Published online Dec 27, 2010. doi: 10.4254/wjh.v2.i12.419
Revised: October 28, 2010
Accepted: November 4, 2010
Published online: December 27, 2010
Intrahepatic cholangiocarcinoma (ICC) arises from the lining epithelium and peribiliary glands of the intrahepatic biliary tree and shows variable cholangiocytic differentiation. To date, ICC was largely classified into adenocarcinoma and rare variants. Herein, we propose to subclassify the former, based on recent progress in the study of ICC including the gross classification and hepatic progenitor/stem cells and on the pathological similarities between biliary and pancreatic neoplasms. That is, ICC is classifiable into the conventional (bile duct) type, the bile ductular type, the intraductal neoplasm type and rare variants. The conventional type is further divided into the small duct type (peripheral type) and large bile duct type (perihilar type). The former is a tubular or micropapillary adenocarcinoma while the latter involves the intrahepatic large bile duct. Bile ductular type resembles proliferated bile ductules and shows a replacing growth of the hepatic parenchyma. Hepatic progenitor cell or stem cell phenotypes such as neural cell adhesion molecule expression are frequently expressed in the bile ductular type. Intraductal type includes papillary and tubular neoplasms of the bile duct (IPNBs and ITNBs) and a superficial spreading type. IPNB and ITNB show a spectrum from a preneoplastic borderline lesion to carcinoma and may have pancreatic counterparts. At invasive sites, IPNB is associated with the conventional bile duct ICC and mucinous carcinoma. Biliary mucinous cystic neoplasm with ovarian-like stroma in its wall is different from IPNB, particularly IPNB showing cystic dilatation of the affected ducts. Rare variants of ICC include squamous/adenosquamous cell carcinoma, mucinous/signet ring cell carcinoma, clear cell type, undifferentiated type, neuroendocrine carcinoma and so on. This classification of ICC may open up a new field of research of ICC and contribute to the clinical approach to ICC.
- Citation: Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010; 2(12): 419-427
- URL: https://www.wjgnet.com/1948-5182/full/v2/i12/419.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i12.419
Intrahepatic cholangiocarcinoma (ICC), a primary malignant neoplasm of the liver secondary to hepatocellular carcinoma, arises from intrahepatic biliary epithelia (lining epithelia and peribiliary glands) and shows a variable cholangiocytic differentiation[1,2]. Recently, the incidence of ICC has been increasing worldwide[3,4]. ICC is heterogenous in clinical features, genotypes and biological behaviors depending on anatomical location and histological differentiation. Generally, ICCs are detected and diagnosed clinically at an inoperative stage and a majority of them show a poor prognosis, even after surgical resection[3,4]. However, some patients show a rather favorable post-operative course[5,6].
ICC is usually classified into peripheral and hilar types grossly and histologically into adenocarcinoma and rare variants[7-10]. The former was simply graded into well, moderately and poorly differentiated adenocarcinomas. Recently, a new gross classification of ICC has been proposed by the Japanese Study Group of Liver Cancer[11]. While ICCs are usually adenocarcinomas, they are heterogeneous in their gross and histological features. Recently, much progress has been made in the study of precancerous and early malignant lesions of ICC in addition to molecular and genetic characteristics[12,13]. Furthermore, ICC with hepatic progenitor cell phenotypes has been proposed[2]. Interestingly, pathological similarities of ICC to pancreatic carcinoma have been proposed and biliary and pancreatic neoplasms are now being studied under the same concept and terminology[14,15].
In this review, we propose a new pathological classification of ICC based on recent progress in the study of ICC and on the pathological similarities between biliary and pancreatic neoplasms. First, the anatomy of the intrahepatic biliary tree will be briefly described.
The biliary tree is dividable into extrahepatic and intrahepatic bile ducts. The gallbladder drains into the extrahepatic bile duct via the cystic duct. The right and left hepatic ducts and their first to third branches are collectively called “hilar and perihilar bile ducts”. The intrahepatic bile ducts, proximal to the right or left hepatic duct, are classified as intrahepatic large and small bile ducts[16]. The former are visible grossly and consist of the first to third branches of right or left hepatic bile ducts. Peribiliary glands are physiologically distributed around the large bile ducts and drain into the duct lumen via their own conduits. The latter are recognizable microscopically and consist of septal and interlobular bile ducts. The interlobular bile ducts are connected to bile ductules. The septal bile duct is surrounded by a fibrous wall and is over 100 μm in size while the external diameter of the interlobular bile duct is less than 100 μm. These two bile ducts are accompanied by hepatic arterial branches of similar size while bile ductules or canals of Hering are located at the periphery of portal tracts and facing hepatocytes[17].
While ICCs usually develop in an apparently normal liver, some are associated with preceding biliary or hepatic diseases and various etiologies (Table 1). ICC may show characteristic features according to the background biliary or hepatic lesions[3,4,18-20]. Chronic inflammation of the bile ducts with sustained stress on biliary epithelial cells is reportedly at least partly responsible for the cholangiocarcinogenesis in which ICC tends to proliferate and spread along the affected intrahepatic bile ducts[3,13,21]. Primary sclerosing cholangitis (PSC) with or without inflammatory bowel disease, usually ulcerative colitis, is a common risk factor for ICC in Western countries. Clinically undetected ICCs or precursor lesions are occasionally encountered in explant livers of these biliary diseases at liver transplantation. Hepatolithiasis, not rare in the Far East, is the primary independent risk factor for ICC and about 7% of patients with hepatolithiasis eventually develop ICC[6]. Stones of most of these cases belong to calcium bilirubinate stones although cholesterol stones also occur. The hepatic lobe or segments containing stones affected by ICC are atrophic in some cases. The stone-containing bile ducts show hyperplasia of the lining epithelium and, not infrequently, premalignant lesions. Liver flukes, especially O. viverrini and C. sinensis, are risk factors for ICC[13,22]. The presence of parasites in the biliary tree leads to a chronic inflammatory response and cellular proliferation of the bile duct epithelium (adenomatous hyperplasia) with an increased risk of ICC[22,23]. As for biliary malformations and other lesions, ICC may arise with congenital segmental or multiple dilatation of the intrahepatic bile ducts (Caroli’s disease) and other biliary malformations such as choledochal cyst, solitary unilocular or multiple liver cysts and congenital hepatic fibrosis[1,22,24]. Non-biliary cirrhosis, particularly hepatitis virus-related cirrhosis, is recognized as part of the background of ICC. Hepatitis C virus (HCV) infections may also play a role in the development of ICC. In Japan, patients with cirrhosis due to HCV have about a 1000-fold higher risk of developing ICC than the general population[18]. The development of ICC seems also to be related to hepatitis B virus (HBV) infections in areas where both HBV and ICC are endemic. Such ICCs are usually of a smaller, mass-forming type when clinically detectable. Hepatic progenitor cells (HPCs) or stem cells may be involved in cholangicarcinogenesis in liver cirrhosis.
| Chronic inflammatory biliary diseases |
| Primary sclerosing cholangitis/Ulcerative colitis |
| Hepatolithiasis |
| Liver flukes and other biliary parasite infections |
| Others |
| Biliary malformation and developmental disorders |
| Caroli’s disease |
| Congenital hepatic fibrosis |
| Biliary-pancreatic maljunction |
| Simple, solitary or multiple, hepatic cyst |
| Polycystic liver |
| Others |
| Chronic advanced, non-biliary, liver diseases |
| Chronic hepatitis/cirrhosis related to HCV and HBV infection |
| Non-alcoholic fatty liver disease |
| Others |
| Thorotrast deposition |
| EB virus infection |
| Others |
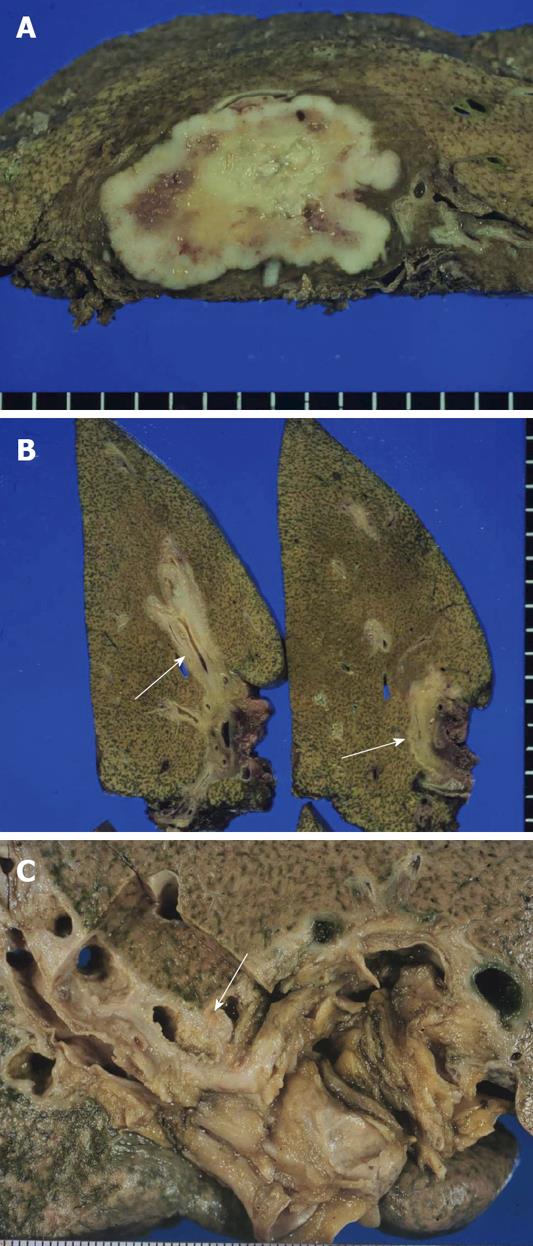
ICC is grossly classifiable into mass-forming (MF), periductal infiltrating (PI) and intraductal growth (IG) types[1,14]. The MF type presents as a nodular lesion or mass in the hepatic parenchyma and the carcinoma is gray to gray-white, firm and solid (Figure 1A). The PI type shows spreading of the carcinoma along the portal tracts with stricture of the affected bile ducts and dilatation of the peripheral bile ducts (Figure 1B). The IG type presents as a polypoid or papillary tumor within the variably dilated bile duct lumen (Figure 1C) and represents the malignant progression of an intraductal papillary neoplasm of the bile duct (IPNB) (see below). ICC arising in the intrahepatic small bile ducts or bile ductules is usually of the MF type while ICC arising in the intrahepatic large bile ducts (perihilar ICC) can be of the PI, MF or IG type. ICC cases involving the hepatic hilum show cholestasis, biliary fibrosis and cholangitis of the intrahepatic bile ducts. MF type ICCs can be quite large. Central necrosis or scarring is common and mucin may be visible on cut surfaces. These three gross types can overlap in a variable combination. At more advanced stages, ICCs consist of variably sized nodules, usually coalescent.
ICCs are pathologically classifiable into conventional ICCs (bile duct ICCs), bile ductular ICCs, intraductal neoplasms and rare variants (Table 2).
| New classification of ICC | Traditional classification of ICC |
| Conventional type (bile duct type type) | Adenocarcinoma |
| Small bile duct type (peripheral type) | Well differentiated |
| Well differentiated | Moderately differentiated |
| Moderately differentiated | Poorly differentiated |
| Poorly differentiated | |
| Large bile duct type (perihilar type) | |
| Well differentiated | |
| Moderately differentiated | |
| Poorly differentiated | |
| Bile ductular type | |
| Intraductal type | |
| Papillary type | |
| Tubular type | |
| Superficial spreading type | |
| Rare variants | Rare variants |
| Squamous/adenosquamous cell type | Squamous/adenosquamous cell type |
| Mucinous/signet ring cell | Mucinous/signet ring cell |
| Clear cell type | Clear cell type |
| Undifferentiated type | Undifferentiated type |
| Lymphoepithelial type | Lymphoepithelial type |
| Others | Others |
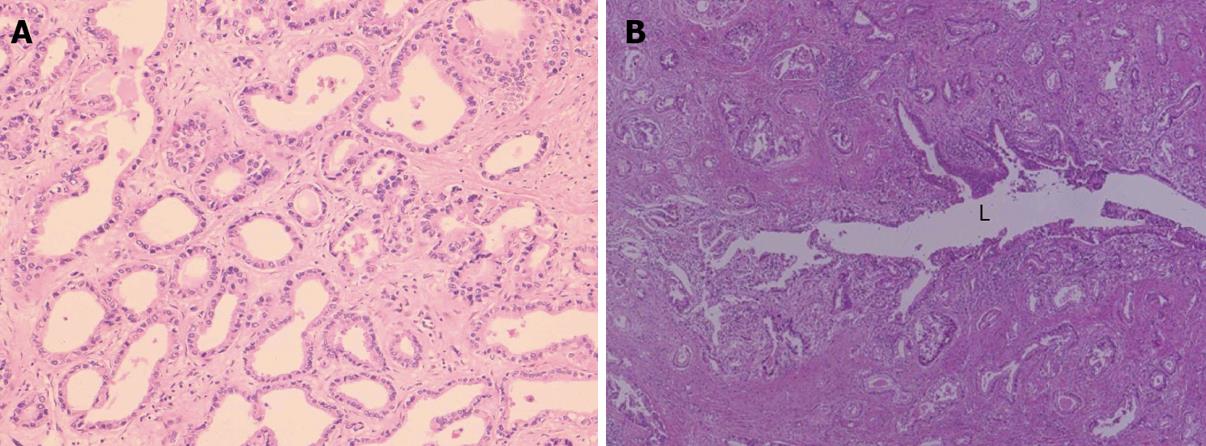
This type is an invasive adenocarcinoma with features of variable sized tubular structures, acini formation and papillary configurations (Figure 2A, B). It is generally a well to moderately differentiated adenocarcinoma composed of columnar to cuboidal epithelial cells with clear or slightly granular, eosinophilic cytoplasm, resembling cholangiocytes (biliary epithelial cells). In addition, poorly differentiated adenocarcinoma is admixed, infrequently and shows solid, cord-like or cribriform growth with variable cellular and nuclear pleomorphism. Coagulative necrosis is not infrequent in the central parts. Mucin production is found in secretions of the lumen, along the luminal sides and in the cytoplasm of carcinoma cells. A prominent desmoplastic reaction is usually found and inflammatory reactions are variable. The adenocarcinoma frequently shows portal venous and lymphatic infiltration. Against the hepatic parenchyma, carcinoma may show a compressive growth although no evident fibrous capsule is formed. There are also frequent bud-like growths of the carcinoma into the surrounding liver and there is direct contact or very local admixture between hepatocytes and carcinoma cells at the interfaces. The intervening hepatic parenchyma containing the portal tracts is forcibly incorporated secondarily between or into the carcinoma tissues. The carcinoma also shows invasion between hepatocytes, appearing to infiltrate the sinusoid. Replacing infiltration of carcinoma is also found variably (see below). Grossly, this ICC is of a MF or MF + PI type or shows multinodular growth in advanced cases.
This ICC is largely dividable into two types according to the level or size of the affected bile ducts: small bile duct and large bile duct types.
Small bile duct type (peripheral type): Grossly, this ICC is of the MF type. Histologically, a variable sized tubular or acinar adenocarcinoma with variable desmoplastic and inflammatory reactions shows a nodular growth and invades the parenchyma with a replacing or compressive pattern (Figure 2A). A small solid cord-like or cribriform pattern is also found in variable combinations. The carcinomatous acini or tubules are usually larger than those of non-neoplastic small bile ducts such as interlobular bile ducts and septal bile ducts.
Large bile duct (perihilar type): Grossly, this ICC belongs to the PI type and PI with MF type. Histologically, the cancerous large bile duct shows luminal spread of carcinoma with papillary, micropapillary and flat configurations along the affected lumen and also variable invasion of carcinoma cells with a tubular, acinar or micropapillary configuration into the duct wall and surrounding parenchyma (Figure 2B). Peribiliary glands and their conduits are also frequently involved. The luminal surface of the cancerous bile duct is not infrequently ulcerated. In the invasive area, particularly in the parenchyma, their histologies are variable and some resemble small bile duct ICCs. This type of ICC might have arisen from the intrahepatic large bile duct (perihilar intrahepatic bile ducts) including the peribiliary glands although some cases might have arisen from the small bile duct ICC with the secondary involvement of intrahepatic large bile ducts (cancerization).
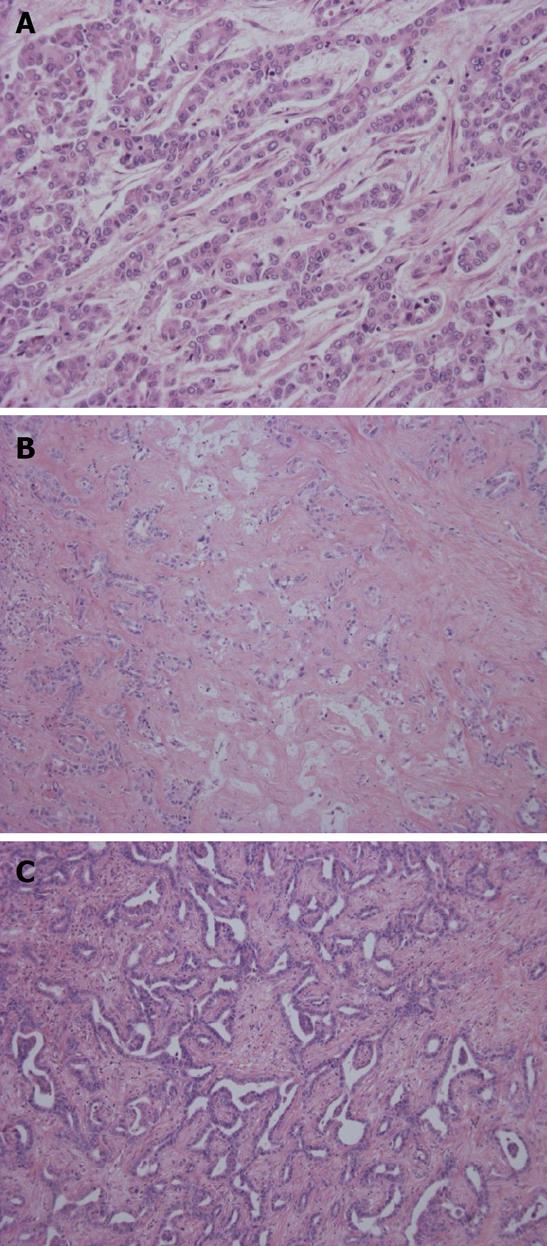
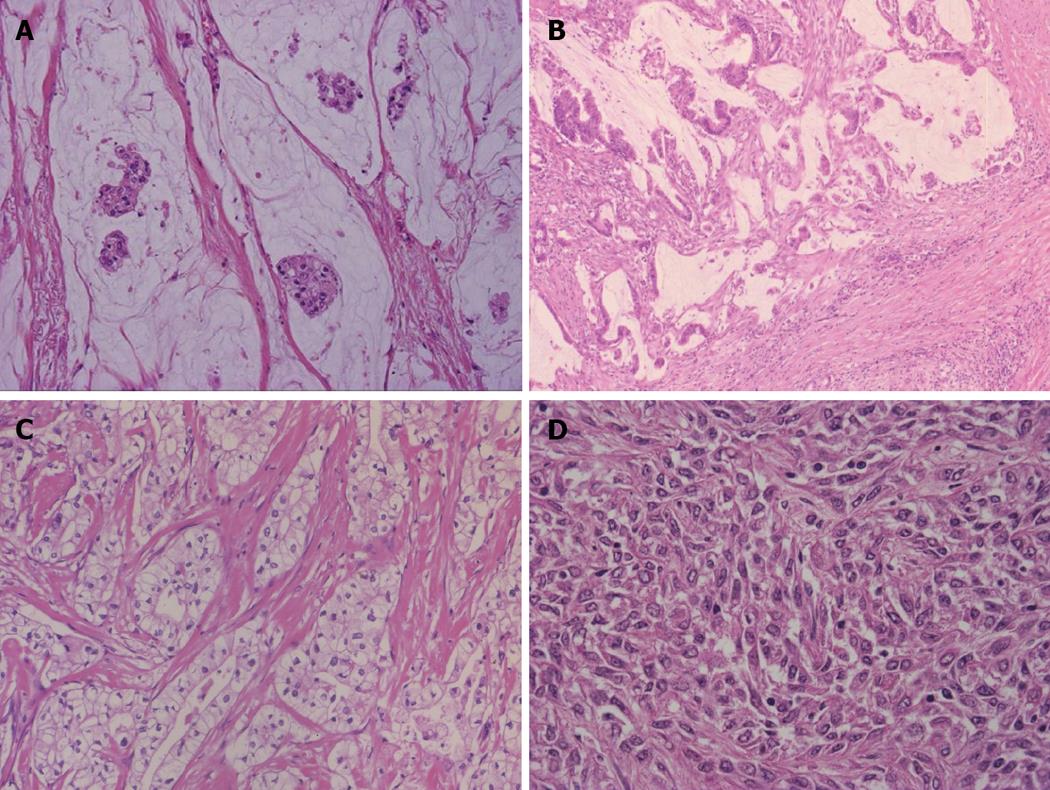
Grossly, this carcinoma belongs to the MF type. The adenocarcinoma cells show well-differentiated, small tubular or acinar patterns or cord-like structures with a slit-like lumen and arborization[2,25]. They resemble bile ductules or proliferating reactive bile ductules (Figure 3A, B). A small-cord like pattern with spindle cell features is occasionally predominant. The size of carcinoma cells is usually small in comparison to conventional ICC. Deposition of collagen fiber around or along the carcinoma cells is significant and carcinoma cells are squeezed here and there. Ghost-like features are found, a fibrous or hyalinous configuration reflecting pre-existing hepatic lobules or regenerative nodules is recognizable and fibrotic portal tracts are distributed regularly within the tumor. Portal vein tumor emboli are absent or else rather focal. This type is characterized by widespread replacing growth which is characterized by (1) direct contact between hepatocytes and carcinoma cells; (2) no or minimal compression of the surrounding hepatic parenchyma by the carcinoma cells; and (3) the apparent replacement of hepatocytes by carcinoma cells[2,25].
Neural cell adhesion molecule (NCAM), a marker of HPCs[2], is characteristically detected mainly on the cell membranes and to a lesser degree in the cytoplasm[25]. Normal and mature bile ducts of various sizes in non-tumorous liver are negative for NCAM while reactive proliferated bile ductules in diseased livers are positive for NCAM.
Ductal plate malformation variant: This type mimics ductal plate malformation (DPM) which is found in Caroli’s disease or congenital hepatic fibrosis. That is, the carcinoma shows an irregular configuration and lumen lined by one columnar or cuboidal layer of carcinoma cells (Figure 3C). A central dot or bridge is found microscopically. A fibrous stroma is sometimes found. They look benign although they show infiltrative growth and occasionally venous invasion. These features are not infrequently admixed with the bile ductular type while some cases are exclusively composed of such DPM features.
This type is usually seen in the intrahepatic large bile ducts and similar lesions are seen in the hilar and extrahepatic bile ducts.
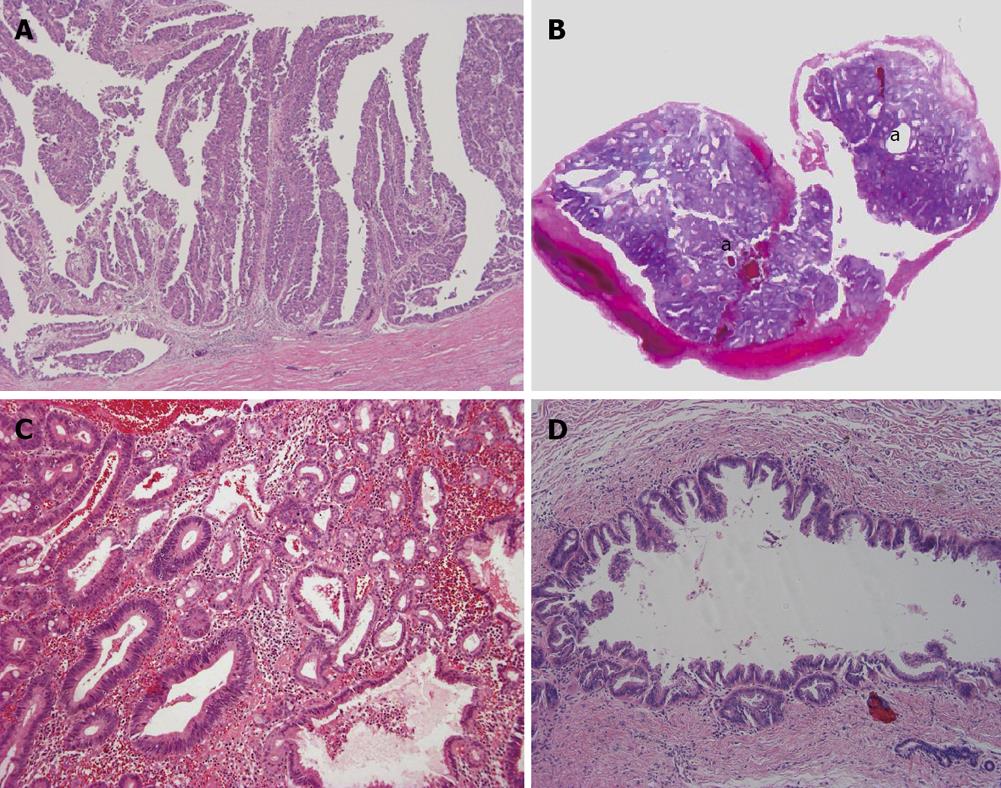
Intraductal papillary neoplasm of bile duct: This tumor shows a spectrum from preneoplastic lesion to non-invasive and invasive carcinoma, and intraductal papillary neoplasm of bile duct (IPNB) of carcinoma corresponds to the IG type of ICC grossly. In the dilated bile duct it shows papillary growth which is grossly visible[5,9]. Histologically, it is a well-differentiated papillary adenocarcinoma which is invasive or non-invasive while some IPNB can be recognized as a preneoplastic borderline lesion (Figure 4A). Intraductal, intraepithelial spread involving the large bile ducts and even small bile ducts is found variably and constantly. IPNB shows four phenotypes: pancreatobiliary type, oncocytic type, gastric type and intestinal type[14,26]. Clinicopathologically, IPNB is classified into a papillomatosis or papilloma type, intraductal growing type, mucin-producing type and cystic type[14,26]. The bile duct affected by IPNB shows variable dilatation, not infrequently cystic dilatation. Such cystic type should be differentiated from a hepatobiliary mucinous cystic neoplasm (MCN) in which an ovarian-like stroma is detectable in the wall of the cystic tumor[14,26] and the mucin-producing type shows massive mucin secretion. At the invasion site of IPNB, mucinous carcinoma and more frequently conventional tubular adenocarcinoma are found[5,6].
Intraductal tubular neoplasm of bile duct: This type is occasionally encountered in the intrahepatic large bile duct[27]. The neoplasm is mainly composed of a tubular component and focally papillary. Mucin secretion is usually absent and appears as a cast in the slightly dilated bile duct (Figure 4B, C). Intraductal tubular neoplasm of bile duct (ITNB) also shows a spectrum from preneoplastic lesion to carcinoma, even within the same tumor.
Superficial spreading type: This is a rare type of ICC showing extensive spread along the luminal surface of the intrahepatic bile duct. While the affected bile ducts show a variable luminal dilatation, the grossly visible nodular or tumor lesions are usually not identifiable or conspicuous in the lumen of the affected biliary tree (Figure 4D). Rarely, they show invasion into the surrounding tissue.
The following variants are only occasionally encountered.
Squamous and adenosquamous cell carcinoma: Both squamous and adenocarcinoma components are mixed, isolated or adjoining in adenosquamous cell carcinoma. Squamous carcinoma is usually well-differentiated and keratinizing. Chronic cholangitis such as hepatolithiasis or liver fluke infection is a common background to these variants and this variant is also reported to occur in polycystic liver.
Mucinous carcinoma/signet ring cell carcinoma: In mucinous carcinoma, carcinoma cells are floating within a mucinous lake. This type is usually found at the invasive parts of IPNB (Figure 5A, B). Signet ring cell carcinoma is occasionally encountered in mucinous carcinoma or conventional ICC but pure signet ring cell carcinoma is extremely rare.
Clear cell carcinoma: This type is occasionally experienced. Tubules or acini composed of columnar epithelial cells with abundant clear cytoplasm and eccentric small nuclei are predominant (Figure 5C). Other histologies of adenocarcinoma such as micropapillary or tubular configuration are focally encountered in this type.
Undifferentiated carcinoma: This carcinoma is heterogeneous in its histology and has several subcategories. Coagulative necrosis is frequently seen. Lymphatic or portal venous emboli are frequent. (1) Sarcomatous type: Spindle cell sarcoma type is common and spindle shaped sarcomatous cells grows medullary (Figure 5D) and these cells express epithelial phenotypes variably. Adenocarcinoma or cord-like growth is usually admixed focally. When a more mature sarcomatous component such as osteosarcoma or angiosarcoma is found in addition to the adenocarcinoma, carcinosarcoma of the liver or biliary tract is the preferred term; (2) Anaplastic type: Pleomorphic carcinomas with loose cell adhesion are one example and anisocytotic carcinoma cells grow with cord or nest-like patterns. Cohesive carcinoma cell nests without glandular differentiation occur here and there. Giant cells are mingled with the carcinoma. Growth may be medullary and no fibrous stroma is found.
Lymphoepithelioma-like carcinoma: Carcinomas with features of undifferentiated tumors and intense lymphoid stroma are classified as lymphoepithelioma-like carcinomas (LELCs). This type is reported in the liver. Like nasopharyngeal carcinoma, most LELC are strongly linked to Epstein-Barr virus (EBV)[28]. The role of EBV implicated in the cholangiocarcinogenesis is not fully delineated but a strong lymphoplasmacytic response to these neoplasms characterizes most of these lesions. The prognosis of LELC-type ICC seems to be better than that for conventional ICC.
Neuroendocrine type: Most cases reported so far are adenocarcinomas accompanying a neuroendocrine component positive for chromogranin A and/or synaptophysin. The neuroendocrine component is usually a well-differentiated neuroendocrine carcinoma (low grade malignancy) or poorly differentiated neuroendocrine carcinoma (high grade malignancy). Histologically, the adenocarcinoma is usually located at the surface of the tumor and the majority of the stromal invasion involves the neuroendocrine component.
ICC has been usually classified grossly into peripheral and hilar types and histologically into well, moderately and poorly differentiated adenocarcinomas and rare variants[7-10]. Herein, we propose a new histopathological classification of ICC based on the recent studies of ICC including the gross classification of ICC and on the pathological similarities between pancreatic and biliary neoplasms[1,5,14]. That is, ICC is largely classified histologically into four categories: a conventional (bile duct) type, a bile ductular type, an intraductal neoplasm type and rare variants.
Conventional ICC is further classified into small and large bile duct types. The former is characterized by a mass forming tumor grossly with or without involvement of the small bile ducts and the latter by evident cancerous large bile duct(s) showing periductal infiltration grossly. However, the differentiation of these two types is occasionally arbitrary, particularly in advanced cases, because the small bile duct type may secondarily involve the large bile duct type (cancerization) appearing as the large bile duct type of ICC and the large bile duct often shows a mass around the cancerous large bile duct at progressive stages.
Bile ductular ICC is proposed based on morphological similarities to proliferating and reactive bile ductules which frequently present with features of HPCs[2,25]. There is extensive replacement of hepatocytes of hepatic lobules or regenerative nodules by infiltrating carcinoma cells. Recent studies of biliary pathology show that HPCs, which exist in bile ductules and/or canals of Hering, can differentiate into hepatocytes and cholangiocytes and are activated in most chronic liver diseases. Interestingly, HPCs are potential targets for carcinogenesis and, eventually, primary liver tumors with HPC features may develop and such neoplasms may correspond to this type of ICC.
Intraductal neoplasm of the bile duct is a new category of intrahepatic biliary neoplasm and is classified into three types: IPNB, ITNB and intraductal superficial spreading type. IPNB is now being accepted as a counterpart of intraductal papillary mucinous neoplasm (IPMN) of the pancreas[5] and IPNB and IPMN share many features such as four types of phenotypes. At present, a majority of IPNB is regarded as the IPMN of main pancreatic duct type. Biliary mucinous cystic neoplasm (biliary MCN) is characterized by an ovarian-like stroma in the wall of the cystic neoplasm[14,26]. This type of neoplasm usually lacks luminal communication with the bile duct lumen. These points are used for the differentiation of this tumor from cystic IPNB. ITNB is only rarely reported and is characterized by a cast-like growth in the dilated duct lumen. ITNB may also correspond to the pancreatic counterpart, intraductal tubular neoplasm of the pancreas (adenoma or carcinoma). Several cases of intraductal, superfical spreading ICC are being reported and a detailed analysis of this type is mandatory.
The biliary tree and pancreas are closely located anatomically and share several physiological functions. Both derive from the foregut at almost the same time and recent studies using animals revealed that they show plasticity to each other during development[14]. Experimental studies using animals suggest that the biliary tract shows some potential for pancreatic differentiation. There are peribiliary glands around the biliary tract in humans and these glands drain into the bile duct lumen. Interestingly, small amounts of pancreatic exocrine acini are intermingled with these glands, raising the possibility that these glands may be abortive pancreatic exocrine acini which are prevented from differentiation into fully exocrine pancreatic acini in humans.
In this context, the biliary pathology is being considered given the similarities between the biliary tract and pancreas[14]. IgG4-related sclerosing cholangitis and autoimmune pancreatitis is one example[29,30]. Mucinous cystic neoplasm is also reported to develop in the pancreas and along the hepatobiliary system. In addition, advanced cholangiocarcinoma and preneoplastic or early intraepithelial neoplasms of the biliary tract show similar morphological or genetical changes to their pancreatic counterparts[6,14]. That is, invasive duct carcinoma of the pancreas and conventional ICC of large bile duct type share many biological and clinical features. In addition, similar intraepithelial neoplasms are also reported in the biliary tract and pancreas: biliary intraepithelial neoplasm (BilIN) and pancreatic intraepithelial neoplasm (PanIN)[15,21,31]. BilIN and PanIN are followed by conventional invasive duct adenocarcinoma. In this context, conventional ICC of large bile duct type can be regarded as a “bilio-pancreatic duct adenocarcinoma” along with extrahepatic and hilar cholangiocarcinomas as well as invasive ductal adenocarcinomas of the pancreas.
Based on recent progress in ICC pathology, including the gross classification of ICC and the similarities between biliary and pancreatic neoplasms, ICC was pathologically classified into a conventional (bile duct) type, a bile ductular type, an intraductal type and rare variants. The conventional type was further divided into small bile duct type (peripheral type) and large bile duct type (perihilar type). This new classification of ICC proposed here needs extensive discussions at an international consensus meeting and clinical and practical applications, especially a correlational study with TNM staging and prognostic study. This classification may also lead to a novel approach for research of ICC.
| 1. | Nakanuma Y, Sripa B, Batanasapt V, Leong ASY, Ponchon T, Ishak KG. Intrahepatic cholangiocarcinoma. Tumours of the Digestive System. World Health Organization of Tumours (Hamilton SR, Aaltonen LA, editors). IARC Press: Lyon 2000; 173-180. |
| 2. | Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen Y, Kosaka K, Harada K. Pathology of peripheral intrahepatic cholangiocarcinoma with reference to tumorigenesis. Hepatol Res. 2008;38:325-334. |
| 3. | Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856-867. |
| 4. | Reddy SB, Patel T. Current approaches to the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2006;8:30-37. |
| 5. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. |
| 6. | Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350-358. |
| 7. | Okuda K, Kubo Y, Okazaki N, Arishima T, Hashimoto M. Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer. 1977;39:232-246. |
| 8. | Goodman ZD, Terraciano L. Tumours and tumour-like lesions of the liver. MacSween’s Pathology of the Liver (Burt AD, Portman BC, Ferrell LD, editors) Churchill Livingstone 5th editors. IARC Press: Lyon 2006; 761-814. |
| 9. | Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658. |
| 10. | Nakanuma Y, Kida T, Minato H, Terada T. Pathology of cholangiocellular carcinoma. Springer-Verlag: Tokyo 1992; 39-50. |
| 11. | Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288-291. |
| 12. | Onodera M, Zen Y, Harada K, Sato Y, Ikeda H, Itatsu K, Sato H, Ohta T, Asaka M, Nakanuma Y. Fascin is involved in tumor necrosis factor-alpha-dependent production of MMP9 in cholangiocarcinoma. Lab Invest. 2009;89:1261-1274. |
| 13. | Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, Sato Y, Harada K, Zen Y, Sato H. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829-841. |
| 14. | Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419-429. |
| 15. | Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts. World J Hepatol. 2009;1:35-42. |
| 16. | Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552-570. |
| 17. | Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739-1745. |
| 18. | Yamamoto M, Takasaki K, Nakano M, Saito A. Minute nodular intrahepatic cholangiocarcinoma. Cancer. 1998;82:2145-2149. |
| 19. | Sasaki M, Tsuneyama K, Ishikawa A, Nakanuma Y. Intrahepatic cholangiocarcinoma in cirrhosis presents granulocyte and granulocyte-macrophage colony-stimulating factor. Hum Pathol. 2003;34:1337-1344. |
| 20. | Terada T, Kida T, Nakanuma Y, Kurumaya H, Doishita K, Takayanagi N. Intrahepatic cholangiocarcinomas associated with nonbiliary cirrhosis. A clinicopathologic study. J Clin Gastroenterol. 1994;18:335-342. |
| 21. | Zen Y, Aishima S, Ajioka Y, Haratake J, Kage M, Kondo F, Nimura Y, Sakamoto M, Sasaki M, Shimamatsu K. Proposal of histological criteria for intraepithelial atypical/proliferative biliary epithelial lesions of the bile duct in hepatolithiasis with respect to cholangiocarcinoma: preliminary report based on interobserver agreement. Pathol Int. 2005;55:180-188. |
| 22. | Ishak KG, Goodman ZD, Stocker JT. Intrahepatic cholangiocrcinoma and other malignant biliary tumors. In: Tumors of the liver and intraheaptic bile ducts. AFIP Series 2001; 245-270. |
| 23. | Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175-183. |
| 24. | Azizah N, Paradinas FJ. Cholangiocarcinoma coexisting with developmental liver cysts: a distinct entity different from liver cystadenocarcinoma. Histopathology. 1980;4:391-400. |
| 25. | Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, Sawada S, Minato H, Matsui O, Nakanuma Y. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: ‘bile ductular carcinoma’. Histopathology. 2007;51:390-400. |
| 26. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. |
| 27. | Sato Y, Osaka H, Harada K, Sasaki M, Nakanuma Y. Intraductal tubular neoplasm of the common bile duct. Pathol Int. 2010;60:516-519. |
| 28. | Chen TC, Ng KF, Kuo T. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like component. Mod Pathol. 2001;14:527-532. |
| 29. | Nakanuma Y, Zen Y. Pathology and immunopathology of immunoglobulin G4-related sclerosing cholangitis: The latest addition to the sclerosing cholangitis family. Hepatol Res. 2007;37 Suppl 3:S478-S486. |
| 30. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193-1203. |
| 31. | Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, Hong SM, Hytiroglou P, Klöppel G, Lauwers GY. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701-709. |
Peer reviewers: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, Rozzano 20089, Milan, Italy; Ruben Ciria, PhD, Uniersity Hospital Reina Sofia, Department of Hepatobiliary Surgery and Liver Transplantation, Avennida Menendez PidalI s/n, Cordoba 14004, Spain
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N