Published online Mar 27, 2010. doi: 10.4254/wjh.v2.i3.103
Revised: January 14, 2010
Accepted: January 21, 2010
Published online: March 27, 2010
Even though Sorafenib has radically changed the natural history of those hepatocellular carcinoma patients who are not amenable for curative treatments, further therapeutic improvements are badly needed. As it was for Sorafenib, our increasingly refined understanding of the complex mechanisms underlying HCC carcinogenesis are the starting point for the future development of such treatments. Presently, a number of molecularly targeted agents are in different stages of development for this once orphan cancer. Indeed, several pathways are presently being explored to identify potentially active drugs, including epidermal growth factor receptor, vascular endothelial growth factor/vascular endothelial growth factor receptors, mammalian target of rapamycin, phosphatidyl-inositol-3-kinase/Akt, insulin growth factor, Aurora kinase, Wnt/β-catenin, retinoic acid receptor and hepatocyte growth factor/C-Met. This review is aimed at addressing the results obtained so far with these newer drugs, also considering the challenges we shall face in the near future, including the issue of response evaluation and identification of predictive/prognostic biomarkers.
- Citation: Porta C, Paglino C. Medical treatment of unresectable hepatocellular carcinoma: Going beyond sorafenib. World J Hepatol 2010; 2(3): 103-113
- URL: https://www.wjgnet.com/1948-5182/full/v2/i3/103.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i3.103
The medical treatment of hepatocellular carcinoma (HCC) has remained a major ‘black hole’ in Oncology for many years. We have lacked systemic therapies that could impact the life expectancy of that 40% of patients who are not candidates for either a potentially curative treatment (surgical resection, liver transplant, or local ablation therapy) or palliation with chemoembolization, which does however have a positive impact on survival.
This discouraging scenario has suddenly changed thanks to the positive results obtained with Sorafenib. This molecularly targeted agent with both antiangiogenic and antiproliferative capabilities[1] was seen to increase the overall survival of these patients versus placebo within a randomized clinical trial[2].
The extent of this advantage in terms of survival, 31% improvement over placebo, was initially underestimated by some. It is, in fact, an extraordinary result, comparable to those obtained with Bevacizumab in carcinoma of the large intestine[3], and with Trastuzumab in breast carcinoma[4].
Such positive results have clearly encouraged research on other molecularly targeted drugs which are selectively directed against the molecular mechanisms specific to HCC. The aim is to further improve, if possible, the results achieved with Sorafenib and to increase the number of patients who can benefit from treatment.
Our increasingly accurate and refined understanding of the complex mechanisms underlying HCC development (carcinogenesis), local growth, angiogenesis mechanisms, and distant spread, therefore offer an opportunity to develop new therapies which will be even more effective.
When dealing with the molecular mechanisms responsible for HCC development and progression, we must consider the extremely heterogeneous nature of this type of tumor.
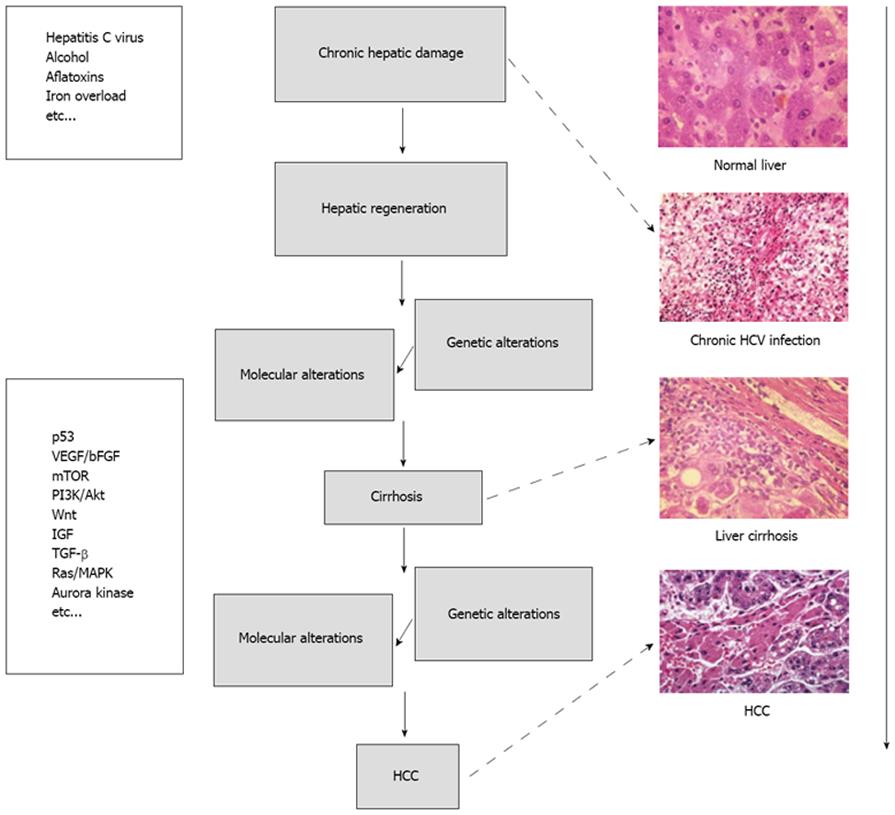
HCC can develop in a healthy liver, in a diseased but not cirrhotic liver or, most frequently, in a frankly cirrhotic liver. Degeneration into cancer can be triggered by various causes, from damage by toxic substances (alcohol, aflatoxins, iron accumulation, and so on) to viruses, as in the case of chronic infections from hepatitis (B or C). In very broad terms, liver carcinogenesis can be schematized as seen in Figure 1.
At the molecular level, the mechanisms responsible for the etiopathogenesis of HCC can be summarized into two major groups. First is the activation of specific pathways triggering cancer development and subsequent proliferation, such as those of the Epidermal Growth Factor Receptor (EGFR)/mitogen activated protein (MAP)-kinase, Wnt, Insulin-like Growth Factor (IGF), or mammalian target of rapamycin (mTOR) and the second group includes the activation of more generic mechanisms/pathways, shared by nearly all types of cancer, which are responsible for the activation of angiogenesis [e.g. Vascular Endothelial Growth Factor (VEGF), Platelet-Derived Growth Factor (PDGF) and relative receptors], insensitivity to apoptosis (Bcl-2, p53, PI3K/Akt), the inactivation of specific cell cycle checkpoints (e.g. p53, Rb, TP53, p21, cycline D1), or for preserving unlimited replicative potential[5,6].
Any of these changes can, at least potentially, be treated either with drugs that are already on the market, although mostly prescribed for other indications, or with molecules undergoing different phases of preclinical and/or clinical development (Table 1).
| Agent | Targets | Development stage | |
| Sorafenib (BAY 43-9006) | Small molecule | VEGFR-2 e -3, PDGFR-β, Raf | Registered |
| Sunitinib (SUO11248) | Small molecule | VEGFR-1, e 2 (-3), PDGFR-α e-β, Flt-3, C-Kit, RET | Phase II |
| Vatalanib (PTK787/ZK222584) | Small molecule | VEGFR-1, -2 e-3, PDGFR, C-Kit, c-Fms | Phase II |
| Cediranib (AZD2171) | Small molecule | VEGFR-1, -2 e-3, C-Kit | Phase II |
| Brivanib (BMS-582664) | Small molecule | VEGFR-2, FGFR-1 | Phase II |
| Bevacizumab | Monoclonal antibody | VEGF-A | Phase II |
| Gefinitib (ZD1839) | Small molecule | EGFR/ErbB1/Her1 | Phase II |
| Erlotinib (OSI774) | Small molecule | EGFR/ErbB1/Her1 | Phase II |
| Lapatinib (GW572016) | Small molecule | EGFR/ErbB1/Her1, ErbB2/Her2neu | Phase II |
| Cetuximab | Monoclonal antibody | EGFR/ErbB1/Her1 | Phase II |
| Everolimus (RAD001) | Small molecule | mTOR | Phase I/II |
| Bortezomib | Small molecule | Proteasome | Phase I/II |
| Belinostat (PXD101) | Small molecule | Histone-deacetylase (HDAC) | Phase II |
| AZD6244 | Small molecule | MEK | Phase II |
| PI-88 | Small molecule | Eparanase | Phase III |
| TAC-101 | Small molecule | RAR-α | Phase II |
As mentioned above, the EGFR pathway significantly contributes to the proliferation, resistance to apoptosis and invasive behavior of HCC cells[7].
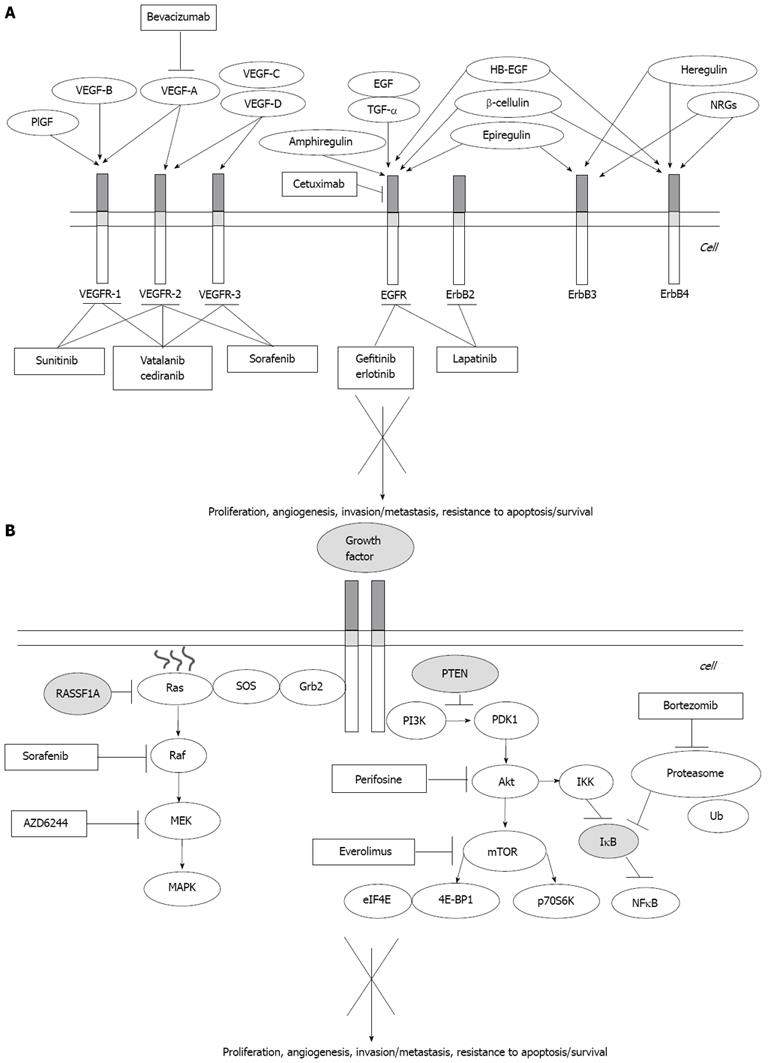
Three small molecules targeting the tyrosine-kinase receptor of the EGFR (Erlotinib, Gefinitib and Lapatinb) and a monoclonal antibody neutralizing the EGFR (Cetuximab) have undergone clinical trials for use in HCC (Table 2, Figure 2).
| Class | Agent | Development | Comments |
| Small molecules | |||
| Erlotinib | As single-agent | Cytostatic more than cytotoxic | |
| In combination with bevacizumab | Active (high ORR), but toxic | ||
| Gefitinib | As single-agent | Not active | |
| Lapatinib | As single-agent | Too early to draw conclusions | |
| Monoclonal antibodies | |||
| Cetuximab | As single-agent | Low ORR (but prolonged survival) | |
| In combination with chemotherapy | High DCR, but toxic |
Erlotinib has been shown to possess some anticancer activity against HCC in both preclinical models and clinical trials.
In a first trial[8], 38 patients with intermediate to advanced HCC according to the Barcelona Clinic Liver Cancer (BCLC) classification[9], 39% of whom already had extra-hepatic metastases, were treated with this EGFR inhibitor, administered per os at the dose of 150 mg/d. The objective response rate was low (9%), which is not very surprising given the cytostatic, rather than cytotoxic, activity of this drug. However, progression-free survival (PFS) at 6 mo was 32%, and median survival was 13 mo. Both these figures are noteworthy, even though they can be at least partly explained by the the fact that a large part (42% of cases) of the enrolled patients had no associated non-cancer liver condition.
In a second trial[10], the combination of Erlotinib and the monoclonal anti-VEGF antibody Bevacizumab, proved to be feasible, even though toxic, and active. The objective of this study was to determine the proportion of HCC patients treated with such a combination who were alive and progression-free at 16 wk (PFS16). The choice of this someway singular timepoint was based on the analysis of several previous trials of different chemotherapeutic agents, which have indeed demonstrated a median PFS of about 16 wk. This choice of timepoint has, not surprisingly, been criticized by many.
Of the 40 patients enrolled, 12 and 26 were from the B and C stages of the BCLC classification respectively, while just 11 had been previously treated with Transcatheter Arterial Chemoembolization(TACE). Further indications that such a patient population was not really representative of the vast majority of HCC patients we see everyday were that only 27 of them had a concomitant cirrhosis and that only 10 and 6 patients were positive for hepatitis C virus (HCV) and hepatitis B virus, respectively.
Median PFS16 was 62.5%, objective response rate was 25%, while overall survival was 68 wk. On the other hand, toxicity was a significant issue, with several grade 3 or 4 adverse events, including fatigue (20%), hypertension (15%), gastrointestinal bleeding episodes (12.5%), diarrhea (10%), increase of transaminases (10%), and infections/wound healing complications (5%).
Overall, even though this study has been criticized, probably with some justification, it clearly suggests that the combination of Erlotinib plus Bevacizumab deserves further evaluation on larger and less selected, (i.e. biased), case series.
Gefitinib appeared to prevent HCC development in experimental models. However, a single phase-II trial on 31 patients[11] failed to show any significant therapeutic benefit, with a median survival of 6.5 mo, a mean PFS of only 2.8 mo, no objective response, and a single instance of disease stabilization. Therefore, in contrast to its ‘twin’ Erlotinib, this EGFR inhibitor appears unsuitable for further clinical trials for HCC, although the reasons for this lack of efficacy are quite elusive.
Though overexpression/amplification of Her2/neu[12] and EGFR mutations[13] are quite uncommon events in HCC, Lapatinib, a double inhibitor of EGFR and Her2 is currently on trial for this type of cancer[6].
Cetuximab, a chimeric anti-EGFR antibody, was seen to exhibit antiproliferative and pro-apoptotic activity in preclinical models of HCC, but failed to provide any objective response in two trials[14,15]. Time to progression (TTP) was as low as 8 wk in one trial (32 patients)[14], although the authors of the second trial[15] reported a fairly good median survival of 9.6 mo (with a PFS of only 1.4 mo), which suggests the need to test this drug further and in larger series[6].
In another trial[16] Cetuximab was combined with Gemcitabine and Oxaliplatin chemotherapy (the GemOx regimen). This combination provided a 23% objective response (43 patients), 65% of disease control rate (DCR), and a decrease in α1-fetoprotein higher than 50% in half of the patients. On the other hand, the toxicity profile was not neglectable (60% of grade 3 or 4 toxicity), although still acceptable.
HCC is known to be a highly vascular tumor and angiogenesis plays a major role in its pathogenesis[17]. Consequently, angiogenesis and the growth factors that contribute to its regulation are the preferred target in this type of cancer, at least theoretically.
In addition to Sorafenib, which exhibits both anti-neoangiogenic and antiproliferative activity by inhibiting the MAP-kinase pathway[1], many other drugs have been studied in HCC. These include Bevacizumab, the anti-VEGF monoclonal antibody, and Sunitinib, Brivanib, Vatalanib and Cediranib, small molecules inhibiting different kinases (Figure 2).
No activity or even tolerability data on Brivanib, Vatalanib and Cediranib are yet available as the relevant clinical trials are still underway.
A first trial, updated yearly from 2005 to 2007 and then published in extenso in 2008[18], clearly showed that Bevacizumab is safe when administered at the dosage of 5 and 10 mg/kg to patients with localized but unresectable HCC who exhibit adequate residual liver function and have no esophageal varices at high risk of bleeding. As a whole, these results indicate a positive impact of this monoclonal antibody on the natural history of the disease, the DCR being 80%, and the median TTP exceeding 6 mo. However, one of the most relevant, and troublesome, findings of this trial is an 11% increase in the risk of bleeding, possibly fatal, of esophageal varices[18].
The activity and toxicity results of Bevacizumab have been subsequently confirmed by a small French phase-II study[19].
Another recent trial[20] demonstrated Bevacizumab to be active and tolerated also when administered by an intra-arterial route, at the dose of 5 mg/kg. Of 10 patients, 2 achieved a complete response lasting 4 mo, while 6 others had a partial response and the remaining 2 a 6-mo disease stabilization. Seven of 10 patients also exhibited a serological response, defined as a decrease in a1-fetoprotein values greater than 50%, relative to baseline. These encouraging results obviously need confirmation from lager series of patients.
We have already mentioned the promising combination with Erlotinib[10] but would point out that Bevacizumab has also been combined, mostly within small phase-II trials, with chemotherapy agents exhibiting some, albeit small, activity against HCC, namely Capecitabine and/or Oxaliplatin and/or Gemcitabine.
One trial investigated the combination of Capecitabine [825 mg/m2, per os, b.i.d., from d 1 to d 14], Oxaliplatin (130 mg/m2, IV, on d 1, every 21 d) and Bevacizumab (5 mg/kg, IV, on d 1, every 21 d)[21]. Of 30 patients receiving this regimen, 11% had a partial response and 78% achieved disease stabilization, adding up to an overall DCR of 89%. The mean PFS was 5.4 mo, with 70% and 40% PFS at 3 and 6 mo, respectively. As for tolerance, 33% of the patients had grade 2 or 3 Oxaliplatin-induced neuropathy and 11% had grade 2/3 Capecitabine-induced hand-foot syndrome. One patient experienced intestinal perforation after the first administration of Bevacizumab (and Oxaliplatin), and two others experienced bleeding from preexisting esophageal varices.
Another phase-II trial[22] carried out on 45 patients receiving 6 cycles of Capecitabine (800 mg/m2per os, BID, from day 1 to day 14, every 3 wk) and Bevacizumab (7.5 mg/kg, IV, every 3 wk) provided 16% objective responses, 60% DCR, median PFS of 4.1 mo and median survival of 10.7 mo. Toxicity was as expected and mild (grade 3 at the most), even though there was one case of acute bleeding from a gastric ulcer.
Another phase-II trial[23] investigated the combination of Gemcitabine (1000 mg/m2, IV infusion of 10 mg/m2/min, on days 2 and 16 of each 28-d cycle), Oxaliplatin (85 mg/m2, IV, on days 2 and 16) and Bevacizumab (10 mg/kg, IV, every 15 d) on 27 HCC patients. It may be considered somewhat surprising that this trial provided quite poor results, with only 2 minor responses (no objective responses), and 5 disease stabilizations. The clinical study was related to a trial investigating the treatment effect on tumor perfusion by means of dynamic contrast-enhanced magnetic resonance imaging, which demonstrated a transient and reversible decrease in tumor blood supply only after Bevacizumab administration.
In conclusion, despite the small numbers of cases available, which come from selected series and from very different studies, we believe that Bevacizumab does exhibit some anticancer activity in HCC and that this does not appear to be especially increased by its combination with chemotherapy. As a whole, the results obtained so far with Bevacizumab, alone or in combination, are summarized in Table 3.
| Development | Comments | |
| Bevacizumab | As single agent, i.v. route | Active (high DCR), but increased risk of bleeding from esophageal varices |
| As single agent, i.a. route | Promising early results | |
| In combination with other molecularly targeted agents, e.g. erlotininib | Active (high ORR), but toxic | |
| In combination with chemotherapy | Not particularly active and toxic |
On the other hand, Bevacizumab may cause severe, and even fatal, bleeding in these patients. Although expected, this problem obviously limits the use of this agent to patients without any esophageal varices at risk of bleeding and, realistically, also without thrombocytopenia.
To date, three phase-II trials have investigated the activity and tolerability of this agent, an inhibitor of several tyrosine kinases [Vascular Endothelial Growth Factor Receptor (VEGFR)-1 and -2, Platelet-Derived Growth Factor Receptor (PDGFR), C-Kit, RET, Flt3, and others], for HCC.
One trial[24] carried out on 37 patients receiving a full dose (50 mg/d, per os) and following the classic treatment schedule (4 wk on and 2 wk off) provided one single partial response and 13 disease stabilizations (39%), with signs of tumor necrosis and decreased tumor perfusion in a significant number of patients (46% of cases exhibited necrosis > 50%). However, side-effects were severe, with frequent grade 3-4 toxicities (thrombocytopenia in 43% of cases, neutropenia in 24%, neurological symptoms in 24%, asthenia in 22%, and bleeding in 14%), with as many as 5 toxic deaths. Moreover, 27% of patients needed a dosage decrease during treatment.
Given these tolerance problems with a full drug dose, another trial[25] scheduled 34 patients to receive 37.5 mg (again for 4 wk on plus 2 wk off). Similarly to what has been observed in renal cancer, Sunitinib at this dosage was seen to have mild anticancer activity (only 1 partial response and 8 disease stabilizations), but a fair tolerability profile, i.e. a decrease in anticancer activity upon a decrease in the drug Area Under the Curve (AUC)[26]. This trial also demonstrated that at least two circulating angiogenic markers, IL-6 and endothelial precursor cells, correlate with survival[25], providing the rational basis for future research.
Similar results in terms of activity and tolerability were obtained in another trial[27] carried out on 23 patients who also received the lower dosage, 37.5 mg for 4 in every 6 wk.
These results, especially those relating to tolerability (at least at the most active drug doses), make the actual practical use of such a powerful but toxic treatment questionable in such delicate patients as cirrhotics[6]. Nontheless, Sunitinib deserves to be further investigated in HCC[28].
As already mentioned, no clinical data are available on these three drugs. However, there is preclinical evidence that they may exert not only high antiangiogenic, but also antiproliferative or at any rate angiogenesis-independent, activity in HCC[29-31].
Brivanib alaninate, an inhibitor of both the VEGFR and the Fibroblast Growth Factor Receptor pathways[32], appears to be a particularly promising agent. It is the latter activity that makes this compound so interesting, at least theoretically, since the Fibroblast Growth Factor is known to play a major role in the etiopathogenesis of HCC[33].
About 50% of HCCs exhibit activation of the mTOR pathway, as demonstrated by immunohistochemical analysis of the phosphorylation of ribosomal protein S6. This is a direct consequence of the upstream activation of the pathways of the IGF, of the EGFR, or of the dysregulation of PTEN[34]. PTEN is a phosphatase exhibiting tumor suppressor activity[35], which can both inhibit cell proliferation[36] and increase cell sensitivity to apoptosis and anoiki. This latter is a very special type of apoptosis, typical of epithelial cells, which is triggered by changes in the relationship between some membrane integrins and the extracellular matrix[37].
mTOR appears to make a potentially very interesting target in HCC and we have acquired some preclinical evidence of HCC xenotransplant growth inhibition by the mTOR inhibitor Everolimus[38]. It is not, therefore, surprising that mTOR inhibitors are currently undergoing clinical trials in HCC.
The pathway of phosphatidylinositol-3-kinase (PI3K)/Akt is crucial for cell proliferation and, especially, survival in both normal and abnormal conditions. Physiologically, the PI3K/Akt pathway is an essential regulator of cell survival under stress; since tumors, by definition, develop in an environment characterized by severe cell stress from different causes, e.g. a low pH, decreased availability of oxygen and nutrients, this pathway appears to be key to the complex mechanisms of carcinogenesis[39].
Activation of the PI3K/Akt pathway ultimately leads to severe disturbance in the control of cell growth and survival, which results in the competitive proliferative advantage, metastatic competence and resistance to apoptosis that characterize cancer. PI3K/Akt makes therefore a very attractive target for cancer therapy, also in HCC[40,41]. Many compounds that can inhibit this pathway are currently under development[39]. Among them, Perifosine, an oral alkyl phospholipid[42], is considered the most promising agent, even though its use in HCC is not expected in the near future.
Correct cell progression through the different cell cycle phases is strictly regulated by the presence of checkpoints whose purpose is to safeguard genomic stability and ultimately prevent transformation into cancer cells[43].
The checkpoint regulating the formation of the mitotic spindle is particularly important because it is the first defense against the possible development of an aneuploid clone and is the controller of correct chromosome segregation. Among the many kinases that regulate this checkpoint, the family of serine/threonine kinases called Aurora is emerging as an extremely important controller of cell mitosis, which is essential to maintaining genomic stability. Aberrant expression of one, or more, of the three members of the Aurora family (Aurora A, B or C) has been observed in many solid and hematological cancers[43].
As for HCC, overexpression of Aurora kinase B, which specifically regulates chromosome segregation, cytokinesis, protein localization to centromere and kinetochore, as well as correct microtubule anchoring to the kinetochore itself[44], is correlated with the genetic instability of HCC and has been identified as an independent predictor of recurrence in this cancer[45,46].
We can thus speculate that some small molecules having inhibitory activity on Aurora kinase, particularly Aurora kinase B (VX-680, PHA-680632, AT9283, AZD1152 and others), which are mostly undergoing phase-I trials in different solid tumors[47], may eventually make good candidates for use in HCC.
The IGF family, which plays a major role in the regulation of many normal cell functions, has also been implicated in the genesis of many cancers[48].
In HCC, even though IGF-I can potentially improve cirrhosis[6], as suggested by some experimental trials, IGF-II appears to be overexpressed in about 30% of human HCCs, while IGF-binding proteins (IGF-BP-1, 3 and -4), which can act as oncosuppressors, are downregulated[49]. The oncosuppressor Insulin-like Growth Factor Receptor (IGFR)-II, which is mainly involved in IGF-II binding and degradation, is also downregulated in a subgroup of HCCs, as the direct result of mutations/deletions in the long arm of chromosome 6[50].
Many compounds targeting IGFR-II, both monoclonal antibodies and small molecules, are currently on trial in various solid tumors.
As for the Wnt/β-catenin pathway, its activation has been implicated in the etiopathogenesis of over one third of HCCs, especially those related to HCV[6], making this pathway an extremely attractive one from a therapeutic viewpoint. However, this pathway is currently considered the worst possible candidate for the development of drugs targeting it at any level and has thus been defined as “undruggable”[51].
TAC-101 4-[3, 5-bis (trimethyl-silyl) benzamido] benzoic acid is certainly one of the most interesting new compounds currently tested in HCC.
TAC-101 is a synthetic retinoid for oral administration that binds the receptor of retinoic acid and activates its transcriptional activity. This triggers many biological events, such as stimulation of cell differentiation (common to many retinoids, stimulation of apoptosis, inhibition of DNA-activator protein (DNA-AP-1) binding (with consequent inhibition of angiogenesis and of extracellular matrix degradation), inhibition of phosphorylation of the retinoblastoma gene product, and cell cycle arrest. The latter is correlated with modulation of the activity of cyclin-dependent kinase 2 inhibitors[52-57].
A first phase-I trial[58] on 29 patients defined the dose to be used in subsequent trials (24 mg/m2) and indicated specific drug toxicities, such as muscle pain, hypertriglyceridemia, and especially venous thromboembolism, observed in 7 of 21 patients unscreened for thrombophilic factors.
A subsequent Phase-I/II trial on 33 HCC patients[59] confirmed this toxicity profile and demonstrated mainly cytostatic drug activity in this cancer. Indeed, no objective responses were achieved during treatment although 57% of patients exhibited long disease stabilization, with an extremely interesting overall survival of 19.2 mo. Surprisingly, two patients exhibited a late response, appearing after drug discontinuation, which would seem to be a specific characteristic of TAC-101.
Unfortunately, an international randomized, phase II, study aimed at comparing TAC-101 versus placebo in HCC patients pre-treated with Sorafenib, has been recently closed to the enrollment due to the occurrence of an unexpectedly high incidence of thromboembolic events. It is therefore possible that these events, already observed also in earlier phases of development, could significantly slow the development of what is, nevertheless, a potentially highly interesting compound, at least in HCC.
C-Met, a tyrosine kinase receptor, is presently the only known receptor for the HGF, also known as scatter factor.
The binding of HGF with the high-affinity extracellular domain of its receptor C-Met, causes a multimerization of the receptor itself and results in the phosphorylation of multiple tyrosine residues, localized within the intracellular portion of C-Met and, ultimately leads to signal transduction to the nucleus. This pathway regulates several biological events which are highly involved in the processes of cancerogenesis. These include the appearance of a more invasive phenotype, the stimulation of mitogenic and motogenic activity, increased resistance to apoptosis and increased angiogenesis[60]. It is therefore easy to guess how such a pathway is frequently deregulated in a number of human tumors, including HCC[60].
ARQ-197 is an extremely interesting first-in-class compound, which selectively inhibits C-Met. It is presently under clinical evaluation, within a randomized, placebo-controlled, phase II study, in HCC patients pre-treated with Sorafenib.
The assessment of response is unquestionably one of the main problems emerging with the increasingly frequent use of the new molecularly targeted drugs. As seen, first in gastrointestinal stromal tumors (GIST) treated with Imatinib[61] and then in the phase-II trial of Sorafenib in HCC[62], the classic response criteria used in Oncology, from WHO to RECIST, which were originally developed to assess response to conventional chemotherapeutic drugs, are difficult to apply to molecularly targeted agents and have a high risk of underestimating drug activity.
In order to address this issue, which will become increasingly important in the near future, some authors have developed new and different guidelines for response assessment. For GIST, Choi[63,64] based assessment on changes in tumor density as demonstrated by computed tomography (CT) scan, and on those by the EORTC, determined by changes in glucide metabolism as demonstrated by positron emission tomography (PET) with fluorodeoxyglucose. No specific response criteria are yet available for fusion CT/PET techniques, while new PET tracers aimed at depicting specific molecular or metabolic pathways are under evaluation[65].
Since in clinical practice we still rely on inadequate morphologic techniques or not fully validated functional techniques, the need for the development of new response assessment criteria is real and this research field will certainly boom in the next few years.
Despite the current revolution represented by the addition of Sorafenib to our currently poor therapeutic armamentarium and the promise shown by experimental treatments, HCC remains an incurable disease unless it can be treated with (non)surgical radical ablation or transplantation. This lack of curative treatment options is accompanied by the growing issue of the cost of new molecularly targeted agents, which is especially important now that financial resources are limited. These factors underline the need to identify really reliable prognostic and predictive factors, another important line of research which is undergoing major progress.
As for Sorafenib, we now know that the amount of basal phosphorylation of ERK a protein downstream of Ras in the MAP-kinase pathway, is correlated with PFS in patients treated with this drug[62]. We need to identify and carefully validate other and more reliable biomarkers to be able to select the patients who could benefit, ornot, from these expensive treatments. This will allow us to allocate the scarce resources available in the most appropriate, and accurate, possible way.
Therapy aimed at specific, though sometimes multiple, molecular targets has rapidly grown in Oncology, to become the most innovative and promising approach to the treatment of many solid tumors. This approach also appears extremely promising in HCC thanks to the development of Sorafenib, the first medical treatment proven to impact on HCC survival[2].
Nevertheless, the results obtained so far must be improved. We will have to pursue this goal by better defining and characterizing the molecular mechanisms underlying carcinogenesis and by consequently developing increasingly specific, active and tolerated molecularly targeted agents. Studies must be designed that combine different agents of this type with one another and/or with conventional chemotherapy or locoregional ablation. New predictive and prognostic factors need to be identified, possibly directly related to the molecular mechanisms inhibited by the different drugs (biomarkers). We also need better means of understanding and describing the cytotoxic or cytostatic activity of the various agents.
Although are certainly on the verge of an exciting era there is much work ahead. Specialists from different fields, from molecular biology to biochemistry, hepatology, oncology, radiology, and nuclear medicine must join in a common effort to try to achieve these ambitious but indispensable goals.
| 1. | Keating GM, Santoro A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223-240. |
| 2. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. |
| 3. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. |
| 4. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. |
| 5. | Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72 Suppl 1:30-44. |
| 6. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. |
| 7. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. |
| 8. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657-6663. |
| 9. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. |
| 10. | Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843-850. |
| 11. | O’Dwyer PJ, Giantonio BJ, Levy DE, Kauh JS, Fitzgerald DB, Benson AB. Gefitinib in advanced unresectable hepatocellular carcinoma: Results from the Eastern Cooperative Oncology Group’s Study E1203. J Clin Oncol. 2006;24:A4143. |
| 12. | Vlasoff DM, Baschinsky DY, De Young BR, Morrison CD, Nuovo GJ, Frankel WL. C-erb B2 (Her2/neu) is neither overexpressed nor amplified in hepatic neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:237-241. |
| 13. | Wong CI, Yap HL, Lim SG, Guo JY, Goh BC, Lee SC. Lack of somatic ErbB2 tyrosine kinase domain mutations in hepatocellular carcinoma. Hepatol Res. 2008;38:838-841. |
| 14. | Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, Enzinger PC, Bhargava P, Meyerhardt JA, Horgan K. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581-589. |
| 15. | Louafi S, Hebbar M, Rosmorduc O, Tesmoingt C, Asnacios A, Romano O, Fartoux L, Artru P, Poynard T, Taieb J. Gemcitabine, oxaliplatin (GEMOX) and cetuximab for treatment of hepatocellular carcinoma (HCC): Results of the phase II study ERGO. J Clin Oncol. 2007;25:A4594. |
| 16. | Pang RW, Joh JW, Johnson PJ, Monden M, Pawlik TM, Poon RT. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962-971. |
| 17. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. |
| 18. | Malka D, Dromain C, Farace F, Horn S, Pignon J, Ducreux M, Boige V. Bevacizumab in patients (pts) with advanced hepatocellular carcinoma (HCC): Preliminary results of a phase II study with circulating endothelial cell (CEC) monitoring. J Clin Oncol. 2007;25:A4570. |
| 19. | El-Shami K. Pilot study of intra-arterial bevacizumab for hepatocellular carcinoma (HCC). J Clin Oncol. 2008;26:A15681. |
| 20. | Sun W, Haller DG, Mykulowycz K, Rosen M, Soulen M, Capparo M, Faust T, Giantonia B, Olthoff K. Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): A phase II study. J Clin Oncol. 2007;25:A4574. |
| 21. | Hsu C, Yang T, Hsu C, Toh H, Epstein RJ, Hsiao L, Cheng A. Modified-dose capecitabine + bevacizumab for the treatment of advanced/metastatic hepatocellular carcinoma (HCC): A phase II, single-arm study. J Clin Oncol. 2007;25:A15190. |
| 22. | Zhu AX, Sahani D, Norden-Zfoni A, Holalkere NS, Blaszkowsky L, Ryan DP, Clark JW, Taylor K, Heymach JV, Stuart K. A Phase II Study of Gemcitabine, Oxaliplatin in Combination with Bevacizumab (GEMOX-B) in Patients with Hepatocellular Carcinoma. J Clin Oncol. 2005;23:A4120. |
| 23. | Faivre SJ, Raymond E, Douillard J, Boucher E, Lim HY, Kim JS, Lanzalone S, Lechuga MJ, Sherman L, Cheng A. Assessment of safety and drug-induced tumor necrosis with sunitinib in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2007;25:A3546. |
| 24. | Zhu AX, Sahani DV, di Tomaso E, Duda DG, Catalano OA, Ancukiewicz M, Blaszkowsky LS, Abrams TA, Ryan DP, Jain PK. Sunitinib monotherapy in patients with advanced hepatocellular carcinoma (HCC): Insights from a multidisciplinary phase II study. J Clin Oncol. 2008;26:A4521. |
| 25. | Houk BE, Bello CL, Michaelson MD, Bukowski RM, Redman BG, Hudes GR, Wilding G, Motzer RJ. Exposure-response of sunitinib in metastatic renal cell carcinoma (mRCC): A population pharmacokinetic/pharmacodynamic (PKPD) approach. J Clin Oncol. 2007;25:A5027. |
| 26. | Hoda D, Catherine C, Strosberg J, Valone T, Jump H, Campos T, Halina G, Wood G, Hoffe S, Garrett CR. Phase II study of sunitinib malate in adult pts (pts) with metastatic or surgically unresectable hepatocellular carcinoma (HCC). Gastrointestinal Cancers Symposium. 2008;A267. |
| 27. | Zhu AX, Raymond E. Early development of sunitinib in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2009;9:143-150. |
| 28. | Liu Y, Poon RT, Li Q, Kok TW, Lau C, Fan ST. Both antiangiogenesis- and angiogenesis-independent effects are responsible for hepatocellular carcinoma growth arrest by tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2005;65:3691-3699. |
| 29. | Yang ZF, Poon RT, Liu Y, Lau CK, Ho DW, Tam KH, Lam CT, Fan ST. High doses of tyrosine kinase inhibitor PTK787 enhance the efficacy of ischemic hypoxia for the treatment of hepatocellular carcinoma: dual effects on cancer cell and angiogenesis. Mol Cancer Ther. 2006;5:2261-2270. |
| 30. | Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer. 2008;112:250-259. |
| 31. | Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146-6153. |
| 32. | Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004;130:307-319. |
| 33. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-1983. |
| 34. | Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29-41. |
| 35. | Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA, Eng C. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 1999;59:5808-5814. |
| 36. | Lu Y, Lin YZ, LaPushin R, Cuevas B, Fang X, Yu SX, Davies MA, Khan H, Furui T, Mao M. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034-7045. |
| 37. | Huynh H, Chow KH, Soo KC, Toh HC, Choo SP, Foo KF, Poon D, Ngo VC, Tran E. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009;13:1371-1380. |
| 38. | Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol. 2009;182:2569-2577. |
| 39. | He X, Zhu Z, Johnson C, Stoops J, Eaker AE, Bowen W, DeFrances MC. PIK3IP1, a negative regulator of PI3K, suppresses the development of hepatocellular carcinoma. Cancer Res. 2008;68:5591-5598. |
| 40. | Li W, Tan D, Zhang Z, Liang JJ, Brown RE. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep. 2008;20:713-719. |
| 41. | van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14:2061-2074. |
| 42. | Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60-65. |
| 43. | Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798-812. |
| 44. | Tanaka S, Noguchi N, Ochiai T, Kudo A, Nakamura N, Ito K, Kawamura T, Teramoto K, Arii S. Outcomes and recurrence of initially resectable hepatocellular carcinoma meeting Milan criteria: Rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204:1-6. |
| 45. | Tanaka S, Arii S, Yasen M, Mogushi K, Su NT, Zhao C, Imoto I, Eishi Y, Inazawa J, Miki Y. Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. Br J Surg. 2008;95:611-619. |
| 46. | Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119:3664-3675. |
| 47. | Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505-518. |
| 48. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. |
| 49. | Tovar V, Alsinet C, Sole M. Role of insulin-growth factor signaling pathway in hepatocellular carcinoma. Molecular targeted therapies blocking IGF pathway in vitro and in vivo. J Hepatol. 2010;in press. |
| 50. | Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691-701. |
| 51. | Fujimoto K, Hosotani R, Doi R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Imamura M. Induction of cell-cycle arrest and apoptosis by a novel retinobenzoic-acid derivative, TAC-101, in human pancreatic-cancer cells. Int J Cancer. 1999;81:637-644. |
| 52. | Murakami K, Sakukawa R, Sano M, Hashimoto A, Shibata J, Yamada Y, Saiki I. Inhibition of angiogenesis and intrahepatic growth of colon cancer by TAC-101. Clin Cancer Res. 1999;5:2304-2310. |
| 53. | Shibata J, Murakami K, Aoyagi Y, Oie S, Hashimoto A, Suzuki K, Sano M, Wierzba TT, Yamada Y. The induction of apoptosis and inhibition of AP-1 activity by TAC-101 (4-[3,5-bis(trimethylsilyl) benzamido] benzoic acid) may result in life prolonging effect in animals bearing metastasizing cancer. Anticancer Res. 2000;20:3583-3590. |
| 54. | Minagawa N, Nakayama Y, Inoue Y, Onitsuka K, Katsuki T, Tsurudome Y, Shibao K, Hirata K, Sako T, Nagata N. 4-[3,5-Bis(trimethylsilyl)benzamido] benzoic acid inhibits angiogenesis in colon cancer through reduced expression of vascular endothelial growth factor. Oncol Res. 2004;14:407-414. |
| 55. | Sako T, Nakayama Y, Minagawa N, Inoue Y, Onitsuka K, Katsuki T, Tsurudome Y, Shibao K, Hirata K, Nagata N. 4-[3,5-Bis(trimethylsilyl)benzamido] benzoic acid (TAC-101) induces apoptosis in colon cancer partially through the induction of Fas expression. In Vivo. 2005;19:125-132. |
| 56. | Inoue Y, Nakayama Y, Sako T, Minagawa N, Abe Y, Nagato M, Kadowaki K, Katsuki T, Matsumoto K, Tsurudome Y. 4-[3,5-bis(trimethylsilyl)benzamido] benzoic acid (TAC-101) induced fas expression and activated caspase-3 and -8 in a DLD-1 colon cancer cell line. In Vivo. 2007;21:381-387. |
| 57. | Rizvi NA, Marshall JL, Ness E, Hawkins MJ, Kessler C, Jacobs H, Brenckman WD Jr, Lee JS, Petros W, Hong WK. Initial clinical trial of oral TAC-101, a novel retinoic acid receptor-alpha selective retinoid, in patients with advanced cancer. J Clin Oncol. 2002;20:3522-3532. |
| 58. | Higginbotham KB, Lozano R, Brown T, Patt YZ, Arima T, Abbruzzese JL, Thomas MB. A phase I/II trial of TAC-101, an oral synthetic retinoid, in patients with advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1325-1335. |
| 59. | Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3:219-234. |
| 60. | Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760-1764. |
| 61. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. |
| 62. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. |
| 63. | Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist. 2008;13 Suppl 2:4-7. |
| 64. | Pantaleo MA, Nannini M, Lopci E, Castellucci P, Maleddu A, Lodi F, Nanni C, Allegri V, Astorino M, Brandi G. Molecular imaging and targeted therapies in oncology: new concepts in treatment response assessment. A collection of cases. Int J Oncol. 2008;33:443-452. |
| 65. | Tanaka S, Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1-8. |
Peer reviewer: Eileen M O'Reilly, Associate Member, MSKCC, Associate Professor, Cornell University Medical Center, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 324, New York, NY 10065, United States