Revised: December 29, 2010
Accepted: November 6, 2010
Published online: February 27, 2011
AIM: To examine the consequences of cellular fibronectin (cFn) accumulation during alcohol-induced injury, and investigate whether increased cFn could have an effect on hepatocytes (HCs) by producing factors that could contribute to alcohol-induced liver injury.
METHODS: HCs were isolated from rats fed a control or ethanol liquid diet for four to six weeks. Exogenous cFn (up to 7.5 μg/mL) was added to cells cultured for 20 h, and viability (lactate dehydrogenase,LDH), apoptosis (caspase activity) and secretion of proinflammatory cytokines (tumor necrosis factor alpha, TNF-α and interleukin 6 IL-6), matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs) was determined. Degradation of iodinated cFn was determined over a 3 h time period in the preparations.
RESULTS: cFn degradation is impaired in HCs isolated from ethanol-fed animals, leading to its accumulation in the matrix. Addition of exogenous cFn did not affect viability of HCs from control or ethanol-fed animals, and apoptosis was affected only at the higher concentration. Secretion of MMPs, TIMPs, TNF-α and IL-6, however, was increased by exogenously added cFn, with HCs from ethanol-fed animals showing increased susceptibility compared to the controls.
CONCLUSION: These results suggest that the elevated amounts of cFn observed in alcoholic liver injury can stimulate hepatocytes to produce factors which promote further tissue damage.
- Citation: Aziz-Seible RS, McVicker BL, Kharbanda KK, Casey CA. Cellular fibronectin stimulates hepatocytes to produce factors that promote alcohol-induced liver injury. World J Hepatol 2011; 3(2): 45-55
- URL: https://www.wjgnet.com/1948-5182/full/v3/i2/45.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i2.45
Chronic alcohol consumption is a major health problem in the United States, and a leading cause of end-stage liver disease. Though much evidence exists which suggests that ethanol itself and/or its metabolites directly induce the cascade of pathophysiological responses from the cells of the liver that contribute to the development of steatosis, inflammation and fibrosis, the precise mechanism(s) behind these events has not yet been completely described[1-3]. To this end, our laboratory has largely focused on determining the specific processes underlying aberrations in hepatocellular protein trafficking resulting from ethanol administration. In recent studies, we have demonstrated that ethanol-induced impairments occur in the receptor-mediated endocytosis (RME) of the hepatocyte-specific asialoglycoprotein receptor (ASGP-R)[4-7].
Of interest, cellular fibronectin (cFn), a purported natural ligand for the hepatic ASGP-R, is a matrix component known to activate hepatic stellate cells (HSCs), and has been found, by our laboratory, as well as by others, to be increased in the livers of ethanol-fed animals[8,9]. Fibronectins are high molecular weight glycoproteins that exist as soluble dimers circulating in the blood and other tissue fluids, or as insoluble multimeric fibrils that are incorporated into the extracellular matrix (ECM). They are involved in numerous cellular processes that include cytoskeletal organization, cell adhesion, migration, growth and differentiation, that are mediated by interactions with integrin and non-integrin cell surface receptors[10]. Two major types of fibronectin are found in vivo, having distinct structures that define their respective functions. Plasma fibronectin (pFn), which predominantly exists as a soluble dimer produced by hepatocytes, has terminal carbohydrate residues capped by sialic acid. In contrast, 80%-85% of cFn, which is synthesized in soluble form by mesenchymal, epithelial and inflammatory cells prior to deposition in the ECM to form fibrils, contains terminal galactose residues that are not capped by sialic acid[11]. Studies by Rotundo et al[9] show the direct participation of the ASGP-R in the rapid in vivo removal of this desialylated fibronectin from the blood. In their study, Gillis and Nagy[8] suggest that accumulating levels of cFn after long-term ethanol administration in a rat model may be an early response to liver injury, and could lead to the activation of a fibrogenic response in hepatic stellate cells (HSCs). Overall, it is believed that changes in cFn levels prompt the remodeling of the hepatic ECM, which in turn may lead to the stimulation of factors involved in the initiation and/or progression of liver fibrosis[12,13]. The presence and accumulation of cellular fibronectin after ethanol administration may be involved in a variety of autocrine and paracrine responses within liver cells. Specifically, accumulating fibronectin generated in part by altered ASGP-R clearance may further stimulate the activity of cells to release chemokines, cytokines or matrix factors that are associated with ethanol-induced pathological changes in the liver[8,14-16]. This accumulation of cFn during liver injury may be attributed not only to impaired ASGP-receptor mediated clearance and increased cellular output, but also to alterations in the balanced interaction between matrix metalloproteinases (MMPs) that are largely responsible for the proteolytic degradation of the ECM, and their associated inhibitors, the tissue inhibitors of metalloproteinases (TIMPs)[17,18].
Matrix metalloproteinases (MMPs) are the principal enzymes involved in the remodeling of ECM components. Expressed in a cell or tissue-specific pattern, these highly specialized proteases are important in many biological and pathological processes. Of specific interest to our laboratory is the knowledge that, under certain conditions, the balanced interaction between TIMPs and the associated MMPs is altered with consequent changes to the ECM. More specifically, liver fibrosis, which is characterized by changes in the composition and extent of the ECM, is regulated by the activity of MMPs and TIMPs both of which play a role in healing after acute injury[17,18]. As previously mentioned, fibronectin deposition has been suggested to be an early indicator of liver fibrosis, and, as such, it may be a trigger for certain mechanisms that lead to changes in MMP/TIMP expression[8,13]. Cytokines have also been implicated in the regulation of MMP/TIMP activity, and the consequent remodeling of the ECM distinctive in liver injury[19-21]. Notably, production of these soluble ligands is influenced by cellular interactions with ECM molecules[22].
To date, no studies have examined the effect of cellular fibronectin accumulation following chronic ethanol administration on the functionality of hepatocytes in the liver. Any associations that have been drawn focus on non-parenchymal cellular activity, with little parenchymal cell involvement. In the present study, our goal is to examine the effect of exogenous cFn on hepatocyte performance including cytokine release and the MMP/TIMP relationship. We demonstrate, for the first time in a model of ethanol induced liver injury, a response by hepatocytes to elevated concentrations of exogenous cFn, such as is found in tissue exposed to alcohol, that reveals the ability of these cells to play an active role in the promotion of damage.
William’s Eagle culture medium, Percoll, lipopolysaccharide (LPS), type IV collagen, HEPES, BSA (fraction V), collagenase, phosphotungstic acid (PTA), trichloroacetic acid (TCA), human plasma fibronectin (pFn), and NADH were purchased from Sigma Chemical Co. (St. Louis, MO). Fetal Bovine Serum (FBS) was obtained from Gemini (West Sacramento, CA). Human cFn was received from Millipore (Temecula, CA). Penicillin/streptomycin was obtained from Cellgro (Manassas, VA) and L-glutamine and gentamicin were purchased from Gibco BRL (Grand Island, NY). Na125I was obtained from Amersham-Pharmacia. The antibodies for MMP-2 and TIMP-2 came from Calbiochem (San Diego, CA), and ICN (Costa Mesa, CA) supplied the cFn antibody. All other materials not specifically identified were of reagent grade. Nutritionally adequate liquid diets were formulated according to the method of Lieber and DeCarli[23] and purchased from Dyets Inc. (Allentown, PA). The caloric distribution of the ethanol-containing diet was 18% as protein, 35% as fat, 11% as carbohydrate and 36% as ethanol. In the isocaloric control diet, additional carbohydrates replaced ethanol.
Male Wistar rats weighing 175-200 g, purchased from Charles River Labs (Portage, Michigan) were paired according to weight, and housed in individual cages in the Animal Research Facility at the Omaha Veterans Affairs Medical Center. After three days acclimatization on a control Lieber-DeCarli liquid diet, one animal from each pair was gradually introduced to a liquid diet containing 6.4% ethanol by volume as 36% of total calories. Each counterpart was pair-fed the isocaloric control diet. This feeding regimen was carried out for 12 wk for one group of rats, and 4-6 wk for all others, after which time the animals were sacrificed. As the purpose of this study was to ascertain whether cFn contributes to the development of advanced liver injury, animals were fed for a shorter duration of 4-6 wk, sufficient for the development of the early stages of alcoholic liver injury, but not prolonged enough for substantial cFn accumulation to have already taken place[8]. All animals received humane care in accordance with the guidelines established by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Animal Studies Subcommittee of the Omaha Department of Veterans Affairs Medical Center. At necropsy, the livers of these animals were either removed completely and frozen for immunohistochemical analysis (12 wk fed group) or perfused for hepatocyte isolation in subsequent studies (4-6 wk fed group).
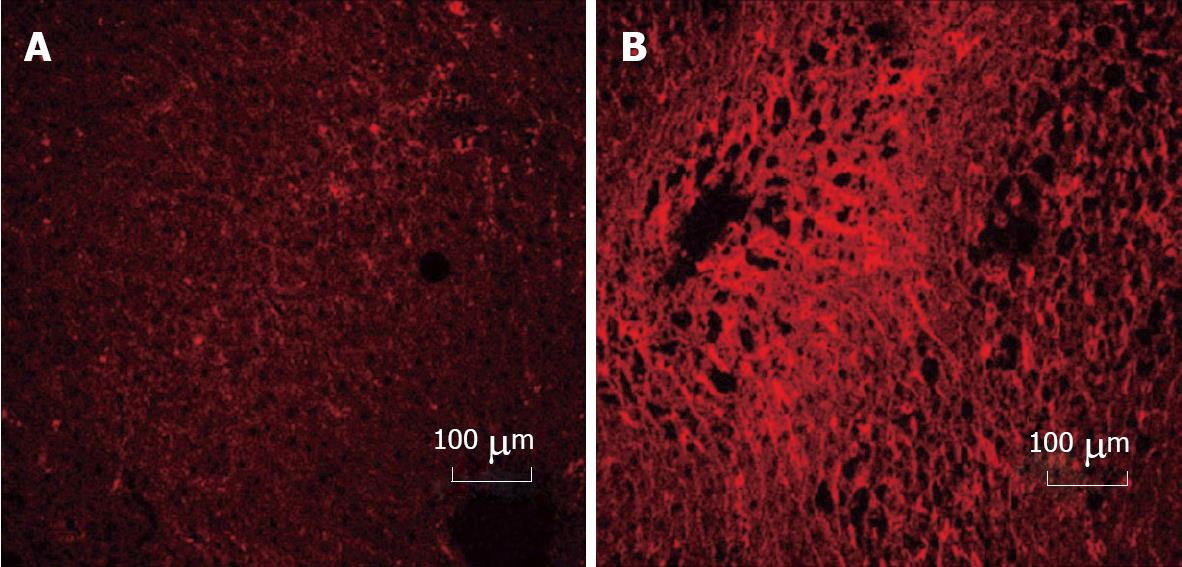
Cellular fibronectin was detected in control and ethanol rat livers using monoclonal antibody (Clone DH1) specific to the extra domain of cellular fibronectin (EDA-sequence). Liver tissue was removed from the rats that were fed for 12 wk, and flash frozen in liquid N2. A piece of frozen tissue was embedded in freezing media and frozen tissue sections were sliced into 6-micron sections and affixed to slides (Fisher, Superfrost Plus). The sections were fixed in acetone for 10 min at -20°C and subsequently washed with TBS (15 mmol/L Tris, 150 mmol/L NaCl, pH 7.6) at room temperature. Monoclonal antibody at 1:50 dilution in TBS was added to each section and incubated overnight at room temperature in a humidified chamber. Mouse IgG1 (Sigma, St. Louis, MO) was used as a negative control. After a series of washes with TBS the sections were further incubated with rhodamine (TRITC) conjugated anti-mouse IgG (H + L) (Jackson Immunoresearch, West Grove, PA) at 1:50 dilution at room temperature for 3 h in a humidified chamber. The slides were further washed, then mounted using Vectashield (Vector Laboratories, Burlingame, CA), viewed, and quantified, using a confocal-laser scanning microscope (Carl Zeiss LSM 410 inverted microscope with an argon-krypton laser with DIC capabilities) at the appropriate wavelengths.
Hepatocytes (HC) were obtained from control and ethanol-fed rats by the collagenase perfusion method[24] as described in our previous work[25]. The isolated cells were washed with Seglen suspension buffer and purified over a 35% (controls) or 33% (ethanol) Percoll gradient. Hepatocyte viability, determined by trypan blue dye exclusion was routinely > 85% for all the experimental groups. These cells were used for cFn degradation studies, or were cultured overnight to determine the biological effects of exogenously added cFn.
Preparation of iodinated cellular fibronectin: Iodinated cellular fibronectin was prepared according to the procedure of Fraker and Speck[26]. Briefly, 10 μL of a 1 mg/mL solution of 1, 3, 4, 6-tetrachloro-3a, 6a-diphenylglycoluril was evaporated under nitrogen gas in a glass tube (12 mm × 75 mm). To the tube (on ice) was then added 200 μL of a solution of cFn (1 mg/mL) in phosphate-buffered saline (PBS) followed by 7.5 μL of Na125I (750 μCi). This mixture was swirled on ice for 10 min; after mixing, the sample was loaded onto a column (1 cm × 12 cm) of Sephadex G-25 (medium) and eluted at room temperature with PBS.
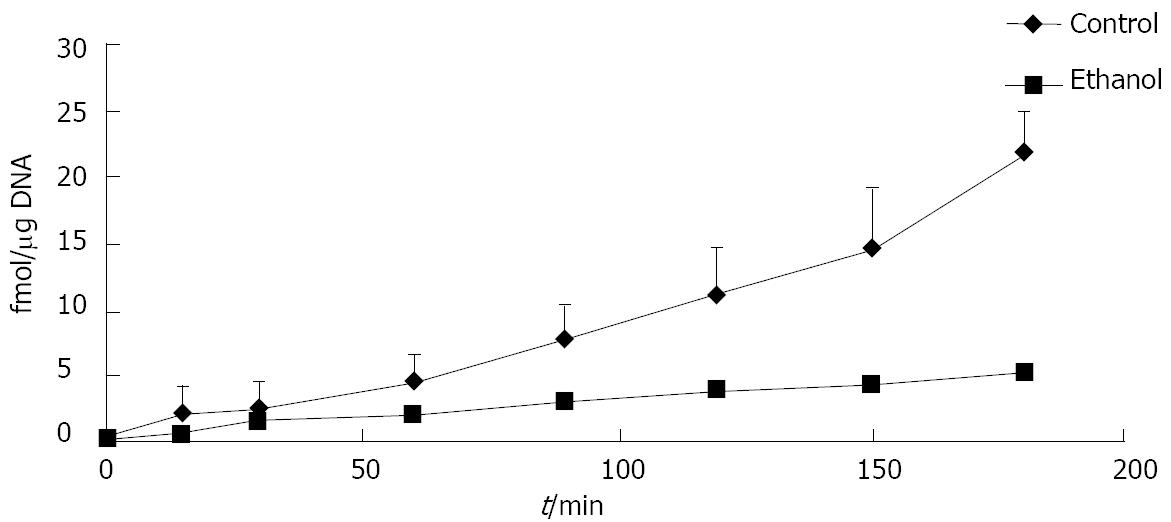
Measurements of 125I-cFn degradation: Isolated hepatocytes were pre-incubated in suspension buffer with 2% BSA at 37°C for 30 min in a metabolic shaker before use in order to increase and equilibrate the number of cell surface receptors. The hepatocytes were then suspended in Williams Eagle’s medium, pH 7.4, with 10 mol/L HEPES and 0.5% BSA at a concentration of 2 × 106 cells/mL. 125I-cFn, at a final concentration of 2.5 μg/mL, was added to the cell suspension, which was subsequently incubated at 37°C with gentle swirling. At 0, 15, 25, 60, 85, 120, 150, and 180 min respectively, an aliquot was removed to an ice-cold suspension buffer, pH 7.4, with 25 mmol/L EDTA, and incubated on ice to remove surface-bound ligand. After a minimum of 10 min on ice, the cells were pelleted (900 g, 4°C, 3 min), and an aliquot of the supernatant was placed in an equal volume of ice-cold 2% PTA in 20% TCA. The PTA/TCA mixture was incubated for a further 10 min on ice, then centrifuged (900 g, 4°C, 10 min), after which the radioactivity of an aliquot of the resulting supernatant was determined (125I-cFn degradation).
Plating and incubation: Hepatocytes were plated a density of 7.5 × 105 viable cells/well on type IV collagen coated 6-well plates as detailed in Tuma et al[27] in Williams Eagle media supplemented with 10% FBS, penicillin/streptomycin (100 IU), 2 mmol/L L-glutamine and 40 mg/L gentamicin, and allowed to equilibrate at 37°C, 5% CO2 for 2 h. Non-adherent cells were aspirated, and fresh serum-free Williams E media was added to the wells. The hepatocytes were subsequently treated with different concentrations of cFn (0 μg/mL, 0.2 μg/mL, 0.75 μg/mL, 4.0 μg/mL and 7.5 μg/mL), and 40 ng/mL LPS, a known activator of liver cells, as a positive control, and incubated at 37°C, 5% CO2 for 20 h.
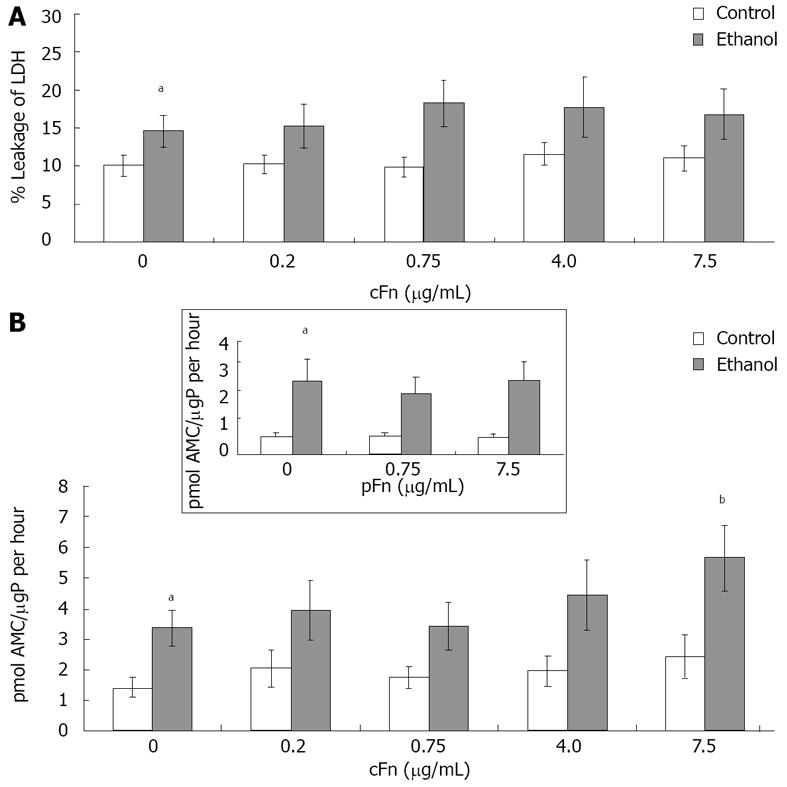
Viability assessment: Hepatocyte viability was assessed following the 20-h incubation for necrotic cell death as indicated by lactate dehydrogenase (LDH) leakage, as well as for caspase-3 activity, which is an upstream indicator of an activated programmed cell death (apoptosis) cascade. The leakage of LDH from the cells into the cell culture supernatant was measured by assaying the rate of change in the absorbance of NADH as it is oxidized (through the enzymatic activity of LDH) to NAD+ in the culture media and comparing this value to that activity originally in the cells. The cell lysate was further assayed for caspase-3 activity, by measuring the ability to cleave a fluorogenic substrate Ac (N-acetyl)-DEVD-AMC (7-amino-4-methylcoumarin) (BD Biosciences, San Jose, CA), and quantified by spectrofluorometric analysis as previously demonstrated[28].
Cytokine assay: BD OptEIA rat TNF and BD OptEIA rat IL-6 ELISA sets (BD Biosciences, San Jose, CA) were used to measure TNF-alpha and IL-6 levels in the media obtained from HC cultures. The assays were performed according to the manufacturer’s directions.
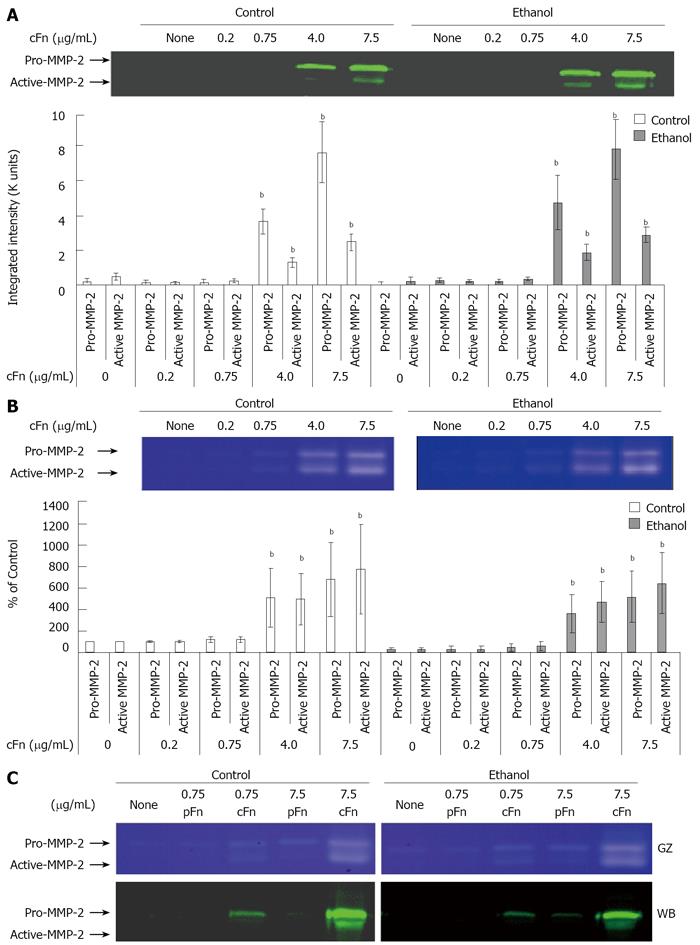
Western blot analysis: Cell culture supernatant was prepared in Laemmli[29] denaturing sample buffer and electrophoresed on 15% polyacrylamide gels using Mini-Protean II Cell (Bio-Rad, Hercules, CA). Proteins were transferred (30 V, 4°C, 16 h) onto 0.2-μm nitrocellulose membrane using the Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA) and probed with specific antibodies against MMP-2 and TIMP-2. Briefly, the nitrocellulose blots were incubated at room temperature for 1 h in Odyssey blocking buffer (Licor Biosciences, Lincoln, NE), followed by exposure to either MMP-2 or TIMP-2 antibody (1:500 or 1:250 respectively diluted in blocking buffer) overnight at 4°C. After washing in PBS with 1% Tween-20, the blots were incubated in 1:5000 diluted IRDye 800 CW labeled-goat anti mouse IgG for 1 h at room temperature. Subsequent to a final wash, the immunoreactive proteins were visualized and quantified using LICOR Odyssey Infrared Imaging System.
Gelatin zymography: The total activity of MMP-2 released in the cell culture supernatant was measured by gelatin zymography. Culture media was prepared in non-reducing sample buffer without boiling, then electrophoresed on 7.5% polyacrylamide gels containing 0.1% gelatin using Mini-Protean II Cell (Bio-Rad, Hercules, CA). Proteins were renatured (30 min to 1 h) in 2.5% Triton-X100 (Sigma, St. Louis, MO), after which the gels were incubated in an activation buffer (50 mmol/L Tris, 200 mmol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij-350, pH 8.0) for 24 h at 37°C. The gels were washed in deionized water and stained with Coomassie Blue (40% Methanol, 10% Acetic Acid, 0.5% Brilliant Blue R-250), and subsequently dried. MMP activity, represented by unstained bands was quantified by scanning densitometry using Quantity-One analysis software (Bio-Rad, Hercules, CA).
Results refer to the average from 3-15 experiments reported as mean ± SEM. Groups were compared using Student t-test, with values P < 0.05 considered significant.
Ethanol administration alters the amount of cFn degraded by hepatocytes. When compared with the cells from control animals, the cells isolated from ethanol-fed rats degraded significantly less iodinated cFn (50%-75%) (Figure 1). Soluble pFn, however, was not degraded by any of the cells (data not shown). Immunohistochemical data revealed a dramatic up-regulation in cFn accumulation after ethanol feeding in our rat model of liver injury (Figure 2). Little or no staining was evident in the liver sections of animals fed control diets, while a dramatic increase in cFn staining (60%) was observed in the liver sections from the ethanol-fed rats.
Exogenous cellular fibronectin has minimal effect on hepatocyte necrosis but increased apoptotic caspase-3 activity: The viability of isolated hepatocytes from control and ethanol-fed animals incubated with different concentrations of cFn as described in Material and Methods was characterized to determine whether cFn treatment produced a cytotoxic effect. The present data (Figure 3A) reveals a significantly higher percentage of LDH in the media of cultured cells from ethanol-fed animals when compared with the control group. However, the presence of exogenously added cFn did not induce an additional effect. The activity of caspase 3, a death protease, in the cell lysates of hepatocytes from control and ethanol-fed animals was determined by quantifying the release of the fluorogenic AMC, which was produced by cleavage of the highly specific Ac-DEVD-AMC synthetic tetrapeptide caspase-3 substrate. From our data (Figure 3B), it is apparent that a significantly elevated level of basal caspase-3 activity exists in cultured hepatocytes from ethanol-fed rats, compared to that of the control rats. Similar results were obtained from assays of freshly isolated hepatocytes (data not shown). Furthermore, the hepatocytes from ethanol-fed animals were more susceptible to the effect of cFn at the high concentration of 7.5 μg/mL. However, cFn appeared to have no effect on the viability of cells derived from the control animals. We also included data of another isoform of fibronectin, plasma fibronectin (pFn), that is increased after alcohol administration but is neither a ligand of the ASGP-R, nor does it activate non-parenchymal cells. This isoform did not have an effect on either cell type.
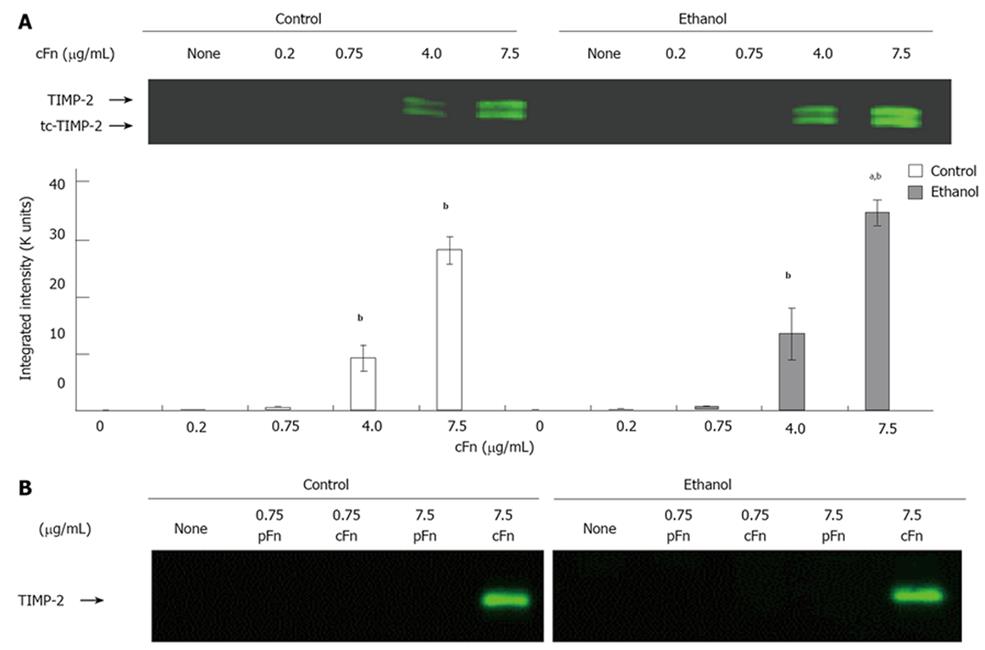
Cellular fibronectin stimulates the secretion of MMP-2 and its corresponding inhibitor TIMP-2 by hepatocytes isolated from control and ethanol-fed rats: As outlined in the Material and Methods section, the media from cultured hepatocytes was collected after a 20-h incubation, clarified and assayed on SDS-PAGE gels, followed by Western blot analysis for MMP-2 and its corresponding inhibitor, TIMP-2 expression, and via gelatin zymography for MMP-2 activity. Data from Figure 4A and B show th-at MMP-2 expression and activity levels from both control and ethanol-fed animals, in a basal condition of incubation, are very low in hepatocytes. At low concentrations of exogenous cFn, there is very little response. However, at the higher concentrations of 4 and 7.5 μg/mL cFn, secreted levels of MMP-2 are significantly increased in the media of both cultured cell-types. This increase in expression corresponds with the gelatin zymography data, which shows a higher level of MMP-2 activity in the media of hepatocytes from both control and ethanol-fed rats. However, there appears to be no distinction in the response between the two cell types. Similarly, in the absence of cFn and at low concentrations, the secretion of TIMP-2 by hepatocytes from both control and ethanol-fed animals is very low (Figure 5). In the presence of higher concentrations of exogenous cFn (4 μg/mL and 7.5 μg/mL), there is a substantial response from both cell types, with hepatocytes from ethanol-fed animals secreting significantly more TIMP-2 in the presence of 7.5 μg/mL cFn than in the corresponding control animals. As shown in Figures 4C and 5B, overall, pFn did not produce an effect.
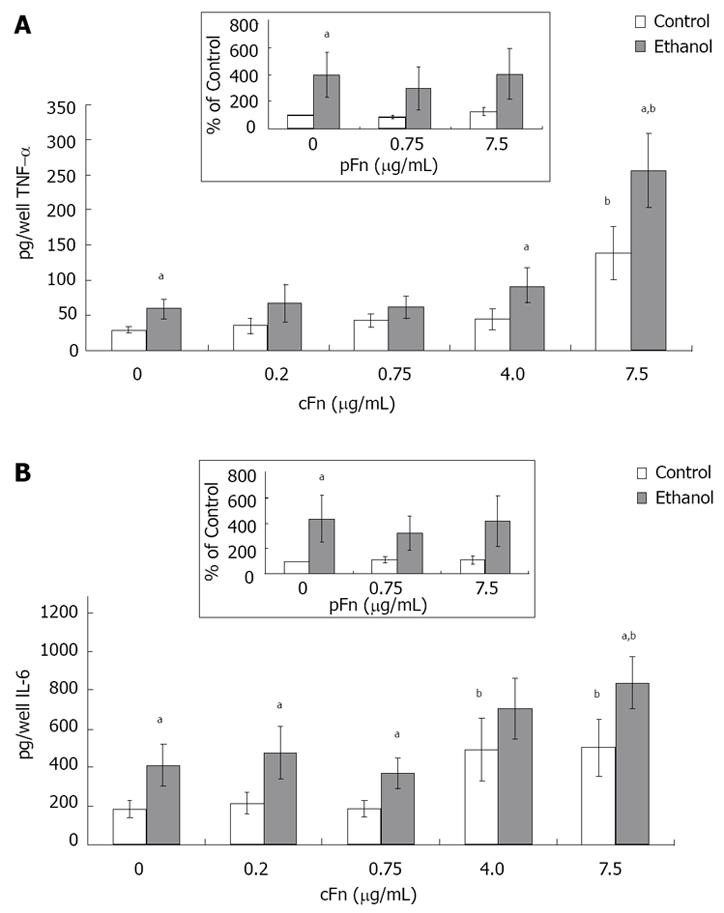
Production of pro-inflammatory cytokines by hepatocytes isolated from control and ethanol-fed rats increased in response to cellular fibronectin: Hepatocytes were cultured in the presence of low (0.2 and 0.75 μg/mL ) and high (4 and 7.5 μg /mL) concentrations of cellular fibronectin, and the secretion of TNF-α and IL-6 into the cell culture media was determined by ELISA, as described in Material and Methods. The results show that hepatocytes cultured in the presence of exogenous cFn are stimulated to release TNF-α and IL-6 (Figure 6A and B). In the absence of cFn, cells from ethanol-fed rats produced significantly higher amounts of both cytokinesin comparison with hepatocytes from control-fed animals. The level of IL-6 in the media of cultured hepatocytes from the ethanol-fed rats was consistently higher, regardless of the presence of cFn. However, only at the higher concentrations of cFn (4 and 7.5 μg/mL), did chronic ethanol administration result in a significant enhancement of the secretion of TNF-α. There was a marked difference in the levels of cytokines secreted by hepatocytes from control animals and ethanol-fed animals in the presence of 7.5 μg/mL cFn, in comparison with corresponding untreated cells (4-fold increase in TNF-α and 2-fold increase in IL-6). Treatment of both cell types with pFn did not produce an effect (inserts Figure 6A and B).
The results of this study demonstrate that the accumulation of cellular fibronectin in the liver tissue of rats subject to chronic ethanol administration elicits a response in the parenchymal cells that leads to the increased secretion of factors that could contribute to the progression of alcohol-induced liver injury. Prior studies from our laboratory have shown that chronic ethanol administration can alter the function and expression of the hepatocyte-specific asialoglycoprotein receptor[4-7]. A consequence of the impaired function of this receptor is the inability to adequately bind and internalize respective ligands for vesicular transport to the lysosome where they are degraded. Of particular interest is cellular fibronectin, cFn, a ligand for the ASGP-R and a protein that is known to participate in alcohol-induced fibrogenesis. We first examined the degradation of iodinated cellular fibronectin in isolated hepatocytes from control and ethanol-fed rats and found a significant disparity in the ability of each cell type to degrade cFn, in that hepatocytes from ethanol-fed animals were markedly impaired. The impaired degradation of cFn would contribute to the previously identified accumulation of cFn in the livers of ethanol-fed animals, and may be a factor in alcohol-induced liver pathology[30-34]. In addition, impaired degradation could lead to an increase in the concentration of circulating cFn, which has been identified in patients, and reflects a corresponding increase in tissue matrix changes and endothelial cell activation in the liver, events which are known to occur during ethanol-induced liver injury[27,34].
In our investigation, we investigated the addition of exogenous cFn to isolated HC preparations that had been obtained from rats fed ethanol or a control diet for 4-6 wk. We used a range of cFn from 0.2-7.5 μg/mL, for which the low concentration (0.2 μg/mL) represented circulating cFn levels in healthy subjects, while the higher concentrations (4 and 7.5 μg/mL) represented characteristic pathological levels of cFn as determined by previous studies[35,36].
In our study, HCs from both control and ethanol-fed livers were fairly resistant to any additional death by either necrosis or apoptosis in the presence of added cFn, so the cFn was not considered to be toxic on its own. The increased level of caspase-3 activity observed in hepatocytes from ethanol-fed rats at the highest concentration of cFn tested, suggest that this ECM protein may contribute in some way to the induction of apoptosis. It is thus plausible that, at elevated levels, cFn could facilitate apoptosis in hepatocytes whose viability has already been otherwise compromised by the effects of ethanol.
The increased deposition of cFn is implicated in the induction of matrix remodeling activity, that may contribute to the progression of liver injury towards fibrosis[8,13]. Correspondingly, we observed an increase in secreted MMP-2 levels and activity when excess cFn was introduced to cultured HCs. This output was observed in both cell types, with little variance between the two populations, and was seen as a likely regulatory response to excess cFn. The secreted proteases should break down the fibronectin molecule, rendering it non-functional, and decreasing its accumulation about the cell. However, even fragments of fibronectin have been reported to be involved in signal transduction events, thus the effect of excess cFn may not necessarily be neutralized[37-39].
Our data also show that the cells from ethanol-fed animals exposed to high concentrations of cFn, secreted substantially more TIMP-2 than corresponding control and untreated cells. These cells from the ethanol-fed animals appear to be more susceptible to cFn-induced up-regulation of TIMP-2 secretion, and are thus more likely to produce a disproportionate amount of TIMP-2 relative to MMP-2, that could lead to inhibited metalloproteinase activity and facilitate further build-up of cFn, as well as other ECM proteins characteristic of the early stages of fibrosis. An additional form of TIMP-2, two-chain (tc)-TIMP-2, a more potent inhibitor of MMP-2 function, was also detected at elevated levels[40]. The increased presence of tc-TIMP-2 would also contribute to a further reduction in the number of active-MMP-2 that could be involved in ECM degradation.
It should be noted that although our data demonstrates a seemingly similar release of MMP-2 and TIMP-2 by cells from both control and ethanol-fed animals, in normal control livers cFn accumulation does not occur. By introducing cFn to control cell cultures, we are reproducing conditions found in livers subject to ethanol-induced injury. This analysis allows us to identify ethanol-induced alterations that may exist in a cell’s response to homeostatic challenge.
Generally, fibrosis and inflammation are coordinate events in liver injury; mediators of fibrosis may operate in concert with pro-inflammatory factors[20]. Although the output of pro-inflammatory factors TNF-α and IL-6, have been previously observed from primary cultured hepatocytes in other models[20,41], our results demonstrate for the first time the in vitro secretion of these cytokines by rat hepatocytes in a study of alcohol liver disease. We demonstrate a greater responsiveness by hepatocytes from ethanol-fed animals, that appears to be exacerbated in the presence of the higher concentrations of cFn.
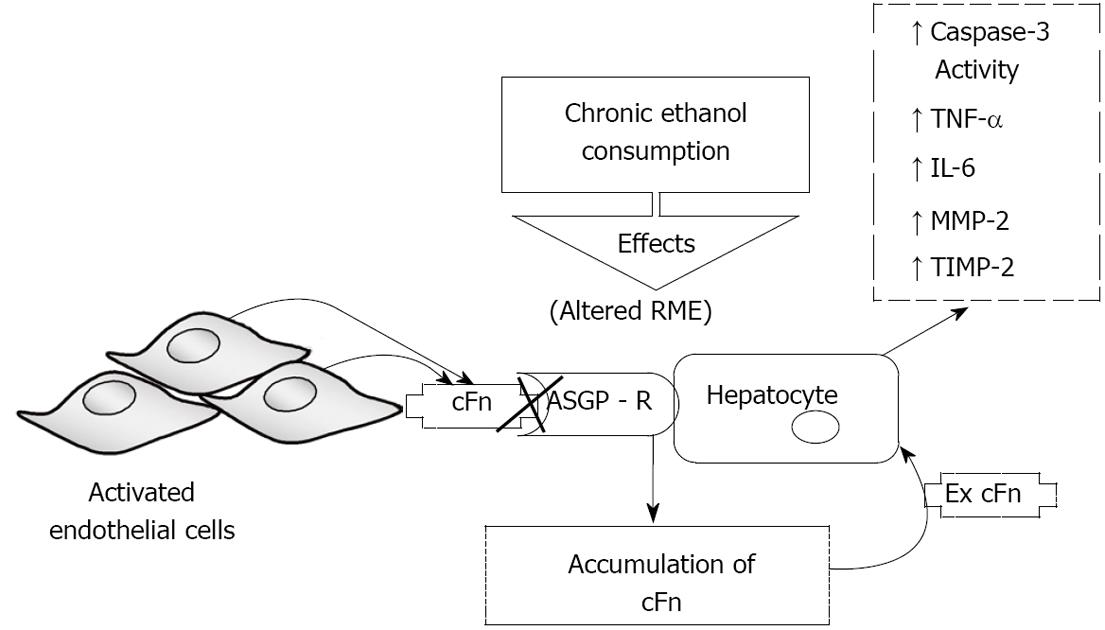
Collectively, the data is summarized in Figure 7, which depicts a model of ethanol-induced liver injury linking altered asialoglycoprotein receptor clearance of cellular fibronectin with hepatocyte activation by the accumulating protein. Activation leads to a subsequent increase in caspase-3 activity, and an elevated secretion of pro-inflammatory cytokines, TNF-α and IL-6, as well as that of MMP-2 and its corresponding inhibitor, TIMP-2. These results, in conjunction with those from the aforementioned studies, suggest that autocrine and paracrine effects could exist that would produce a feedback relationship as a result of fibronectin-mediated changes in metalloproteinases and associated factors. For example, TNF-α has been shown to induce signaling that leads to apoptosis, thus it is plausible that the cFn-induced activation of caspase-3, observed at the higher cFn concentration, in cells from ethanol-fed animals, may in part be attributed to the associated cFn-induced increase in the secretion of this proinflammatory cytokine[42]. As a consequence of such reinforcing interactions, the promotion of tissue injury can be enhanced.
Conventionally, hepatocytes have been regarded merely as recipients and respondents of action taken by their other more reactive non-parenchymal counterparts; the results described here have been mainly ascribed to non-parenchymal cells only. Though it has been shown that in vitro conditions can evoke changes in cell-signaling and protein expression profiles in hepatocytes that cause them to exhibit behavior less representative of intact in vivo tissue, this study supports the contention that hepatocytes may be more involved in the orchestration of liver injury than previously considered.
In summary, our results demonstrate an effect resulting from accumulating cellular fibronectin, due in part to altered ASGP-R mediated clearance, on hepatocytes, that is enhanced by ethanol-induced injury. Further characterization of these responses, especially in the non-parenchymal cells of the liver, as well as further investigation of the mechanisms governing these responses, will help elucidate the specific role cFn has in promoting the progression of alcoholic liver disease.
Alcohol abuse is the leading risk factor for terminal liver disease worldwide. Though this association has long been established, the mechanisms of alcohol-induced liver injury remain poorly understood. To date, no effective strategies exist to counter the progression of alcoholic liver disease. It is known that alcohol-induced alterations to the character and function of the cells of the liver contribute to the build-up of cellular fibronectin in hepatic tissue. On the other hand, the significance of this excess cellular fibronectin, and its effect on the liver during a condition of prolonged alcohol-induced damage, is not known.
The increased deposition of cellular fibronectin in the liver is implicated in the development of liver fibrosis, the onset of which is characterized by remodeling of the extracellular matrix, as well as the myofibroblastic transformation of hepatic stellate cells. The composition of the extracellular matrix is regulated by the balanced interaction between matrix metalloproteinases and their inhibitors. Alterations in this balance often have pathological consequences. This fibrotic response is also preceded by an increase in pro-inflammatory cytokine levels, which in turn may contribute to the activation of stellate cells, as well asinfluencing the activity of extracellular matrix regulatory factors. The increased production of these cytokines may occur as a direct response to higher cellular fibronectin levels. The evaluation of these effects of cellular fibronectin and their potential roles in the development and progression of alcoholic liver disease is the focus of this research.
In the present study, we identifythese increased levels of cellular fibronectin not only as a symptom of progressive disease, but also as a contributing event to the development of alcohol-induced injury. Moreover, we demonstrate, for the first time in a model of alcohol-induced liver injury, a response by hepatocytes to elevated concentrations of exogenous cellular fibronectin, such as is found in tissue exposed to alcohol, that reveals the ability of these cells to play an active role in the promotion of damage.
Understanding how elevated levels of cellular fibronectin affect the cells of the liver, specifically, in this study, hepatocytes, during a condition of alcohol abuse could yield additional targets for the development of new strategies to prevent and treat alcoholic liver disease.
Hepatocytes are the chief functioning cells of the liver, and key targets for mediators of injury. Cellular fibronectin is an extracellular matrix glycoprotein that is normally present at low levels in the body, and is only briefly up-regulated during tissue repair. A sustained elevation in cellular fibronectin levels has been observed during several pathological conditions, including that of alcoholic liver disease.
This paper describes the effect of cellular fibronectin on hepatocytes isolated from control and ethanol-fed rats. Overall, this paper is interesting, well written and provides new information for the field, though several points need clarification and/or correction.
Peer reviewers: Felix Dias Carvalho, Professor, University of Porto, Faculty of Pharmacy, Toxicology Department, Rua Anibal Cunha, 164, Porto 4099-033, Portugal; Shannon Marie Bailey, Associate Professor, Department of Environmental Health Sciences, University of Alabama at Birmingham, 1665 University Blvd, Ryals Building Room 623, Birmingham, Alabama 35294, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
| 1. | Mailliard ME, Sorrell MF, Volentine GD, Tuma DJ. Impaired plasma membrane glycoprotein assembly in the liver following acute ethanol administration. Biochem Biophys Res Commun. 1984;123:951-982. |
| 2. | Sorrell MF, Nauss JM, Donohue TM Jr, Tuma DJ. Effects of chronic ethanol administration on hepatic glycoprotein secretion in the rat. Gastroenterology. 1983;84:580-586. |
| 3. | Tuma DJ, Casey CA, Sorrell MF. Effects of alcohol on hepatic protein metabolism and trafficking. Alcohol Alcohol Suppl. 1991;1:297-303. |
| 4. | Casey CA, Sorrel MF, Tuma DJ. Effect of Ethanol on Asialoglycoprotein Receptor Function. Wu GY and Wu CH, editors. Mariel Decker, Inc. : Farmington 1991; 189-213. |
| 5. | McCashland TM, Tuma DJ, Sorrell MF, Casey CA. Zonal differences in ethanol-induced impairments in hepatic receptor binding. Alcohol. 1993;10:549-554. |
| 6. | Tworek BL, Tuma DJ, Casey CA. Decreased binding of asialoglycoproteins to hepatocytes from ethanol-fed rats. Consequence of both impaired synthesis and inactivation of the asialoglycoprotein receptor. J Biol Chem. 1996;271:2531-2538. |
| 7. | Tworek BL, Wiegert RL, Jeanette JP 2nd, Tuma DJ, Casey CA. Differential effects of monensin on asialoglycoprotein receptor function after short-term ethanol administration. Biochem Pharmacol. 1998;55:1603-1609. |
| 8. | Gillis SE, Nagy LE. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol Clin Exp Res. 1997;21:857-861. |
| 9. | Rotundo RF, Rebres RA, Mckeown-Longo PJ, Blumenstock FA, Saba TM. Circulating cellular fibronectin may be a natural ligand for the hepatic asialoglycoprotein receptor: possible pathway for fibronectin deposition and turnover in the rat liver. Hepatology. 1998;28:475-485. |
| 10. | Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126-d146. |
| 11. | Peterson TE, Skorstengaard K, Vibe-Pedersen K. Primary Structure of Fibronectin. Mosher DF, editor. Academic Press: San Diego 1989; 1-24. |
| 12. | Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985;53:166-186. |
| 13. | Odenthal M, Neubauer K, Meyer zum Büschenfelde KH, Ramadori G. Localization and mRNA steady-state level of cellular fibronectin in rat liver undergoing a CCl4-induced acute damage or fibrosis. Biochim Biophys Acta. 1993;1181:266-272. |
| 14. | Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120-1130. |
| 15. | McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205-219. |
| 16. | McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497-G502. |
| 17. | Arthur MJ. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825-833. |
| 18. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. |
| 19. | Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114:131-139α. |
| 20. | Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48-60. |
| 21. | Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139:1338-1345. |
| 22. | Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7:89-101. |
| 23. | Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523-531. |
| 24. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. |
| 25. | Casey CA, Kragskow SL, Sorrell MF, Tuma DJ. Chronic ethanol administration impairs the binding and endocytosis of asialo-orosomucoid in isolated hepatocytes. J Biol Chem. 1987;262:2704-2710. |
| 26. | Fraker PJ, Speck JC Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849-857. |
| 27. | Tuma DJ, Smith TE, Schaffert CS, Kharbanda KK, Sorrell MF. Ethanol feeding selectively impairs the spreading of rat perivenous hepatocytes on extracellular matrix substrates. Alcohol Clin Exp Res. 1999;23:1673-1680. |
| 28. | McVicker BL, Tuma DJ, Kubik JA, Hindemith AM, Baldwin CR, Casey CA. The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology. 2002;36:1478-1487. |
| 29. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. |
| 30. | Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793-805. |
| 31. | Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49:203-206. |
| 32. | Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497-505. |
| 33. | Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858-5869α. |
| 34. | Xu D, Sorrell MF, Casey CA, Tuma DJ. Impaired attachment of hepatocytes to extracellular matrix components after chronic ethanol administration. Lab Invest. 1992;67:186-190. |
| 35. | Kanters SD, Banga JD, Algra A, Frijns RC, Beutler JJ, Fijnheer R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care. 2001;24:323-327. |
| 36. | Haglund C, Ylätupa S, Mertaniemi P, Partanen P. Cellular fibronectin concentration in the plasma of patients with malignant and benign diseases: a comparison with CA 19-9 and CEA. Br J Cancer. 1997;76:777-783. |
| 37. | Thiele GM, Duryee MJ, Freeman TL, Sorrell MF, Willis MS, Tuma DJ, Klassen LW. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593-1600. |
| 38. | Kapila YL, Kapila S, Johnson PW. Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol. 1996;15:251-261. |
| 39. | Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229-10233. |
| 40. | Miyazaki K, Funahashi K, Numata Y, Koshikawa N, Akaogi K, Kikkawa Y, Yasumitsu H, Umeda M. Purification and characterization of a two-chain form of tissue inhibitor of metalloproteinases (TIMP) type 2 and a low molecular weight TIMP-like protein. J Biol Chem. 1993;268:14387-14393. |
| 41. | Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, Blanchard J, Wong HR, Lentsch AB. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514-C520. |
| 42. | Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350-364. |