Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.109965
Revised: June 30, 2025
Accepted: August 12, 2025
Published online: September 27, 2025
Processing time: 122 Days and 2.9 Hours
The gut–liver-pancreas axis (GLPA) is a critical network shaped by gut microbiota (GM) and their metabolites, essential for maintaining metabolic and immune balance. Disruption of this microbial equilibrium, known as dysbiosis, contributes to the development and progression of various hepatic and pancreatic diseases. Through mechanisms such as increased intestinal permeability and exposure to microbial products-including lipopolysaccharide, trimethylamine-N-oxide, and secondary bile acids-dysbiosis promotes inflammation, oxidative stress, insulin resistance, and carcinogenesis. These changes are linked to conditions including metabolic dysfunction-associated steatotic liver disease, alcohol-associated liver disease, cirrhosis, hepatocellular carcinoma, pancreatitis, pancreatic ductal ade
Core Tip: This editorial highlights the central role of gut microbiota in regulating the gut-liver-pancreas axis, a dynamic network critical for metabolic and immune homeostasis. Dysbiosis-driven disruptions-via microbial metabolites, barrier dysfunction, and immune imbalance-underlie the pathogenesis of liver and pancreatic diseases. While gut-liver interactions are well studied, gut-pancreas crosstalk remains underexplored. We emphasize emerging biomarkers, microbial-based therapies, and the urgent need for longitudinal studies and metabolomic profiling to translate microbiome science into precision diagnostics and treatments for hepatopancreatic disorders.
- Citation: Abdelwahab MM, Ghattas AS, Tawheed A. Implications of gut microbiota in hepatic and pancreatic diseases: Gut-liver-pancreas axis. World J Hepatol 2025; 17(9): 109965
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/109965.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.109965
The human body hosts trillions of microorganisms, predominantly in the gut, forming a complex ecosystem crucial for health. These microbes, including bacteria, viruses, and fungi, interact symbiotically with the host, influencing me
Dysbiosis-an imbalance in microbial composition-disrupts GLPA homeostasis, leading to disease. A compromised intestinal barrier permits harmful molecules like lipopolysaccharide (LPS) to enter the liver, triggering inflammation via toll-like receptor 4 (TLR4) and promoting conditions such as metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD)[3]. Similarly, diseases like type 2 diabetes (T2D) involve gut barrier dysfunction and microbial translocation, promoting inflammation and insulin resistance[4]. These processes, along with harmful metabolites like secondary BAs and trimethylamine-N-oxide (TMAO), impair β-cell function and contribute to diabetes, pancreatitis, and cancers such as hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (PDAC).
Immune dysregulation plays a pivotal role, with dysbiosis skewing T-cell responses toward pro-inflammatory Th17 cells and away from regulatory T-cells (Tregs). Triggers of inflammasome activation exacerbate inflammation, while oxidative stress from microbial byproducts through reactive oxygen and nitrative species damages tissues, accelerating disease progression. Clinically, microbial signatures (e.g., Proteobacteria overgrowth) and metabolomic profiles (e.g., SCFA depletion) serve as biomarkers for diseases like cirrhosis and PDAC[5].
Emerging therapies targeting the gut microbiota (GM), such as probiotics and fecal microbiota transplantation (FMT), aim to restore microbial balance. However, challenges in standardization and in-depth understanding of gut-pancreas interactions persist. While the gut-liver axis has been extensively studied, the direct interaction between the gut and the pancreas remains insufficiently explored[6]. Most existing studies focus on hepatic outcomes of gut dysbiosis, often overlooking how gut-derived metabolites and immune signaling pathways influence pancreatic function. This editorial aims to address that gap by offering an integrated view of the GLPA, emphasizing how GM contribute to both hepatic and pancreatic pathophysiology. By dissecting microbial metabolites, immune cross-talk, and therapeutic potential, we aim to illuminate strategies for restoring GLPA equilibrium and mitigating the global burden of chronic hepatopancreatic diseases.
The GLPA represents a sophisticated network of anatomical and functional interactions that integrate metabolic, immune, and endocrine signaling across organs. Central to this axis is the bidirectional communication mediated by microbial metabolites, which orchestrate systemic homeostasis or, under dysbiotic conditions, drive pathophysiology[2].
The liver derives approximately 75% of its blood supply from the intestinal portal circulation, facilitating direct exposure to gut-derived nutrients, microbial metabolites, and endotoxins. This vascular linkage ensures that BAs, synthesized in the liver and secreted into the intestines, undergo enterohepatic recirculation-a process modulated by microbial metabolism[7]. The pancreas, anatomically connected to the duodenum via the pancreatic duct, interfaces with the GLPA through endocrine secretion (e.g., insulin, glucagon) and susceptibility to microbial translocation via mesenteric circulation[2].
SCFAs, such as acetate, propionate, and butyrate, are produced by the microbial fermentation of dietary fiber. These metabolites play crucial roles in maintaining intestinal barrier integrity, regulating hepatic gluconeogenesis and glycogen storage, and enhancing glucose-stimulated insulin secretion via G-protein-coupled receptors (e.g., FFAR2/3) on pancreatic β-cells[8]. Similarly, BAs undergo microbial modification, transforming primary BAs into secondary forms like de
Dysbiosis-defined as a pathological imbalance in gut microbial composition marked by reduced diversity, overgrowth of harmful pathobionts, and depletion of beneficial taxa[11] serves as the pivotal disruptor of the GLPA. The upcoming section explores how these disruptions drive fibrosis, cancer, and multi-organ disease.
The gut liver pancreas axis contributes to liver and pancreatic diseases through a network of interconnected mechanisms, including intestinal barrier disruption, imbalanced microbial metabolites, and immune system dysregulation.
A compromised intestinal barrier (“leaky gut”) is a hallmark of GLPA axis disruption. Dysbiosis degrades tight junction proteins (e.g., occludin, zonula occludens-1) and thins the mucus layer, enabling translocation of microbial products like LPS into the portal circulation. LPS, a gram-negative bacterial endotoxin, activates hepatic TLR4, triggering Kupffer cells, the liver’s resident macrophages, to release pro-inflammatory cytokines (TNF-α, IL-6) and reactive oxygen species (ROS)[4,12]. Chronic endotoxemia perpetuates systemic inflammation and promotes fibrotic remodeling across multiple organs. Alcohol, high-fat diets, and pathobionts (e.g., Proteobacteria) exacerbate barrier dysfunction, while reduced SCFAs, worsen permeability[13]. In the pancreas, LPS translocation promotes β-cell apoptosis and chronic pancreatitis (CP), linking “leaky gut” to pancreatic dysfunction[2].
Harmful metabolites include TMAO, derived from microbial choline metabolism, which impairs reverse cholesterol transport, promotes hepatic steatosis, and exacerbates insulin resistance[12]; ethanol produced by bacteria (e.g., Klebsiella) that induces oxidative stress and lipid peroxidation, mimicking alcohol-induced liver injury[14]; and excess of secondary bile such as DCA promotes hepatocyte senescence, cholangiocyte damage, and pro-tumorigenic STAT3 signaling[2].
Immune dysregulation is a central mechanism by which gut dysbiosis contributes to liver and pancreatic pathology. Disrupted microbial composition skews host immunity through several interconnected pathways.
One key pathway involves the imbalance between pro-inflammatory T-helper 17 (Th17) cells and regulatory T cells (Tregs). Dysbiosis favors the expansion of Th17 cells while suppressing Treg development. Th17 cells secrete cytokines such as IL-17 and IL-22, which recruit neutrophils and activate inflammatory cascades that contribute to tissue injury. This Th17/Treg imbalance has been implicated in the development of MASLD, autoimmune liver diseases like primary biliary cholangitis (PBC), and pancreatic inflammation[15].
Another major mechanism is the activation of inflammasomes by gut-derived microbial components, including LPS and other pathogen-associated molecular patterns. These molecules activate inflammasome complexes-particularly NLRP3 and NLRP6-in both hepatic and intestinal tissues. The inflammasomes regulate the maturation and secretion of pro-inflammatory cytokines, such as IL-1β and IL-18, which drive chronic inflammation in the liver and pancreas. When dysbiosis disrupts normal inflammasome signaling, it can impair mucosal healing, increase intestinal permeability, and facilitate microbial translocation, thereby perpetuating a vicious cycle of inflammation[14,16].
Oxidative and nitrative stress represent additional mechanisms by which gut-derived microbial products contribute to tissue injury. ROS, generated in response to microbial metabolites such as ethanol and LPS, activate hepatic stellate cells, promote extracellular matrix deposition, and initiate fibrogenesis. ROS also inflicts direct damage on mitochondrial DNA, lipids, and proteins, exacerbating cellular injury. Moreover, nitrative species like peroxynitrite (ONOO—), formed from the reaction between nitric oxide and superoxide, impair mitochondrial function and protein integrity, thereby am
The mechanistic pillars described above do not function in isolation. The GLPA is a tightly interconnected system, where dysfunction in one organ often propagates reciprocal pathology in others[2]. Liver cirrhosis disrupts BA synthesis, altering microbial composition and weakening the gut barrier, which enhances microbial translocation and promotes pancreatic inflammation[18]. Conversely, pancreatic exocrine insufficiency impairs nutrient digestion and BA signaling, fostering gut dysbiosis that accelerates liver disease[19]. Inflammatory mediators and microbial metabolites such as TMAO and secondary BAs can circulate systemically, compounding insulin resistance and immune activation in both hepatic and pancreatic contexts[20]. These feedback loops underscore the need to treat GLPA dysfunction as a unified, axis-wide pathology rather than isolated organ diseases.
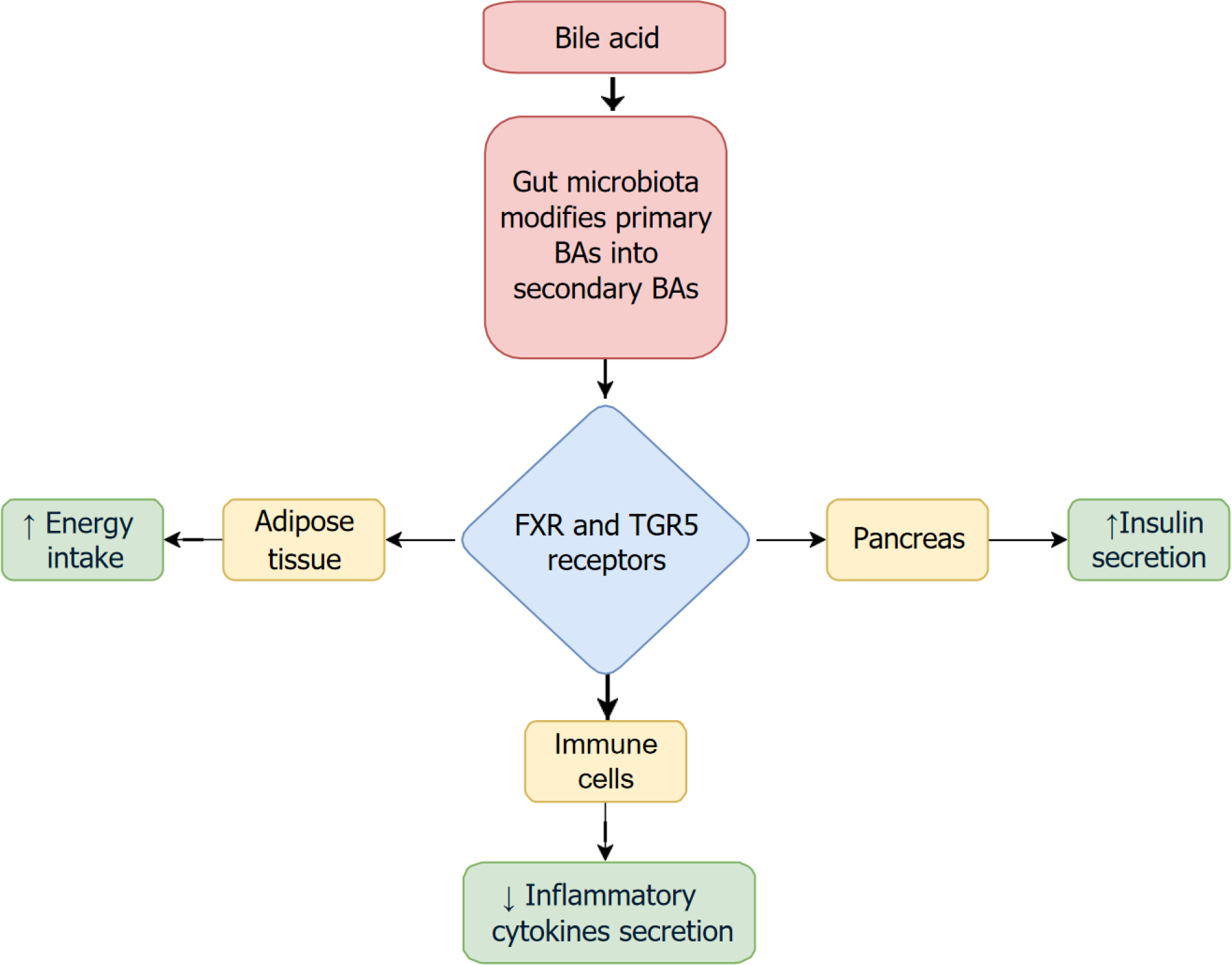
The interplay between GM, BAs, and the pancreas constitutes a key component of the GLPA. The GM modifies primary BAs into secondary BAs through various enzymatic reactions, including deconjugation and dehydroxylation, thereby altering the composition and function of the BA pool[9]. These microbially modified BAs engage receptors such as FXR and TGR5, which are expressed in multiple tissues, including the pancreas. Activation of TGR5 and FXR not only regulates glucose and lipid metabolism but also enhances insulin secretion and improves pancreatic endocrine function, they also have multiple effects as shown in Figure 1[21]. Dysbiosis, by disrupting BA composition, can impair this signaling, contributing to β-cell dysfunction, insulin resistance, and the development of metabolic disorders like T2D. Thus, the microbiota-BA-pancreas axis plays a critical regulatory role in maintaining pancreatic metabolic homeostasis and represents a potential target for therapeutic intervention[2].
MASLD progression correlates with Bacteroides enrichment, Firmicutes depletion, and ethanol-producing taxa. Impaired BA conversion reduces FXR activation, promoting lipogenesis, while LPS-TLR4 signaling drives steatohepatitis[22].
ALD is characterized by Lactobacillus/Bifidobacterium depletion and Proteobacteria expansion. Alcohol metabolism by gut microbes generates acetaldehyde, exacerbating intestinal permeability and Kupffer cell-derived TGF-β-mediated fibrosis[23,24].
Primary sclerosing cholangitis (PSC) is marked by bile duct inflammation and scarring and is associated with intestinal microbiota dysbiosis. Studies have shown reduced microbial diversity and an overrepresentation of proinflammatory genera such as Enterococcus, Veillonella, Streptococcus, and Lactobacillus, regardless of the presence of inflammatory bowel disease. Additionally, there is enrichment of Barnesiellaceae and depletion of Clostridiales. These microbial alterations may contribute to PSC pathogenesis by promoting bacterobilia, which activates proinflammatory responses in cholangiocytes and leads to fibrosis. Endotoxemia may also drive molecular mimicry, triggering antibody production and immune-mediated bile duct damage[14].
PBC is characterized by progressive bile duct destruction and is associated with significant intestinal microbiota dysbiosis. Studies have shown that PBC patients, especially those not yet treated with ursodeoxycholic acid, exhibit reduced levels of beneficial bacteria (e.g., Bacteroidetes, Ruminococcus bromii) and increased levels of opportunistic pathogens (e.g., Veillonella, Streptococcus, Klebsiella, Proteobacteria). These microbial changes correlate with elevated markers of liver injury and inflammation, suggesting that intestinal microbiota alterations may contribute to PBC path
Cirrhosis, characterized by severe fibrosis and hepatocyte loss, is associated with intestinal microbiota dysbiosis: Reduced Bacteroidetes and beneficial taxa (Blautia, Lachnospiraceae) alongside pathogenic overgrowth (Proteobacteria, Enterococcus)[25]. Hepatic encephalopathy correlates with elevated Alcaligenaceae, Enterobacteriaceae, and endotoxemia, while Ruminococcaceae depletion impairs ammonia detoxification. Urease-producing bacteria (e.g., Klebsiella) increase intestinal ammonia, overwhelming hepatic clearance[26].
Pro-inflammatory taxa [Escherichia coli (E. coli), Helicobacter hepaticus] activate TLR4/STAT3, while genotoxins from Enterococcus faecalis induce DNA damage. DCA accumulation promotes stromal inflammation and hepatocarcinogenesis[27,28].
Chronic HBV/HCV infections correlate with reduced beneficial taxa (Lactobacillus, Bifidobacterium) and increased pathogens (E. coli, Enterobacteriaceae), driving inflammation via LPS-TLR activation and impaired butyrate production. HBV progression involves reduced Lachnospiraceae (anti-inflammatory) and elevated LPS-producing bacteria, while HCV dysbiosis elevates Bacteroidetes and endotoxemia[29].
The pancreas is indirectly influenced by GM through metabolic, immune, and inflammatory pathways described in Section “Mechanistic Pillars of Axis-Mediated Injury”. Dysbiosis can lead to bacterial translocation, alter metabolite signaling, and compromise endocrine function, contributing to a range of pancreatic disorders.
Acute pancreatitis (AP) and CP are exacerbated by gut-derived microbial disturbance[30]. In AP Increasing evidence indicates that intestinal microecological disorder might promote the progression of AP through multiple mechanisms[31]. those mechanisms include reduced zymogen secretion which leads to GM imbalance, disruption of acinar cells and infiltrating immune cells. This, in turn, results in increased excessive production of ROSs, which further exacerbates the impairment of the intestinal barrier and microbial dysbiosis such as the overgrowth of Proteobacteria and Actinobacteria phyla through the TLR4/NF-κB signaling pathways[32]. Mitochondrial dysfunction caused by several gut bacterial metabolites including hydrogen sulfide, indole-3-acetic acid, and LPS, whereas beneficial microbiota-derived nicotinamide mononucleotide could reduce mitochondrial oxidative injury via promoting the activation of mitochondrial deacetylase SIRT3[33].
Other possible mechanisms include increased oxidative stress, imbalance of immunological homeostasis, intestinal microcirculation and barrier disturbance[34]. Therefore, regulating microbial metabolites may represent a promising strategy to protect AP-mediated oxidative damage[35]. While in CP the GM dysbiosis play critical role, particularly the depletion of SCFA-producing bacteria, in the progression of CP. Disruption of Gram-positive (G+) bacteria, such as Ruminococcus, Lactobacillus, and Roseburia, reduces SCFA levels, impairing gut barrier function and exacerbating pancreatic fibrosis, monocyte recruitment, and M2 macrophage polarization thereby aggravating the progression of CP[36]. Small intestinal bacterial overgrowth (SIBO) is common in CP and worsens exocrine insufficiency. Dysbiosis also promotes chronic inflammation and fibrosis via barrier breach and inflammatory triggers[37].
PDAC is marked by an altered tumor-associated microbiota, which modulates the immune microenvironment and contributes to chemoresistance. Certain taxa may influence tumor signaling, while microbial metabolites affect the efficacy of immune checkpoint inhibitors. Dysbiosis-induced inflammation and BA imbalance support tumor progression[38].
GM alterations contribute to both type 1 and T2D by: Inducing β-cell inflammation and apoptosis, and by modifying GLP-1 secretion and incretin responsiveness, and Promoting insulin resistance.
Low butyrate levels correlate with pancreatic steatosis and impaired glucose metabolism. SCFA supplementation and dietary modulation show promise in preserving pancreatic endocrine function[39].
The gut microbiome plays a key role in liver and pancreatic diseases, offering new ways to diagnose and predict outcomes. Advanced tools like stool metagenomics and blood metabolomics help identify microbial imbalances linked to conditions such as fatty liver disease, cirrhosis, and pancreatic cancer. These microbial and metabolic patterns could lead to earlier detection and personalized treatments, though more research is needed (Table 1).
| Disease | Key microbiota findings in the diseased person | Therapeutic strategies using probiotics/symbiotics |
| Chronic hepatitis B | Reduced SCFA-producers (e.g., Roseburia, Dialister); increased Escherichia–Shigella and Proteobacteria | Restoration of microbial balance via lactobacillus and bifidobacterium strains |
| Liver cirrhosis | Overgrowth of Enterobacteriaceae; reduced Lachnospiraceae, Ruminococcaceae; decreased SCFA producers | Rebalancing microbiota using synbiotics; targeted probiotics |
| Hepatocellular crcinoma | Enrichment of Proteobacteria, Rothia; depletion of Bifidobacterium, Roseburia | Prevention in aflatoxin exposure models using probiotic mixtures |
| Across CLD spectrum | Gradual decrease in SCFA-producing bacteria; increased gram-negative pathogens | General microbiota modulation via diet and probiotics |
| Acute pancreatitis | ↑ Enterococcus, ↓ Bifidobacterium in severe cases; increased IL-6 with dysbiosis | Lactobacillus plantarum reduced necrosis in RCTs; caution due to PROPATRIA trial findings |
| Chronic pancreatitis | ↑ Proteobacteria, ↓ Firmicutes; SIBO in approximately 36% of patients | Symbiotics (e.g., Lactobacillus + FOS); PERT restores Akkermansia muciniphila |
| Pancreatic cancer | Oral: ↑ Porphyromonas gingivalis; Tumor: ↑ Fusobacterium, Proteobacteria | Probiotics may enhance chemotherapy; antibiotics may reduce gemcitabine-degrading bacteria |
| Diabetes mellitus | ↓ Faecalibacterium and butyrate levels; ↑ LPS-producing taxa | SCFA supplementation; dietary prebiotics |
In chronic liver diseases, there is a consistent decrease in beneficial bacteria such as Firmicutes, Roseburia, and Blautia, alongside an increase in potentially pathogenic taxa including Proteobacteria, E. coli, and Enterococcus. These microbial shifts correlate with disease progression and severity, as reflected by the Child-Pugh classification[40].
In pancreatic diseases, AP is associated with increased Enterococcus and decreased Bifidobacterium, while approximately 36% of patients with CP exhibit SIBO. In pancreatic cancer, a high Firmicutes/Bacteroidetes ratio and the presence of Fusobacterium nucleatum have been linked to increased disease risk[41].
Pancreatic cancer is associated with distinct microbial alterations, including enrichment of Streptococcus, Proteobacteria, and Fusobacterium nucleatum in tumor tissues. These shifts are linked to tumor progression, liver metastasis, and poor prognosis. Conversely, higher abundance of Akkermansia has been associated with better response to neoadjuvant therapy. These microbial patterns are being investigated as potential diagnostic and prognostic biomarkers, as well as therapeutic targets in microbiome-modulating interventions[42].
In HCC, altered GM composition includes increased Veillonella, Dialister, and Fusobacterium, correlating with disease recurrence and severity. Metabolomic shifts, such as reduced stercobilin and elevated triterpenoids, further support diagnostic utility. Certain taxa, such as Clostridium leptum and Bacteroides stercoris, have been linked to favorable prognosis and improved response to immunotherapy. These findings underscore the microbiome’s relevance in HCC progression and therapeutic outcomes[43].
High-fiber and Mediterranean-style diets have been shown to promote the production of SCFAs by beneficial microbes such as Bifidobacterium and Lactobacillus, thereby strengthening intestinal barrier integrity, reducing systemic inflammation, and modulating hepatic metabolism and autophagy. Nutritional strategies that emphasize increased dietary fiber and reduced saturated fat intake play a foundational role in shaping GM composition and function. Additionally, diets enriched with polyphenols, omega-3 fatty acids, and complex carbohydrates are associated with enhanced microbial diversity and improved metabolic profiles[44,45].
Microbiota-targeted therapies are emerging as promising interventions aimed at restoring gut microbial balance and modulating host-microbiota interactions. The administration of beneficial bacterial strains or fermentable fibers, such as probiotics and prebiotics, helps re-establish a favorable microbial profile. Specific strains like Lactobacillus and Bifidobacterium have been shown to reduce intestinal inflammation, enhance barrier integrity, and modulate immune responses, while prebiotics like inulin and fructooligosaccharides selectively promote the growth of SCFA-producing bacteria. FMT introduces a diverse, healthy microbiota from a donor to a recipient, aiming to outcompete dysbiotic species and restore microbial diversity, and has shown success in treating recurrent Clostridioides difficile infection, with ongoing investigations into its use for metabolic and inflammatory conditions. BA modulators, including BA sequestrants, FXR agonists, and dietary strategies that alter enterohepatic circulation, can influence BA synthesis or receptor signaling (FXR, TGR5), thereby reshaping microbial composition and metabolic output. Supplementation with SCFAs such as butyrate, propionate, and acetate may help compensate for microbial deficits, offering anti-inflammatory benefits, strengthening epithelial barriers, and regulating immune signaling. Finally, next-generation microbiome modulators, including engineered probiotics, microbial consortia, and postbiotics, are being developed to deliver precise therapeutic effects, with emerging technologies like CRISPR-edited bacteria and synthetic microbial ecosystems targeting specific pathogenic pathways or metabolite profiles.
This study highlights several important avenues for future research, including the need for larger, longitudinal cohorts to validate the progression-linked microbial shifts observed from chronic hepatitis B through cirrhosis to HCC. Further investigations using metabolomics are critical to clarify the role of microbial by-products such as SCFAs, BAs, and LPSs in disease progression. Experimental studies on butyrate or acetate supplementation, particularly in animal models or clinical trials, could help define therapeutic targets. Additionally, greater focus should be placed on exploring the GLPA, as interactions between intestinal microbiota and pancreatic function remain under-investigated and may significantly influence the pathogenesis of both hepatic and pancreatic diseases. Addressing these future directions will be essential for advancing microbiome-based diagnostic and therapeutic strategies for chronic liver disease and its association with pancreatic dysfunction.
The GLPA plays a crucial role in maintaining metabolic and immune balance, with the GM acting as a central regulator. Dysbiosis disrupts this axis, contributing to liver and pancreatic diseases through mechanisms such as barrier dysfunction, harmful metabolites, and immune imbalance. While emerging tools like metagenomics offer diagnostic promise, and therapies such as probiotics and dietary interventions show potential, clinical application remains limited. Future research should focus on clarifying microbial mechanisms and standardizing interventions to harness the GLPA for effective, personalized treatment strategies.
| 1. | Wang L, Cao ZM, Zhang LL, Li JM, Lv WL. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front Immunol. 2022;13:923599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Marroncini G, Naldi L, Martinelli S, Amedei A. Gut-Liver-Pancreas Axis Crosstalk in Health and Disease: From the Role of Microbial Metabolites to Innovative Microbiota Manipulating Strategies. Biomedicines. 2024;12:1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1456] [Article Influence: 242.7] [Reference Citation Analysis (1)] |
| 4. | Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 454] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 5. | Silveira MAD, Bilodeau S, Greten TF, Wang XW, Trinchieri G. The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer. 2022;8:583-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Zhang T, Gao G, Sakandar HA, Kwok LY, Sun Z. Gut Dysbiosis in Pancreatic Diseases: A Causative Factor and a Novel Therapeutic Target. Front Nutr. 2022;9:814269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Xiao K, Li K, Xiao K, Yang J, Zhou L. Gut Microbiota and Hepatocellular Carcinoma: Metabolic Products and Immunotherapy Modulation. Cancer Med. 2025;14:e70914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2603] [Article Influence: 260.3] [Reference Citation Analysis (1)] |
| 9. | Lucas LN, Barrett K, Kerby RL, Zhang Q, Cattaneo LE, Stevenson D, Rey FE, Amador-Noguez D. Dominant Bacterial Phyla from the Human Gut Show Widespread Ability To Transform and Conjugate Bile Acids. mSystems. 2021;6:101128msystems0080521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 10. | Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 1286] [Article Influence: 183.7] [Reference Citation Analysis (0)] |
| 11. | Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 775] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 12. | Liu J, Yang D, Wang X, Asare PT, Zhang Q, Na L, Shao L. Gut Microbiota Targeted Approach in the Management of Chronic Liver Diseases. Front Cell Infect Microbiol. 2022;12:774335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Brandl K, Kumar V, Eckmann L. Gut-liver axis at the frontier of host-microbial interactions. Am J Physiol Gastrointest Liver Physiol. 2017;312:G413-G419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Schwenger KJ, Clermont-Dejean N, Allard JP. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep. 2019;1:214-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Zhang S, Gang X, Yang S, Cui M, Sun L, Li Z, Wang G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front Immunol. 2021;12:678355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Zhao T, Gu J, Tang F, Zhu L. Gut microbiota and inflammasome-mediated pyroptosis: a bibliometric analysis from 2014 to 2023. Front Microbiol. 2024;15:1413490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Che Z, Zhou Z, Li SQ, Gao L, Xiao J, Wong NK. ROS/RNS as molecular signatures of chronic liver diseases. Trends Mol Med. 2023;29:951-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 18. | Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021;75 Suppl 1:S67-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 19. | Guo Y, Cao F, Li F. Impacts of pancreatic exocrine insufficiency on gut microbiota. J Zhejiang Univ Sci B. 2024;25:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1185] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 21. | Hao Y, Han L, Wu A, Bochkis IM. Pioneer Factor Foxa2 Mediates Chromatin Conformation Changes for Activation of Bile Acid Targets of FXR. Cell Mol Gastroenterol Hepatol. 2024;17:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30:675-688.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 23. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 610] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 24. | Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: pathogenetic role and therapeutic perspectives. World J Gastroenterol. 2014;20:16639-16648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6:34055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 433] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 27. | Krüttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G, Luedde T, Roderburg C. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Tao X, Wang N, Qin W. Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Tumors. 2015;2:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Sehgal R, Bedi O, Trehanpati N. Role of Microbiota in Pathogenesis and Management of Viral Hepatitis. Front Cell Infect Microbiol. 2020;10:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 30. | Zhou J, Li ML, Zhang DD, Lin HY, Dai XH, Sun XL, Li JT, Song LY, Peng H, Wen MM. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology. 2016;16:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | van den Berg FF, van Dalen D, Hyoju SK, van Santvoort HC, Besselink MG, Wiersinga WJ, Zaborina O, Boermeester MA, Alverdy J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut. 2021;70:915-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | Mei QX, Hu JH, Huang ZH, Fan JJ, Huang CL, Lu YY, Wang XP, Zeng Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol Sin. 2021;42:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Liu LW, Xie Y, Li GQ, Zhang T, Sui YH, Zhao ZJ, Zhang YY, Yang WB, Geng XL, Xue DB, Chen H, Wang YW, Lu TQ, Shang LR, Li ZB, Li L, Sun B. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br J Pharmacol. 2023;180:647-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (1)] |
| 34. | Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, Yuan H, Liu C, Li Y, Xu K. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 35. | Du Z, Li G, Luo Y, Bai X, Sun B. The role of gut microbiota in acute pancreatitis: new perspectives in pathogenesis and therapeutic approaches. J Pancreat. 2024;7:61-71. [DOI] [Full Text] |
| 36. | Pan LL, Ren ZN, Yang J, Li BB, Huang YW, Song DX, Li X, Xu JJ, Bhatia M, Zou DW, Zhou CH, Sun J. Gut microbiota controls the development of chronic pancreatitis: A critical role of short-chain fatty acids-producing Gram-positive bacteria. Acta Pharm Sin B. 2023;13:4202-4216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1933] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 38. | Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, Tang HY, Liu Q, Auslander N, Dang CV, Abdel-Mohsen M, Kossenkov A, Stanger BZ, Shinde RS. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. 2022;7:eabn0704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 39. | Kirsoy F, Yalniz M, Bahçecioğlu İH, Artaş H, Türkoğlu S, Solmaz O, Tawheed A. The gut-pancreas axis: investigating the relationship between microbiota metabolites and pancreatic steatosis. Intern Emerg Med. 2024;19:1887-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Ma C, Yang J, Fu XN, Luo JY, Liu P, Zeng XL, Li XY, Zhang SL, Zheng S. Microbial characteristics of gut microbiome dysbiosis in patients with chronic liver disease. World J Hepatol. 2025;17:106124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (4)] |
| 41. | Qin HL, Zheng JJ, Tong DN, Chen WX, Fan XB, Hang XM, Jiang YQ. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Pourali G, Kazemi D, Chadeganipour AS, Arastonejad M, Kashani SN, Pourali R, Maftooh M, Akbarzade H, Fiuji H, Hassanian SM, Ghayour-Mobarhan M, Ferns GA, Khazaei M, Avan A. Microbiome as a biomarker and therapeutic target in pancreatic cancer. BMC Microbiol. 2024;24:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 43. | Lee MS, Son MY, Cho HS. The Gut Microbiome in Hepatocellular Carcinoma: Proliferation, Inhibition, Diagnosis, and Immunotherapy. J Microbiol Biotechnol. 2025;35:e2412075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Llauradó G, Cedó L, Climent E, Badia J, Rojo-Martínez G, Flores-Le Roux J, Yanes O, Vinaixa M, Granado-Casas M, Mauricio D, Fernández-Veledo S, Vendrell J. Circulating short-chain fatty acids and Mediterranean food patterns. A potential role for the prediction of type 2 diabetes risk: The Di@bet.es Study. BMC Med. 2025;23:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 45. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/