Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.110652
Revised: June 25, 2025
Accepted: August 15, 2025
Published online: September 27, 2025
Processing time: 105 Days and 20.6 Hours
Acute-on-chronic liver failure (ACLF) is a life-threatening syndrome associated with high short-term mortality. Accurate risk stratification is crucial for the mana
To evaluate the prognostic value of the C-reactive protein to albumin ratio (CAR) and its dynamic changes in patients with ACLF defined by the Chinese Group on Study of Severe Hepatitis B (COSSH) criteria.
A total of 126 consecutive patients diagnosed with COSSH-ACLF were pros
The 28-day and 90-day mortality rates were 27.8% and 40.5%, respectively. Baseline CAR was significantly higher in 28-day non-survivors than in survivors (2.68 vs 1.42, P < 0.001). The dynamic change in CAR from baseline to day 7 (ΔCAR-7) showed stronger predictive power for 28-day mortality [area under the receiver operating characteristic curve (AUC) = 0.765] than baseline CAR (AUC = 0.698), ΔCAR-4 (AUC = 0.706) or ΔCAR-14 (AUC = 0.712). Multivariate analysis identified ΔCAR-7 (HR = 1.53), baseline Model for End-Stage Liver Disease-Sodium (MELD-Na) score (HR = 1.08), and hepatic encephalopathy grade (HR = 1.92) as independent predictors of 28-day mortality (all P < 0.05). The COSSH-CAR model, which incorporated these parameters, showed superior predictive performance (AUC = 0.832) for 28-day mortality compared with established prognostic scores, including Child-Pugh (AUC = 0.721), MELD-Na (AUC = 0.768) and COSSH-ACLF (AUC = 0.786) and effectively stratified patients into three risk categories with significantly different survival rates (P < 0.001).
Dynamic changes in CAR during the first week provide important prognostic information in patients with COSSH-ACLF, surpassing baseline values and conventional inflammatory markers. The novel COSSH-CAR model improves risk stratification and may support clinical decision-making in the management of ACLF, pending ex
Core Tip: This study demonstrates that dynamic changes in C-reactive protein to albumin ratio (CAR) during the first week after admission provide superior prognostic value compared to baseline measurements in patients with acute-on-chronic liver failure (ACLF). The optimal predictive timepoint was day 7 change from baseline (ΔCAR-7). A novel Chinese Group on Study of Severe Hepatitis B (COSSH)-CAR prognostic model, incorporating ΔCAR-7, baseline Model for End-Stage Liver Disease-Sodium (MELD-Na) score, and hepatic encephalopathy grade, significantly outperformed established scoring systems including Child-Pugh, MELD-Na, and COSSH-ACLF scores. This dynamic biomarker approach offers enhanced risk stratification and may improve clinical decision-making in ACLF management.
- Citation: Zhu ZY, Yan LJ. Prognostic value of dynamic changes in C-reactive protein to albumin ratio in patients with acute-on-chronic liver failure. World J Hepatol 2025; 17(9): 110652
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/110652.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.110652
Acute-on-chronic liver failure (ACLF) is a complex clinical syndrome characterized by the acute deterioration of liver function in patients with pre-existing chronic liver disease, leading to hepatic and extrahepatic organ failures and high short-term mortality[1]. Despite improvements in critical care management, the prognosis of ACLF remains poor, with mortality rates ranging from 40% to 70% within three months of diagnosis, posing significant clinical and economic cha
The definition and diagnostic criteria for ACLF have evolved over the past decade, with various regional associations proposing differing standards based on geographical variations in etiology and clinical presentation[3-5]. In China and many other Asian countries, hepatitis B virus (HBV) infection, is the predominant cause of chronic liver disease and ACLF, in contrast to Western countries, where alcoholic liver disease and non-alcoholic steatohepatitis are more pre
The pathophysiology of ACLF involves complex interactions among hepatic inflammation, systemic inflammatory response, oxidative stress, and immune dysfunction, ultimately leading to multi-organ failure[7-9]. Systemic inflammation plays a pivotal role in the development and progression of ACLF, with studies showing elevated levels of pro-inflammatory cytokines and innate immune activation in ACLF patients compared to those with stable chronic liver disease[10]. This systemic inflammatory state contributes to hepatocellular injury, impaired liver regeneration, circulatory dysfunction, and extrahepatic organ damage through various mechanisms, including cytokine-mediated tissue damage, mitochondrial dysfunction, and endothelial activation[11,12].
Given the key role of inflammation in the pathogenesis of ACLF, various inflammatory biomarkers have been investigated for their prognostic significance. Among these, C-reactive protein (CRP), an acute-phase protein synthesized by hepatocytes in response to inflammatory stimuli, has emerged as a readily available marker of systemic inflammation[13,14]. However, the utility of CRP in liver disease may be limited by impaired hepatic synthetic capacity in patients with advanced cirrhosis or ACLF[15]. To address this limitation, the CRP to albumin ratio (CAR), which incorporates both an inflammatory marker (CRP) and a negative acute-phase protein and marker of hepatic synthetic function (albumin), has been proposed as a more comprehensive indicator that reflects both inflammation and liver dysfunction[16-18].
Several prognostic models have been developed for ACLF, including the Child-Turcotte-Pugh (CTP), Model for End-stage Liver Disease (MELD), MELD-sodium (MELD-Na)[19], CLIF-Consortium Organ Failure[20], and COSSH-ACLF scores[21]. While these models incorporate various clinical and laboratory parameters at baseline, they generally do not reflect the dynamic nature of ACLF and or account for temporal changes in inflammatory markers during the disease course[22]. Recent studies suggest that dynamic assessment of inflammatory parameters may provide superior pro
Although several studies have examined the prognostic value of CAR in various liver diseases, including hepatocellular carcinoma[25,26] and decompensated cirrhosis[27], data regarding its utility in ACLF remain limited-especially when ACLF is defined using the COSSH criteria. Moreover, the prognostic significance of dynamic changes in CAR during hospitalization for ACLF has not been comprehensively evaluated.
Therefore, this prospective study aimed to: (1) Evaluate the prognostic value of baseline CAR in patients with COSSH-defined ACLF; (2) Determine whether dynamic changes in CAR during hospitalization offer additional prognostic information beyond baseline values; (3) Identify the optimal timing for CAR reassessment; (4) Develop a novel prognostic model incorporating both baseline and dynamic CAR parameters; and (5) Compare the performance of this new model with existing prognostic scores. We hypothesized that dynamic changes in CAR would significantly enhance mortality prediction compared to baseline values alone and might offer improved risk stratification for ACLF patients.
This prospective data collection and retrospective analysis observational cohort study enrolled 126 consecutive patients diagnosed with ACLF according to COSSH criteria who were hospitalized at the Handan Central Hospital between February, 2022 and March, 2025. A novel prognostic model (COSSH-CAR) incorporating dynamic CAR changes change in CAR from baseline to day 7 (ΔCAR-7), and independent predictors identified in multivariate analysis was developed and internally validated using bootstrap resampling. The predictive accuracy of COSSH-CAR was evaluated by receiver operating characteristic (ROC) curve analysis and compared with established prognostic scores (CTP, MELD, MELD-Na and COSSH-ACLF). The study adhered to the principles of Declaration of Helsinki and followed the STROBE reporting guidelines. The study protocol was approved by the Ethics Committee of Handan Central Hospital (Approval number: 2025070) and written informed consent was obtained from all participants or their legal representatives.
Patients who were hospitalized at the Handan Central Hospital from February, 2022 to March, 2025 with acute deterioration of chronic liver disease for at least 1 day were initially screened in this study. Acute deterioration was defined according to the COSSH criteria: For non-cirrhotic patients, total bilirubin (TBIL) ≥ 5 mg/dL (≥ 85.5 μmol/L) and international normalized ratio (INR) ≥ 1.5; for cirrhotic patients: Acute decompensation was defined as new onset or worsening of hepatic encephalopathy (HE), ascites, variceal bleeding, or bacterial infection within the preceding 4 weeks. All patients received standard medical treatment in accordance with current ACLF practice guidelines, including identification and treatment precipitating factors, organ supportive care and prevention/treatment of complications.
HE was diagnosised and graded according to the 2024 Chinese Guidelines on the Management of HE in cirrhosis[28]. Ascites was classified as mild, moderate, or severe based on clinical and ultrasonographic assessments. Bacterial infection was diagnosed as previously described[29]. ACLF was defined and diagnosed according to the COSSH criteria[6]. Discharged patients were followed up at 28 days and 90 days after admission through outpatient visits or telephone interviews to confirm their survival status. The primary study outcome was 28-day mortality, and the secondary study outcome was 90-day mortality. Exclusion criteria included: (1) Age < 18 years or > 80 years; (2) Pregnancy or breastfeeding; (3) Hepatocellular carcinoma or other malignancies; (4) Severe extraheptaic diseases; (5) Human immunodeficiency virus infection; (6) Receiving immunosuppressive drugs for reasons other than severe alchoholic hepatics; and (7) Refusal to sign the informed consent form.
Demographic, clinical, and laboratory data were collected at baseline (time of enrollment), and on days 4, 7, and 14 during hospitalization. Demographic and clinical data included age, sex, etiology of chronic liver disease, precipitating events for ACLF, presence and grade of ascites and HE, vital signs, and comorbidities.
Laboratory parameters included complete blood count, liver function tests (TBIL, alanine aminotransferase, aspartate aminotransferase, albumin), coagulation profile (prothrombin time, INR), renal function tests (creatinine, blood urea nitrogen), electrolytes, and high-sensitivity CRP (hs-CRP).
The CAR was calculated as the ratio of serum hs-CRP (mg/L) to albumin (g/dL). Dynamic changes in CAR were defined as the absolute difference between values at different time points and baseline (e.g., ΔCAR-4 = CAR day 4 - CAR baseline, ΔCAR-7 = CAR day 7 - CAR baseline, ΔCAR-14 = CAR day 14 - CAR baseline). Established prognostic scores, including MELD, MELD-Na, CTP, and COSSH-ACLF scores, were calculated at baseline according to their respective formulas. The COSSH-ACLF score was calculated as: 0.741 × INR + 0.523 × HBV-sequential organ failure assessment (SOFA) + 0.026 × age + 0.003 × TBIL, where HBV-SOFA is a modified Sequential Organ Failure Assessment score. HBV-SOFA components include: Liver (TBIL: 1-5.9 mg/dL = 1 point, 6-11.9 mg/dL = 2 points, ≥ 12 mg/dL = 3 points), coagulation (INR: 1.5-1.9 = 1 point, 2.0-2.4 = 2 points, ≥ 2.5 = 3 points), circulation (mean arterial pressure and vasopressor requirements), renal (creatinine levels), and central nervous system (HE grade)[6].
For patients who died before day 7 or day 14 assessments, the last available CAR value was used to calculate dynamic changes. Patients with missing baseline CAR values were excluded from analysis. For ΔCAR-7 calculations, patients who died before day 7 (n = 8) had their last available CAR measurement used (day 4 for 6 patients, day 2-3 for 2 patients). Sensitivity analyses excluding patients with early deaths showed consistent results for model performance.
The selection of day 7 as the optimal time point for CAR reassessment was based on several considerations: (1) Preliminary analysis showed maximal divergence between survivor and non-survivor trajectories at day 7; (2) Day 7 provides sufficient time for initial treatment response while remaining clinically actionable; (3) Most patients remain hospitalized at day 7, making reassessment feasible; and (4) Day 7 balances the need for early risk stratification with adequate observation time for inflammatory trajectory assessment.
Continuous variables were presented as mean ± SD or median interquartile range (IQR) depending on the data distribution, while categorical variables were summarized as frequencies and percentages. Normality of data distribution was assessed using the Shapiro-Wilk test. Comparisons between survivors and non-survivors were performed using Student's t-test or Mann-Whitney U test for continuous variables, and χ2 test or Fisher's exact test for categorical variables, as ap
The prognostic value of baseline CAR and its dynamic changes for 28-day and 90-day mortality was evaluated using univariate and multivariate Cox proportional hazards regression models. Variables with P < 0.05 in univariate analysis were included in multivariate analysis using a backward stepwise selection approach.
The discriminative ability of CAR, its dynamic changes, and prognostic scores was assessed using ROC curve analysis with calculation of the area under the ROC curve (AUC). Kaplan-Meier survival curves were constructed for patients stratified by the optimal cutoff values, and differences between groups were compared using the log-rank test.
Based on the results of multivariate Cox proportional hazards regression analysis, a novel prognostic model (COSSH-CAR) was developed by integrating baseline and dynamic parameters that were independently associated with mortality. The performance of this model was compared with existing prognostic scores using DeLong test for paired ROC curves.
Bootstrap resampling was performed with 1000 iterations using bias-corrected and accelerated method. Each bootstrap sample was randomly drawn with replacement from the original dataset, maintaining the same sample size (n = 126). Model coefficients and performance metrics were calculated for each bootstrap sample, and 95% confidence intervals were derived from the 2.5th and 97.5th percentiles of the bootstrap distribution.
All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States) and R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P < 0.05 was considered statistically significant.
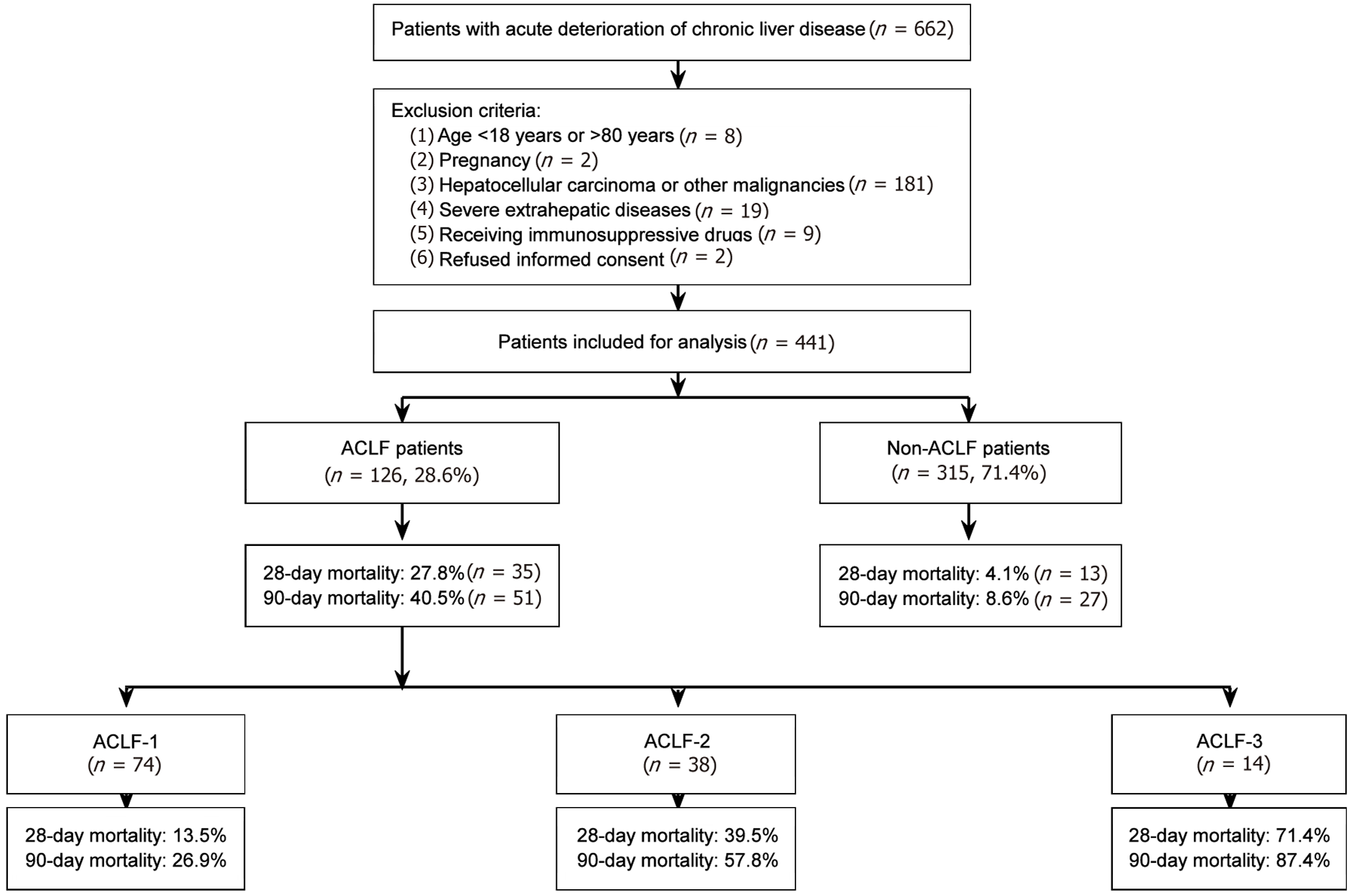
Among 441 patients presenting with acute deterioration of chronic liver disease, 126 (28.6%) were diagnosed with ACLF, and 315 (71.4%) were diagnosed with non-ACLF based on the COSSH-ACLF criteria (Figure 1). A total of 126 consecutive patients with COSSH-ACLF were included for the final analysis. The clinical characteristics of all enrolled ACLF patients are summarized in Table 1. The mean age of the enrolled patients was 51.8 ± 12.7 years, and the majority of patients were male (75.4%). Chronic HBV infection was the most common etiology of underlying chronic liver disease (67.5%), followed by alcohol-related liver disease (19.0%), chronic hepatitis C virus infection (6.3%), autoimmune liver disease (4.8%), and other etiologies (2.4%). Identified precipitating events for ACLF development included HBV reactivation (38.9%), alcohol (25.4%) and bacterial infection (18.3%), while no obvious precipitating event was identified in 17.5% of patients. The most frequent organ failures in patients with COSSH-ACLF were liver failure (83.7%) and coagulation failure (28.6%), followed by circulatory failure (17.8%), renal failure (10.3%), brain failure (5.6%) and respiration failure (3.4%).
| Characteristic | All patients (n = 126) | Survivors (n = 91) | Non-survivors (n = 35) | P value |
| Demographics | ||||
| Age, years | 51.8 ± 12.7 | 49.2 ± 13.0 | 54.2 ± 11.9 | 0.023 |
| Male | 95 (75.4) | 69 (75.8) | 26 (74.3) | 0.682 |
| Etiology of chronic liver disease | 0.398 | |||
| HBV infection | 85 (67.5) | 63 (69.2) | 22 (62.9) | |
| Alcohol | 24 (19.0) | 15 (16.5) | 9 (25.7) | |
| HCV infection | 8 (6.3) | 6 (6.6) | 2 (5.7) | |
| Autoimmune | 6 (4.8) | 5 (5.5) | 1 (2.9) | |
| Others | 3 (2.4) | 2 (2.2) | 1 (2.9) | |
| Precipitating events | 0.176 | |||
| HBV reactivation | 49 (38.9) | 35 (38.5) | 14 (40.0) | |
| Alcohol | 32 (25.4) | 22 (23.8) | 10 (28.6) | |
| Bacterial infection | 23 (18.3) | 16 (17.6) | 7 (20.0) | |
| Indeterminate | 22 (17.5) | 15 (16.5) | 7 (20.0) | |
| Complications | ||||
| Ascites | 82 (64.9) | 52 (57.1) | 30 (85.7) | 0.002 |
| Acute variceal bleeding | 41 (32.5) | 26 (28.6) | 15 (42.9) | 0.083 |
| Hepatic encephalopathy | 29 (23.0) | 14 (15.4) | 15 (42.9) | < 0.001 |
| Grade 1-2 | 22 (17.5) | 12 (13.2) | 10 (28.6) | 0.037 |
| Grade 3-4 | 7 (5.6) | 2 (2.2) | 5 (14.3) | 0.009 |
| Organ failures | ||||
| Liver | 105 (83.7) | 72 (79.1) | 33 (94.3) | 0.020 |
| Coagulation | 36 (28.6) | 18 (19.8) | 18 (51.4) | 0.034 |
| Circulation | 22 (17.8) | 9 (9.9) | 13 (37.1) | 0.027 |
| Renal | 13 (10.3) | 5 (5.5) | 8 (22.9) | 0.004 |
| Cerebral | 7 (5.6) | 2 (2.2) | 5 (14.3) | 0.008 |
| Respiration | 4 (3.4) | 1 (1.1) | 3 (8.6) | 0.032 |
| Laboratory parameters | ||||
| Total bilirubin (mg/dL) | 26.0 ± 9.0 | 22.1 ± 7.2 | 29.5 ± 9.3 | < 0.001 |
| Albumin (g/dL) | 2.8 ± 0.5 | 3.0 ± 0.5 | 2.6 ± 0.4 | 0.023 |
| ALT (U/L) | 275 (139-657) | 293 (157-678) | 258 (124-642) | 0.217 |
| AST (U/L) | 243 (149-528) | 251 (158-541) | 239 (137-516) | 0.386 |
| INR | 2.26 ± 0.67 | 1.98 ± 0.47 | 2.51 ± 0.72 | 0.029 |
| Creatinine (mg/dL) | 1.41 ± 0.75 | 1.18 ± 0.53 | 1.62 ± 0.87 | 0.014 |
| Sodium (mEq/L) | 134.2 ± 5.8 | 135.9 ± 5.0 | 132.6 ± 6.1 | 0.006 |
| Neutrophil count (109/L) | 7.8 (5.2-11.8) | 6.5 (4.6-9.2) | 9.2 (6.7-13.5) | 0.073 |
| Lymphocyte count (109/L) | 0.87 (0.58-1.31) | 1.04 (0.72-1.47) | 0.76 (0.52-1.12) | 0.134 |
| Platelet count (109/L) | 79 (54-115) | 86 (60-126) | 73 (51-107) | 0.213 |
| Inflammatory indices | ||||
| White blood cell count (109/L) | 9.8 (6.9-14.2) | 8.7 (6.3-11.8) | 11.3 (8.2-16.1) | 0.041 |
| CRP (mg/L) | 25.8 (14.6-42.9) | 18.2 (11.4-29.5) | 35.7 (22.1-51.9) | 0.028 |
| NLR | 9.0 (8.8-9.2) | 6.1 (4.1-9.4) | 11.5 (7.8-17.2) | 0.039 |
| CAR | 2.03 (1.52-3.27) | 1.42 (0.95-2.31) | 2.68 (1.82-4.23) | 0.031 |
| Prognostic scores | ||||
| MELD | 28.6 (23.9-33.4) | 25.2 ± 5.5 | 31.8 ± 6.2 | < 0.001 |
| MELD-Na | 30.7 (25.8-35.3) | 27.6 ± 5.1 | 33.6 ± 5.8 | < 0.001 |
| CTP | 11.3 ± 1.8 | 10.5 ± 1.6 | 12.0 ± 1.7 | 0.019 |
| COSSH-ACLF | 7.3 (6.1-8.7) | 6.4 ± 1.2 | 8.2 ± 1.5 | < 0.001 |
| Mortality | < 0.001 | |||
| ACLF 1 (n = 74) | 10 (13.5) | 64 (86.5) | 10 (28.6) | |
| ACLF 2 (n = 38) | 15 (39.5) | 23 (60.5) | 15 (42.9) | |
| ACLF 3 (n = 14) | 10 (71.4) | 4 (28.6) | 10 (28.6) | |
At baseline, the median MELD score was 28.6 (IQR 23.9-33.4), median MELD-Na score was 30.7 (IQR 25.8-35.3), and median COSSH-ACLF score was 7.3 (IQR 6.1-8.7). The majority of patients presented with ascites (64.9%), and HE was observed in 23.0% of patients, with 17.5% having grade 1–2 and 5.6% having grade 3-4 encephalopathy.
During the 90-day follow-up period, 51 patients (40.5%) died, with mortality rates varying by ACLF grade: 26.9% for ACLF grade 1, 57.8% for grade 2, and 87.4% for grade 3. The 28-day mortality rate was 27.8% (35 patients), with rates of 13.5%, 39.5%, and 71.4% for ACLF grades 1, 2, and 3, respectively. Comparison of baseline characteristics between 28-day survivors and non-survivors showed that non-survivors were significantly older (54.2 ± 11.9 vs 49.2 ± 13.0 years, P = 0.023) and had higher MELD scores (31.8 ± 6.2 vs 25.2 ± 5.5, P < 0.001), MELD-Na scores (33.6 ± 5.8 vs 27.6 ± 5.1, P < 0.001), and COSSH-ACLF scores (8.2 ± 1.5 vs 6.4 ± 1.2, P < 0.001) compared to survivors. In addition, non-survivors had significantly higher levels of TBIL (29.5 ± 9.3 vs 22.1 ± 7.2 mg/dL, P < 0.001), creatinine (1.62 ± 0.87 vs 1.18 ± 0.53 mg/dL, P = 0.014), and INR (2.51 ± 0.72 vs 1.98 ± 0.47, P = 0.029), along with a higher prevalence of grade 3-4 HE (14.3% vs 2.2%, P = 0.009) (Table 1).
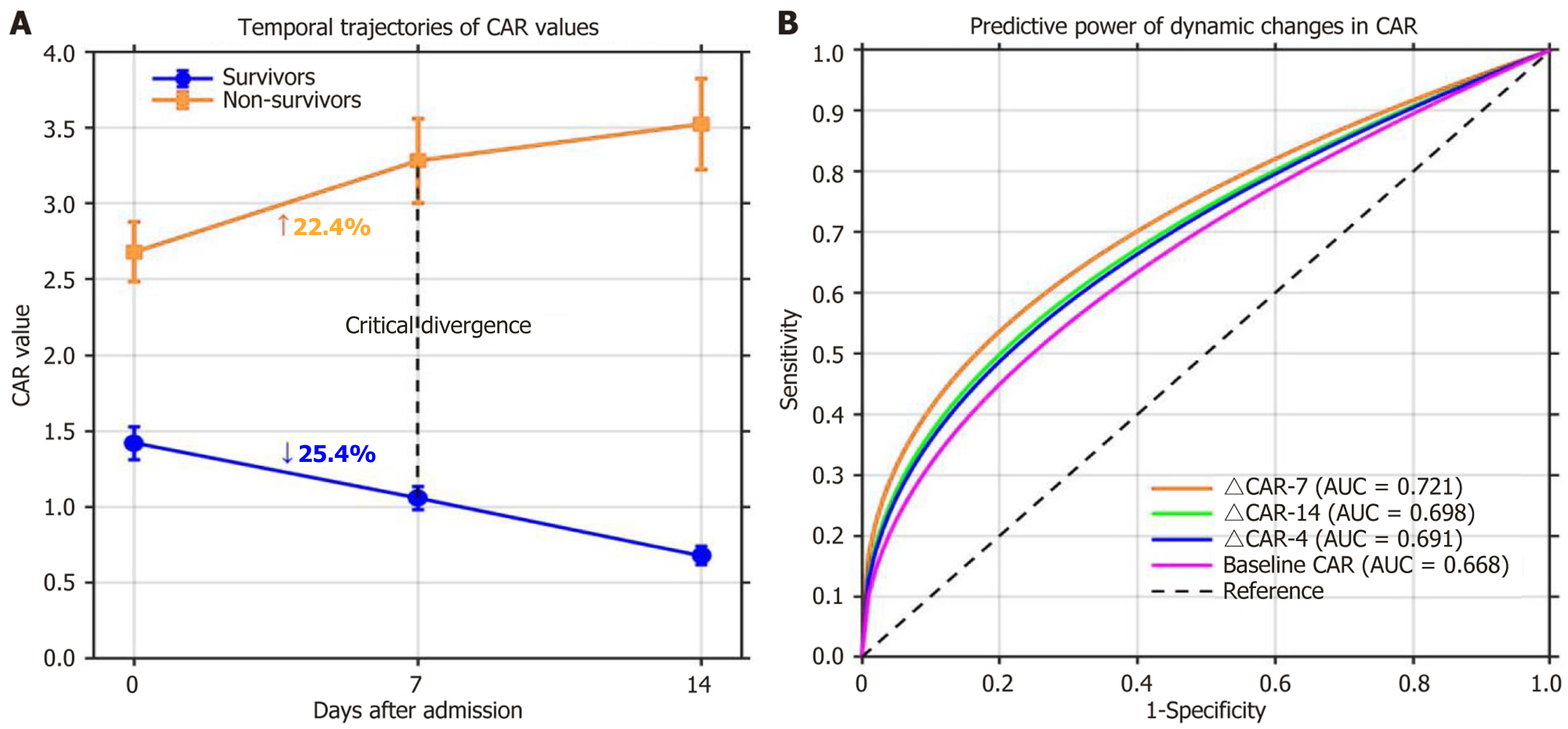
At baseline, non-survivors exhibited significantly higher white blood cell (WBC) counts [11.3 (IQR 8.2-16.1) vs 8.7 (IQR 6.3-11.8) × 109/L, P = 0.041], neutrophil counts [9.2 (IQR 6.7-13.5) vs 6.5 (IQR 4.6-9.2) × 109/L, P = 0.073], neutrophil-to-lymphocyte ratio (NLR) [11.5 (IQR 7.8-17.2) vs 6.1 (IQR 4.1-9.4), P = 0.039], and CRP levels [35.7 (IQR 22.1-51.9) vs 18.2 (IQR 11.4-29.5) mg/L, P = 0.028] compared to survivors. Serum albumin levels were significantly lower in non-survivors (2.6 ± 0.4 vs 3.0 ± 0.5 g/dL, P = 0.023). Baseline CAR was significantly higher in non-survivors than in survivors [2.68 (IQR 1.82-4.23) vs 1.42 (IQR 0.95-2.31), P = 0.031]. Baseline CAR consistently demonstrated superior discriminative power (28-day AUC = 0.698; 90-day AUC = 0.662) compared to other inflammatory markers (CRP, WBC and NLR) at both time points (Table 2).
| Inflammatory marker | 28-day mortality, AUC (95%CI) | 90-day mortality, AUC (95%CI) | 28-day, P value vs baseline CAR | 90-day, P value vs baseline CAR |
| Baseline inflammatory markers | ||||
| C-reactive protein | 0.652 (0.560-0.744)a | 0.624 (0.534-0.714)a | 0.032a | 0.041a |
| White blood cell count | 0.635 (0.542-0.728)a | 0.617 (0.527-0.707)a | 0.025a | 0.035a |
| Neutrophil-lymphocyte ratio | 0.645 (0.552-0.738)a | 0.619 (0.529-0.709)a | 0.028a | 0.038a |
| Baseline CAR | 0.698 (0.610-0.786) | 0.662 (0.575-0.749) | Reference | Reference |
| Dynamic changes in CAR | ||||
| ΔCAR-4 | 0.706 (0.618-0.794) | 0.687 (0.602-0.772) | 0.068 | 0.072 |
| ΔCAR-7 | 0.765 (0.684-0.846) | 0.723 (0.641-0.805) | 0.003 | 0.006 |
| ΔCAR-14 | 0.712 (0.625-0.799) | 0.695 (0.611-0.779) | 0.056 | 0.065 |
CAR trajectories showed markedly divergent patterns between 28-day survivors and non-survivors during hospitalization. While survivors exhibited a progressive decline in CAR (baseline: 1.42 ± 0.76; day 7: 1.06 ± 0.54; day 14: 0.68 ± 0.42), non-survivors experienced a continuous increase (baseline: 2.68 ± 1.07; day 7: 3.28 ± 1.52; day 14: 3.52 ± 1.64). By day 7, survivors showed a mean 25.4% decrease in CAR from baseline, whereas non-survivors showed a 22.4% increase, marking a critical point of divergence (Figure 2A). Notably, the absolute change in ΔCAR-7 emerged as the most powerful predictor of mortality, showing significantly stronger discriminative ability (AUC = 0.721 for 28-day mortality; AUC = 0.693 for 90-day mortality) than baseline CAR (AUC = 0.668 and 0.632, respectively), ΔCAR-4 (AUC = 0.691 and 0.657), and ΔCAR-14 (AUC = 0.698 and 0.665) (Figure 2B).
Univariate Cox regression analysis identified multiple baseline and dynamic parameters associated with 28-day mortality (Table 3). Multivariate Cox regression analysis identified serum sodium (adjusted HR = 0.99, 95%CI: 0.96-1.02, P = 0.038), TBIL (adjusted HR = 1.03, 95%CI: 0.98-1.08, P = 0.024), creatinine (adjusted HR = 1.22, 95%CI: 0.91-1.63, P = 0.031), INR (adjusted HR = 1.31, 95%CI: 0.87-1.97, P = 0.039), ΔCAR-7 (adjusted HR = 1.53, 95%CI: 1.29-1.82, P = 0.036) and HE grade (adjusted HR = 1.92, 95%CI: 1.43-2.58, P = 0.027) as independent predictors of 28-day mortality (Table 3). After adjusting for these variables, baseline CAR was no longer significantly associated with mortality (adjusted HR = 1.12, 95%CI: 0.94-1.33, P = 0.203). In addition, some existing prognostic scores, including baseline MELD score (adjusted HR = 1.03, 95%CI: 0.97-1.09, P = 0.031), MELD-Na score (adjusted HR = 1.08, 95%CI: 1.04-1.12, P = 0.022) and COSSH-ACLF score (adjusted HR = 1.24, 95%CI: 0.95-1.62, P = 0.019), were also independent predictors of 28-day mortality.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Demographics | ||||
| Age (per year) | 1.03 (1.00-1.05) | 0.025 | 1.01 (0.98-1.04) | 0.472 |
| Male sex | 0.82 (0.41-1.64) | 0.578 | — | — |
| Complications | ||||
| Ascites | 1.65 (1.15-2.36) | 0.026 | 1.18 (0.78-1.79) | 0.431 |
| HE grade | 2.15 (1.63-2.84) | < 0.001 | 1.92 (1.43-2.58) | 0.027 |
| AVB | 1.53 (1.09-2.15) | 0.079 | — | — |
| Laboratory parameters | ||||
| White blood cell count (per × 109/L) | 1.07 (1.06-1.09) | < 0.001 | 1.02 (0.99-1.05) | 0.214 |
| Hemoglobin (per g/L) | 0.99 (0.98-1.00) | 0.035 | 1.00 (0.99-1.01) | 0.837 |
| Platelet count (per × 109/L) | 0.997 (0.994-1.000) | 0.127 | — | — |
| NLR | 1.05 (1.02-1.08) | 0.023 | 1.02 (0.98-1.06) | 0.106 |
| C-reactive protein (per mg/L) | 1.015 (1.011-1.020) | 0.031 | 1.004 (0.998-1.010) | 0.183 |
| Serum albumin (per g/dL) | 0.35 (0.18-0.67) | 0.043 | 0.78 (0.37-1.65) | 0.518 |
| ALT (per U/L) | 1.000 (1.000-1.001) | 0.652 | — | — |
| Serum potassium (per mmol/L) | 1.54 (1.32-1.81) | 0.036 | 1.14 (0.93-1.39) | 0.213 |
| Serum sodium (per mmol/L) | 0.96 (0.94-0.98) | 0.012 | 0.99 (0.96-1.02) | 0.038 |
| Total bilirubin (per mg/dL) | 1.09 (1.05-1.13) | 0.019 | 1.03 (0.98-1.08) | 0.024 |
| Creatinine (per mg/dL) | 1.68 (1.32-2.14) | 0.025 | 1.22 (0.91-1.63) | 0.031 |
| INR (per unit) | 2.41 (1.72-3.38) | 0.032 | 1.31 (0.87-1.97) | 0.039 |
| CAR parameters | ||||
| Baseline CAR | 1.24 (1.12-1.37) | 0.037 | 1.12 (0.94-1.33) | 0.203 |
| ΔCAR-4 | 1.38 (1.21-1.58) | 0.025 | 1.17 (0.98-1.39) | 0.079 |
| ΔCAR-7 | 1.67 (1.43-1.95) | 0.004 | 1.53 (1.29-1.82) | 0.036 |
| ΔCAR-14 | 1.45 (1.26-1.67) | 0.085 | — | — |
| Prognostic scores | ||||
| CTP | 1.12 (1.07-1.17) | 0.037 | 1.02 (0.95-1.06) | 0.146 |
| MELD | 1.12 (1.07-1.17) | < 0.001 | 1.03 (0.97-1.09) | 0.031 |
| MELD-Na | 1.15 (1.09-1.21) | < 0.001 | 1.08 (1.04-1.12) | 0.022 |
| COSSH-ACLF | 1.89 (1.54-2.32) | < 0.001 | 1.24 (0.95-1.62) | 0.019 |
As serum sodium, TBIL, creatinine and INR are all the variables included in the MELD-Na score, we developed a novel COSSH-CAR model incorporating MELD-Na score, ΔCAR-7 and HE grade: COSSH-CAR = 0.077 × MELD-Na + 0.652 × HE grade + 0.426 × ΔCAR-7. In this formula, HE grade was defined as 0 (none), 1 (grade 1-2), or 2 (grade 3-4), and ΔCAR-7 represented the change in ΔCAR-7.
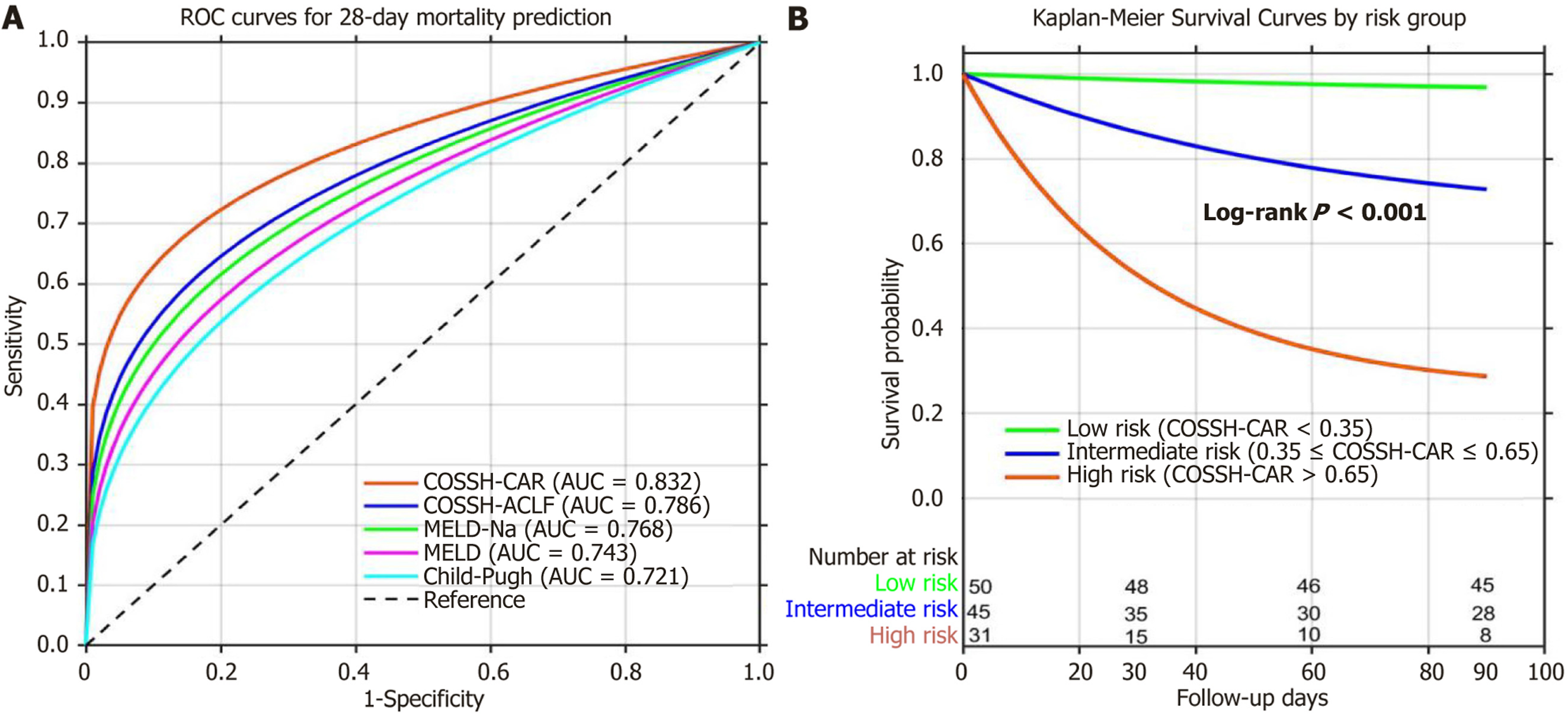
The COSSH-CAR model demonstrated excellent discriminative ability for both 28-day mortality (AUC = 0.832) and 90-day mortality (AUC = 0.811) (Table 4). Compared with existing prognostic scores, the COSSH-CAR model showed sig
| Prognostic model | 28-day mortality original AUC (95%CI) | 28-day mortality bootstrap corrected AUC (95%CI) | 90-day mortality original AUC (95%CI) | 90-day mortality bootstrap corrected AUC (95%CI) | P value Original | P value Bootstrap |
| COSSH-CAR | 0.832 (0.756-0.895) | 0.821 (0.793-0.849) | 0.811 (0.732-0.874) | 0.798 (0.767-0.829) | Reference | Reference |
| MELD | 0.743 (0.658-0.818) | 0.731 (0.698-0.764) | 0.725 (0.640-0.800) | 0.712 (0.679-0.745) | 0.008 | 0.005 |
| MELD-Na | 0.768 (0.685-0.840) | 0.752 (0.721-0.783) | 0.751 (0.667-0.825) | 0.736 (0.703-0.769) | 0.015 | 0.012 |
| CTP | 0.721 (0.635-0.798) | 0.709 (0.675-0.743) | 0.708 (0.622-0.785) | 0.693 (0.658-0.728) | 0.002 | < 0.001 |
| COSSH-ACLF | 0.786 (0.704-0.856) | 0.769 (0.737-0.801) | 0.772 (0.690-0.842) | 0.758 (0.726-0.790) | 0.027 | 0.023 |
Using optimal cutoff values, patients were stratified into three risk groups: Low-risk (COSSH-CAR < 0.35, n = 50), intermediate-risk (COSSH-CAR 0.35-0.65, n = 45), and high-risk (COSSH-CAR > 0.65, n = 31). The 28-day mortality rates were 4.0%, 22.2%, and 48.4%, and the 90-day mortality rates were 10.0%, 37.8%, and 74.2% in the low, intermediate, and high-risk groups, respectively (P < 0.001) (Figure 3B). Clinical example: A 45-year-old patient with: MELD-Na score = 32, HE grade 2 (HE grade = 1), baseline CAR = 2.1, day 7 CAR = 2.8 (ΔCAR-7 = 0.7), the COSSH-CAR score = 0.077 × 32 + 0.652 × 1 + 0.426 × 0.7 = 2.464 + 0.652 + 0.298 = 3.414. This score places the patient in the high-risk category (> 0.65), indi
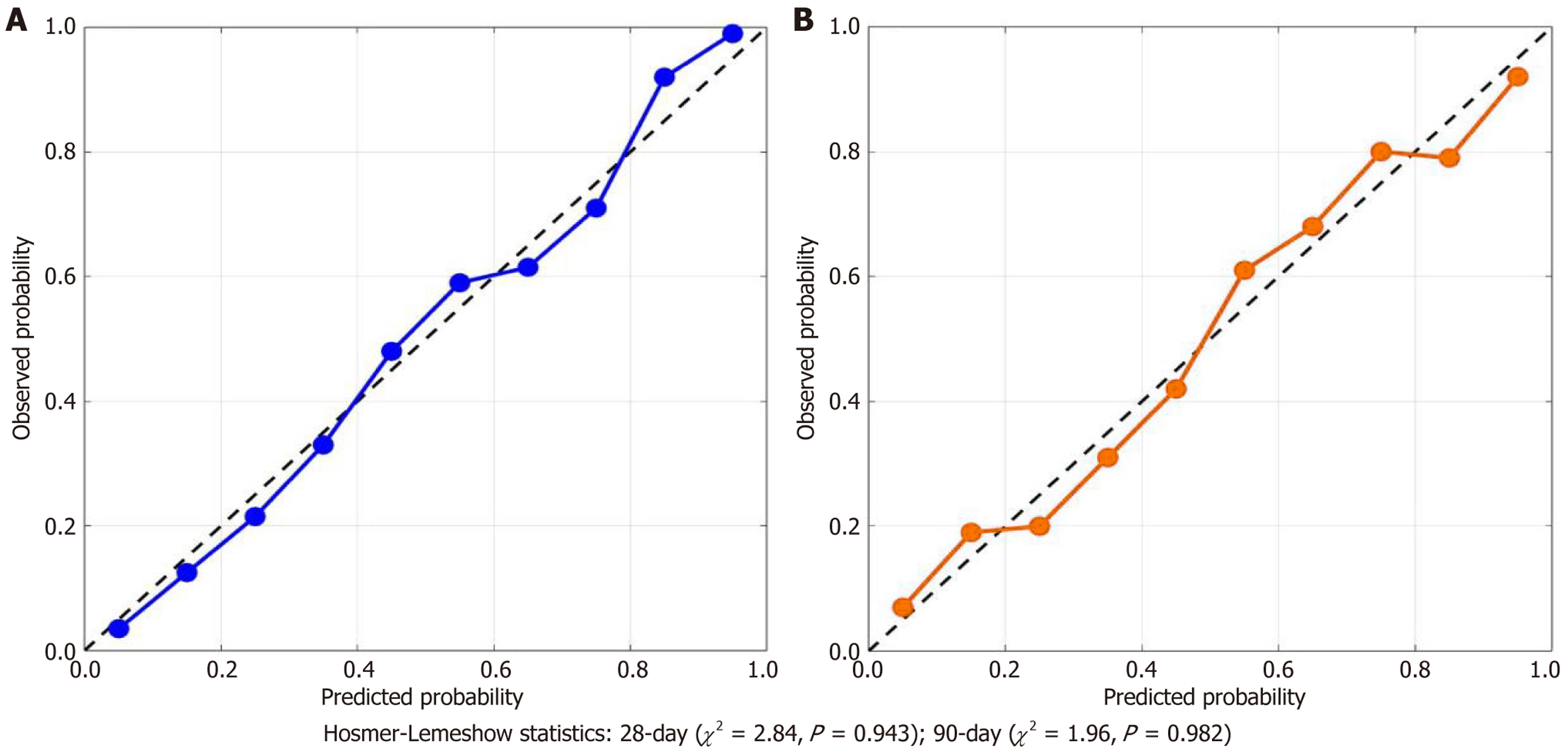
The calibration curves show generally good agreement between predicted and observed probabilities, with some deviation in the intermediate risk range (30%-60% predicted probability). These deviations may reflect the relatively small sample size in specific risk strata and emphasize the need for external validation. Despite these variations, Hosmer-Lemeshow statistics remain non-significant for both timepoints (28-day: χ² = 3.56, P = 0.895; 90-day: χ² = 4.29, P = 0.830), indicating acceptable overall model calibration (Figure 4).
To assess the internal validity and potential optimism of the COSSH-CAR model, we conducted bootstrap resampling with 1000 replications. This rigorous validation technique involves random sampling with replacement from the original dataset to create multiple bootstrap samples of the same size as the original cohort.
The bootstrap-corrected AUC for the COSSH-CAR model was 0.821 (95%CI: 0.793-0.849) for 28-day mortality and 0.798 (95%CI: 0.767-0.829) for 90-day mortality, indicating minimal optimism of 0.011 and 0.013, respectively. The model maintained excellent discriminative ability after correction for potential overfitting.
The bootstrap validation also confirmed the stability of the model coefficients, with narrow confidence intervals for MELD-Na (coefficient: 0.077; 95%CI: 0.061-0.093), HE grade (coefficient: 0.652; 95%CI: 0.513-0.791), and ΔCAR-7 (coefficient: 0.426; 95%CI: 0.351-0.501). Notably, ΔCAR-7 remained a significant predictor in 97.3% of bootstrap replications, underscoring its robust contribution to the model.
The bootstrap-derived calibration slope was 0.873 (95%CI: 0.824-0.916) for 28-day mortality predictions and 0.812 (95%CI: 0.785-0.893) for 90-day mortality predictions, with values close to 1 indicating good calibration. The calibration-in-the-large parameters were -0.08 (95%CI: -0.21-0.05) and -0.11 (95%CI: -0.25-0.03), respectively, with values close to 0 supporting proper calibration.
These findings confirm the internal validity of the COSSH-CAR model and suggest that its performance estimates are likely to be reliable when applied to new patients from similar clinical settings. Importantly, bootstrap validation dem
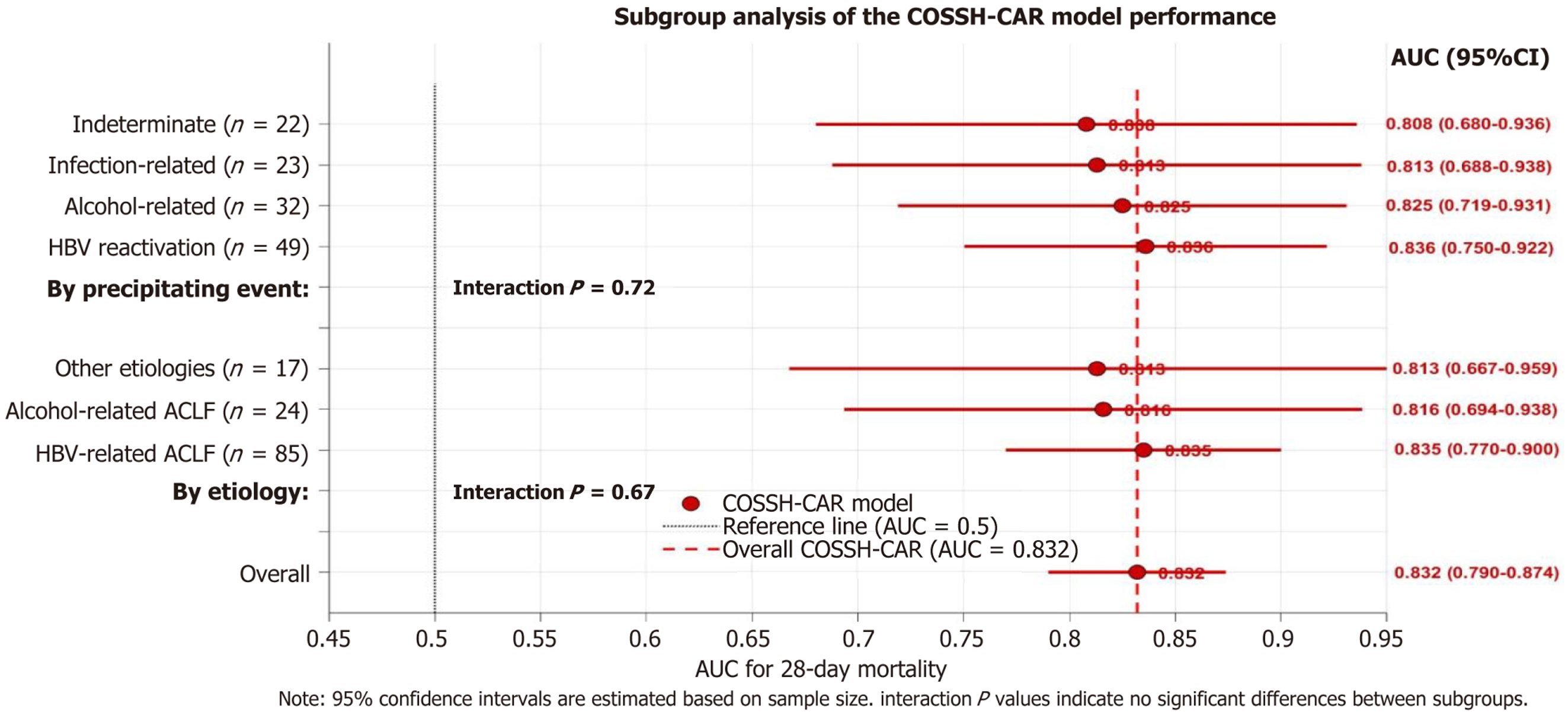
Subgroup analyses were performed to assess the performance of the COSSH-CAR model in patients with different etiologies of chronic liver disease and precipitating event for ACLF. The model maintained good discriminative ability across all major etiological subgroups, with AUCs for 28-day mortality of 0.835 in HBV-related ACLF (n = 85), 0.816 in alcohol-related ACLF (n = 24), and 0.813 in ACLF of other etiologies (n = 17). The predictive value of COSSH-CAR for 28-day mortality remained significant across different precipitating factors, with AUCs of 0.836 in HBV reactivation (n = 49), 0.825 in alcohol-related (n = 32), 0.813 in infection-related (n = 23), and 0.808 in ACLF with indeterminate precipitating events (n = 22). The differences in AUCs between subgroups for both of chronic liver disease etiologies and ACLF precipitating event were not statistically significant (interaction P > 0.05) (Figure 5).
In this prospective cohort study of patients with COSSH-ACLF, we demonstrated that both baseline CAR and, more importantly, its dynamic changes during the first week of hospitalization are strong predictors of short-term mortality. The novel COSSH-CAR model, which incorporates ΔCAR-7 along with baseline MELD-Na score and HE grade, showed superior prognostic accuracy compared to established prognostic scores and effectively stratified patients into distinct risk categories with markedly different survival rates. To our knowledge, this is the first study to comprehensively evaluate the prognostic significance of dynamic changes in CAR in ACLF patients defined by COSSH criteria and to dev
Our findings highlight the central role of systemic inflammation in the pathogenesis and prognosis of ACLF. The significantly higher baseline CAR in non-survivors compared to survivors is consistent with previous studies that have reported associations between inflammatory markers and adverse outcomes in liver diseases. Wang et al[30] reported that elevated CAR was associated with 30-day mortality in HBV-related decompensated cirrhosis, while Oikonomou et al[27] found that CAR predicted medium to long-term mortality in patients with decompensated cirrhosis. However, our study goes for the by demonstrating that dynamic changes in CAR during hospitalization provide additional prognostic value beyond baseline measurements in patients with COSSH-ACLF.
The divergent trajectories of CAR between survivors and non-survivors reflect the dynamic nature of the inflammatory response in ACLF[31]. While survivors showed progressive decline in CAR, indicating resolution of inflammation and/or improvement in hepatic synthetic function, non-survivors exhibited persistent elevation or further increases, suggesting ongoing, uncontrolled systemic inflammation. This pattern became apparent as early as day 4 after admission and was most pronounced by day 7, suggesting that early reassessment of inflammatory status can yield valuable prognostic in
The superior predictive performance of ΔCAR-7 compared to baseline CAR underscores the importance of monitoring the temporal evolution of inflammation rather than relying solely on a single measurement at admission. This finding has important clinical implications, as it suggests that the trajectory of the inflammatory response may be more relevant to prognosis than its initial magnitude[32]. Selecting day 7 for CAR reassessment offers an optimal balance between clinical feasibility and prognostic utility, enabling clinicians to enabling clinicians to evaluate treatment response and adjust management strategies accordingly. While ΔCAR-4 showed earlier divergence, day 7 provided superior discrimination (AUC 0.765 vs 0.706) and is clinically feasible as most ACLF patients remain hospitalized during the first week. Day 7 assessment allows clinicians to evaluate treatment response and adjust management strategies, including consideration for liver transplantation listing or intensive care unit transfer. Patients with persistently elevated inflammatory markers despite standard medical therapy may represent a high-risk group that could benefit from more intensive monitoring, aggressive interventions, or early consideration for liver transplantation.
Several mechanisms may underlie the association between persistent inflammation (as reflected by increasing CAR) and mortality in ACLF. First, ongoing inflammation can directly aggravate hepatocellular injury through mechanisms such as various pathways, including oxidative stress, mitochondrial dysfunction, and activation of death receptors, leading to progressive liver failure[7-9]. Second, systemic inflammation contributes to circulatory dysfunction, including splanchnic vasodilation and hyperdynamic circulation, which may further impair organ perfusion and exacerbate mul
The integration of ΔCAR-7 with baseline MELD-Na and HE grade in the COSSH-CAR model captures multiple dimensions of ACLF pathophysiology, including liver dysfunction (MELD-Na)[19], neurological complications (HE), and systemic inflammation (ΔCAR-7)[17,34]. This comprehensive approach likely accounts for the superior performance of the COSSH-CAR model compared to existing prognostic scores, which primarily focus on organ dysfunction without adequately capturing the inflammatory component.
The COSSH-CAR model demonstrated excellent discriminative ability across different etiological subgroups and precipitating factors, suggesting its broad applicability in diverse ACLF populations. This consistent performance is particularly noteworthy given that COSSH criteria were initially developed for HBV-related ACLF but are increasingly applied to ACLF of various etiologies in clinical practice in China and other Asian countries[35]. Our findings suggest that the combination of baseline disease severity and dynamic inflammatory response captured in the COSSH-CAR model provides robust prognostic information regardless of the underlying etiology or precipitating event.
Future validation and implementation strategies. While our bootstrap internal validation demonstrated model stability, external validation in diverse populations remains essential before widespread clinical implementation. Future multicenter validation should include: (1) Prospective cohorts from multiple geographic regions to assess model generalizability across different healthcare systems; (2) Diverse etiological populations, including non-HBV ACLF patients from Western countries where alcoholic and metabolic liver diseases predominate; and (3) Validation in different ACLF classification systems (e.g., EASL-CLIF criteria) to determine broader applicability. Collaborative international networks could facilitate rapid validation across diverse ACLF populations. The COSSH-CAR model can be integrated into clinical practice through multiple platforms: (1) Electronic Health Record integration with automated calculation using existing laboratory values and clinical assessments, with alerts for high-risk scores; (2) Web-based calculators and mobile applications for point-of-care risk assessment; and (3) Incorporation into clinical decision support systems to guide tran
Our research has several limitations. First, the single-center design and relatively modest sample size (n = 126) may limit the generalizability of the results to broader populations and healthcare settings. While our sample size meets established guidelines for clinical prediction model development (approximately 10 events per predictor variable), larger cohorts would strengthen model reliability. Ideally, we would have utilized separate training and validation cohorts, but our sample size constraints precluded this approach. To address this limitation, we employed bootstrap resampling with 1000 replications for internal validation, which demonstrated minimal model optimism and confirmed the stability of our findings. Nevertheless, external validation in larger, multicenter cohorts with diverse etiological backgrounds is essential before the model can be widely applied. Second, our model requires reassessment at day 7, enhancing predictive accuracy but limiting utility for very early prognostication. This trade-off between immediacy and precision is justified by the substantially improved discriminative performance provided by incorporating temporal inflammatory changes. Third, the observational design precludes causal inferences about whether interventions targeting the inflammatory response would improve outcomes. Prospective interventional studies are needed to determine if CAR-guided therapeutic strategies could translate into improved survival among high-risk patients.
This study demonstrates that dynamic changes in CAR during the first week of hospitalization provide significant prognostic information in patients with COSSH-ACLF beyond baseline values and established prognostic scores. The novel COSSH-CAR model, which incorporates both baseline disease severity (MELD-Na, HE) and the trajectory of systemic inflammation (ΔCAR-7), offers improved risk stratification and may aid clinical decision-making in the management of ACLF patients. Future research should focus on external validation of the COSSH-CAR model in diverse populations and exploration of targeted anti-inflammatory interventions for patients with persistent elevation of inflammatory markers to further refine prognostic assessment in ACLF.
| 1. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2258] [Article Influence: 173.7] [Reference Citation Analysis (6)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023;79:461-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 3. | Ngu NLY, Flanagan E, Bell S, Le ST. Acute-on-chronic liver failure: Controversies and consensus. World J Gastroenterol. 2023;29:232-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 4. | Hernaez R, Li H, Moreau R, Coenraad MJ. Definition, diagnosis and epidemiology of acute-on-chronic liver failure. Liver Int. 2025;45:e15670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Aggarwal A, Biswas S, Arora U, Vaishnav M, Shenoy A, Swaroop S, Agarwal A, Elhence A, Kumar R, Goel A, Shalimar. Definitions, Etiologies, and Outcomes of Acute on Chronic Liver Failure: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024;22:2199-2210.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Chen Y, Li H, Huang Y, Xie Q, Lin S, Li L, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (2)] |
| 7. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (1)] |
| 8. | Clària J, Arroyo V, Moreau R. Roles of systemic inflammatory and metabolic responses in the pathophysiology of acute-on-chronic liver failure. JHEP Rep. 2023;5:100807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 9. | Qiang R, Liu XZ, Xu JC. The Immune Pathogenesis of Acute-On-Chronic Liver Failure and the Danger Hypothesis. Front Immunol. 2022;13:935160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Br VK, Sarin SK. Acute-on-chronic liver failure: Terminology, mechanisms and management. Clin Mol Hepatol. 2023;29:670-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 11. | Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3:100176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Casulleras M, Zhang IW, López-Vicario C, Clària J. Leukocytes, Systemic Inflammation and Immunopathology in Acute-on-Chronic Liver Failure. Cells. 2020;9:2632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 13. | Zhou HH, Tang YL, Xu TH, Cheng B. C-reactive protein: structure, function, regulation, and role in clinical diseases. Front Immunol. 2024;15:1425168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 14. | Rizo-Téllez SA, Sekheri M, Filep JG. C-reactive protein: a target for therapy to reduce inflammation. Front Immunol. 2023;14:1237729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 15. | Ding Z, Wei Y, Peng J, Wang S, Chen G, Sun J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines. 2023;11:2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 16. | Karagoz I, Ozer B, Ital I, Turkoglu M, Disikirik A, Ozer S. C-reactive protein-to-serum albumin ratio as a marker of prognosis in adult intensive care population. Bratisl Lek Listy. 2023;124:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Gao Y, Liang B, Liang Z. The prognostic value of C-reactive protein to albumin ratio in patients with sepsis: a systematic review and meta-analysis. Aging Male. 2023;26:2261540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Kunutsor SK, Laukkanen JA. Serum C-reactive protein-to-albumin ratio is a potential risk indicator for pneumonia: Findings from a prospective cohort study. Respir Med. 2022;199:106894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Freitas ACT, Rampim AT, Nunes CP, Coelho JCU. IMPACT OF MELD SODIUM ON LIVER TRANSPLANTATION WAITING LIST. Arq Bras Cir Dig. 2019;32:e1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (2)] |
| 21. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Zhou X, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Goyes D, Trivedi HD, Curry MP. Prognostic Models in Acute-on-Chronic Liver Failure. Clin Liver Dis. 2023;27:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Patange AP, Desai JV, Pujari B, Marwah A, Dey A. Dynamic Assessment of Hematological Parameters as Predictive Biomarkers for Disease Severity and Prognosis in COVID-19 Patients: A Longitudinal Study. Cureus. 2024;16:e63593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Varga NI, Benea AT, Suba MI, Bota AV, Avram CR, Boru C, Dragomir TL, Prisca M, Sonia T, Susan M, Horhat FG. Predicting Mortality in Sepsis: The Role of Dynamic Biomarker Changes and Clinical Scores-A Retrospective Cohort Study. Diagnostics (Basel). 2024;14:1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Naganuma A, Aoki T, Koizumi Y, Nakamura S, Joko K, Hiasa Y, Kudo M. C-reactive protein to albumin ratio predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Sci Rep. 2022;12:8421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Wang S, Xu S, Wang J, Ye H, Zhang K, Fan X, Xu X. Preoperative C-reactive protein to albumin ratio may be a good prognostic marker in patients undergoing hepatectomy for hepatocellular carcinoma: a meta-analysis. Front Nutr. 2024;11:1444352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Oikonomou T, Goulis I, Kiapidou S, Tagkou N, Akriviadis E, Papatheodoridis G, Cholongitas E. The significance of C-reactive protein to albumin ratio in patients with decompensated cirrhosis. Ann Gastroenterol. 2020;33:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Xu X, Ding H, Li W, Han Y, Guan Y, Xu J, Han Y, Jia J, Wei L, Duan Z, Nan Y, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association. Chinese Guidelines on the Management of Hepatic Encephalopathy in Cirrhosis (2024). J Clin Transl Hepatol. 2025;13:253-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (2)] |
| 30. | Wang CJ, Wu JP, Zhou WQ, Mao WL, Huang HB. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Patients with HBV-Related Decompensated Cirrhosis. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 32. | Sundaram V, Kogachi S, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Levitsky J, Rahimi RS, Jalan R. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Trebicka J, Hernaez R, Shawcross DL, Gerbes AL. Recent advances in the prevention and treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF) and the role of biomarkers. Gut. 2024;73:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Piano S, Mahmud N, Caraceni P, Tonon M, Mookerjee RP. Mechanisms and treatment approaches for ACLF. Liver Int. 2025;45:e15733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Choudhury A, Kulkarni AV, Arora V, Soin AS, Dokmeci AK, Chowdhury A, Koshy A, Duseja A, Kumar A, Mishra AK, Patwa AK, Sood A, Roy A, Shukla A, Chan A, Krag A, Mukund A, Mandot A, Goel A, Butt AS, Sahney A, Shrestha A, Cárdenas A, Di Giorgio A, Arora A, Anand AC, Dhawan A, Jindal A, Saraya A, Srivastava A, Kumar A, Kaewdech A, Pande A, Rastogi A, Valsan A, Goel A, Kumar A, Singal AK, Tanaka A, Coilly A, Singh A, Meena BL, Jagadisan B, Sharma BC, Lal BB, Eapen CE, Yaghi C, Kedarisetty CK, Kim CW, Panackel C, Yu C, Kalal CR, Bihari C, Huang CH, Vasishtha C, Jansen C, Strassburg C, Lin CY, Karvellas CJ, Lesmana CRA, Philips CA, Shawcross D, Kapoor D, Agrawal D, Payawal DA, Praharaj DL, Jothimani D, Song DS, Kim DJ, Kim DS, Zhongping D, Karim F, Durand F, Shiha GE, D'Amico G, Lau GK, Pati GK, Narro GEC, Lee GH, Adali G, Dhakal GP, Szabo G, Lin HC, Li H, Nair HK, Devarbhavi H, Tevethia H, Ghazinian H, Ilango H, Yu HL, Hasan I, Fernandez J, George J, Behari J, Fung J, Bajaj J, Benjamin J, Lai JC, Jia J, Hu JH, Chen JJ, Hou JL, Yang JM, Chang J, Trebicka J, Kalf JC, Sollano JD, Varghese J, Arab JP, Li J, Reddy KR, Raja K, Panda K, Kajal K, Kumar K, Madan K, Kalista KF, Thanapirom K, Win KM, Suk KT, Devadas K, Lesmana LA, Kamani L, Premkumar M, Niriella MA, Al Mahtab M, Yuen MF, Sayed MH, Alla M, Wadhawan M, Sharma MK, Sahu M, Prasad M, Muthiah MD, Schulz M, Bajpai M, Reddy MS, Praktiknjo M, Yu ML, Prasad M, Sharma M, Elbasiony M, Eslam M, Azam MG, Rela M, Desai MS, Vij M, Mahmud N, Choudhary NS, Marannan NK, Ormeci N, Saraf N, Verma N, Nakayama N, Kawada N, Oidov Baatarkhuu, Goyal O, Yokosuka O, Rao PN, Angeli P, Parikh P, Kamath PS, Thuluvath PJ, Lingohr P, Ranjan P, Bhangui P, Rathi P, Sakhuja P, Puri P, Ning Q, Dhiman RK, Kumar R, Vijayaraghavan R, Khanna R, Maiwall R, Mohanka R, Moreau R, Gani RA, Loomba R, Mehtani R, Rajaram RB, Hamid SS, Palnitkar S, Lal S, Biswas S, Chirapongsathorn S, Agarwal S, Sachdeva S, Saigal S, Kumar SE, Violeta S, Singh SP, Mochida S, Mukewar S, Alam S, Lim SG, Alam S, Shalimar, Venishetty S, Sundaram SS, Shetty S, Bhatia S, Singh SA, Kottilil S, Strasser S, Shasthry SM, Maung ST, Tan SS, Treeprasertsuk S, Asthana S, Manekeller S, Gupta S, Acharya SK, K C S, Maharshi S, Asrani S, Dadhich S, Taneja S, Giri S, Singh S, Chen T, Gupta T, Kanda T, Tanwandee T, Piratvishuth T, Spengler U, Prasad VGM, Midha V, Rakhmetova V, Arroyo V, Sood V, Br VK, Wong VW, Pamecha V, Singh V, Dayal VM, Saraswat VA, Kim W, Jafri W, Gu W, Jun WY, Qi X, Chawla YK, Kim YJ, Shi Y, Abbas Z, Kumar G, Shiina S, Wei L, Omata M, Sarin SK; APASL-ACLF Research Consortium (AARC) for APASL-ACLF working party. Acute-on-chronic liver failure (ACLF): the 'Kyoto Consensus'-steps from Asia. Hepatol Int. 2025;19:1-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (1)] |