Published online Jun 27, 2010. doi: 10.4254/wjh.v2.i6.246
Revised: May 17, 2010
Accepted: May 24, 2010
Published online: June 27, 2010
To investigate the simultaneous occurrence of hepatocellular carcinoma and non-Hodgkin’s lymphoma, we report the case of a 70 year old patient with a primary diagnosis of non-Hodgkin’s lymphoma in 2002. In a routine follow up investigation of his chronic lymphocytic leukemia a newly detected mass in the Couinaud's segments 2 and 3 was found. No hepatitis C virus or hepatitis B virus infection or cirrhosis was evident. After laparoscopic segmentectomy the histological examination revealed a hepatocellular carcinoma. While the relation between liver parenchyma damages and hepatocellular carcinoma or non-Hodgkin’s lymphoma is well known, only a few publications have focused on the coexistence of hepatocellular carcinoma and non-Hodgkin’s lymphoma. With this case we demonstrate the coexistence of these diseases without having a predamaged liver parenchyma.
- Citation: Heidecke S, Stippel DL, Hoelscher AH, Wedemeyer I, Dienes HP, Drebber U. Simultaneous occurrence of a hepatocellular carcinoma and a hepatic non-Hodgkin’s lymphoma infiltration. World J Hepatol 2010; 2(6): 246-250
- URL: https://www.wjgnet.com/1948-5182/full/v2/i6/246.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i6.246
Hepatocellular carcinoma (HCC) is a common type of primary liver cancer and is well investigated concerning its development and risk factors. In most of the cases HCC arises from chronic liver disease and cirrhosis. As a main risk factor the chronic infection by hepatitis C virus (HCV) or hepatitis B virus (HBV) is known[1-2].
The invasion of the liver by non-Hodgkin’s lymphoma (NHL) has also been part of several clinical investigations. Hepatitis infection has also been reported frequently as a risk factor for NHL[3-5].
Only a few publications have focused on the coexistence of HCC and NHL. For both conditions the risk factors of cirrhosis and hepatitis infection have been described, which lead to a damage of liver parenchyma. HCV has been suggested to be lymphotrophic as well as hepatotrophic and has therefore attracted speculation about a causative role in some cases of lymphoma. With this case we demonstrate the coexistence of HCC and NHL without having a predamaged liver parenchyma.
A 70 year old patient with a primary diagnosis of NHL in 2002 was assigned to our hospital in October 2008 for further treatment of a newly detected mass in the left lateral liver lobe. Prior to this he underwent routine investigations once a year to follow up the current status of his chronic lymphocytic leukemia (CLL). There was no need for any intervention concerning the CLL given stable blood parameters and hardly any clinical complaints. In the last routine check-up an unknown mass in the left lateral liver lobe was found.
The physical examination on admission showed a slightly overweight patient (170 cm tall and 91 kg bodyweight) in good health. Premedication included amlodipine against arterial hypertension and acetylcysteine and betahistidine relating to therapy for Menière's Disease. Neither the medical history nor the outer appearance gave any hint of alcoholic or nicotine abuse.
No upper abdominal pain was expressed and the patient did not report any other complaints in the previous months.
Viral serological tests were negative for anti-HCV antibodies, anti-HB core antibodies and HB surface antigen. The patient’s previous medical history included hepatitis A virus (HAV) infection revealed by a positive Anti-HAV (IgG/IgM). Alphafetoprotein (AFP) was negative, white blood cells were elevated at 84.000/μL due to the CLL. Further laboratory investigation revealed Glutamic-oxaloacetic transaminase (GOT) 25 U/L, Glutamic-pyruvic transaminase (GPT) 37 U/L, γ-Glutamyltransferase (γ-GT) 29U/L. Furthermore Albumin, Bilirubin and the parameters of coagulation were normal. So there was no indication of any liver parenchyma disorder, which would be reflected in a higher Child-Pugh-Classification.
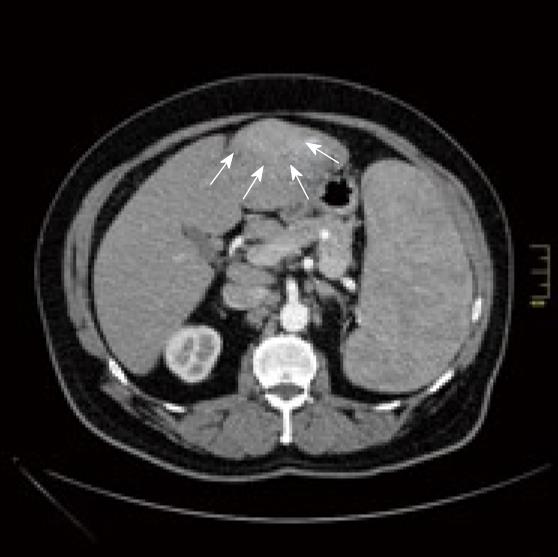
Diagnostic imaging was done by computed tomographic (CT) scan and magnetic resonance imaging. Contrast-CT scan (Figure 1) showed a hyperdense (hypervascularised) lesion in Couinaud's segments 2 and 3, which appeared hyperattenuating in the early arterial phase of contrast enhanced CT.
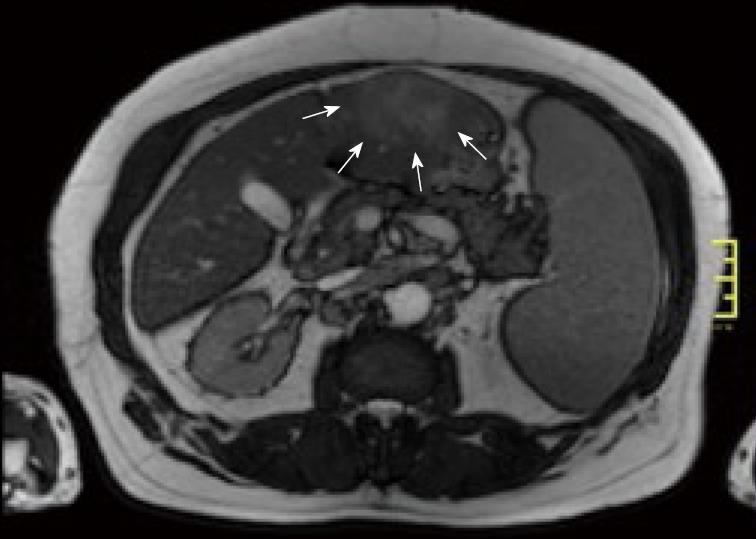
Magnetic resonance imaging of the abdomen (Figure 2) confirmed an early enhancing mass in Couinaud's segments 2 and 3.
The radiological findings suggested that the newly detected mass was not a manifestation of the NHL. In CT scan done with contrast media an arterial enhancement was seen, followed by a venous washout and a late ring enhancement. This characteristic vascular profile led to the differential diagnosis of hepatocellular carcinoma. Imaging indicated no signs of cirrhosis.
Additionally, splenomegaly and huge masses in paraaortic lymph nodes reaching down to the femoral vessels were observed, related to the patient’s already known lymphatic disease. There was no biopsy taken preoperative.
On the sixth of January 2009 the patient underwent a laparoscopic liver resection of Couinaud's segments 2 and 3. No complications occurred after the operation and the further hospital stay was uneventful. He underwent a new check-up to establish the current status of the NHL and was then released 8 d after admission.
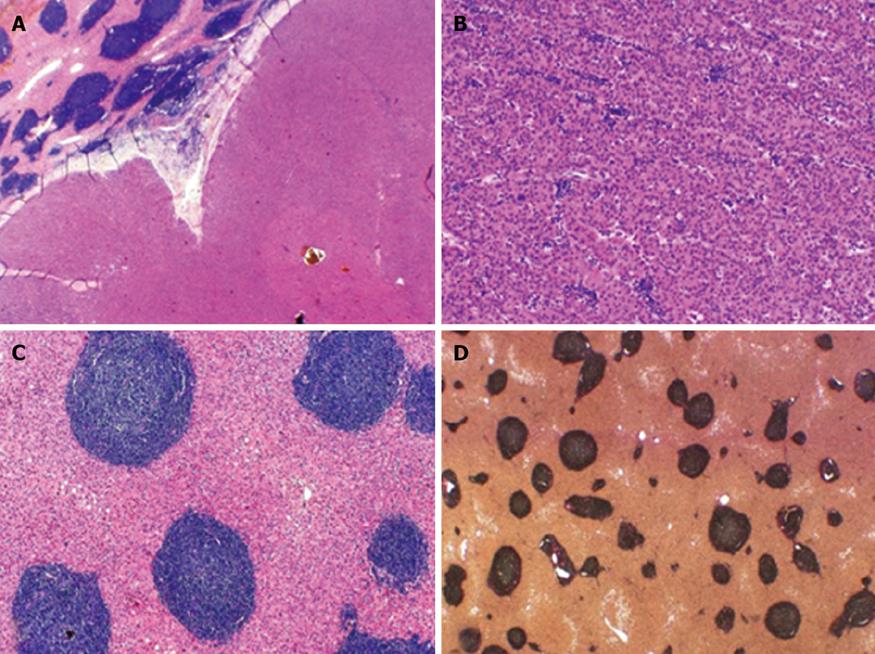
Histological examination of the resected specimen showed that there was no significant parenchymal fibrosis. Pathological findings suggested neither hepatitis, cirrhosis nor any noticeable hepatosteatosis. One could see a 4.8 cm, predominantly trabecular configured HCC with a histopathological grading of G2 and with a tumor staging of pT2, pNx, pMx. No lymphatic vessel invasion (L0) or blood vessel invasion (V0) was found.
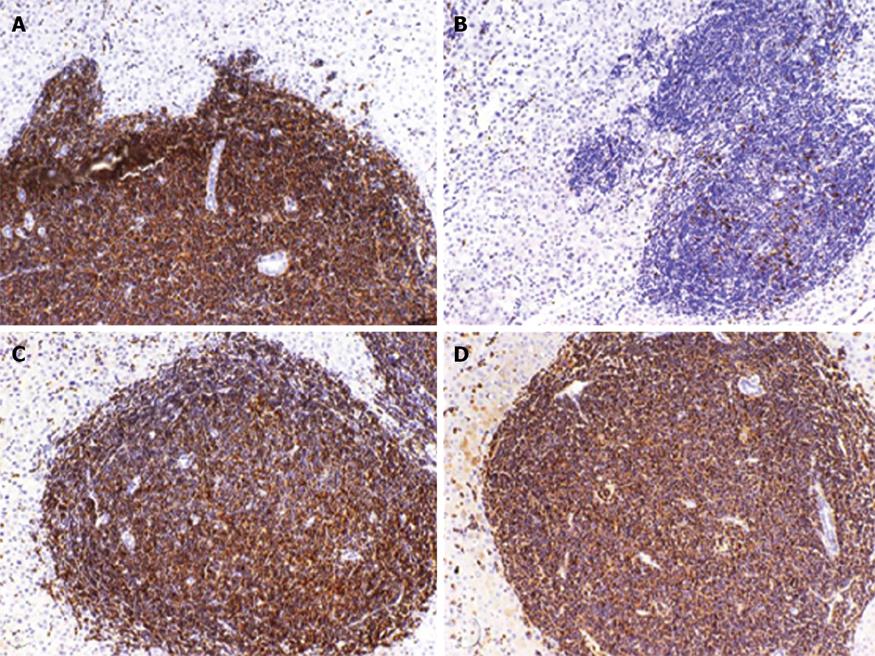
Furthermore, the diffuse infiltration of NHL in the liver parenchyma was confirmed. Portal tracts appeared densely infiltrated by an atypical small lymphocytic population, typically seen in B-CLL[6]. Immunohistochemical examination with positive reactions for the B-cell-marker CD20 and for CD5, CD23 and CD43 confirmed the diagnosis of B-CLL (Figure 3).
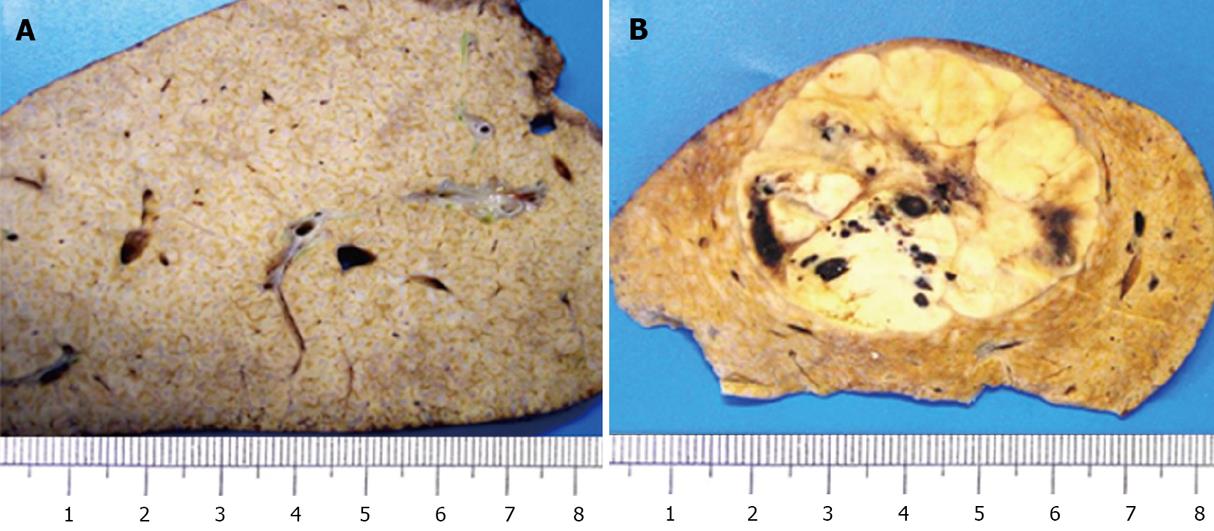
Macroscopically, the liver parenchyma showed prominent enhanced greyish portal tracts and a tumorous nodular lesion measuring 4.8 cm at its widest point (Figure 4).
Microscopically, a border of non-neoplastic liver parenchyma and hepatocellular carcinoma could be seen. Furthermore, a trabecular configured HCC with atypical lymphocytic infiltration by B-CLL was detected. Portal tracts were rounded and showed an atypical dense lymphocytic infiltration by B-CLL. There was neither significant fibrosis, cirrhosis nor steatosis in the liver parenchyma (Figure 5).
The case of this patient was discussed in the hospital oncological conference. The haemato-oncologist re-evaluated the data and confirmed the diagnosis of the NHL. Cytology showed a lymphocytosis with typical smudge cells. Furthermore flow cytometric immunophenotyping of peripheral blood confirmed the diagnosis of CLL, whilst ZAP70 and CD38 were negative. The results indicated a good prognosis and lead to the conclusion that even with infiltration of CLL and simultaneous occurrence of HCC in the liver there would be no indication for chemotherapy.
The patient underwent a follow-up examination every four months for both the CLL and HCC.
To date (17 mo after surgery) the patient is in good health, and no recurrence has been detected by follow-up imaging. His CLL has not required treatment until now.
The end stage of chronic liver disease may be the onset of hepatocellular carcinoma. Therefore, liver cirrhosis could be considered to be a pre-neoplastic lesion. The question arises in this patient of why a HCC occured in the absence of cirrhosis or hepatitis infection. Which other risk factor have to be considered? In this case the HCC was discovered during a routine follow up without clinical complaints. There have been recent investigations concerning the possible relationship between metabolic syndrome and the occurrence of HCC. Although our patient was obese with a Body mass index of 31 kg/m2 and was affected by mild arterial hypertension, there were no other signs of metabolic syndrome such as dyslipidemia or diabetes. One has to consider that metabolic syndrome has been identified as a risk factor for the occurrence of HCC. As mentioned above, there was no alcohol or nicotine abuse with this patient. In addition, the pathological findings did not show any sign of cirrhosis, hepatitis or steatosis which might have resulted in to a predamaged liver parenchyma.
Chronic lymphocytic leukemia of the B-cell-lineage (B-CLL) is the most common form of adult leukemia and predominantly a disease of older individuals. Due to the strong heterogeneity in the clinical course of B-CLL with survival ranging from months to decades, treatment regimens are strongly based upon clinical staging. This includes clinical presentation, laboratory values and lymphocyte doubling time. Until now the CLL in our patient has not been symptomatic and laboratory data have been stable since primary diagnosis.
One might speculate that the occurrence of a HCC in this patient was promoted by the NHL as a cofactor.
In PubMed research one can find 19 positive findings for “NHL and HCC”, and 6 relevant reports for the discussion of our case were found (Table 1).
| Author | Year | Type of cancer(s) | Predamaged parenchyma |
| Cavanna et al | 1994 | HCC and NHL | Chemotherapy |
| Tanaka et al | 1997 | HCC and NHL | Chemotherapy |
| Kataoka et al | 2006 | HCC and NHL | Nodular regenerative hyperplasia |
| Ohtsubo et al | 2006 | HCC and NHL | Hepatitis cinfection |
| Xiong et al | 2008 | HCC and NHL | |
| Utsunomiya et al | 2009 | HCC and NHL | Hepatitis c infection |
In 1994 Cavanna et al reported a patient diagnosed with NHL in the liver, who received chemotherapy. Six years later a needle aspiration was carried out. The histopathological findings showed a HCC, which was completely resected[7]. Three years after this operation a NHL was detected again and the patient died a short time after due to gastrointestinal bleeding. As Cavanna et al stated at that time, this was the first case report concerning the coincidence of NHL and HCC.
In 1997 Tanaka et al[8] demonstrated a higher risk for the development of a primary liver cancer (PLC), in all cases a HCC, if a preexistent NHL was found in the liver. Furthermore, patients who received chemotherapy as NHL treatment had a significantly increased risk of HCC.
In 2006 there was a report about a case of NHL and HCC in the absence of hepatitis infection or any signs of liver cirrhosis, but with nodular regenerative hyperplasia, which is a premalignant lesion[9]. The diagnosis was focused on a CT scan, which showed a simultaneous infiltration by NHL and HCC. After a short clinical period the patient died.
In 2006 Othsubo et al[10] reported on a 66 year old man with HCV-related liver cirrhosis and simultaneous hepatic relapse of NHL and HCC.
In 2009 Xiong et al[11] discussed the influence of the Cdc6 promoter, which plays an important role in DNA replication and carcinogenesis. They reported on the Cdc6 G1321A polymorphism, which lowers the risk for the development of both NHL and HCC.
Last year a case report was published about the coexistence of HCC and NHL, although this was again combined with an HCV infection[12].
In our case report the patient did not undergo chemotherapy, thereby excluding any possible influence of chemotherapy on outcomes. Furthermore, we have no evidence of any chronic hepatitis infection, cirrhosis or alcohol abuse. So this case report is in fact the first one to describe the simultaneous occurrence of HCC and NHL without having any other risk factors such as hepatitis infection or other premalignant lesions. Our pathological findings support the clinical history which indicated the absence of cirrhosis or any other liver parenchyma disorders.
The publications indicating a coincidence of NHL and HCC in the liver showed no proper evidence of the pathogenesis.
Future studies are required to establish firm evidence of the relationship between the occurrence of NHL and HCC.
| 1. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83. |
| 2. | Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80-92. |
| 3. | Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin‘s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745-752. |
| 4. | Nath A, Agarwal R, Malhotra P, Varma S. Prevalence of Hepatitis B virus infection in Non-Hodgkin‘s lymphoma. A systematic review and meta-analysis. Intern Med J. 2009;Epub ahead of print. |
| 5. | Fan HB, Zhu YF, Chen AS, Zhou MX, Yan FM, Ma XJ, Zhou H. B-cell clonality in the liver of hepatitis C virus-infected patients. World J Gastroenterol. 2009;15:1636-1640. |
| 6. | Loddenkemper C, Longerich T, Hummel M, Ernestus K, Anagnostopoulos I, Dienes HP, Schirmacher P, Stein H. Frequency and diagnostic patterns of lymphomas in liver biopsies with respect to the WHO classification. Virchows Arch. 2007;450:493-502. |
| 7. | Cavanna L, Civardi G, Fornari F, Vallisa D, Berte R, Buscarini E, Sbolli G, Paties C, Foroni R, Di Stasi M. Simultaneous relapse of liver cell carcinoma and non-Hodgkin‘s lymphoma in the liver. Report of a case with diagnosis by ultrasonically guided fine needle aspiration biopsy. Acta Cytol. 1994;38:451-454. |
| 8. | Tanaka H, Tsukuma H, Teshima H, Ajiki W, Koyama Y, Kinoshita N, Masaoka T, Oshima A. Second primary cancers following non-Hodgkin‘s lymphoma in Japan: increased risk of hepatocellular carcinoma. Jpn J Cancer Res. 1997;88:537-542. |
| 9. | Kataoka TR, Tsukamoto Y, Kanazawa N, Izumi T, Awata N, Nishizawa Y, Ohsawa M, Ishiguro S. Concomitant hepatocellular carcinoma and non-Hodgkin‘s lymphoma in a patient with nodular regenerative hyperplasia. Pathol Int. 2006;56:279-282. |
| 10. | Ohtsubo K, Oku E, Imamura R, Seki R, Hashiguchi M, Osaki K, Yakushiji K, Yoshimoto K, Ogata H, Nagamatsu H. Simultaneous hepatic relapse of non-Hodgkin‘s lymphoma and hepatocellular carcinoma in a patient with hepatitis C virus-related cirrhosis. Acta Haematol. 2006;116:266-271. |
| 11. | Xiong XD, Qiu FE, Fang JH, Shen Y, Liang C, Jiang W, Zhuang SM. Association analysis between the Cdc6 G1321A polymorphism and the risk for non-Hodgkin lymphoma and hepatocellular carcinoma. Mutat Res. 2009;662:10-15. |
| 12. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83. |
Peer reviewers: Luca Vigano, MD, Department of HPB and Digestive Surgery, Ospedale Mauriziano Umberto I, Largo Turati 62, Torino 10128, Italy; Derek Anthony O’Reilly, PhD, FRCS, Department of Surgery, North Manchester General Hospital, Delaunays Road, Manchester, M8 5RB, United Kingdom