa M, Yoshihara H, Naito M, Miyamoto T. Circulating matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as serum markers of fibrosis in patients with chronic hepatitis C. Relationship to interferon response. J Hepatol. 1997;26:574-583. [PubMed] |
| 93. | Tillmann HL, Manns MP, Rudolph KL. Merging models of hepatitis C virus pathogenesis. Semin Liver Dis. 2005;25:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Park CH, Moon Y, Shin CM, Chung JH. Cyclic AMP suppresses matrix metalloproteinase-1 expression through inhibition of MAPK and GSK-3beta. J Invest Dermatol. 2010;130:2049-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Son KN, Hwang J, Kwon BS, Kim J. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem Biophys Res Commun. 2006;340:498-504. [PubMed] |
| 96. | Masson V. [Roles of serine proteases and matrix metalloproteinases in tumor invasion and angiogenesis]. Bull Mem Acad R Med Belg. 2006;161:320-326. [PubMed] |
| 97. | Türkay C, Yönem O, Arici S, Koyuncu A, Kanbay M. Effect of angiotensin-converting enzyme inhibition on experimental hepatic fibrogenesis. Dig Dis Sci. 2008;53:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrinolysis in chronic liver injury (with special emphasis on MMPs and TIMPs). Z Gastroenterol. 2007;45:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Zhou X, Hovell CJ, Pawley S, Hutchings MI, Arthur MJ, Iredale JP, Benyon RC. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271-279. [PubMed] |
| 101. | Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA, Kisseleva T. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179:189-198. [PubMed] |
| 102. | Bisht S, Khan MA, Bekhit M, Bai H, Cornish T, Mizuma M, Rudek MA, Zhao M, Maitra A, Ray B. A polymeric nanoparticle formulation of curcumin (NanoCurc™) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest. 2011;91:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Blazejewski S, Preaux AM, Mallat A, Brocheriou I, Mavier P, Dhumeaux D, Hartmann D, Schuppan D, Rosenbaum J. Human myofibroblastlike cells obtained by outgrowth are representative of the fibrogenic cells in the liver. Hepatology. 1995;22:788-797. [PubMed] |
| 104. | Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol. 2010;21:896-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Nielsen BS, Egeblad M, Rank F, Askautrud HA, Pennington CJ, Pedersen TX, Christensen IJ, Edwards DR, Werb Z, Lund LR. Matrix metalloproteinase 13 is induced in fibroblasts in polyomavirus middle T antigen-driven mammary carcinoma without influencing tumor progression. PLoS One. 2008;3:e2959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Inatomi O, Andoh A, Yagi Y, Ogawa A, Hata K, Shiomi H, Tani T, Takayanagi A, Shimizu N, Fujiyama Y. Matrix metalloproteinase-3 secretion from human pancreatic periacinar myofibroblasts in response to inflammatory mediators. Pancreas. 2007;34:126-132. [PubMed] |
| 107. | Lin W, Tsai WL, Shao RX, Wu G, Peng LF, Barlow LL, Chung WJ, Zhang L, Zhao H, Jang JY. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138:2509-2518, 2518.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 108. | Robinson RT, Wang J, Cripps JG, Milks MW, English KA, Pearson TA, Gorham JD. End-organ damage in a mouse model of fulminant liver inflammation requires CD4+ T cell production of IFN-gamma but is independent of Fas. J Immunol. 2009;182:3278-3284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Nozato E, Shiraishi M, Nishimaki T. Up-regulation of hepatocyte growth factor caused by an over-expression of transforming growth factor beta, in the rat model of fulminant hepatic failure. J Surg Res. 2003;115:226-234. [PubMed] |
| 110. | Akahoshi T, Hashizume M, Tanoue K, Shimabukuro R, Gotoh N, Tomikawa M, Sugimachi K. Role of the spleen in liver fibrosis in rats may be mediated by transforming growth factor beta-1. J Gastroenterol Hepatol. 2002;17:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Grasl-Kraupp B, Rossmanith W, Ruttkay-Nedecky B, Müllauer L, Kammerer B, Bursch W, Schulte-Hermann R. Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. Hepatology. 1998;28:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Satish L, Gallo PH, Baratz ME, Johnson S, Kathju S. Reversal of TGF-β1 stimulation of α-smooth muscle actin and extracellular matrix components by cyclic AMP in Dupuytren's-derived fibroblasts. BMC Musculoskelet Disord. 2011;12:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Kamitani S, Yamauchi Y, Kawasaki S, Takami K, Takizawa H, Nagase T, Kohyama T. Simultaneous stimulation with TGF-β1 and TNF-α induces epithelial mesenchymal transition in bronchial epithelial cells. Int Arch Allergy Immunol. 2011;155:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 115. | Thirunavukkarasu C, Watkins S, Harvey SA, Gandhi CR. Superoxide-induced apoptosis of activated rat hepatic stellate cells. J Hepatol. 2004;41:567-575. [PubMed] |
| 116. | Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764-5774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1318] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 117. | Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 118. | Sugioka Y, Watanabe T, Inagaki Y, Kushida M, Niioka M, Endo H, Higashiyama R, Okazaki I. c-Jun NH2-terminal kinase pathway is involved in constitutive matrix metalloproteinase-1 expression in a hepatocellular carcinoma-derived cell line. Int J Cancer. 2004;109:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Matsuzaki K, Seki T, Okazaki K. TGF-beta during human colorectal carcinogenesis: the shift from epithelial to mesenchymal signaling. Inflammopharmacology. 2006;14:198-203. [PubMed] |
| 121. | Matsuzaki K, Okazaki K. Transforming growth factor-beta during carcinogenesis: the shift from epithelial to mesenchymal signaling. J Gastroenterol. 2006;41:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, Ruhland C, Adler G, Gress TM. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222-4228. [PubMed] |
| 123. | Park EJ, Chun JN, Kim SH, Kim CY, Lee HJ, Kim HK, Park JK, Lee SW, So I, Jeon JH. Schisandrin B suppresses TGFβ1 signaling by inhibiting Smad2/3 and MAPK pathways. Biochem Pharmacol. 2012;83:378-384. [PubMed] |
| 124. | Hachimine D, Uchida K, Asada M, Nishio A, Kawamata S, Sekimoto G, Murata M, Yamagata H, Yoshida K, Mori S. Involvement of Smad3 phosphoisoform-mediated signaling in the development of colonic cancer in IL-10-deficient mice. Int J Oncol. 2008;32:1221-1226. [PubMed] |
| 125. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4401] [Article Influence: 191.3] [Reference Citation Analysis (8)] |
| 126. | Pavio N, Battaglia S, Boucreux D, Arnulf B, Sobesky R, Hermine O, Brechot C. Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene. 2005;24:6119-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 127. | Cheng PL, Chang MH, Chao CH, Lee YH. Hepatitis C viral proteins interact with Smad3 and differentially regulate TGF-beta/Smad3-mediated transcriptional activation. Oncogene. 2004;23:7821-7838. [PubMed] |
| 128. | Rossmanith W, Schulte-Hermann R. Biology of transforming growth factor beta in hepatocarcinogenesis. Microsc Res Tech. 2001;52:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Colombo M. Hepatitis C virus and hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:519-528. [PubMed] |
| 130. | Mohamed AA, Loutfy SA, Craik JD, Hashem AG, Siam I. Chronic hepatitis c genotype-4 infection: role of insulin resistance in hepatocellular carcinoma. Virol J. 2011;8:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 131. | Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516-e522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 132. | Pecic V, Stankovic-Djordjevic D, Nestorovic M, Radojkovic M, Marjanovic H, Ilic B, Milojkovic M. Hepatitis C virus-related hepatocellular carcinoma and liver cirrhosis. J BUON. 2011;16:277-281. [PubMed] |
| 133. | Ramirez AG, Weiss NS, Holden AE, Suarez L, Cooper SP, Munoz E, Naylor SL. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One. 2012;7:e35573. [PubMed] |
| 134. | Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012;24:840-848. [PubMed] |
| 135. | Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 136. | Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One. 2011;6:e27496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 137. | Wang L, Huang J, Jiang M, Lin H. Tissue-specific transplantation antigen P35B (TSTA3) immune response-mediated metabolism coupling cell cycle to postreplication repair network in no-tumor hepatitis/cirrhotic tissues (HBV or HCV infection) by biocomputation. Immunol Res. 2012;52:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 138. | Herzer K, Weyer S, Krammer PH, Galle PR, Hofmann TG. Hepatitis C virus core protein inhibits tumor suppressor protein promyelocytic leukemia function in human hepatoma cells. Cancer Res. 2005;65:10830-10837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Machida K, Liu JC, McNamara G, Levine A, Duan L, Lai MM. Hepatitis C virus causes uncoupling of mitotic checkpoint and chromosomal polyploidy through the Rb pathway. J Virol. 2009;83:12590-12600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Tsukiyama-Kohara K, Toné S, Maruyama I, Inoue K, Katsume A, Nuriya H, Ohmori H, Ohkawa J, Taira K, Hoshikawa Y. Activation of the CKI-CDK-Rb-E2F pathway in full genome hepatitis C virus-expressing cells. J Biol Chem. 2004;279:14531-14541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Cho J, Baek W, Yang S, Chang J, Sung YC, Suh M. HCV core protein modulates Rb pathway through pRb down-regulation and E2F-1 up-regulation. Biochim Biophys Acta. 2001;1538:59-66. [PubMed] |
| 142. | Hoshikawa Y, Kanki K, Ashla AA, Arakaki Y, Azumi J, Yasui T, Tezuka Y, Matsumi Y, Tsuchiya H, Kurimasa A. c-Jun N-terminal kinase activation by oxidative stress suppresses retinoid signaling through proteasomal degradation of retinoic acid receptor α protein in hepatic cells. Cancer Sci. 2011;102:934-941. [PubMed] |
| 143. | Lin CC, Hwang JM, Tsai MT, Su WW, Chen LM, Lai TY, Hsu HH, Yen SK, Huang CY, Liu JY. Protein kinase C alpha location and the expression of phospho-MEK and MDR1 in hepatitis virus-related hepatocellular carcinoma biopsies. Chin J Physiol. 2010;53:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 144. | Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, Majano PL, Benedicto I, Rosado JA, Salido GM, Gonzalez-Gallego J. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50:872-882. [PubMed] |
| 145. | Selimović D, Hassan M. Inhibition of hepatitis C virus (HCV) core protein- induced cell growth by non-structural protein 4A (NS4A) is mediated by mitochondrial dysregulation. Bosn J Basic Med Sci. 2008;8:4-11. [PubMed] |
| 146. | Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921-4933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 147. | Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J. 2004;378:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 148. | Zhang Q, Gong R, Qu J, Zhou Y, Liu W, Chen M, Liu Y, Zhu Y, Wu J. Activation of the Ras/Raf/MEK pathway facilitates hepatitis C virus replication via attenuation of the interferon-JAK-STAT pathway. J Virol. 2012;86:1544-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 149. | Yi Z, Pan T, Wu X, Song W, Wang S, Xu Y, Rice CM, Macdonald MR, Yuan Z. Hepatitis C virus co-opts Ras-GTPase-activating protein-binding protein 1 for its genome replication. J Virol. 2011;85:6996-7004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 150. | Yi Z, Fang C, Pan T, Wang J, Yang P, Yuan Z. Subproteomic study of hepatitis C virus replicon reveals Ras-GTPase-activating protein binding protein 1 as potential HCV RC component. Biochem Biophys Res Commun. 2006;350:174-178. [PubMed] |
| 151. | Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 573] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 152. | Mankouri J, Griffin S, Harris M. The hepatitis C virus non-structural protein NS5A alters the trafficking profile of the epidermal growth factor receptor. Traffic. 2008;9:1497-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 153. | El-Bassiouni A, Nosseir M, Zoheiry M, El-Ahwany E, Ghali A, El-Bassiouni N. Immunohistochemical expression of CD95 (Fas), c-myc and epidermal growth factor receptor in hepatitis C virus infection, cirrhotic liver disease and hepatocellular carcinoma. APMIS. 2006;114:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 154. | Divella R, Daniele A, Gadaleta C, Tufaro A, Venneri MT, Paradiso A, Quaranta M. Circulating transforming growth factor-β and epidermal growth factor receptor as related to virus infection in liver carcinogenesis. Anticancer Res. 2012;32:141-145. [PubMed] |
| 155. | Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Häussinger D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 156. | Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Williams M, McFaline JL, Essigmann JM, Schauer DB. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59:88-97. [PubMed] |
| 157. | Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 158. | Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 159. | Merle P, Kim M, Herrmann M, Gupte A, Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la Monte S. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854-862. [PubMed] |
| 160. | Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 161. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. [PubMed] |
| 162. | Ravinayagam V, Jaganathan R, Panchanadham S, Palanivelu S. Potential Antioxidant Role of Tridham in Managing Oxidative Stress against Aflatoxin-B(1)-Induced Experimental Hepatocellular Carcinoma. Int J Hepatol. 2012;2012:428373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, Taylor JA. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol Carcinog. 2001;31:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 164. | Schmidt MK, Tommiska J, Broeks A, van Leeuwen FE, Van't Veer LJ, Pharoah PD, Easton DF, Shah M, Humphreys M, Dörk T. Combined effects of single nucleotide polymorphisms TP53 R72P and MDM2 SNP309, and p53 expression on survival of breast cancer patients. Breast Cancer Res. 2009;11:R89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 165. | Jablkowski M, Bocian A, Bialkowska J, Bartkowiak J. A comparative study of P53/MDM2 genes alterations and P53/MDM2 proteins immunoreactivity in liver cirrhosis and hepatocellular carcinoma. J Exp Clin Cancer Res. 2005;24:117-125. [PubMed] |
| 166. | Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773-7779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 409] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 167. | Hsieh TC, Nicolay BN, Frolov MV, Moon NS. Tuberous sclerosis complex 1 regulates dE2F1 expression during development and cooperates with RBF1 to control proliferation and survival. PLoS Genet. 2010;6:e1001071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 168. | Zhang J, Lee EY, Liu Y, Berman SD, Lodish HF, Lees JA. pRB and E2F4 play distinct cell-intrinsic roles in fetal erythropoiesis. Cell Cycle. 2010;9:371-376. [PubMed] |
| 169. | Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis. 2003;24:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 170. | Chen Z, Xiao Z, Gu WZ, Xue J, Bui MH, Kovar P, Li G, Wang G, Tao ZF, Tong Y. Selective Chk1 inhibitors differentially sensitize p53-deficient cancer cells to cancer therapeutics. Int J Cancer. 2006;119:2784-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 171. | Mazière C, Marcheux V, Louandre C, Mazière JC. Oxidized low density lipoprotein induces the cyclin-dependent kinase inhibitor p21(waf1) and the tumor suppressor Rb. Biochem Biophys Res Commun. 2002;293:1327-1332. [PubMed] |
| 172. | Morel AP, Unsal K, Cagatay T, Ponchel F, Carr B, Ozturk M. P53 but not p16INK4a induces growth arrest in retinoblastoma-deficient hepatocellular carcinoma cells. J Hepatol. 2000;33:254-265. [PubMed] |
| 173. | Aoki H, Hayashi J, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J Virol. 2000;74:1736-1741. [PubMed] |
| 174. | Santoni-Rugiu E, Jensen MR, Thorgeirsson SS. Disruption of the pRb/E2F pathway and inhibition of apoptosis are major oncogenic events in liver constitutively expressing c-myc and transforming growth factor alpha. Cancer Res. 1998;58:123-134. [PubMed] |
| 175. | Kasai Y, Takeda S, Takagi H. Pathogenesis of hepatocellular carcinoma: a review from the viewpoint of molecular analysis. Semin Surg Oncol. 1996;12:155-159. [PubMed] |
| 176. | Chung YM, Park KJ, Choi SY, Hwang SB, Lee SY. Hepatitis C virus core protein potentiates TNF-alpha-induced NF-kappaB activation through TRAF2-IKKbeta-dependent pathway. Biochem Biophys Res Commun. 2001;284:15-19. [PubMed] |
| 177. | You LR, Chen CM, Lee YH. Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha. J Virol. 1999;73:1672-1681. [PubMed] |
| 178. | Giambartolomei S, Covone F, Levrero M, Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene. 2001;20:2606-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 179. | Spaziani A, Alisi A, Sanna D, Balsano C. Role of p38 MAPK and RNA-dependent protein kinase (PKR) in hepatitis C virus core-dependent nuclear delocalization of cyclin B1. J Biol Chem. 2006;281:10983-10989. [PubMed] |
| 180. | Li ZH, Tang QB, Wang J, Zhou L, Huang WL, Liu RY, Chen RF. Hepatitis C virus core protein induces malignant transformation of biliary epithelial cells by activating nuclear factor-kappaB pathway. J Gastroenterol Hepatol. 2010;25:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 181. | Sato Y, Kato J, Takimoto R, Takada K, Kawano Y, Miyanishi K, Kobune M, Sato Y, Takayama T, Matunaga T. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor alpha expression via activation of nuclear factor-kappaB. Gut. 2006;55:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 182. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. [PubMed] |
| 183. | Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA. 2001;98:15089-15094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 184. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 185. | Jeannot E, Boorman GA, Kosyk O, Bradford BU, Shymoniak S, Tumurbaatar B, Weinman SA, Melnyk SB, Tryndyak V, Pogribny IP. Increased incidence of aflatoxin B1-induced liver tumors in hepatitis virus C transgenic mice. Int J Cancer. 2012;130:1347-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 186. | Tazi el M, Essadi I, M'rabti H, Touyar A, Errihani PH. Systemic treatment and targeted therapy in patients with advanced hepatocellular carcinoma. N Am J Med Sci. 2011;3:167-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 187. | Wörns MA, Schuchmann M, Düber C, Otto G, Galle PR, Weinmann A. Sunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatment. Oncology. 2010;79:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 188. | Bekaii-Saab T, Markowitz J, Prescott N, Sadee W, Heerema N, Wei L, Dai Z, Papp A, Campbell A, Culler K. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009;15:5895-5901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 189. | Barbare JC, Bouché O, Bonnetain F, Dahan L, Lombard-Bohas C, Faroux R, Raoul JL, Cattan S, Lemoine A, Blanc JF. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: a phase III multicentre, randomised, double blind placebo-controlled study. Eur J Cancer. 2009;45:1788-1797. [PubMed] |
| 190. | Montella L, Addeo R, Caraglia M, Faiola V, Guarrasi R, Vincenzi B, Palmeri A, Capasso E, Nocera V, Tarantino L. Vascular endothelial growth factor monitoring in advanced hepatocellular carcinoma patients treated with radiofrequency ablation plus octreotide: a single center experience. Oncol Rep. 2008;20:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 191. | Higginbotham KB, Lozano R, Brown T, Patt YZ, Arima T, Abbruzzese JL, Thomas MB. A phase I/II trial of TAC-101, an oral synthetic retinoid, in patients with advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 192. | Wenger JB, Santos N, Liu Y, Dallas J, Subbiah S, Hochwald S, Huang EH, Dang DT, Allegra CJ, Luesch H. Can we develop effective combination antiangiogenic therapy for patients with hepatocellular carcinoma? Oncol Rev. 2011;5:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 193. | Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2012;8:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 194. | Woo HY, Heo J. Sorafenib in liver cancer. Expert Opin Pharmacother. 2012;13:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 195. | Yang Y, Lu Y, Wang C, Bai W, Qu J, Chen Y, Chang X, An L, Zhou L, Zeng Z. Cryotherapy is associated with improved clinical outcomes of Sorafenib therapy for advanced hepatocellular carcinoma. Cell Biochem Biophys. 2012;63:159-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 196. | Shao YY, Lin ZZ, Chen TJ, Hsu C, Shen YC, Hsu CH, Cheng AL. High circulating endothelial progenitor levels associated with poor survival of advanced hepatocellular carcinoma patients receiving sorafenib combined with metronomic chemotherapy. Oncology. 2011;81:98-103. [PubMed] |
| 197. | Duan F, Wang MQ, Liu FY, Wang ZJ, Song P, Wang Y. Sorafenib in combination with transarterial chemoembolization and bronchial arterial chemoinfusion in the treatment of hepatocellular carcinoma with pulmonary metastasis. Asia Pac J Clin Oncol. 2012;8:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 198. | Ibrahim N, Yu Y, Walsh WR, Yang JL. Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies (review). Oncol Rep. 2012;27:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 199. | Kaseb AO, Garrett-Mayer E, Morris JS, Xiao L, Lin E, Onicescu G, Hassan MM, Hassabo HM, Iwasaki M, Deaton FL. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology. 2012;82:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 200. | Philip PA, Mahoney MR, Holen KD, Northfelt DW, Pitot HC, Picus J, Flynn PJ, Erlichman C. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer. 2012;118:2424-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 201. | Finn RS, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, Baudelet C, Manekas D, Park JW. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2090-2098. [PubMed] |
| 202. | Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146-6153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 203. | Chen W, Shen X, Xia X, Xu G, Ma T, Bai X, Liang T. NSC 74859-mediated inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinoma. Liver Int. 2012;32:70-77. [PubMed] |
| 204. | Sanoff HK, Bernard S, Goldberg RM, Morse MA, Garcia R, Woods L, Moore DT, O'Neil BH. Phase II Study of Capecitabine, Oxaliplatin, and Cetuximab for Advanced Hepatocellular Carcinoma. Gastrointest Cancer Res. 2011;4:78-83. [PubMed] |
| 205. | Michielsen P, Ho E, Francque S. Does antiviral therapy reduce the risk of hepatocellular carcinoma in patients with chronic hepatitis C? Minerva Gastroenterol Dietol. 2012;58:65-79. [PubMed] |
| 206. | Hung CH, Lu SN, Wang JH, Hu TH, Chen CH, Huang CM, Lee CM. Sustained HCV clearance by interferon-based therapy reduces hepatocellular carcinoma in hepatitis B and C dually-infected patients. Antivir Ther. 2011;16:959-968. [PubMed] |
| 207. | Masuzaki R, Yoshida H, Omata M. Interferon reduces the risk of hepatocellular carcinoma in hepatitis C virus-related chronic hepatitis/liver cirrhosis. Oncology. 2010;78 Suppl 1:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 208. | Akamatsu M, Yoshida H, Shiina S, Teratani T, Tateishi R, Obi S, Sato S, Koike Y, Fujishima T, Ishikawa T. Neither hepatitis C virus genotype nor virus load affects survival of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004;16:459-466. [PubMed] |
| 209. | Yoshida H, Onda M, Tajiri T, Umehara M, Mamada Y, Matsumoto S, Yamamoto K, Kaneko M, Kumazaki T. Treatment of spontaneous ruptured hepatocellular carcinoma. Hepatogastroenterology. 1999;46:2451-2453. [PubMed] |
| 210. | Imai Y, Tamura S, Tanaka H, Hiramatsu N, Kiso S, Doi Y, Inada M, Nagase T, Kitada T, Imanaka K. Reduced risk of hepatocellular carcinoma after interferon therapy in aged patients with chronic hepatitis C is limited to sustained virological responders. J Viral Hepat. 2010;17:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 211. | Welzel TM, Katki HA, Sakoda LC, Evans AA, London WT, Chen G, O'Broin S, Shen FM, Lin WY, McGlynn KA. Blood folate levels and risk of liver damage and hepatocellular carcinoma in a prospective high-risk cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:1279-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 212. | Moriyama M, Matsumura H, Aoki H, Shimizu T, Yamagami H, Shioda A, Kaneko M, Goto I, Tanaka N, Arakawa Y. Decreased risk of hepatocellular carcinoma in patients with chronic hepatitis C whose serum alanine aminotransferase levels became less than twice the upper limit of normal following interferon therapy. Liver Int. 2005;25:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Almasio PL, Venezia G, Craxì A. The impact of antiviral therapy on the course of chronic HCV infection. A systematic review. Panminerva Med. 2003;45:175-182. [PubMed] |
| 214. | Alberstein M, Zornitzki T, Zick Y, Knobler H. Hepatitis C core protein impairs insulin downstream signalling and regulatory role of IGFBP-1 expression. J Viral Hepat. 2012;19:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 215. | Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. Focal overexpression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer. 2003;88:733-739. [PubMed] |
| 216. | Mee CJ, Farquhar MJ, Harris HJ, Hu K, Ramma W, Ahmed A, Maurel P, Bicknell R, Balfe P, McKeating JA. Hepatitis C virus infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. 2010;138:1134-1142. [PubMed] |
| 217. | Ivanovska I, Zhang C, Liu AM, Wong KF, Lee NP, Lewis P, Philippar U, Bansal D, Buser C, Scott M. Gene signatures derived from a c-MET-driven liver cancer mouse model predict survival of patients with hepatocellular carcinoma. PLoS One. 2011;6:e24582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 218. | Crnic KA, Greenberg MT. Minor parenting stresses with young children. Child Dev. 1990;61:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Peer reviewers: Dr. Rosa Divella, Laboratory of Experimental Oncology, National Cancer Institute, Viale Orazio Flacco, 65, 70100 Bari, Italy; Maria-Cristina Navas, Professor, Grupo de Gastrohepatologia, Universidad de Antioquia, Medellin, Colombia
S- Editor Song XX L- Editor A E- Editor Li JY