Published online Aug 27, 2011. doi: 10.4254/wjh.v3.i8.205
Revised: June 15, 2011
Accepted: July 28, 2011
Published online: August 27, 2011
The treatment of choice for patients with severe alcoholic hepatitis (AH) is use of corticosteroids. Many randomized well designed studies have been reported from all over the world on the use of corticosteroids in the treatment of AH. However, the data on the efficacy of corticosteroids in these patients have been conflicting. Initial meta-analyses also failed to show beneficial effects of corticosteroids. Based on individual data meta-analysis showing clear benefit of corticosteroids amongst patients with severe AH (modified discriminant function of 32 or more), led American College of Gastroenterology to recommend use of corticosteroids as the first line treatment option amongst patients with severe AH. However, corticosteroids are relatively contraindicated amongst patients with severe AH and coexistent sepsis, gastrointestinal bleeding, and acute pancreatitis. These patients may be candidates for second line treatment with pentoxifylline. Further, specific treatment of AH with corticosteroids far from satisfactory with as many as 40%-50% of patients failing to respond to steroids, thus classified as non-responsive to steroids. The management of these patients is a continuing challenge for physicians. Better treatment modalities need to be developed for this group of patients in order to improve the outcome of patients with severe AH. This article describes at length the available trials on use of corticosteroids and pentoxifylline with their current status. Route of administration, dosage, adverse effects, and mechanisms of action of these two drugs are also discussed. Finally, an algorithm with clinical approach to management of patients who present with clinical syndrome of AH is described.
- Citation: Singal AK, Walia I, Singal A, Soloway RD. Corticosteroids and pentoxifylline for the treatment of alcoholic hepatitis: Current status. World J Hepatol 2011; 3(8): 205-210
- URL: https://www.wjgnet.com/1948-5182/full/v3/i8/205.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i8.205
Patients with severe alcoholic hepatitis (AH) have a short-term mortality of about 40%-50%[1]. Therefore, these patients should be identified early and treated appropriately. Two established specific agents for treating severe AH are corticosteroids and pentoxifylline.
The choice for treating patients with established and diagnosed cases of severe AH is corticosteroids[1]. There have been 12 randomized placebo controlled trials (RCT) to assess the benefit of corticosteroids in AH patients (Table 1). Results from these RCTs, conducted during the last 40 years, have varied with the sample size, inclusion/exclusion criteria, disease severity, end-points, type of corticosteroid used and treatment duration. These studies have shown conflicting data on the benefit of steroids with only five studies showing a survival benefit (Table 1).
| Ref. | Study design | Sample size | Mean age (yr) males (%) | Drug schedule | Main outcome /findings | Secondary findings | Causes of death |
| Helman et al[19] 1971 | Randomized controlled trial: 3 groups: severe, moderate without encephalopathy, and ambulatory | 37 (20) | 48 (32) | Prednisolone 40 mg/d × 4 wk | Mortality benefit seen only for group I with severe alcoholic hepatitis (1/15 in treated vs 6/15 in untreated, P < 0.01) | No difference on histology at 4 wk and no effect on prevention to cirrhosis. Improved caloric intake was seen with steroids | Treated: (n = 1): liver failure. Untreated: (n = 6): hepatorenal syndrome (4), lower gastrointestinal bleed (1), variceal bleed (1) |
| Porter et al[20] 1971 | Double blind Randomized controlled trial | 20 (11) | 45 (64) | 6-Methylprednisolone 40 mg/d in 3 d × 10 d followed by oral if possible | Survival 45% vs 22%, P = NS | No effect on biochemical parameters | Treated: (n = 1): tuberculosis |
| Campra et al[21] 1973 | Prospective controlled trial | 45 (20) | 43 (75) | Prednisone 0.5 mg/kg per day × 3 wk then 0.25 mg/kg per day × 3 wk | Survival was no different (36% vs 35%). Trend for improved survival with encephalopathy (P = 0.2) | No effect on biochemical parameters | Treated: (n = 7): hepatic failure. Untreated (n = 9): hepatic failure, GIB (5), renal failure (4) |
| Blitzer et al[22] 1977 | Prospective double blind | 28 (16) | 48.4 (not reported) | Prednisolone 40 mg × 14 d then tapering × 2 wk | Overall mortality higher in the treated group | No effect on biochemical parameters. Prothrombin time higher in non-survivors | Treated: (n = 11): GIB, Hepatorenal syndrome, spontaneous bacterial peritonitis fungal infections (33% cases): disseminated aspergillosis, candidemia disclosed on autopsy; Untreated (31%): GIB, hepatorenal syndrome, spontaneous bacterial peritonitis, fungal infections |
| Shumaker et al[23] 1978 | Randomized controlled trial | 27 (12) | 44 (75) | 6 -methyl prednisolone 80 mg/d × 4-7 d po | No change in survival (50% vs 53%) | Patients with contraindication to steroids had higher mortality. Causes of death in the two groups were similar with > 50% dying from GIB | Treated: (n = 3): GIB, (n = 2): hepatic failure, (n = 1): acute pancreatitis. Untreated: (n = 3): GIB, (n = 2): sepsis, (n = 1): not reported |
| Maddrey et al[24] 1978 | Randomized controlled trial | 55 | 40 (60) | prednisolone 40 mg/d × 28-32 d | Improved short-term mortality but no effect on development of portal hypertension even in short term | Serum bilirubin > 20, prothrombin time > 8 s, prolonged and encephalopathy predicted mortality | Treated: (n = 2): hepatic failure, (n = 1): cytomegalic inclusion disease and pneumocystis carinii pneumonia mono-lineal esophagitis, (n = 2): severe liver disease. Untreated: (n = 5): hepatic failure, coma and hepatorenal syndrome |
| Lesesne et al[25] 1978 | Randomized controlled trial | 14 (7) | 49 (not reported) | Prednisolone 40 mg/d × 30 d then 2 wk of tapering | Improved survival of the treated group. Improved nutrition alone is not a factor for better survival | Infrequent complications from steroids could be cause of death | Treated: (n = 2): hepatic failure and hepatorenal syndrome, (n = 1): hemorrhagic pancreatitis, (n = 1): pneumococcal pneumonia. Untreated: (n = 7): hepatic failure, (n = 4): GIB, (n = 3): hepatorenal syndrome, (n = 1): aspiration pneumonia, (n = 1): klebsiella bacteremia |
| Depew et al[26] 1980 | Randomized controlled trial | 28 (15) | 49 (66) | Prednisolone 40 mg/d × 28 d then taper × 14 d | Mortality in both groups similar (53% vs 54%). LOS: 66 d with prednisolone and 56 d with placebo | No effect on biochemical parameters and complications higher with steroids | Treated: (n = 7): urinary tract infection, (n = 3) pneumonia, (n = 2) septicemia, (n = 1) perinephric abscess. Untreated: (n = 6): urinary tract infection, (n = 1) pneumonia |
| Ramond et al[27] 1992 | Randomized controlled trial | 61 (32) | 48 (not reported) | prednisolone 40 mg/d × 28 d (IV if unable to take orally) | Improved survival at 6 mo (84% vs 45%, P = 0.002) irrespective of encephalopathy for patients with discriminant function > 32 (21/23 vs 10/19, P < 0.001) | Death in steroids group occurred early. Patients should be started on steroids while awaiting biopsy results | Treated: (n = 2): gastritis and GIB, (n = 2): septicemia lung. Untreated: (n = 16): GIB, ascites, variceal rupture, pancreatitis, (n = 1 each): septicemia |
| Theodossi et al[28] 1982 | Randomized controlled trial | 55 (27) | Not reported, 70% (treatment group), 30% (control group) | Methylprednisolone 1 g/d × 3 d | Patients survival predicted by: encephalopathy, discriminant function > 93, bilirubin 20 mg/dL, creatinine 3 mg/dL, and histological evidence of cirrhosis | Not reported | Treated: (n = 7):septicemia, (n = 2): pancreatitis. Untreated: (n = 6): septicemia, (n = 2): pancreatitis % mortality in patients of hepatic encephalopathy: 94% (treatment group) 69% (control group) |
| Richardet et al[29] 19931 | Randomized controlled trial | 23 (12) | Not reported | Prednisolone 40 mg/d × 8 d | Tumor necrosis factor, interleukin-6, interleukin-8 decreased in treated group significantly at Day 8 from their baseline Day 0 levels | Not reported | Not reported |
Meta-analyses of RCTs provide the best evidence for efficacy. To date, four meta-analyses have been published on the efficacy of steroids in AH[2-5]. The latest Cochrane analysis concluded that there is no clear evidence that steroids are effective in the management of AH. The potential for bias is due to heterogeneous data[5]. However, the same meta-analyses concluded that steroids do have survival benefit for patients with severe AH (discriminant function index, DFI ≥ 32)[5].
One of the means to tackle the issue of heterogeneity is to perform meta-analysis on the individual patient data from each study[6]. This had been performed earlier by Mathurin and colleagues from France where they analyzed individual patient data from 3 RCTs. The results of this meta-analysis showed that steroids have survival advantage for severe AH with 28 d survival of 85% among treated patients and 65% for patients receiving placebo (P = 0.001). This was also associated with improvement of liver function starting within the first week of starting the steroids[4].
Corticosteroids act by reducing inflammatory cytokines such as tumor necrosis factor-α (TNF-α), intercellular adhesion molecule 1, interlukin (IL)-6 and IL-8[7,8]. Inflammation is a major component of AH pathogenesis. In fact, in one study, peripheral white blood cell count > 5500/cm and the amount of polymorphonuclear leucocytic infiltration on the liver biopsy specimen were independent predictors for response and survival on steroid treatment[9].
Although, many agents have been used across different studies, prednisolone is preferred (but not demonstrated to be better) over prednisone as the latter requires conversion within the liver to its active form, prednisolone. The drug is given orally in a dose of 40-60 mg/d for a total duration of 4 wk. The treatment is then tapered over next 2-3 wk. If the patient is unable to take it orally due to nausea, vomiting or altered sensorium, an intravenous preparation such as methylprednisolone may be used until the patient is capable to take medication by mouth.
It is prudent to screen patients for any contraindication prior to starting steroids. One of the most important contraindications is the presence of infection which is fairly common among patients with severe AH. This used to be considered an absolute contraindication for steroids[10]. However, the latest data from France have shown that if a patient is adequately treated for an established infection, steroids can be safely started and even improve the outcome in these patients. In this study, all 246 patients studied prospectively were treated with steroids. Patients with infection (25% of the group) were treated adequately with antibiotics prior to starting steroids. Survival with steroids at 2 mo was similar, irrespective of the presence of infection prior to starting steroids (71% vs 72%, P = 0.99)[11].
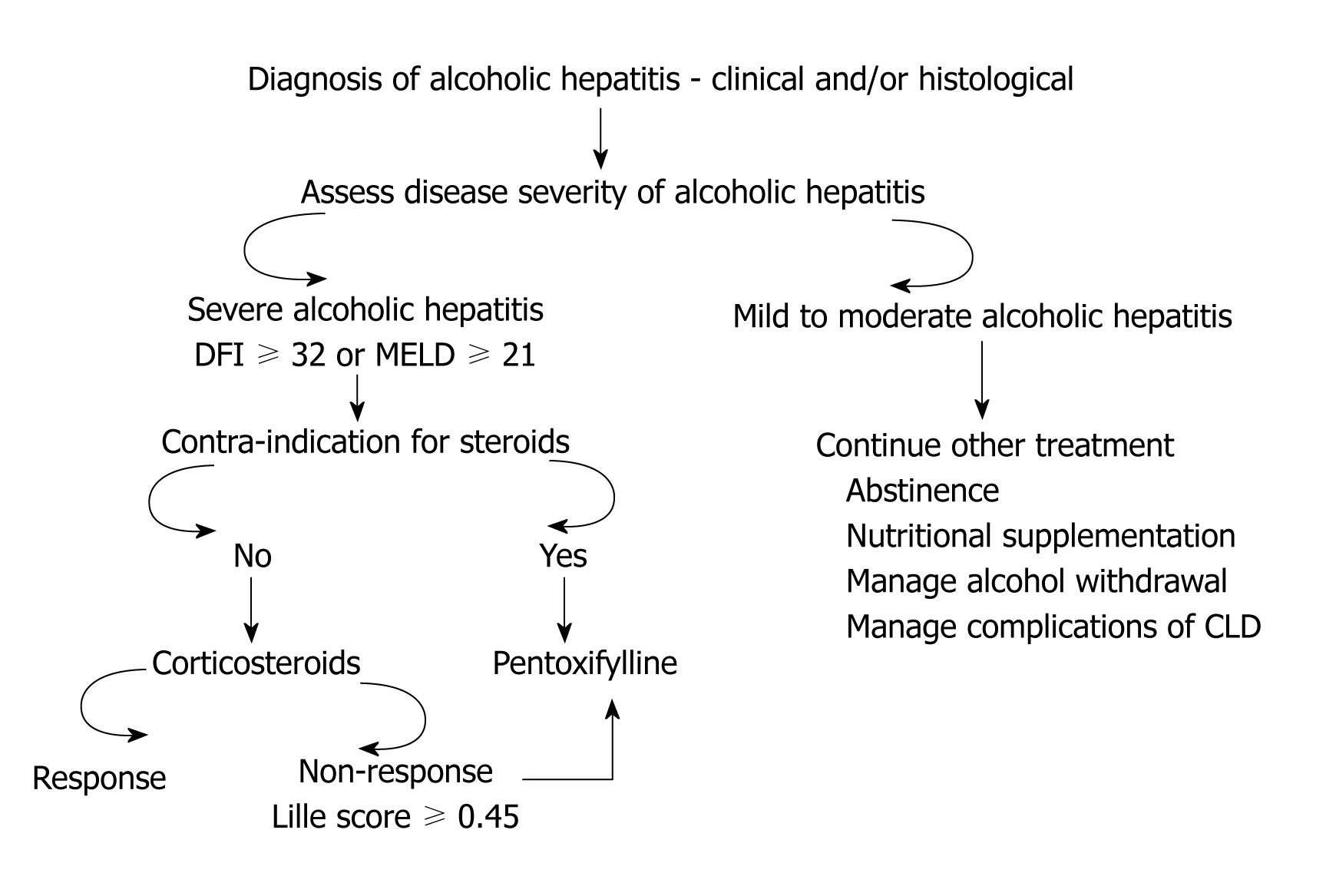
Other contra-indications are an active gastrointestinal bleeding, renal failure, acute pancreatitis, active tuberculosis, uncontrolled diabetes and psychosis. Patients should be assessed for response to steroids. It has been shown that a decrease in bilirubin at 1 wk (early change in bilirubin, ECBL) is a reliable and specific marker for response. Patients who achieved ECBL had a better survival at 6 mo compared to patients who did not achieve ECBL (98.3% vs 23%, P < 0.0001)[12]. Based on ECBL and other variables, French workers have derived a score (Lille score) based on the patient’s age, serum albumin, ECBL, renal insufficiency and prothrombin time. Patients with a Lille score of ≥ 0.45 are defined as non-responders to steroids (NRS). This score, with a cut off at 0.45, has an accuracy of 75% in predicting death at 3-6 mo[13]. Patients should also be screened for infective complications while on steroids. Occurrence of sepsis and infective complications while the patient is on steroids is a poor prognostic sign. A total of 57 patients developed infection after starting steroids which occurred more frequently among NRS than responders to steroids (42% vs 11%, P < 0.000001)[11]. Lille score was an independent predictor for the occurrence of infection after starting steroids.
For patients who have contraindications to steroids, the second option for treatment is oral pentoxifylline (PTX), a phosphodiesterase and a possible TNF-α inhibitor[14] (Figure 1). The drug was first shown to have beneficial effect in AH in a double blind placebo controlled RCT. The study showed survival benefit at 1 mo with the use of PTX compared to placebo (76% vs 54%)[15]. This benefit was attributed mainly to the prevention of the hepatorenal syndrome (HRS) among patients treated with PTX as compared to placebo (50% vs 92%, P < 0.05)[15]. Later, many studies (reported as abstracts) confirmed this observation of beneficial effect of PTX in the prevention of HRS (Table 2). A study published recently comparing steroids to PTX showed superiority of PTX in the treatment of AH patients with better survival rate at 3 mo (85% vs 65%, P = 0.04)[16]. This was again mainly due to prevention of HRS by PTX (6 of 34 patients receiving steroids developed HRS compared to none of 34 receiving PTX)[16]. However, the latest Cochrane systematic review of 5 RCTs (4 reported as abstracts) concluded that there is not enough evidence for survival benefit of PTX in the treatment of AH. However, the problem with these studies is a small sample size. Further, four of these five studies are reported as abstracts[17].
| Ref. | Sample size | Mean age (yr) males (%) | Drug schedule | Main outcome/findings | Secondary findings | Causes of death |
| McHutchison et al[30] 19911 | 22 (12 pentoxifylline) | Not reported | Pentoxifylline 400 mg tid× 10 d | Renal impairment more with placebo (mean creatinine change -0.3 vs +2.1 | No difference on other biochemical parameters. Plasma tumor necrosis factor increased in controls only. Survival trend better with pentoxifylline (3 deaths vs 1 death) | Not reported |
| Akriviadis et al[15] 2000 | 101 (49 pentoxifylline) | 42 (71% males) | Pentoxifylline 400 mg tid× 28 d | Mortality during the admission 25% vs 46% (P = 0.037) | Age, creatinine at randomization, and pentoxifylline treatment predicted survival. Tumor necrosis factor levels were no different with pentoxifylline and placebo. However, among non-survivors tumor necrosis factor levels decreased more in pentoxifylline group | Hepatorenal syndrome: treated vs untreated (50% vs 92%, P = 0.009) |
| Paladugu et al[31] 20061 | 30 (14) | 50 (100%) | Pentoxifylline | Mortality at 28 d: 29% vs 46% (P = 0.09). Time to death 21 d vs 18 d (P = 0.041) | Tumor necrosis factor levels unchanged in both groups | Hepatorenal syndrome: treated vs untreated (50% vs 86%, P = 0.1) |
| Sidhu et al[32] 20061 | 50 | Not reported | Pentoxifylline 400 mg tid× 28 d | Mortality at 28 d (24% vs 40%, P = NS) | Pentoxifylline reduced creatinine, tumor necrosis factor, discriminant function index, prothrombin time | Hepatorenal syndrome: treated vs untreated (83% vs 60%) |
| Lebrec et al[33] 20071 | 132 | Not reported | Pentoxifylline | Mortality at 2 mo (14% vs 16%, P = 0.77) and at 6 mo (27% vs 31%, P = 0.3) were similar | No difference for serious adverse effects between the 2 groups. Subgroup with renal dysfunction also did not get benefit with pentoxifylline | Not reported |
The question of whether PTX is a salvage option for patients with NRS was answered by a study in which patients were identified as NRS at 1 wk (Lille score ≥ 0.45) and randomized to PTX or placebo. Steroids were continued in both the groups for 28 d[18]. The use of additional PTX failed to demonstrate survival advantage at 2 mo (36% vs 31%, P > 0.05). Further, there was no benefit shown on the biochemical parameters. Clearly, PTX was not shown to be an option for patients with NRS[18]. However, the drug has not been studied in patients with NRS without additional steroids. Since patients with NRS are prone to develop infectious complications, this might have abrogated the benefit of PTX. Occurrence of infective complications and/or sepsis as cause of death was not reported in this study. Although the evidence for efficacy of PTX for severe AH is weak, this drug may be a second line option and is worth considering until newer and more effective agents are developed.
PTX is given orally at a dose of 400 mg three times a day for a total duration of 28 d. Although an anti-TNF agent, the TNF levels were not shown to be different among patients receiving PTX and those receiving placebo[15]. The exact mechanism of action of PTX is not entirely clear. Neutralization of TNF-α by PTX may explain the protective effect of this drug on HRS although the exact mechanism is not clear.
| 1. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 698] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 2. | Daures JP, Peray P, Bories P, Blanc P, Yousfi A, Michel H, Gremy F. [Corticoid therapy in the treatment of acute alcoholic hepatitis. Results of a meta-analysis]. Gastroenterol Clin Biol. 1991;15:223-228. [PubMed] |
| 3. | Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299-307. [PubMed] |
| 4. | Mathurin P, Mendenhall CL, Carithers RL, Ramond MJ, Maddrey WC, Garstide P, Rueff B, Naveau S, Chaput JC, Poynard T. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 6. | Sutton AJ, Higgins JP. Recent developments in meta-analysis. Stat Med. 2008;27:625-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 358] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Spahr L, Rubbia-Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, Hadengue A. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol. 2000;32:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, Benhamou JP, Chaput JC, Rueff B, Poynard T. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996;110:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 202] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | McCullough AJ, O'Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, Mathurin P. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Mathurin P, Abdelnour M, Ramond MJ, Carbonell N, Fartoux L, Serfaty L, Valla D, Poupon R, Chaput JC, Naveau S. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. 2003;38:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 531] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 14. | Reuter BK, Wallace JL. Phosphodiesterase inhibitors prevent NSAID enteropathy independently of effects on TNF-alpha release. Am J Physiol. 1999;277:G847-G854. [PubMed] |
| 15. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 514] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev. 2009;CD007339. [PubMed] |
| 18. | Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, Deltenre P, Canva V, Plane C, Mathurin P. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311-321. [PubMed] |
| 20. | Porter HP, Simon FR, Pope CE, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med. 1971;284:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 99] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Campra JL, Hamlin EM, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Ann Intern Med. 1973;79:625-631. [PubMed] |
| 22. | Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double-blind randomized study. Am J Dig Dis. 1977;22:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Shumaker JB, Resnick RH, Galambos JT, Makopour H, Iber FL. A controlled trial of 6-methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. Am J Gastroenterol. 1978;69:443-449. [PubMed] |
| 24. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] |
| 25. | Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology. 1978;74:169-173. [PubMed] |
| 26. | Depew W, Boyer T, Omata M, Redeker A, Reynolds T. Double-blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology. 1980;78:524-529. [PubMed] |
| 27. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut. 1982;23:75-79. [PubMed] |
| 29. | Richardet JD, Mal M, Roulot FD et al. Influence of corticosteroids (CS) on plasma cytokines concentrations in patients with severe alcoholic hepatitis (HA): Results of a randomized study. J Hepatol. 1993;18:S75. |
| 30. | McHutchison JR, Draduesku BA et al. Pentoxifylline may prevent renal impairment (hepatorenal syndrome) in severe acute alcoholic hepatitis. Hepatology. 1991;14:96A. |
| 31. | Paladugu HS, Dalvi P, Kudalkar L. Role of pentoxifylline in treatment of severe acute alcoholic hepatitis - a randomized controlled trial. J Gastrenterol Hepatol. 2006;21:A459. |
| 32. | Sidhu S, Singla M, Bhatia KL. Pentoxifylline reduces disease severity and prevents renal impairment in severe acute alcoholic hepatitis: a double blind placebo controlled trial. Hepatology. 2006;44:373A. |
| 33. | Lebrec D, Dominique T, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, Carbonell N, Renard P, Ramond MJ. Pentoxifylline for the treatment of patients with advanced cirrhosis. A randomized placebo controlled double blind trial. Hepatology. 2007;46:A249-A250. |
Peer reviewers: Shivendra D Shukla, PhD, Professor, Department of Medical Pharmacology & Physiology, University of Missouri, School of Medicine M517B Medical Sciences Building One Hospital Drive Columbia, Missouri 65212, United States; Henning Grønbæk, MD, PhD, Associate Professor, Medical Department V, Aarhus University Hospital, Norrebrogade 44, DK-8000 Aarhus C, Denmark
S- Editor Zhang SJ L- Editor Roemmele A E- Editor Zheng XM