Published online Feb 27, 2026. doi: 10.4254/wjh.v18.i2.115563
Revised: November 5, 2025
Accepted: December 24, 2025
Published online: February 27, 2026
Processing time: 115 Days and 19.5 Hours
In this article, we discuss the recently published study by Wang et al, which in
Core Tip: This article discusses the potential of levodopa as a novel therapeutic prospect for liver diseases, particularly liver fibrosis and metabolic liver disease. Levodopa, which is traditionally used in the treatment of Parkinson’s disease, may influence liver fibrosis through the modulation of dopamine receptor D1 signaling and the activation of Hippo/Yes-associated protein 1 pathway. In addition, its potential impact on eating behavior and metabolic regulation may propose it a broader application for patients with stress-exacerbated liver disease.
- Citation: Xu J, Qian Y, Wang JM, Wu XL, Zheng YY. Levodopa: A novel therapeutic prospect for liver disease. World J Hepatol 2026; 18(2): 115563
- URL: https://www.wjgnet.com/1948-5182/full/v18/i2/115563.htm
- DOI: https://dx.doi.org/10.4254/wjh.v18.i2.115563
We read with great interest the recent study by Wang et al[1], which explored that levodopa, a drug traditionally used in the treatment of Parkinson’s disease (PD), demonstrated a capacity to alleviate liver fibrosis in a CCl4-induced rat model. This discovery represents a significant advancement in the application field of levodopa, proposing an innovative repurposing method and opening a new therapeutic avenue for liver diseases.
Levodopa, a precursor to dopamine, plays an essential role in regulating a wide array of physiological functions within the central nervous system (CNS), including motor control, reward processing, mood regulation, and cognitive processes[2]. Since its introduction in the 1960s as the principal treatment for PD, levodopa has been regarded as the gold standard for alleviating motor symptoms such as tremors, rigidity, bradykinesia, and postural instability[3]. After levodopa crosses the blood-brain barrier, the enzyme dopa decarboxylase converts it into dopamine. This process compensates for the loss of dopamine-producing neurons in the substantia nigra. As a result, levodopa helps restore motor function[4]. Although levodopa is primarily known for its effects on the CNS, emerging research has begun to explore its influence on peripheral systems. Notably, its potential role in modulating metabolic disorders and tissue fibrosis is of growing interest[5,6].
The study conducted by Wang et al[1] represents a substantial advancement by demonstrating that levodopa can reduce liver fibrosis through the modulation of the dopamine receptor D1 (DRD1)-Hippo/Yes-associated protein 1 (YAP1) signaling pathway. This finding is particularly innovative, as it repurposes an extensively studied drug that is tra
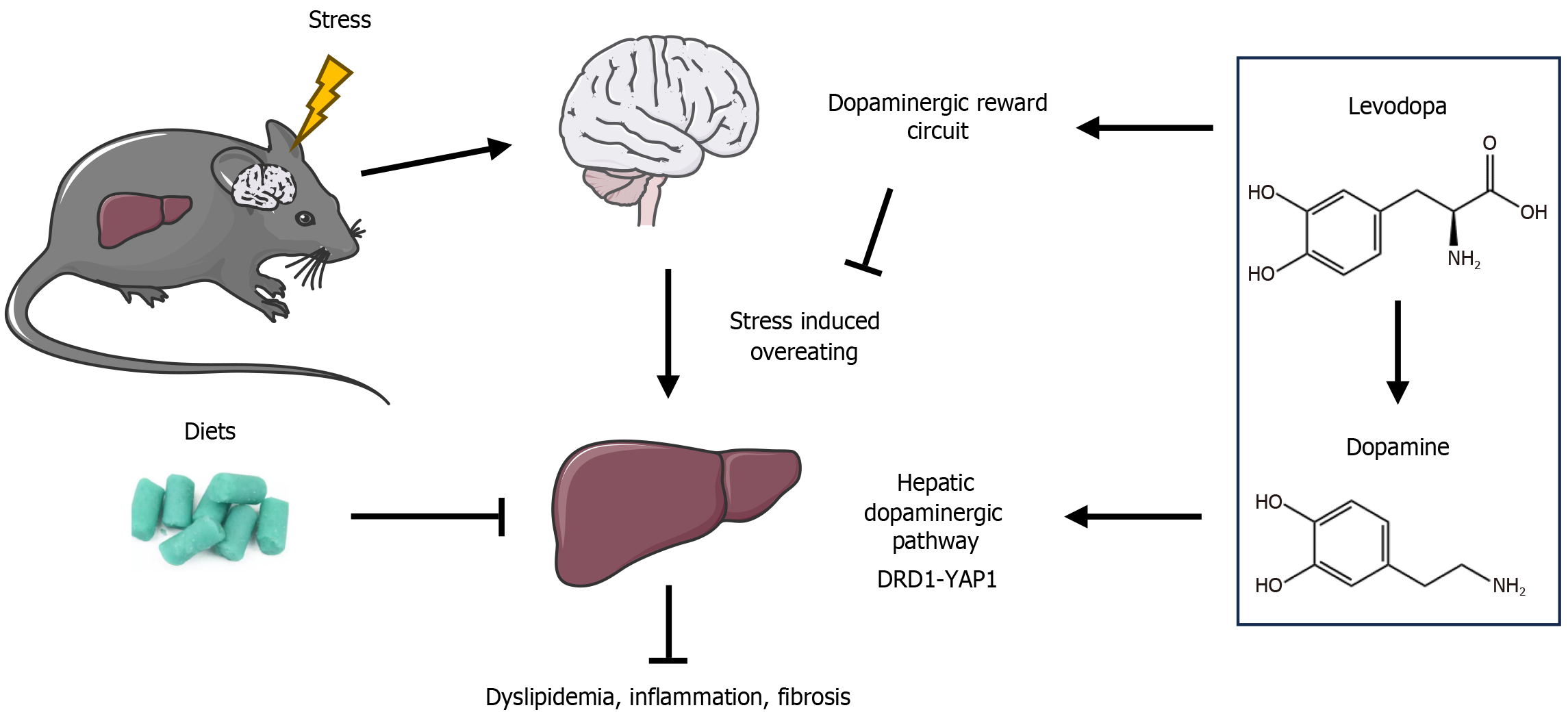
A pivotal innovation of this research lies in the identification of dopamine receptors in peripheral tissues. Specifically, DRD1, a member of the G-protein-coupled receptor family, is expressed in various tissues, including liver tissue. Activation of DRD1 triggers downstream signaling events via coupling to the Gαs protein, which activates the cAMP-PKA signaling axis[7]. These pathways have been implicated in regulating cellular processes such as inflammation, fibrosis, and cell proliferation. Furthermore, DRD1 signaling has been shown to activate the Hippo/YAP1 pathway, a critical regulator of cellular responses to stress and injury[8]. Thus, the levodopa’s ability to modulate DRD1 signaling provides a novel approach to managing liver fibrosis and inflammation.
Moreover, the results of this study highlight the capacity of levodopa to modulate peripheral dopamine signaling, suggesting that this drug may have broader therapeutic implications. The exploration of DRD1 receptor activation and its downstream effects on liver fibrosis introduces a novel mechanism for fibrosis management, especially in patients with chronic liver diseases such as cirrhosis and metabolic dysfunction-associated steatotic liver disease (MASLD), for whom fibrosis progression remains a major clinical challenge[9].
While the findings presented in this study are compelling, several important limitations must be addressed in future investigations.
First, this research predominantly employs a single animal model of liver fibrosis induced by CCl4 administration. Although this model is a useful tool for studying liver fibrosis, it may not fully reflect the complexities inherent in patients with other chronic liver diseases such as MASLD and alcoholic liver disease. Therefore, it would be beneficial for future studies to assess the effects of levodopa in models of MASLD, alcoholic liver disease, or even viral hepatitis to determine whether its therapeutic potential extends to these diverse liver pathologies.
Moreover, while the study elucidates the role of levodopa in modulating fibrosis through the DRD1-Hippo/YAP1 pathway, further research is necessary to explore its interactions with other molecular pathways. A more comprehensive understanding of how levodopa interacts with these pathways will be crucial for determining its broader therapeutic applicability in patients with liver diseases.
In addition to its potential for treating liver fibrosis, emerging evidence suggests that levodopa may be beneficial in addressing other chronic liver diseases, such as MASLD[10]. Dopamine signaling has been shown to influence immune cell activation, particularly in macrophages, suggesting a significant anti-inflammatory effect[11]. In the context of MASLD, levodopa may alleviate liver inflammation, improve insulin sensitivity, and potentially prevent the progression from simple steatosis to hepatitis.
Interestingly, the potential therapeutic effects of levodopa on MASLD may arise not only from its ability to improve fibrosis and inflammation, but also from its influence on metabolism and eating behaviors. In particular, the dopamine system plays a key role in regulating reward-driven behaviors, which control food motivation and pleasure derived from eating[12,13]. In the nucleus accumbens and hypothalamus, the reward circuit tightly control both appetite and how the body spends energy. Disruptions in the circuit will contribute to maladaptive eating behaviors, such as overeating, which are often associated with metabolic dysfunction in conditions like MASLD. Meanwhile, emerging evidence has revealed that, in individuals with MASLD, especially comorbid with pressure related disorders, the dopamine signaling is often dysregulated[14]. Stress may induce disruptions in the dopamine system, thereby leading to maladaptive eating be
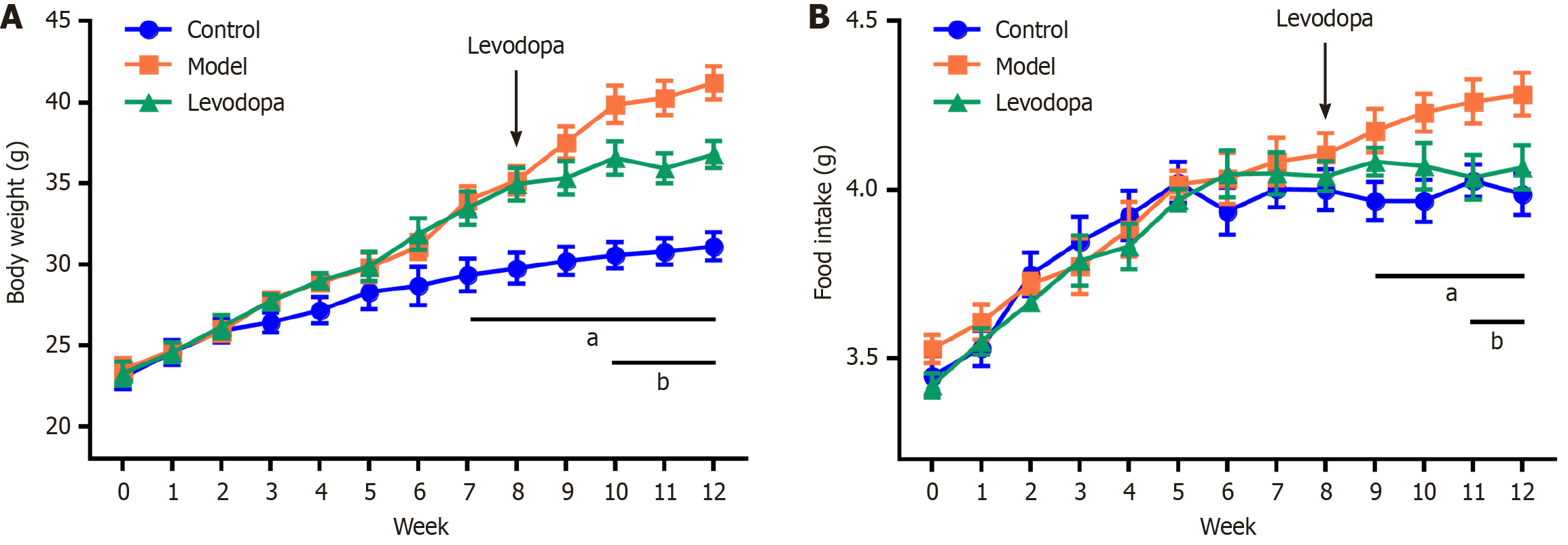
It is noteworthy that our exploration is based on the findings of a prior animal study. In our experiment, a murine model was constructed by a chronic restraint stress experiment combined with high fat, high fructose, and high cholesterol diets, while the levodopa (10 mg/kg) was intraperitoneally injected daily as intervention from the eighth week. Our research has revealed that levodopa effectively ameliorates alterations in feeding behavior induced by chronic restraint stress combined with high fat, high fructose, high cholesterol diets (Figure 2). This finding points toward dopamine’s role in controlling cravings for high-calorie foods by regulating brain reward circuits. As a result, correcting these behaviors may help reduce excessive calorie intake and prevent liver fat buildup.
In conclusion, levodopa holds considerable promise as a therapeutic agent for liver diseases. The innovative discovery that levodopa modulates the Hippo/YAP signaling pathway to reduce liver fibrosis opens new possibilities for repurposing this well-established drug for the treatment of liver diseases. Furthermore, its potential to influence eating behaviors could have profound implications for managing stress-exacerbated metabolic disorders. Given the increasing prevalence of liver diseases, particularly MASLD, levodopa’s ability to modulate both hepatic fibrosis and reward-driven eating behaviors offers a dual therapeutic approach for managing these conditions.
| 1. | Wang HY, Qi MM, Zhang K, Zhu YZ, Zhang J. Dopamine receptor D1-mediated suppression of liver fibrosis via Hippo/Yes-associated protein 1 signaling in levodopa treatment. World J Gastroenterol. 2025;31:108617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 2. | Probst D, Batchu K, Younce JR, Sode K. Levodopa: From Biological Significance to Continuous Monitoring. ACS Sens. 2024;9:3828-3839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Riederer P, Strobel S, Nagatsu T, Watanabe H, Chen X, Löschmann PA, Sian-Hulsmann J, Jost WH, Müller T, Dijkstra JM, Monoranu CM. Levodopa treatment: impacts and mechanisms throughout Parkinson's disease progression. J Neural Transm (Vienna). 2025;132:743-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 4. | Liang K, Yang L, Kang J, Liu B, Zhang D, Wang L, Wang W, Wang Q. Improving treatment for Parkinson's disease: Harnessing photothermal and phagocytosis-driven delivery of levodopa nanocarriers across the blood-brain barrier. Asian J Pharm Sci. 2024;19:100963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364:eaau6323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 499] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 6. | Jameson KG, Hsiao EY. A novel pathway for microbial metabolism of levodopa. Nat Med. 2019;25:1195-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Sun X, Wu C, Tian X, Wang P, Guo J, Shao Z, Wei Q. Activation of Dopamine Receptor D1 and Downstream Cellular Functions by Polydopamine. ACS Biomater Sci Eng. 2024;10:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Papavassiliou KA, Sofianidi AA, Spiliopoulos FG, Gogou VA, Gargalionis AN, Papavassiliou AG. YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis. Cells. 2024;13:1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Haak AJ, Kostallari E, Sicard D, Ligresti G, Choi KM, Caporarello N, Jones DL, Tan Q, Meridew J, Diaz Espinosa AM, Aravamudhan A, Maiers JL, Britt RD Jr, Roden AC, Pabelick CM, Prakash YS, Nouraie SM, Li X, Zhang Y, Kass DJ, Lagares D, Tager AM, Varelas X, Shah VH, Tschumperlin DJ. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med. 2019;11:eaau6296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 10. | Liang S, Yu Z, Song X, Wang Y, Li M, Xue J. Reduced Growth Hormone Secretion is Associated with Nonalcoholic Fatty Liver Disease in Obese Children. Horm Metab Res. 2018;50:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Pei D, Zeng Z, Geng Z, Cai K, Lu D, Guo C, Guo H, Huang J, Gao B, Yu S. Modulation of macrophage polarization by secondary cross-linked hyaluronan-dopamine hydrogels. Int J Biol Macromol. 2024;270:132417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Baik JH. Dopaminergic Control of the Feeding Circuit. Endocrinol Metab (Seoul). 2021;36:229-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Li H, Wang S, Wang D, Li J, Song G, Guo Y, Yin L, Tong T, Zhang H, Dong H. Dopamine Drives Feedforward Inhibition to Orexin Feeding System, Mediating Weight Loss Induced by Morphine Addiction. Adv Sci (Weinh). 2025;12:e2411858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res. 2010;210:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Pardo-Garcia TR, Gu K, Woerner RKR, Dus M. Food memory circuits regulate eating and energy balance. Curr Biol. 2023;33:215-227.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/