Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106795
Revised: April 19, 2025
Accepted: June 18, 2025
Published online: July 27, 2025
Processing time: 140 Days and 19.9 Hours
Liver diseases are progressive conditions driven by multiple factors, including molecular regulators such as nonprotein-coding RNAs, which orchestrate genetic and epigenetic processes across various biological levels. Long noncoding RNAs (lncRNAs), RNA molecules longer than 200 nucleotides, have been identified as key modulators in both cancerous and noncancerous liver diseases. Among them, taurine-upregulated gene 1 (TUG1), one of the earliest discovered lncRNAs, has emerged as a tumor promoter in hepatocellular carcinoma. Functionally, TUG1 exerts its regulatory effects primarily through microRNA sponging as a com
Core Tip: Taurine-upregulated gene 1 (TUG1), a long noncoding RNA, plays a critical role in liver pathogenesis by regulating gene expression through diverse mechanisms, including acting as a microRNA sponge, interacting with proteins, and modulating signaling pathways. TUG1 is involved in various liver diseases, including hepatocellular carcinoma, liver injury, fibrosis/cirrhosis, and metabolic dysfunction–associated steatotic liver disease, making it a potential therapeutic target.
- Citation: Boonto T, Ariyachet C. Deciphering the role of taurine-upregulated gene 1 in liver diseases: Mechanisms, clinical relevance, and emerging therapeutic opportunities. World J Hepatol 2025; 17(7): 106795
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106795.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106795
Liver diseases pose an overwhelmingly high mortality burden, with cirrhosis, hepatitis, and liver cancer accounting for approximately 2 million deaths annually[1]. According to predictive modeling studies, the incidence and mortality rates are projected to continue to rise for metabolic dysfunction–associated steatotic liver disease (MASLD) and hepatocellular carcinoma (HCC) in the coming decades[2-4]. It is anticipated that liver disease-related mortality may increase due to the obesity epidemic and the progressive aging of the global population[5,6]. In addition to compromising patient quality of life, liver diseases also impose a substantial burden on both individual finances and the national economy[7,8].
Over decades of research, studies have emphasized the pivotal role of protein-coding genes as "driver genes" in disease progression[9,10]. However, protein-coding regions constitute only a small fraction of the human genome (approximately 2%), while the majority of these regions are transcribed into noncoding RNAs (ncRNAs)[11]. These ncRNAs can be broadly classified into two main groups: (1) Housekeeping ncRNAs (e.g., ribosomal RNAs, transfer RNAs, small nuclear RNAs, and small nucleolar RNAs); and (2) Regulatory ncRNAs, which are tightly regulated [e.g., microRNAs (miRNAs) and long ncRNAs (lncRNAs)][12,13]. These ncRNAs play diverse roles in regulating gene expression across multiple levels under both physiological and pathological conditions[14-16], including liver diseases[17-19].
LncRNAs, which are generally defined as transcripts longer than 200 nucleotides with no protein-coding potential, have emerged as important regulators of gene expression under both physiological and pathological conditions[20]. Their genetic diversity is approximately twice as high as that of protein-coding transcripts[21]. Most lncRNAs are generated by RNA polymerase II (Pol II), and many of them undergo splicing and polyadenylation, which leads to their description as “mRNA-like”[22]. Moreover, loci expressing lncRNAs could exhibit some characteristics of protein-coding genes, such as multiple exons and promoters[23].
In contrast to mRNAs, lncRNAs generally exhibit more restricted expression patterns, are highly cell lineage specific, and are less evolutionarily conserved[22,24,25]. They have also been described to be closely related to developmental stage or disease-stage-specific patterns[26,27]. Through interactions with DNA, RNA, and proteins, lncRNAs regulate gene expression at multiple levels via a diverse array of molecular mechanisms[28,29]. These interactions allow lncRNAs to participate in critical processes such as chromatin organization, transcriptional regulation, and posttranscriptional modification[29,30]. Consequently, lncRNAs have garnered significant attention as pivotal elements in understanding the complexities of cell biology. Although lncRNAs are often expressed at low levels, their regulatory potential remains biologically significant and warrants further investigation to clarify how lncRNAs exert their regulatory effects[31].
Dysregulated expression of lncRNAs is intricately linked to the development of human diseases[32,33]. Similarly, numerous studies have emphasized the role of lncRNAs in initiating and advancing liver disease, aiming to provide insights into their involvement in key physiological and pathological processes in the liver[34,35]. Therefore, significant efforts have been made to explore lncRNA candidates as potential therapeutic targets, aiming to bridge knowledge gaps and develop therapeutic approaches[36-40]. In this review, we provide a focused exploration of the long noncoding RNA taurine-upregulated gene 1 (TUG1), detailing its mechanisms of action and emerging evidence supporting its regulatory roles in liver diseases.
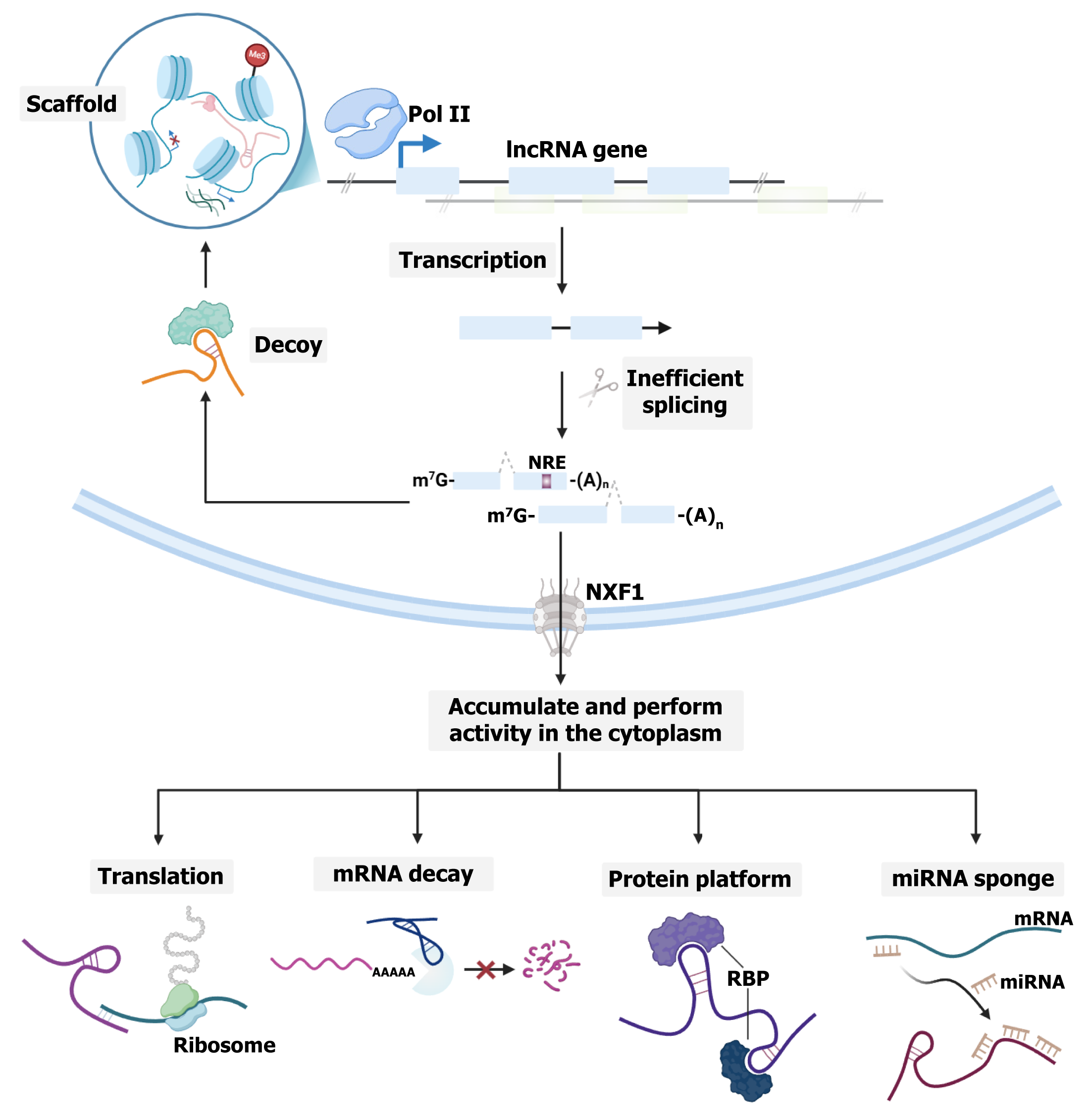
Most lncRNAs are transcribed by RNA Pol II and resemble mRNAs in features such as 5' capping, 3' polyadenylation, and splicing (Figure 1)[29]. Despite these similarities, they are spliced less efficiently[41,42], possibly because of weaker internal splicing signals and larger distances between the branch point and 3’ splice site[43]. Additionally, chromatin-associated lncRNAs harbor multiple U1 small nuclear RNA binding sites, promoting Pol II activity through U1 small nuclear ribonucleoprotein[44]. Some also contain sequence motifs or repeat elements. For example, some contain a nuclear retention element[45], whereas others have an Alu repeat with a C-rich sequence that facilitates interaction with the nuclear matrix protein heterogeneous nuclear ribonucleoprotein K[46]. Moreover, repeating RNA domains can promote association with heterogeneous nuclear ribonucleoprotein U[47]. These features likely explain their primary nuclear localization. However, localization is not strictly nuclear. Under specific conditions, some lncRNAs are exported and accumulate under specific conditions to perform their molecular functions[29]. Notably, a previous study revealed that most lncRNA transcripts, particularly those with fewer, longer exons or high A/U content relative to mRNAs, preferentially rely on nuclear RNA export factor 1 for nuclear export[48]. However, the specific sorting mechanism responsible for lncRNA cytoplasmic localization is not fully understood.
Initially discovered through microarray screening, the long intergenic RNA TUG1, an approximately 7-kb transcript localized to chromosome 22q12.2, was identified by its upregulation upon taurine treatment, a cysteine derivative crucial for neural development[49,50]. Its early functional role was demonstrated in the development of mouse retinal cells[50]. The functional repertoire of TUG1 subsequently expanded, as studies revealed regulatory roles in male fertility and the presence of a coding isoform affecting the mitochondrial membrane potential[51]. Like other lncRNAs, TUG1 employs diverse molecular mechanisms to regulate gene expression. It has been shown to interact with polycomb repressive complex 2 (PRC2) to control the cell cycle[52,53] and with peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) to modulate mitochondrial bioenergetics[54] or miRNAs to inhibit their biological functions[49]. Given these diverse activities, TUG1 has been implicated as one of the key contributing factors in various human diseases, such as Friedreich ataxia[55], particulate matter-induced epithelial injury[56], myocardial infarction[57], infantile hemangioma[58], and atherosclerosis[59]. In the context of cancer, accumulating evidence indicates that TUG1 primarily acts as a competitive endogenous RNA (ceRNA), sequestering tumor suppressor miRNAs and impairing their regulatory acti
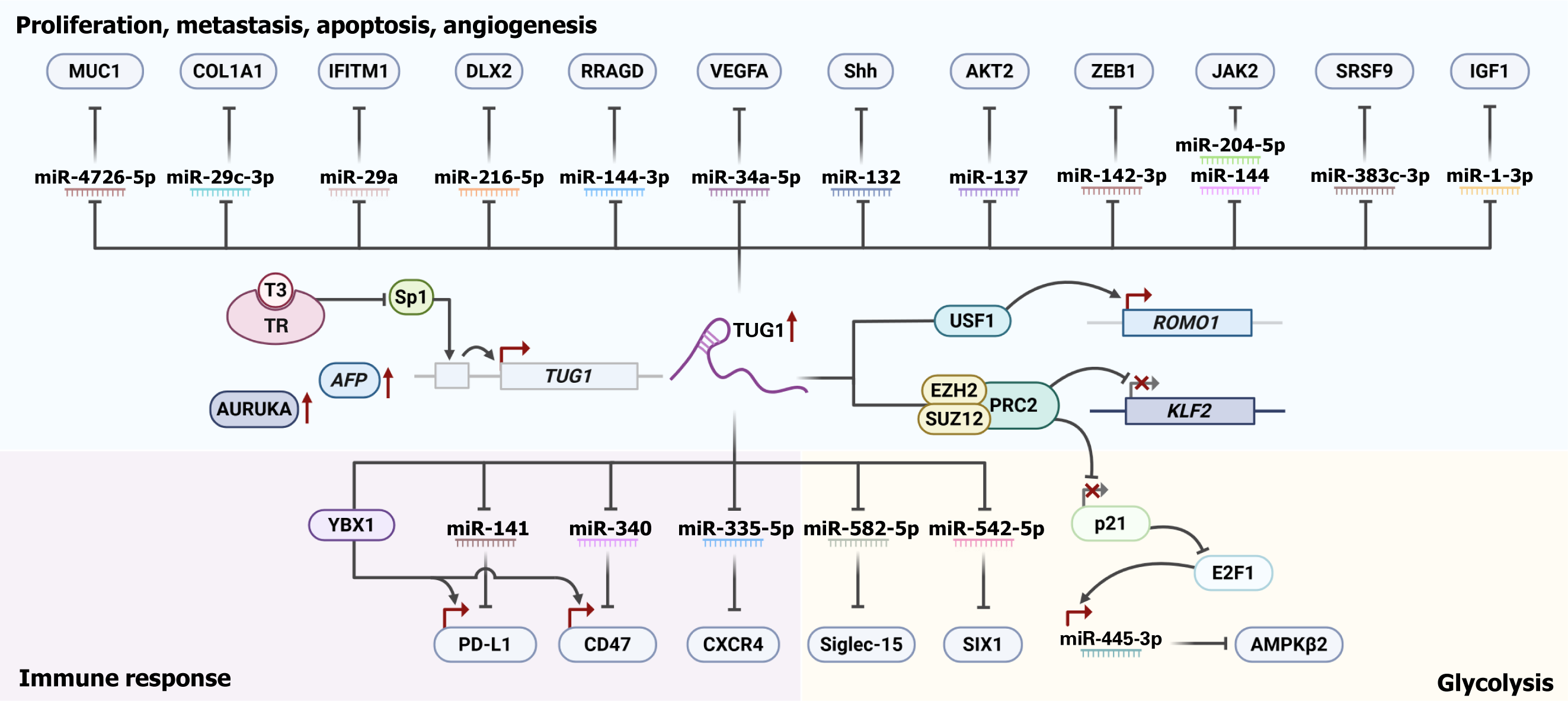
As one of the leading high-mortality cancers, HCC remains a global health threat due to its biological heterogeneity, poor postsurgical prognosis, and risk of tumor recurrence, emphasizing the need for further research into its biology. Table 1 summarizes the diverse molecular functions and regulatory axes of TUG1[65-87]. Figure 2 illustrates its regulatory interactions.
| Function | Pathway | Target miRNA | Target protein | Molecular mechanism | TUG1 role | Ref. |
| Proliferation, apoptosis, metastasis, angiogenesis | ||||||
| LncRNA-protein interaction | - | - | EZH2/SUZ12/KLF2 | SP1-upregulated TUG1 interacts with PRC2 subunit, EZH2 and SUZ12, to suppress KLF2 | Tumor-promoting | Huang et al[68] |
| - | - | - | AFP | Repression of TUG1 mediated by Triiodothyronine reduce AFP expression | Tumor-promoting | Lin et al[69] |
| LncRNA-protein interaction | - | - | USF1/ROMO1 | TUG1 recruits USF1 to ROMO1 promoter, activating its transcription | Tumor-promoting | Liu et al[70] |
| MiRNA sponge/ceRNA | - | MiR-34a-5p | VEGFA | TUG1 sequesters miR-34a-5p, a negative regulator of VEGFA | Tumor-promoting | Dong et al[71] |
| MiRNA sponge/ceRNA | Hedgehog signaling pathway | MiR-132 | Shh | TUG1 sequesters miR-132, a negative regulator of Shh | Tumor-promoting | Li et al[72] |
| - | - | - | AURKA | Overexpression of TUG1 is positively correlated with expression of AURKA | Tumor-promoting | Kang et al[73] |
| MiRNA sponge/ceRNA | Phosphatidylinositol 3-kinase/AKT pathway | MiR-137 | AKT2 | TUG1 sequesters miR-137, a negative regulator of AKT2 | Tumor-promoting | Li et al[74] |
| MiRNA sponge/ceRNA | - | MiR-142-3p | ZEB1 | TUG1 sequesters miR-142-3p, a negative regulator of ZEB1 | Tumor-promoting | He et al[75] |
| MiRNA sponge/ceRNA | JAK2/STAT3 pathway | MiR-144 | JAK2 | TUG1 sequesters miR-144, a negative regulator of JAK2 | Tumor-promoting | Lv et al[76] |
| MiRNA sponge/ceRNA | JAK2/STAT3 pathway | MiR-204-5p | JAK2 | TUG1 sequesters miR-204-5p, a negative regulator of JAK2 | Tumor-promoting | Yuan et al[77] |
| MiRNA sponge/ceRNA | Mammalian target of rapamycin/S6K pathway | MiR-144-3p | RRAGD | TUG1 sequesters miR-144-3p, a negative regulator of RRAGD | Tumor-promoting | Chen et al[78] |
| MiRNA sponge/ceRNA | - | MiR-216b-5p | DLX2 | TUG1 sequesters miR-216b-5p, a negative regulator of DLX2 | Tumor-promoting | Dai et al[66] |
| MiRNA sponge/ceRNA | - | MiR-29a | IFITM3 | TUG1 sequesters miR-29a, a negative regulator of IFITM3 | Tumor-promoting | Liu et al[79] |
| MiRNA sponge/ceRNA | - | MiR-29c-3p | COL1A1 | TUG1 sequesters miR-29c-3p, a negative regulator of COL1A1 | Tumor-promoting | Zhao et al[80] |
| MiRNA sponge/ceRNA | - | MiR-328-3p | SRSF9 | TUG1 sequesters miR-328-3p, a negative regulator of SRSF9 | Tumor-promoting | Liu et al[65] |
| MiRNA sponge/ceRNA | - | MiR-1-3p | IGF1 | TUG1 sequesters miR-1-3p, a negative regulator of IGF1 | Tumor-promoting | Tang et al[81] |
| MiRNA sponge/ceRNA | - | MiR-4726-5p | MUC1 | TUG1 sequesters miR-4726-5p, a negative regulator of MUC1 | Tumor-promoting | Tang et al[82] |
| Immune response | ||||||
| MiRNA sponge/ceRNA | - | MiR-335-5p | CXCR4 | TUG1 sequesters miR-335-5p, a negative regulator of CXCR4 | Tumor-promoting | Xie et al[83] |
| MiRNA sponge/ceRNA | - | MiR-582-5p | Siglec-15 | TUG1 sequesters miR-582-5p, a negative regulator of Siglec-15 | Tumor-promoting | Ren et al[84] |
| - | JAK2/STAT3 pathway | - | PD-L1 | Overexpression of TUG1 is positively correlated with expression of PD-L1 | Tumor-promoting | Wu et al[85] |
| MiRNA sponge/ceRNA | - | MiR-141, miR-340 | PD-L1, CD47 | TUG1 sequesters miR-141 and miR-340, which serve as a negative regulator of PD-L1 and CD47, respectively | Tumor-promoting | Xi et al[86] |
| LncRNA-protein interaction | - | - | YBX1/PD-L1/CD47 | TUG1 directly interacts with YBX1 and recruits to the PD-L1 and CD47 promoters, activating their transcription | Tumor-promoting | Xi et al[86] |
| Glycolysis | ||||||
| LncRNA-protein interaction | - | MiR-455-3p | EZH2/p21/AMPKβ2 | By interacting with EZH2 of PRC2, TUG1 silences the p21 promoter, thereby reducing p21 regulation on E2F transcription factor 1, which controls the level of tumor-promoting miR-455-3p targeting AMPKβ2 | Tumor-promoting | Lin et al[87] |
| MiRNA sponge/ceRNA | - | MiR-524-5p | SIX1 | TUG1 sequesters miR-524-5p, a negative regulator of SIX1 | Tumor-promoting | Lu et al[67] |
Early studies revealed that the TUG1 promoter contains several binding sites for specificity protein 1 (Sp1), a transcription factor that regulates numerous key factors in cancer progression[88]. Through direct binding, Sp1 enhances TUG1 transcription, contributing to TUG1 overexpression. Functionally, TUG1 interacts with the enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) and suppressor of zeste 12 (SUZ12) to epigenetically silence the Krüppel-like factor 2 (KLF2) gene, thereby inhibiting apoptosis and promoting HCC cell proliferation and metastasis[68]. KLF2 itself has been demonstrated to suppress the growth and tumorigenesis of HCC through modulating the hedgehog (Hh) signaling pathway[89]. Interestingly, triiodothyronine (T3) and its receptor interaction have been shown to negatively regulate TUG1 expression by partially counteracting Sp1 activation[69]. T3 treatment also reduces the protein expression of EZH2, SUZ12, and cell cycle markers such as proliferating cell nuclear antigen and cyclin E in HCC cells, inhibiting proliferation and enhancing apoptosis[69]. Furthermore, TUG1 expression is positively correlated with alpha-fetoprotein (AFP), a key HCC biomarker, which is also downregulated by T3 treatment[69].
In addition to these mechanisms, TUG1 also regulates HCC progression through the modulation of diverse signaling pathways. For example, reactive oxygen species (ROS) modulator 1 (ROMO1) has been described as a key modulator of ROS, which contribute to HCC invasiveness[90]. Through interaction with upstream stimulatory factor 1 (USF1), TUG1 facilitates its recruitment to the ROMO1 promoter, thereby facilitating HCC cell proliferation and invasion[70]. TUG1 also contributes to tumor angiogenesis via dysregulation of vascular endothelial growth factor A (VEGFA), a central regulator of neovascularization involved in the progression of tumors, including HCC[91]. In hepatoblastoma (HB), TUG1 upregulation promotes tumor growth and hypervascularity by acting as a sponge for miR-34a-5p, thereby relieving the repr
Beyond the PI3K/AKT pathway, TUG1 also regulates other oncogenic signaling networks in HCC. One such factor is zinc finger E-box binding homeobox 1 (ZEB1), whose aberrant expression is observed in various liver diseases[94]. Notably, ZEB1 facilitates HCC tumorigenesis by enhancing proliferation and invasion through the Wingless-Int (Wnt)/β-catenin signaling pathway[94]. Reduced expression of miR-142-3p has been closely associated with poor prognosis in HCC patients. These studies demonstrate the oncogenic function of TUG1, which sequesters miR-142-3p, a tumor suppressor that targets ZEB1, thereby facilitating HCC proliferation and EMT[75]. As a critical signaling axis, the Janus kinase (JAK)/signal transducer and activator of the transcription (STAT) signaling pathway plays a pivotal role in both normal and cancer cells[95]. Consequently, aberrant activation and dysregulation of the JAK/STAT pathway has been implicated in various diseases, including HCC[96]. TUG1, bearing MREs for miR-144[76] and miR-204-5p[77], facilitates deregulation of their downstream targets, which advances HCC progression. JAK2, which contains potential binding sites for miR-144 and miR-204-5p, is upregulated through TUG1's miRNA sponge activity, leading to increased proliferation, migration, angiogenesis, and tumorigenesis via activation of the JAK/STAT pathway[76,77].
In addition to its role in JAK/STAT signaling, TUG1 also contributes to HCC progression via the mammalian target of rapamycin (mTOR) pathway. As a crucial mediator of mTOR signaling, the overexpression of Ras related GTP binding D (RRAGD) has been implicated in driving aerobic glycolysis, enhancing the malignant features of HCC cells, and correlating with poor prognosis in HCC patients[97]. Elevated levels of phosphorylated mTOR and p70S6K have also been observed in HCC cells[78]. TUG1 promotes HCC progression by sequestering miR-144-3p, resulting in dysregulated RRAGD expression. This leads to increased proliferation, invasion, and resistance to apoptosis via hyperactivation of the mTOR/S6K pathway[78]. Distal-less homeobox 2 (DLX2), a critical regulator of the cell cycle, has been shown to increase HCC proliferation via the upregulation of cell cycle-related genes[98]. In the context of therapy resistance, elevated DLX2 levels are also associated with sorafenib resistance, which is correlated with a poorer prognosis in HCC patients[98]. Mechanistically, the knockdown of TUG1 relieves the suppression of miR-216b-5p, restoring the regulatory activity of DLX2, which suppresses growth and metastasis while sensitizing HCC cells to apoptosis[66]. Additionally, TUG1-mediated upregulation of interferon (IFN)-inducible transmembrane protein 3 (IFITM3) promotes HCC tumorigenesis by competitively binding to miR-29a. This effect is reversed upon the restoration of miR-29a expression achieved by TUG1 depletion[79]. Furthermore, IFITM3 levels are significantly elevated and associated with poor prognosis in HCC patients[99,100].
Apart from modulating a wide array of signaling cascades, TUG1 also facilitates HCC progression through the regulation of extracellular matrix (ECM) components. Collagen type I alpha 1 (COL1A1), a key factor in liver fibrosis[101], has been implicated as a biomarker for poor overall survival in HCC patients[102]. Notably, miR-29c-3p, which is inversely associated with both TUG1 and COL1A1 expression, can be restored upon TUG1 depletion, thereby reducing COL1A1 levels and suppressing HCC cell proliferation and motility[80]. Furthermore, TUG1 also regulates splicing factor expression. The serine/arginine-rich splicing factor (SRSF) family is aberrantly expressed in HCC, and elevated levels correlate with poor clinical outcomes[103]. Specifically, hypomethylation of the serine and arginine rich splicing factor 9 (SRSF9) promoter is linked to increased SRSF9 transcription, which contributes to the malignant behavior of HCC cells[104]. Further investigation into the oncogenic role of SRSF9 revealed that TUG1 acts as a miR-328-3p sponge, thereby alleviating the negative regulation of SRSF9 and driving the aggressive progression of HCC cells[65]. Additionally, TUG1-mediated downregulation of miR-1-3p has been shown to promote proliferation and inhibit apoptosis in HCC cells[81]. This activity likely results in increased expression of insulin like growth factor 1 (IGF1), a putative downstream target of miR-1-3p, which plays a key role in liver pathophysiology[81]. In support of this hypothesis, luciferase reporter assays conducted in colorectal cancer models have confirmed the interaction between miR-1-3p and IGF1, underscoring its broader relevance[105].
A study reported that the coexpression of the lncRNA HOXA transcript at the distal tip (HOTTIP) and TUG1 promotes HCC tumor growth by upregulating mucin 1 (MUC1)[82]. Notably, both lncRNAs harbor MREs for miR-4726-5p, sug
As an immunologically privileged organ, the liver maintains a delicate balance between immunosuppression and immune activation to preserve physiological equilibrium. This balance is disrupted in HCC, where tumor cells acquire mutations that confer resistance to apoptosis and senescence, reprogram cellular metabolism, and modify the microenvironment, enabling evasion of host immune surveillance[106,107]. Emerging evidence highlights the involvement of lncRNAs, including HCC, in mediating cancer immunity[108,109]. In HB-associated HCC, TUG1 is notably elevated in HB-infected patients carrying beta-catenin mutations, a gene encoding β-catenin, which serves as a crucial regulator of the Wnt signaling pathway[83]. Additionally, TUG1 expression is positively correlated with C-X-C motif chemokine receptor 4 (CXCR4) expression[83], which is linked to poor prognosis in HCC patients and an increased presence of protumor immunocytes, including macrophages, neutrophils, and dendritic cells, as well as their respective biomarkers[110]. TUG1 appears to increase CXCR4 expression by competitively binding to miR-335-5p, underscoring its potential as both a biomarker and therapeutic target in immune-related HCC progression[83].
In addition to its association with CXCR4, TUG1 also contributes to broader HCC immune suppression. For example, TUG1 expression is positively correlated with sialic acid-binding immunoglobulin-like lectin-15 (Siglec-15), a crucial immune suppressor that facilitates the migration of HCC cells[111]. A recent study further demonstrated that silencing TUG1 enhances T-cell activity and the production of cytokines, such as IFN-γ and interleukin (IL)-2, resulting in increased cytotoxicity in HCC cells, whereas TUG1 overexpression reverses these effects[84]. Mechanistically, TUG1 sponges miR-582-5p, a miRNA that negatively regulates Siglec-15, thereby upregulating Siglec-15 expression and suppressing T-cell-mediated responses[84]. Furthermore, TUG1 has been implicated in regulating the programmed cell death receptor-1 (PD-1)/programmed death-ligand 1 (PD-L1) immune checkpoint axis, a key immunosuppressive pathway that enables tumors to evade immune detection and destruction[112]. Notably, PD-L1 is highly expressed on the surface, where it interacts with PD-1 on the surface of T cells, effectively suppressing their activation and proliferation and thus compromising host immune defenses against tumor cells[112,113]. In HCC patients, the expression pattern of TUG1 is positively correlated with that of PD-L1 and other immune checkpoint molecules [such as tumor necrosis factor (TNF) ligand superfamily member 4, cluster of differentiation (CD) 200, and CD274] and is closely associated with a poor prognosis[85]. Functional assays revealed that TUG1 knockdown significantly reduced the mRNA, protein, and phosphorylation levels of JAK2 and STAT3, whereas TUG1 overexpression partially restored PD-L1 levels[85]. Furthermore, treatment with a JAK2 inhibitor (AZ960) or a STAT3 inhibitor (A12232) also reduces the mRNA and protein levels of PD-L1, indicating that TUG1 may regulate PD-L1 expression through the JAK2/STAT3 pathway to modulate immune evasion in HCC[85].
Beyond transcriptional regulation, TUG1 also contributes to immune suppression through posttranscriptional modifications. The m6A RNA modification is the most abundant type of mRNA modification that regulates the biological fate of target mRNAs, influencing their cellular dynamics[114]. A key component of the m6A methyltransferase complex, methyltransferase-like 3 (METTL3), which normally functions as a “writer”, is closely related to the poor prognosis of HCC patients and enhances HCC tumorigenicity[115]. A study revealed a positive correlation between TUG1 and METTL3 in HCC patients, suggesting that METTL3-mediated m6A modification promotes TUG1 upregulation[86]. Consistently, METTL3 silencing suppressed TUG1 expression and reduced the m6A modification of TUG1 in HepG2 cells. Intriguingly, TUG1 knockdown in HCC livers increases the presence of M1-like macrophages and CD8+ and CD4+ T cells, which exhibit antitumor immunity with increased production of IFN-γ, IL-2, and TNF-α[86]. Further analysis revealed that TUG1 contains putative MREs for miR-141 and miR-340, which target PD-L1 and CD47, respectively[86]. CD47 is widely recognized as an antiphagocytic signal that tumor cells exploit to escape phagocytosis[116]. In addition, RNA structure analysis has identified potential docking sites for the transcription factor Y-box binding protein 1 (YBX1) within the TUG1 sequence, implicating it in transcriptional regulation[117]. YBX1 directly binds to the promoters of PD-L1 and CD47 to activate their transcription, a process that is significantly impaired upon TUG1 depletion due to reduced YBX1 recruitment[86]. Overall, this study demonstrated that TUG1 overexpression, potentially through m6A modification, enhances PD-L1 and CD47 expression by sequestering miR-141 and miR-340, respectively, thereby alleviating their negative regulation[86]. Furthermore, TUG1 facilitates YBX1 recruitment to the PD-L1 and CD47 promoters, driving their transcription and enhancing HCC immune evasion, tumor growth, and metastatic potential[86]. However, further investigations are warranted to clarify the extent to which METTL3 contributes to TUG1 regulation across different HCC contexts.
The concept of cancer metabolism arises from the observation that diverse tumors often exhibit metabolic signatures distinct from those of noncancerous tissues[118]. These signatures influence the choice of therapies to match these metabolic profiles to maximize patients’ benefits[118]. Growing evidence indicates that lncRNAs act as crucial modulators of metabolic reprogramming in numerous cancers, including HCC[119,120]. The metabolic switch in cancer cells, first described by Warburg[121-123], is characterized by rerouting energy metabolism mainly to glycolysis despite the presence of sufficient oxygen, a phenomenon termed the “Warburg effect” or “aerobic glycolysis”. Along with increasing adenosine triphosphate (ATP) output, the buildup of glycolytic intermediates could be diverted into various biosynthetic pathways, thus enabling the production of essential building blocks for cell proliferation[124,125]. TUG1 was revealed to promote methylation (H3K27me3) of the cyclin-dependent kinase inhibitor 1A (p21) promoter, an antiproliferative modulator[126], through direct interaction with EZH2 of PRC2[87]. TUG1-mediated disruption of p21 enhances the binding of the transcription factor E2F transcription factor 1, which was previously demonstrated to be the target of p21 regulation[127], to the miR-445-3p promoter region[87]. Notably, miR-445-3p was markedly diminished in TUG1-knockdown HCC cells. In line with earlier observations, depletion of miR-445-3p led to increased migration, invasion, cell proliferation, and glycolysis, whereas its overexpression in TUG1-knockdown cells reversed these effects, supporting its tumor-promoting role in HCC[87]. Further investigation revealed that AMP-activated protein kinase beta 2 (AMPKβ2), a well-characterized metabolic tumor suppressor[128], contains a putative binding site for miR-445-3p, indicating that AMPKβ2 is suppressed by TUG1-mediated upregulation of miR-445-3p in HCC[87]. Compared with their less metastatic counterparts, metastatic HCC cells exhibit higher levels of hexokinase 2 (HK2), phosphorylated mTOR, and glycolysis[87]. This observation suggests an association between HCC metastasis and increased glycolysis.
In addition to its role in intrinsic tumor regulation, TUG1 also participates in shaping the tumor microenvironment (TME). Emerging evidence highlights the essential role of cancer-associated fibroblasts (CAFs) as key players in tumor development, nurturing the TME through the secretion of ECM components, growth factors, chemokines, and exosomal miRNAs[129,130]. HCC cells supplemented with CAF-derived exosomes exhibit increased migration, invasion (increased MMP-2 and MMP-9), and glycolysis [increased HK2 and lactate dehydrogenase (LDHA)]. LncRNA profiling of CAF-derived exosomes revealed that TUG1 was highly expressed compared with other elevated lncRNAs (NEAT1, H19, and MALAT1)[67]. Bioinformatics analysis further indicated that TUG1 harbors a potential binding site for miR-524-5p, suggesting that TUG1 sequesters miR-524-5p, thereby diminishing its regulatory influence[67]. Notably, miR-524-5p directly targets the 3’ UTR of SIX homeobox 1 (SIX1), an oncogene implicated in various types of tumors[131,132], thereby promoting HCC development via SIX1 upregulation[67]. Indeed, a recent study suggested that SIX1 is a key transcription factor that directly induces the expression of many glycolytic genes and promotes the Warburg effect in cancer cells[133]. Taken together, these findings highlight the critical role of the TME in HCC progression and suggest that TUG1 is a crucial glycolysis-regulated lncRNA.
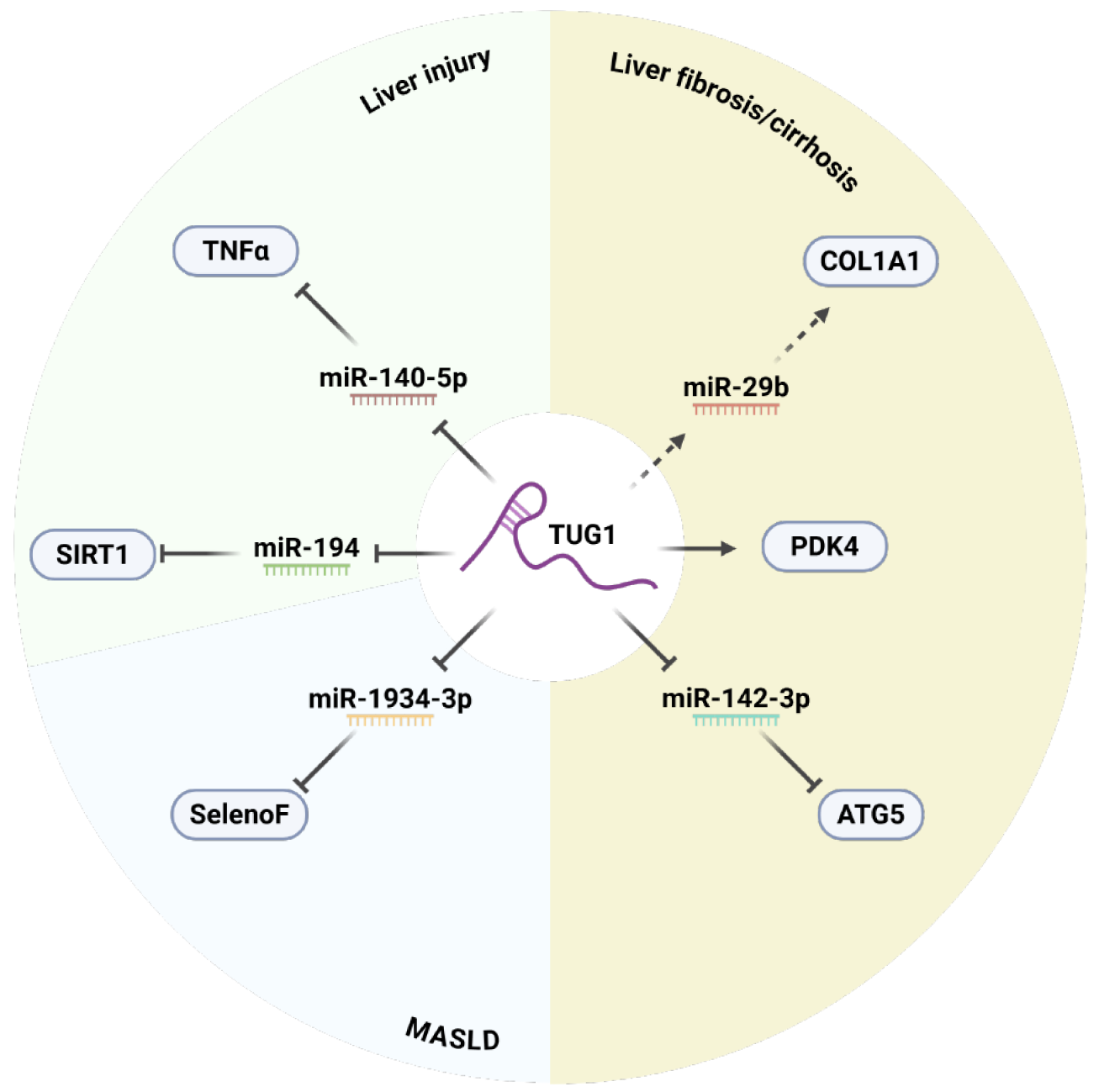
HCC commonly arises from noncancerous liver diseases. Given the potent regulatory role of TUG1, it may influence fibrosis, cirrhosis, liver injury, and MASLD. Since cirrhosis frequently transitions into HCC, investigating the role of TUG1 in early liver pathologies is vital for developing preventive and therapeutic strategies. Table 2 presents the molecular mechanisms and associated target genes of TUG1[134-140]. Figure 3 depicts its regulatory interactions in various liver diseases.
| Function | Target miRNA | Target protein | Molecular mechanism | TUG1 role | Ref. |
| Liver injury | |||||
| - | - | - | Overexpression of TUG1 suppresses endoplasmic reticulum stress, reduces apoptosis, and upregulates anti-apoptotic proteins | Cryoprotective | Su et al[134] |
| MiRNA sponge/ceRNA | MiR-194 | SIRT1 | TUG1 sequesters miR-194, a negative regulator of SIRT1 | Anti-apoptotic, anti-inflammatory | Gu et al[135] |
| MiRNA sponge/ceRNA | MiR-140-5p | TNF | TUG1 sequesters miR-140-5p, a negative regulator of TNF-α | Pro-apoptotic, pro-inflammatory | Liu et al[136] |
| Liver fibrosis/cirrhosis | |||||
| - | MiR-29b | COL1A1/α-SMA | Overexpression of TUG1 is positively correlated with expression of COL1A1 and α-SMA while negatively correlated with miR-29b | Pro-fibrotic | Han et al[137] |
| - | - | PDK4 | Overexpression of TUG1 is positively correlated with expression of PDK4 | Anti-ferroptotic | Zhang et al[138] |
| MiRNA sponge/ceRNA | MiR-142-3p | ATG5 | TUG1 sequesters miR-142-3p, a negative regulator of ATG5 | Pro-autophagic endothelial-mesenchymal transition-promoting | Zhang et al[139] |
| Metabolic dysfunction–associated steatotic liver disease | |||||
| MiRNA sponge/ceRNA | MiR-1934-3p | SelenoF | TUG1 sequesters miR-1934-3p, a negative regulator of SelenoF | Glucose-lipid metabolism regulator | Wang et al[140] |
One of the most remarkable aspects of the liver is its extraordinary ability to regenerate following injury. As the center of metabolic homeostasis and a critical signaling hub, the liver regulates essential metabolic processes, including glucose, lipid, and amino acid metabolism[141,142]. Liver diseases vary widely but are generally characterized by hepatocyte damage, immune cell infiltration, and activation of hepatic stellate cells (HSCs), all of which compromise liver function and structure[141,142]. The development of these conditions is influenced by several factors, including hepatotropic viral infections, drug-induced liver injury, chronic alcohol abuse, hepatitis B virus and hepatitis C virus infections, and MASLD[142,143]. Interestingly, certain lncRNAs, such as Gm2199[144], Mir122hg[145], and Airn[146], which play protective roles against liver damage[147], have been implicated as key regulators of liver injury.
TUG1 has emerged as a potent modulator of liver injury responses. During liver preservation for transplantation, cold ischemia–reperfusion injury is induced by cold-induced damage to hepatic sinusoidal endothelial cells and microvascular dysfunction, significantly compromising transplant viability[148]. LncRNA profiling revealed a 6-fold downregulation of TUG1 in cold-injured mouse livers. Lentivirus-mediated TUG1 overexpression inhibited the endoplasmic reticulum (ER) stress pathway, alleviated cold-induced apoptosis, and increased antiapoptotic protein levels in cold-stressed hepatocytes and liver sinusoidal endothelial cells (LSECs). In vivo, TUG1 exerts protective effects, reducing the levels of hepatic injury markers (alanine aminotransferase, aspartate aminotransferase, LDHA, and glutamate dehydrogenase) and inflammatory cytokines. Furthermore, preinjection of mice with Lenti-TUG1 resulted in prolonged hepatic graft survival compared with that of controls[134]. Dexmedetomidine (DEX), a potent and selective α2-adrenergic agonist used for clinical anesthesia, can alleviate local and systemic inflammation and has been shown to reduce the severity of hepatic injury[149,150]. DEX treatment ameliorated oxygen and glucose deprivation (OGD)-induced hepatocyte injury, suppressed apoptosis, and enhanced cell viability, which correlated with increased TUG1 and sirtuin 1 (SIRT1) expression[135]. SIRT1, a histone deacetylase, exerts hepatoprotective effects by mitigating inflammation and liver injury[151,152]. Bioinformatics analysis identified miR-194 as a predicted TUG1 target, which also contains a putative SIRT1 binding site. Notably, depletion of TUG1 or introduction of a miR-194 mimic abolished the protective effect of DEX on the apoptosis of OGD-treated cells, which was partially rescued by miR-194 inhibition or SIRT1 overexpression[135]. Collectively, these studies demonstrate that TUG1 exerts its protective effects through multiple mechanisms, including the inhibition of ER stress, apoptosis, and inflammation, suggesting a complex interplay between TUG1 and various signaling pathways in liver injury, highlighting its potential as a therapeutic target for improving liver transplantation outcomes.
Conversely, TUG1 has also been shown to promote liver injury. Previous investigations demonstrated TUG1 upregulation in liver tissues following lipopolysaccharide (LPS) treatment, suggesting its involvement in LPS-induced hepatocyte injury[136]. TUG1 silencing significantly reduces hepatocyte damage and inflammatory infiltration[136]. Moreover, miR-140-5p was later identified as a direct target of TUG1, which negatively regulates TNF, a key proinflammatory cytokine[153,154]. In support of the role of TUG1 in miR-140-5p suppression, the overexpression of TUG1, TNF, or miR-140-5p increased the levels of apoptosis (B-cell lymphoma 2 associated X protein, caspase-3), inflammation (TNF, IL-6), and ROS[136]. These effects were partially mitigated by TUG1 or TNF silencing or miR-140-5p mimic overexpression[136]. Although evidence supports the hepatoprotective role of TUG1, some findings suggest that its function may vary depending on the molecular mechanisms underlying different causes of injury.
Liver fibrosis is defined by the pathological accumulation of ECM initiated by repeated repair processes following hepatic injury[155]. Under normal conditions, the liver exhibits astonishing regenerative abilities, partly through HSCs[156]. Upon activation, these subpopulation cell types undergo transdifferentiation into hepatic collagen-producing myofibroblasts, forming a regulated fibrous scar[157]. Generally, this process is transient and is counteracted by antifibrotic pathways[157,158]. However, with prolonged liver injury, the balance between profibrogenic and antifibrotic mechanisms is disrupted, resulting in pathological production of the ECM and the onset of progressive liver problems[157,158]. Investigations using liver fibrosis model mice revealed a positive correlation between TUG1 and key fibrotic markers, including COL1A1, alpha-smooth muscle actin (α-SMA), MMP-2, MMP-9, MMP-10, and tissue inhibitor matrix metalloproteinase 1[137]. Upon treatment with transforming growth factor-β, which plays a central pathogenic role in fibrosis across multiple organs[159-161], these fibrotic markers are significantly elevated and reverse the effect of TUG1 knockdown in HSCs[137]. In contrast, the overexpression of TUG1 in primary murine HSCs increased the expression of these fibrotic markers, whereas the introduction of a miR-29b mimic, a miRNA that was previously implicated in liver fibrosis[162], attenuated the effects of TUG1 overexpression[137]. These findings suggest that TUG1 might modulate fibrogenesis via miR-29b and fibrotic pathways. However, whether miR-29b directly controls the expression of fibrotic genes remains to be explored.
In addition to direct regulation of fibrotic pathways, TUG1 has been shown to modulate HSC ferroptosis, a promising strategy for clearance of liver fibrosis via drug-induced ferroptosis[163,164]. Notably, pyruvate dehydrogenase kinase 4 (PDK4), a critical glycolytic regulator, facilitates ferroptosis resistance by inhibiting pyruvate dehydrogenase-dependent pyruvate oxidation[165]. Depletion of TUG1 diminishes PDK4 expression without affecting other PDK isoforms. Consequently, glycolytic activity and genes (HK2, pyruvate kinase muscle isozyme M2, and LDHA) and ferroptosis [solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4] are also downregulated in TUG1-kno
Cirrhosis is an advanced stage of liver fibrosis marked by the activation of profibrogenic phenotypes in various cell types. The event restricts blood flow, thereby compromising the overall liver’s function and metabolic activities and potentially triggering portal hypertension as a compensatory response[168]. Under normal conditions, LSECs form a barrier between the blood and liver parenchyma and maintain the quiescence of HSCs[157,169]. However, liver damage induces LSEC capillarization, which is characterized by the loss of their native function[157,169]. Consequently, these capillarized LSECs fail to suppress HSC activation, driving the progression of liver fibrosis and cirrhosis[157,169]. LPS from gram-negative bacteria, commonly known as a driver of sepsis, has been implicated in the gut-liver axis in the development and progression of chronic liver diseases[170,171]. Impaired autophagy and endothelial-mesenchymal transition (EndMT) in LSECs have been linked to liver cirrhosis[172,173]. Among the lncRNAs identified to be upregulated following LPS treatment, TUG1 exhibited the strongest dose-dependent expression in starved LSECs. Further analysis revealed the putative binding site for miR-142-3p on autophagy protein 5 (ATG5), a key autophagy-related gene[172], and TUG1[139]. Notably, the overexpression of miR-142-3p lowered ATG5 levels, inhibited migration, and subsequently suppressed EndMT-related genes (light chain 3B and α-SMA) and autophagic markers (P62, VE-cadherin, and CD31)[139]. Collectively, these findings demonstrate that TUG1 directly targets miR-142-3p through sponging activity, thereby impairing ATG5 regulation to promote LSEC EndMT and autophagy. These findings offer perspectives on TUG1-based therapeutic strategies for liver fibrosis and cirrhosis. Interestingly, TUG1 appears to play a profibrotic role in both LSECs and HSCs, albeit through different mechanisms, suggesting that a synergistic approach targeting TUG1 in both cell types could be a promising antifibrotic strategy.
MASLD, formerly known as nonalcoholic fatty liver disease, is a steatotic liver disease that develops alongside cardiometabolic risk factors without excessive alcohol intake[174]. As the primary cause of chronic liver disease, MASLD is strongly associated with obesity, cardiovascular risk, kidney disease, liver failure, and liver cancer[175]. Recent findings highlight the regulatory role of the long noncoding RNA TUG1 in hepatic steatosis. Specifically, the TUG1/miR-1934-3p/selenoprotein F (SelenoF) axis was demonstrated to be a key regulator of hepatocyte steatosis induced by sodium palmitate[140]. SelenoF, an ER-resident protein, is crucial for lipid and glucose metabolism and significantly accelerates energy metabolism[176,177]. These findings suggest that TUG1 acts as a ceRNA, influencing hepatic metabolism through miR-1934-3p, SelenoF, and the insulin receptor substract 1/AKT signaling pathway, highlighting TUG1 as a potent therapeutic target for MASLD treatment[140]. Although this study indicates that TUG1 plays a protective role against MASLD, more studies are warranted to confirm and elucidate the molecular mechanisms involved.
Nevertheless, owing to the multifactorial nature of MASLD, including its links to genetic susceptibility and insulin resistance, further investigation is needed. Notably, insulin-resistant states may increase lipogenesis, increase lipotoxic intermediates, and exacerbate metabolic imbalances[178]. TUG1 has been identified as a regulatory lncRNA capable of mitigating inflammation and improving insulin sensitivity in the adipose tissues of obese mice[179]. This function appears to be mediated by sponging miR-204, which targets SIRT1. TUG1 overexpression leads to derepression of SIRT1, thereby promoting anti-inflammatory signaling through glucose transporter 4, peroxisome proliferator-activated receptor gamma (PPAR-γ), and AKT[179]. Additionally, TUG1 has been associated with gestational diabetes mellitus. Through competitive binding with miR-328-3p, which targets sterol regulatory element binding protein (SREBP)-2 and activates extracellular signal-regulated kinase (ERK) signaling, TUG1 appears to mitigate insulin resistance via suppression of the SREBP-2/ERK axis[180]. Collectively, these findings suggest that TUG1 contributes to insulin regulation during the early stages of metabolic dysfunction and may serve as a preventive target in MASLD development.
In a clinical study, reduced TUG1 levels were observed in the adipose tissues of obese women, with positive correlations with body mass index, waist and hip circumference, insulin levels, and the mRNA levels of key lipogenic transcription factors such as PPAR-γ, PGC1-α, SREBP-1c, fatty acid synthase, and acetyl-CoA carboxylase[181]. However, such correlations were not observed in larger cohort studies, particularly with obesity indices, suggesting potential context-dependent regulation[182,183]. These inconsistencies underscore the importance of additional mechanistic studies to fully define the contribution of TUG1 to lipid metabolic pathways.
LncRNAs have emerged as key regulators of gene expression and biological pathways, significantly contributing to human diseases and cancer progression through various molecular mechanisms. TUG1 has been widely recognized for its role in HCC, primarily facilitating HCC progression by functioning as a ceRNA and competitively binding to tumor-suppressing miRNAs, thereby upregulating oncogenic proteins and driving multiple cancer hallmarks. While the ceRNA model of TUG1 is well supported, comprehensively defining TUG1’s function within specific regulatory axes requires consideration of factors such as ceRNA and miRNA abundance, stoichiometry, the number of shared MREs, and potential indirect interactions[184,185]. Moreover, large-scale patient datasets demonstrating an inverse correlation between TUG1 and its target miRNAs would significantly strengthen its functional validation beyond in vitro studies[184,185]. Apart from its ceRNA function, TUG1 directly interacts with chromatin regulators (e.g., PRC2, EZH2) and transcription factors (e.g., USF1, YBX1), playing a broader role in gene regulation. Given its multifaceted molecular mechanisms, interpreting TUG1’s function on the basis of a single molecular function risks oversimplifying its broader impact in HCC and other disease settings.
In addition to TUG1, a multitude of other ncRNAs, including miRNAs and circular RNAs (circRNAs), have emerged as crucial players in liver diseases[186-190]. These ncRNAs form intricate regulatory networks that interact with each other and with protein-coding genes to influence various cellular processes, such as inflammation, fibrosis, regeneration, and carcinogenesis[191-194]. For example, miRNAs can directly target mRNAs to suppress their translation, whereas circRNAs can act as sponges for miRNAs, modulating their activity[195,196]. These interactions are not limited to the miRNA-mRNA or circRNA-miRNA axes. Notably, lncRNAs and circRNAs can compete for common miRNAs. A bioinformatics-constructed ceRNA network revealed 38 circRNA/miRNA/mRNA axes (7 circRNAs, 8 miRNAs, and 20 mRNAs) and 436 lncRNA/miRNA/mRNA axes (104 lncRNAs, the same 8 miRNAs, and 20 mRNAs). These 20 key differentially expressed mRNAs are targeted by both circRNA and lncRNA axes in HCC, suggesting a more intricate and cooperative mechanism of gene regulation[197]. Understanding the interplay between different ncRNA classes and TUG1 is crucial for deciphering the complex molecular mechanisms underlying liver diseases and for identifying potential therapeutic targets.
Emerging evidence suggests that ncRNAs can also communicate between different cell types within the liver microenvironment, contributing to disease progression[198-200]. For example, ncRNAs can be packaged into exosomes and transported from one cell to another, influencing recipient cell behavior[201,202]. This intercellular communication mediated by ncRNAs adds another layer of complexity to the pathogenesis of liver diseases. Further research is needed to fully elucidate the roles of various ncRNAs and their interactions in different liver diseases, paving the way for the development of novel therapeutic strategies that target these ncRNA networks. Notably, in addition to their established roles in miRNA sponging, recent bioinformatic analyses have identified protein docking sites on TUG1, suggesting that TUG1 directly interacts with transcription factors that may drive HCC progression. These findings emphasize the importance of exploring non-ceRNA mechanisms to fully elucidate the multifaceted functions of TUG1 in liver diseases.
Given that lncRNAs, including TUG1, often exhibit cell type-specific expression patterns, emerging transcriptomic tools such as single-cell RNA sequencing (scRNA-seq) provide promising avenues for exploring their functional roles in heterogeneous tissues such as the liver. Recent advances, such as the ELATUS framework, have optimized lncRNA detection, improving the sensitivity and specificity of scRNA-seq analysis[203]. Leveraging these technologies may help delineate the cell-specific roles of TUG1 and reveal novel regulatory pathways involved in liver pathologies, potentially guiding the development of targeted treatments.
On the basis of the evidence presented in this review, TUG1 has potential as a diagnostic biomarker for HCC. Analyses using large-scale liver HCC (LIHC) datasets such as The Cancer Genome Atlas datasets revealed an inverse correlation between TUG1 and its target miRNAs, which is consistent with its function as a ceRNA. Conversely, TUG1 expression is positively correlated with oncogenic targets negatively regulated by miRNAs, including AFP, ROMO1, AKT2, ZEB1, SRSF9, CXCR4, Siglec-15, PD-L1, CD47, METTL3, and SIX1. Additionally, elevated TUG1 expression has been associated with poorer prognostic outcomes in LIHC patients. These findings suggest that predictive models incorporating TUG1, its miRNA partners, and downstream targets may serve as powerful tools for HCC diagnosis and prognosis.
In the context of HCC, the role of TUG1 in mediating chemoresistance remains insufficiently characterized. Existing evidence suggests that TUG1 expression is significantly upregulated in adriamycin-resistant HCC tissues[204]. Functional silencing of TUG1 has been shown to sensitize cells to adriamycin by promoting apoptosis and repressing the expression of multidrug resistance-associated genes, notably P-glycoprotein and ATP-binding cassette subfamily B member 1 (also known as MDR1)[204]. Similar regulatory roles of TUG1 in modulating drug resistance have been identified in other cancers, including paclitaxel resistance in ovarian cancer[205], 5-fluorouracil resistance in colorectal cancer[206], and cisplatin/adriamycin/etoposide resistance in small cell lung cancer[207]. Despite these observations, conflicting reports from cervical cancer[208] and glioma[209] studies suggest a potential antiresistance role for TUG1, highlighting a possible tissue-specific context for its function. Importantly, studies evaluating the role of TUG1 in resistance to sorafenib, the standard treatment for advanced HCC, are lacking[210]. Notably, only one study reported that solamargine, either alone or in combination with sorafenib, reduces TUG1 expression in vivo in HCC models[82]. Additional investigations are necessary to clarify whether TUG1 is a consistent modulator of drug sensitivity and resistance in HCC.
Owing to their potential for precision targeting, lncRNA-based therapies are gaining attention[211]. Unlike mRNAs, lncRNAs are typically expressed at lower levels and with greater specificity. Even when they vary between individual cells rather than being universally expressed at low levels, lncRNAs exhibit greater cell-type specificity than protein-coding genes do[212]. Interestingly, even ubiquitously expressed lncRNAs may have cell-type-specific functions, suggesting new therapeutic avenues[212]. Therapeutic targeting of tumor-promoting lncRNAs such as TUG1 can be achieved through antisense oligonucleotides, short interfering RNAs, or small molecules, while genome-editing platforms such as clustered regularly interspaced short palindromic repeat interference offer transcriptional silencing capabilities[212]. Clinical trials exploiting lncRNA promoter specificity, such as BC-819 targeting H19, highlight the feasibility of using lncRNAs as vectors for delivering cytotoxic genes to specific tumor tissues[36,213]. Overcoming challenges related to specificity, delivery, and tolerability will be key to realizing the full potential of lncRNA-based therapeutics[211]. On the basis of this review, TUG1 has emerged as a promising target for the treatment of liver cancer and liver disease, showing potential for prevention and early management. Interestingly, TUG1 has been implicated in cancer chemoresistance. However, further investigation into the broader molecular functions of TUG1 is essential to validate its accuracy and effectiveness as a novel therapeutic target in clinical applications.
We would like to thank all members of the Center of Excellence in Hepatitis and Liver Cancer and STAR for Anti-fibrosis Therapeutics and Mechanisms, Faculty of Medicine, Chulalongkorn University, for constructive feedback in writing this review.
| 1. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1224] [Reference Citation Analysis (4)] |
| 2. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1998] [Article Influence: 666.0] [Reference Citation Analysis (3)] |
| 3. | Guo Q, Zhu X, Beeraka NM, Zhao R, Li S, Li F, Mahesh PA, Nikolenko VN, Fan R, Liu J. Projected epidemiological trends and burden of liver cancer by 2040 based on GBD, CI5plus, and WHO data. Sci Rep. 2024;14:28131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1408] [Article Influence: 352.0] [Reference Citation Analysis (1)] |
| 5. | Hahn JW, Woo S, Park J, Lee H, Kim HJ, Ko JS, Moon JS, Rahmati M, Smith L, Kang J, Pizzol D, Tully MA, Dragioti E, Sánchez GFL, Lee K, Ha Y, Lee J, Lee H, Rhee SY, Son Y, Kim S, Yon DK. Global, Regional, and National Trends in Liver Disease-Related Mortality Across 112 Countries From 1990 to 2021, With Projections to 2050: Comprehensive Analysis of the WHO Mortality Database. J Korean Med Sci. 2024;39:e292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 500] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 7. | Ufere NN, Satapathy N, Philpotts L, Lai JC, Serper M. Financial burden in adults with chronic liver disease: A scoping review. Liver Transpl. 2022;28:1920-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Younossi Z, Aggarwal P, Shrestha I, Fernandes J, Johansen P, Augusto M, Nair S. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 2022;4:100525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Chen JC, Alvarez MJ, Talos F, Dhruv H, Rieckhof GE, Iyer A, Diefes KL, Aldape K, Berens M, Shen MM, Califano A. Identification of causal genetic drivers of human disease through systems-level analysis of regulatory networks. Cell. 2014;159:402-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Muro EM, Ibn-Salem J, Andrade-Navarro MA. The distributions of protein coding genes within chromatin domains in relation to human disease. Epigenetics Chromatin. 2019;12:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14554] [Cited by in RCA: 13473] [Article Influence: 962.4] [Reference Citation Analysis (0)] |
| 12. | Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1502] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 13. | Poliseno L, Lanza M, Pandolfi PP. Coding, or non-coding, that is the question. Cell Res. 2024;34:609-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 14. | Bhatti GK, Khullar N, Sidhu IS, Navik US, Reddy AP, Reddy PH, Bhatti JS. Emerging role of non-coding RNA in health and disease. Metab Brain Dis. 2021;36:1119-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 15. | Fu XD. Non-coding RNA: a new frontier in regulatory biology. Natl Sci Rev. 2014;1:190-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 16. | Yin Y, Shen X. Noncoding RNA-chromatin association: Functions and mechanisms. Fundam Res. 2023;3:665-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Mukherjee AG, Wanjari UR, Gopalakrishnan AV, Katturajan R, Kannampuzha S, Murali R, Namachivayam A, Ganesan R, Renu K, Dey A, Vellingiri B, Prince SE. Exploring the Regulatory Role of ncRNA in NAFLD: A Particular Focus on PPARs. Cells. 2022;11:3959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Rowe MM, Kaestner KH. The Role of Non-Coding RNAs in Liver Disease, Injury, and Regeneration. Cells. 2023;12:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Zailaie SA, Khoja BB, Siddiqui JJ, Mawardi MH, Heaphy E, Aljagthmi A, Sergi CM. Investigating the Role of Non-Coding RNA in Non-Alcoholic Fatty Liver Disease. Noncoding RNA. 2024;10:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Gao N, Li Y, Li J, Gao Z, Yang Z, Li Y, Liu H, Fan T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front Oncol. 2020;10:598817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 21. | Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1788] [Cited by in RCA: 2178] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 22. | Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, Gingeras TR, Guttman M, Hirose T, Huarte M, Johnson R, Kanduri C, Kapranov P, Lawrence JB, Lee JT, Mendell JT, Mercer TR, Moore KJ, Nakagawa S, Rinn JL, Spector DL, Ulitsky I, Wan Y, Wilusz JE, Wu M. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 1462] [Article Influence: 487.3] [Reference Citation Analysis (0)] |
| 23. | Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3516] [Cited by in RCA: 4055] [Article Influence: 311.9] [Reference Citation Analysis (0)] |
| 24. | Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 25. | Gloss BS, Dinger ME. The specificity of long noncoding RNA expression. Biochim Biophys Acta. 2016;1859:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1761] [Cited by in RCA: 2137] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 27. | Tsagakis I, Douka K, Birds I, Aspden JL. Long non-coding RNAs in development and disease: conservation to mechanisms. J Pathol. 2020;250:480-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 28. | Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 2803] [Article Influence: 280.3] [Reference Citation Analysis (0)] |
| 29. | Statello L, Guo CJ, Chen LL, Huarte M. Author Correction: Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 30. | Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 1077] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 31. | Unfried JP, Ulitsky I. Substoichiometric action of long noncoding RNAs. Nat Cell Biol. 2022;24:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Sheng N, Huang L, Lu Y, Wang H, Yang L, Gao L, Xie X, Fu Y, Wang Y. Data resources and computational methods for lncRNA-disease association prediction. Comput Biol Med. 2023;153:106527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Sparber P, Filatova A, Khantemirova M, Skoblov M. The role of long non-coding RNAs in the pathogenesis of hereditary diseases. BMC Med Genomics. 2019;12:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Huo X, Han S, Wu G, Latchoumanin O, Zhou G, Hebbard L, George J, Qiao L. Dysregulated long noncoding RNAs (lncRNAs) in hepatocellular carcinoma: implications for tumorigenesis, disease progression, and liver cancer stem cells. Mol Cancer. 2017;16:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Zhao Y, Wu J, Liangpunsakul S, Wang L. Long Non-coding RNA in Liver Metabolism and Disease: Current Status. Liver Res. 2017;1:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Arun G, Diermeier SD, Spector DL. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol Med. 2018;24:257-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 498] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 37. | Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 306] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 38. | Han S, Chen X, Huang L. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharm Sin B. 2023;13:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 39. | Huang SF, Peng XF, Jiang L, Hu CY, Ye WC. LncRNAs as Therapeutic Targets and Potential Biomarkers for Lipid-Related Diseases. Front Pharmacol. 2021;12:729745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Ilieva M, Uchida S. Perspectives of LncRNAs for therapy. Cell Biol Toxicol. 2022;38:915-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Schlackow M, Nojima T, Gomes T, Dhir A, Carmo-Fonseca M, Proudfoot NJ. Distinctive Patterns of Transcription and RNA Processing for Human lincRNAs. Mol Cell. 2017;65:25-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 42. | Chen LL. Towards higher-resolution and in vivo understanding of lncRNA biogenesis and function. Nat Methods. 2022;19:1152-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Melé M, Mattioli K, Mallard W, Shechner DM, Gerhardinger C, Rinn JL. Chromatin environment, transcriptional regulation, and splicing distinguish lincRNAs and mRNAs. Genome Res. 2017;27:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 44. | Yin Y, Lu JY, Zhang X, Shao W, Xu Y, Li P, Hong Y, Cui L, Shan G, Tian B, Zhang QC, Shen X. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature. 2020;580:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 45. | Azam S, Hou S, Zhu B, Wang W, Hao T, Bu X, Khan M, Lei H. Nuclear retention element recruits U1 snRNP components to restrain spliced lncRNAs in the nucleus. RNA Biol. 2019;16:1001-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Lubelsky Y, Ulitsky I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature. 2018;555:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 47. | Hacisuleyman E, Shukla CJ, Weiner CL, Rinn JL. Function and evolution of local repeats in the Firre locus. Nat Commun. 2016;7:11021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Zuckerman B, Ron M, Mikl M, Segal E, Ulitsky I. Gene Architecture and Sequence Composition Underpin Selective Dependency of Nuclear Export of Long RNAs on NXF1 and the TREX Complex. Mol Cell. 2020;79:251-267.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 49. | Zhou H, Sun L, Wan F. Molecular mechanisms of TUG1 in the proliferation, apoptosis, migration and invasion of cancer cells. Oncol Lett. 2019;18:4393-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 51. | Lewandowski JP, Dumbović G, Watson AR, Hwang T, Jacobs-Palmer E, Chang N, Much C, Turner KM, Kirby C, Rubinstein ND, Groff AF, Liapis SC, Gerhardinger C, Bester A, Pandolfi PP, Clohessy JG, Hoekstra HE, Sauvageau M, Rinn JL. The Tug1 lncRNA locus is essential for male fertility. Genome Biol. 2020;21:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 52. | Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667-11672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2182] [Cited by in RCA: 2369] [Article Influence: 139.4] [Reference Citation Analysis (0)] |
| 53. | Lin PC, Huang HD, Chang CC, Chang YS, Yen JC, Lee CC, Chang WH, Liu TC, Chang JG. Long noncoding RNA TUG1 is downregulated in non-small cell lung cancer and can regulate CELF1 on binding to PRC2. BMC Cancer. 2016;16:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Long J, Badal SS, Ye Z, Wang Y, Ayanga BA, Galvan DL, Green NH, Chang BH, Overbeek PA, Danesh FR. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126:4205-4218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 55. | Koka M, Li H, Akther R, Perlman S, Wong D, Fogel BL, Lynch DR, Chandran V. Long non-coding RNA TUG1 is downregulated in Friedreich's ataxia. Brain Commun. 2024;6:fcae170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Li B, Huang N, Wei S, Xv J, Meng Q, Aschner M, Li X, Chen R. lncRNA TUG1 as a ceRNA promotes PM exposure-induced airway hyper-reactivity. J Hazard Mater. 2021;416:125878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Wang YW, Dong HZ, Tan YX, Bao X, Su YM, Li X, Jiang F, Liang J, Huang ZC, Ren YL, Xu YL, Su Q. HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction. Cell Death Discov. 2022;8:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 58. | Zhou L, Jia X, Yang X. LncRNA-TUG1 promotes the progression of infantile hemangioma by regulating miR-137/IGFBP5 axis. Hum Genomics. 2021;15:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc Pathol. 2018;33:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Wang P, Yang Z, Ye T, Shao F, Li J, Sun N, He J. lncTUG1/miR-144-3p affect the radiosensitivity of esophageal squamous cell carcinoma by competitively regulating c-MET. J Exp Clin Cancer Res. 2020;39:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Xu K, Zhang L. Inhibition of TUG1/miRNA-299-3p Axis Represses Pancreatic Cancer Malignant Progression via Suppression of the Notch1 Pathway. Dig Dis Sci. 2020;65:1748-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Wang H, Yu Y, Fan S, Luo L. Knockdown of Long Noncoding RNA TUG1 Inhibits the Proliferation and Cellular Invasion of Osteosarcoma Cells by Sponging miR-153. Oncol Res. 2018;26:665-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Wang X, Bai X, Yan Z, Guo X, Zhang Y. The lncRNA TUG1 promotes cell growth and migration in colorectal cancer via the TUG1-miR-145-5p-TRPC6 pathway. Biochem Cell Biol. 2021;99:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Jiang H, Hu X, Zhang H, Li W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat Oncol. 2017;12:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 65. | Liu Y, Mao X, Ma Z, Chen W, Guo X, Yu L, Deng X, Jiang F, Li T, Lin N, Zhang Y. Aberrant regulation of LncRNA TUG1-microRNA-328-3p-SRSF9 mRNA Axis in hepatocellular carcinoma: a promising target for prognosis and therapy. Mol Cancer. 2022;21:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Dai Q, Deng J, Zhou J, Wang Z, Yuan XF, Pan S, Zhang HB. Long non-coding RNA TUG1 promotes cell progression in hepatocellular carcinoma via regulating miR-216b-5p/DLX2 axis. Cancer Cell Int. 2020;20:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Lu L, Huang J, Mo J, Da X, Li Q, Fan M, Lu H. Exosomal lncRNA TUG1 from cancer-associated fibroblasts promotes liver cancer cell migration, invasion, and glycolysis by regulating the miR-524-5p/SIX1 axis. Cell Mol Biol Lett. 2022;27:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 68. | Huang MD, Chen WM, Qi FZ, Sun M, Xu TP, Ma P, Shu YQ. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 69. | Lin YH, Wu MH, Huang YH, Yeh CT, Lin KH. TUG1 Is a Regulator of AFP and Serves as Prognostic Marker in Non-Hepatitis B Non-Hepatitis C Hepatocellular Carcinoma. Cells. 2020;9:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Liu S, Qiu J, He W, Geng C, He G, Liu C, Cai D, Liu X, Tian B, Pan H. TUG1 long non-coding RNA enlists the USF1 transcription factor to overexpress ROMO1 leading to hepatocellular carcinoma growth and metastasis. MedComm (2020). 2020;1:386-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Dong R, Liu GB, Liu BH, Chen G, Li K, Zheng S, Dong KR. Targeting long non-coding RNA-TUG1 inhibits tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis. 2016;7:e2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Li J, Zhang Q, Fan X, Mo W, Dai W, Feng J, Wu L, Liu T, Li S, Xu S, Wang W, Lu X, Yu Q, Chen K, Xia Y, Lu J, Zhou Y, Xu L, Guo C. The long noncoding RNA TUG1 acts as a competing endogenous RNA to regulate the Hedgehog pathway by targeting miR-132 in hepatocellular carcinoma. Oncotarget. 2017;8:65932-65945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Kang P, Jiang G, Meng Q, Liu W, Wu X. Long non-coding RNA taurine-upregulated gene 1 promotes cells proliferation, migration and invasion while represses apoptosis, and upregulates AURKA expression in hepatocellular carcinoma. Int J Clin Exp Pathol. 2018;11:3199-3207. [PubMed] |
| 74. | Li W, Ge J, Xie J, Yang J, Chen J, He T. LncRNA TUG1 Promotes Hepatocellular Carcinoma Migration and Invasion Via Targeting the miR-137/AKT2 Axis. Cancer Biother Radiopharm. 2021;36:850-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | He C, Liu Z, Jin L, Zhang F, Peng X, Xiao Y, Wang X, Lyu Q, Cai X. lncRNA TUG1-Mediated Mir-142-3p Downregulation Contributes to Metastasis and the Epithelial-to-Mesenchymal Transition of Hepatocellular Carcinoma by Targeting ZEB1. Cell Physiol Biochem. 2018;48:1928-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Lv J, Kong Y, Gao Z, Liu Y, Zhu P, Yu Z. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol. 2018;101:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 77. | Yuan MX, Ji CY, Gao HQ, Sheng XY, Xie WX, Yin Q. lncRNA TUG1 regulates angiogenesis via the miR2045p/JAK2/STAT3 axis in hepatoblastoma. Mol Med Rep. 2021;24:553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Chen W, Bai Z, Bai W, Wang W, Guo J, Guo M, Sai Y, Shi J, Wu J. LncTUG1 contributes to the progression of hepatocellular carcinoma via the miR-144-3p/RRAGD axis and mTOR/S6K pathway. Sci Rep. 2023;13:7500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Liu W, Feng Q, Liao W, Li E, Wu L. TUG1 promotes the expression of IFITM3 in hepatocellular carcinoma by competitively binding to miR-29a. J Cancer. 2021;12:6905-6920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Zhao W, Jiang X, Yang S. lncRNA TUG1 Promotes Cell Proliferation, Migration, and Invasion in Hepatocellular Carcinoma via Regulating miR-29c-3p/COL1A1 Axis. Cancer Manag Res. 2020;12:6837-6847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Tang K, Lv D, Miao L, Mao Y, Yu X. LncRNA TUG1 functions as a ceRNA for miR-1-3p to promote cell proliferation in hepatic carcinogenesis. J Clin Lab Anal. 2022;36:e24415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Tang Q, Li X, Chen Y, Long S, Yu Y, Sheng H, Wang S, Han L, Wu W. Solamargine inhibits the growth of hepatocellular carcinoma and enhances the anticancer effect of sorafenib by regulating HOTTIP-TUG1/miR-4726-5p/MUC1 pathway. Mol Carcinog. 2022;61:417-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | Xie F, Zhang L, Yao Q, Shan L, Liu J, Dong N, Liang J. TUG1 Promoted Tumor Progression by Sponging miR-335-5p and Regulating CXCR4-Mediated Infiltration of Pro-Tumor Immunocytes in CTNNB1-Mutated Hepatoblastoma. Onco Targets Ther. 2020;13:3105-3115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Ren Y, Lyu J, Guo Y, Yao Y, Hu L. Long Noncoding RNA TUG1 Inhibits Tumor Progression through Regulating Siglec-15-Related Anti-Immune Activity in Hepatocellular Carcinoma. J Immunol Res. 2022;2022:9557859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Wu R, Liu W, Yang Q, Zhang J, Hou P, Xiong J, Wu L, Li E. LncTUG1 promotes hepatocellular carcinoma immune evasion via upregulating PD-L1 expression. Sci Rep. 2023;13:16998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 86. | Xi Q, Yang G, He X, Zhuang H, Li L, Lin B, Wang L, Wang X, Fang C, Chen Q, Yang Y, Yu Z, Zhang H, Cai W, Li Y, Shen H, Liu L, Zhang R. M(6)A-mediated upregulation of lncRNA TUG1 in liver cancer cells regulates the antitumor response of CD8(+) T cells and phagocytosis of macrophages. Adv Sci (Weinh). 2024;11:e2400695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 87. | Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY, Lin KH. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 88. | Beishline K, Azizkhan-Clifford J. Sp1 and the 'hallmarks of cancer'. FEBS J. 2015;282:224-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 89. | Lin J, Tan H, Nie Y, Wu D, Zheng W, Lin W, Zhu Z, Yang B, Chen X, Chen T. Krüppel-like factor 2 inhibits hepatocarcinogenesis through negative regulation of the Hedgehog pathway. Cancer Sci. 2019;110:1220-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, Chung YM, Woo SR, Han CJ, Kim SB, Suh KS, Jang JJ, Lee K, Choi DW, Lee S, Lee GY, Hahm KB, Shin JA, Kim BS, Noh KH, Kim TW, Lee KH, Yoo YD. Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012;143:1084-94.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Qi Y, Song Y, Cai M, Li J, Yu Z, Li Y, Huang J, Jiang Y, Peng C, Jiang B, Liu S. Vascular endothelial growth factor A is a potential prognostic biomarker and correlates with immune cell infiltration in hepatocellular carcinoma. J Cell Mol Med. 2023;27:538-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Jeng KS, Jeng CJ, Jeng WJ, Sheen IS, Li SY, Leu CM, Tsay YG, Chang CF. Sonic Hedgehog signaling pathway as a potential target to inhibit the progression of hepatocellular carcinoma. Oncol Lett. 2019;18:4377-4384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 93. | Chen C, Song G, Xiang J, Zhang H, Zhao S, Zhan Y. AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;486:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Li LY, Yang JF, Rong F, Luo ZP, Hu S, Fang H, Wu Y, Yao R, Kong WH, Feng XW, Chen BJ, Li J, Xu T. ZEB1 serves an oncogenic role in the tumourigenesis of HCC by promoting cell proliferation, migration, and inhibiting apoptosis via Wnt/β-catenin signaling pathway. Acta Pharmacol Sin. 2021;42:1676-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 1700] [Article Influence: 340.0] [Reference Citation Analysis (0)] |
| 96. | Hin Tang JJ, Hao Thng DK, Lim JJ, Toh TB. JAK/STAT signaling in hepatocellular carcinoma. Hepat Oncol. 2020;7:HEP18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 97. | Ding L, Liang X. Ras related GTP binding D promotes aerobic glycolysis of hepatocellular carcinoma. Ann Hepatol. 2021;23:100307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Liu J, Cui X, Qu L, Hua L, Wu M, Shen Z, Lu C, Ni R. Overexpression of DLX2 is associated with poor prognosis and sorafenib resistance in hepatocellular carcinoma. Exp Mol Pathol. 2016;101:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Bondok RM, Barakat LA, Elsergany AR, Mahsoub N, Ghattas MH. Interferon-induced transmembrane protein 3 in hepatocellular carcinoma patients. BMC Cancer. 2023;23:584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 100. | Hou Y, Zhang Y, Qin L, Zhang C, Wang S, Chen D, Li A, Lou J, Yu Y, Dong T, Li N, Zhao Y. Interferon-induced transmembrane protein-3 rs12252-CC is associated with low differentiation and progression of hepatocellular carcinoma. Medicine (Baltimore). 2019;98:e13996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Koilan S, Hamilton D, Baburyan N, Padala MK, Weber KT, Guntaka RV. Prevention of liver fibrosis by triple helix-forming oligodeoxyribonucleotides targeted to the promoter region of type I collagen gene. Oligonucleotides. 2010;20:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Hayashi M, Nomoto S, Hishida M, Inokawa Y, Kanda M, Okamura Y, Nishikawa Y, Tanaka C, Kobayashi D, Yamada S, Nakayama G, Fujii T, Sugimoto H, Koike M, Fujiwara M, Takeda S, Kodera Y. Identification of the collagen type 1 α 1 gene (COL1A1) as a candidate survival-related factor associated with hepatocellular carcinoma. BMC Cancer. 2014;14:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Yuan J, Liu Z, Wu Z, Yang J, Lv T. Comprehensive Molecular Analysis Identified an SRSF Family-Based Score for Prognosis and Therapy Efficiency Prediction in Hepatocellular Carcinoma. Cancers (Basel). 2022;14:4727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 104. | Zhang G, Liu B, Shang H, Wu G, Wu D, Wang L, Li S, Wang Z, Wang S, Yuan J. High expression of serine and arginine-rich splicing factor 9 (SRSF9) is associated with hepatocellular carcinoma progression and a poor prognosis. BMC Med Genomics. 2022;15:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 105. | Ye LL, Cheng ZG, Cheng XE, Huang YL. Erratum: Corregendum to: Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation and accelerates apoptosis of colorectal cancer cells. Toxicol Res (Camb). 2021;10:1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 106. | Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 195] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 107. | Chen K, Shuen TWH, Chow PKH. The association between tumour heterogeneity and immune evasion mechanisms in hepatocellular carcinoma and its clinical implications. Br J Cancer. 2024;131:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 108. | Zhang Y, Liu Q, Liao Q. Long noncoding RNA: a dazzling dancer in tumor immune microenvironment. J Exp Clin Cancer Res. 2020;39:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 109. | Guo Y, Xie Y, Luo Y. The Role of Long Non-Coding RNAs in the Tumor Immune Microenvironment. Front Immunol. 2022;13:851004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Neve Polimeno M, Ierano C, D'Alterio C, Simona Losito N, Napolitano M, Portella L, Scognamiglio G, Tatangelo F, Maria Trotta A, Curley S, Costantini S, Liuzzi R, Izzo F, Scala S. CXCR4 expression affects overall survival of HCC patients whereas CXCR7 expression does not. Cell Mol Immunol. 2015;12:474-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 111. | Liu W, Ji Z, Wu B, Huang S, Chen Q, Chen X, Wei Y, Jiang J. Siglec-15 promotes the migration of liver cancer cells by repressing lysosomal degradation of CD44. FEBS Lett. 2021;595:2290-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 112. | Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727-742. [PubMed] |
| 113. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 114. | Song N, Cui K, Zhang K, Yang J, Liu J, Miao Z, Zhao F, Meng H, Chen L, Chen C, Li Y, Shao M, Zhang J, Wang H. The Role of m6A RNA Methylation in Cancer: Implication for Nature Products Anti-Cancer Research. Front Pharmacol. 2022;13:933332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 115. | Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO, Wong CM. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 1024] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 116. | Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα Immune Checkpoint. Immunity. 2020;52:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 506] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 117. | Chao HM, Huang HX, Chang PH, Tseng KC, Miyajima A, Chern E. Y-box binding protein-1 promotes hepatocellular carcinoma-initiating cell progression and tumorigenesis via Wnt/β-catenin pathway. Oncotarget. 2017;8:2604-2616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 118. | Kim J, DeBerardinis RJ. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019;30:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 119. | Lin W, Zhou Q, Wang CQ, Zhu L, Bi C, Zhang S, Wang X, Jin H. LncRNAs regulate metabolism in cancer. Int J Biol Sci. 2020;16:1194-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 120. | Wang K, Lu Y, Li H, Zhang J, Ju Y, Ouyang M. Role of long non-coding RNAs in metabolic reprogramming of gastrointestinal cancer cells. Cancer Cell Int. 2024;24:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 121. | WARBURG O. On respiratory impairment in cancer cells. Science. 1956;124:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1565] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 122. | WARBURG O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 10191] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 123. | Warburg OH, The metabolism of tumours: investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem. United States: Wiley, 1930. |
| 124. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48749] [Article Influence: 3249.9] [Reference Citation Analysis (12)] |
| 125. | Papaneophytou C. The Warburg Effect: Is it Always an Enemy? Front Biosci (Landmark Ed). 2024;29:402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 126. | Shamloo B, Usluer S. p21 in Cancer Research. Cancers (Basel). 2019;11:1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 127. | Porlan E, Morante-Redolat JM, Marqués-Torrejón MÁ, Andreu-Agulló C, Carneiro C, Gómez-Ibarlucea E, Soto A, Vidal A, Ferrón SR, Fariñas I. Transcriptional repression of Bmp2 by p21(Waf1/Cip1) links quiescence to neural stem cell maintenance. Nat Neurosci. 2013;16:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 129. | Nedaeinia R, Najafgholian S, Salehi R, Goli M, Ranjbar M, Nickho H, Haghjooy Javanmard S, A Ferns G, Manian M. The role of cancer-associated fibroblasts and exosomal miRNAs-mediated intercellular communication in the tumor microenvironment and the biology of carcinogenesis: a systematic review. Cell Death Discov. 2024;10:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 130. | Ying F, Chan MSM, Lee TKW. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma. Cell Mol Gastroenterol Hepatol. 2023;15:985-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 131. | Du P, Zhao J, Wang J, Liu Y, Ren H, Patel R, Hu C, Zhang W, Huang G. Sine Oculis Homeobox Homolog 1 Regulates Mitochondrial Apoptosis Pathway Via Caspase-7 In Gastric Cancer Cells. J Cancer. 2017;8:636-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Song W, Ma J, Lei B, Yuan X, Cheng B, Yang H, Wang M, Feng Z, Wang L. Sine oculis homeobox 1 promotes proliferation and migration of human colorectal cancer cells through activation of Wnt/β-catenin signaling. Cancer Sci. 2019;110:608-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 133. | Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, Yan Z, Jin S, Wang T, Zhao W, Mai H, Huang J, Han X, Ji Q, Song Q, Yang C, Zhao S, Xu X, Ye Q. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell. 2018;33:368-385.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 134. | Su S, Liu J, He K, Zhang M, Feng C, Peng F, Li B, Xia X. Overexpression of the long noncoding RNA TUG1 protects against cold-induced injury of mouse livers by inhibiting apoptosis and inflammation. FEBS J. 2016;283:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 135. | Gu XX, Xu XX, Liao HH, Wu RN, Huang WM, Cheng LX, Lu YW, Mo J. Dexmedetomidine hydrochloride inhibits hepatocyte apoptosis and inflammation by activating the lncRNA TUG1/miR-194/SIRT1 signaling pathway. J Inflamm (Lond). 2021;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Liu QM, Liu LL, Li XD, Tian P, Xu H, Li ZL, Wang LK. Silencing lncRNA TUG1 Alleviates LPS-Induced Mouse Hepatocyte Inflammation by Targeting miR-140/TNF. Front Cell Dev Biol. 2020;8:616416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 137. | Han X, Hong Y, Zhang K. TUG1 is involved in liver fibrosis and activation of HSCs by regulating miR-29b. Biochem Biophys Res Commun. 2018;503:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Zhang X, Zhao L, Ying K, Xu J, Huang Y, Zhu R, Ding Y, Cai W, Wu X, Miao D, Xu Q, Zeng Y, Yu F. TUG1 protects against ferroptosis of hepatic stellate cells by upregulating PDK4-mediated glycolysis. Chem Biol Interact. 2023;383:110673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 139. | Zhang R, Huang XQ, Jiang YY, Li N, Wang J, Chen SY. LncRNA TUG1 regulates autophagy-mediated endothelial-mesenchymal transition of liver sinusoidal endothelial cells by sponging miR-142-3p. Am J Transl Res. 2020;12:758-772. [PubMed] |
| 140. | Wang W, Miao Z, Qi X, Wang B, Liu Q, Shi X, Xu S. LncRNA Tug1 relieves the steatosis of SelenoF-knockout hepatocytes via sponging miR-1934-3p. Cell Biol Toxicol. 2023;39:3175-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Ma X, Huang T, Chen X, Li Q, Liao M, Fu L, Huang J, Yuan K, Wang Z, Zeng Y. Molecular mechanisms in liver repair and regeneration: from physiology to therapeutics. Signal Transduct Target Ther. 2025;10:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 142. | Gan C, Yuan Y, Shen H, Gao J, Kong X, Che Z, Guo Y, Wang H, Dong E, Xiao J. Liver diseases: epidemiology, causes, trends and predictions. Signal Transduct Target Ther. 2025;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 82] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 143. | Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, Merz M, Lucena MI, Kaplowitz N, Aithal GP. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 144. | Gao Q, Gu Y, Jiang Y, Fan L, Wei Z, Jin H, Yang X, Wang L, Li X, Tai S, Yang B, Liu Y. Long non-coding RNA Gm2199 rescues liver injury and promotes hepatocyte proliferation through the upregulation of ERK1/2. Cell Death Dis. 2018;9:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | Yu Z, Li Y, Shao S, Guo B, Zhang M, Zheng L, Zhang K, Zhou F, Zhang L, Chen C, Jiang W, Hong W, Han T. Protective effect of hepatocyte-enriched lncRNA-Mir122hg by promoting hepatocyte proliferation in acute liver injury. Exp Mol Med. 2022;54:2022-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 146. | Shao S, Zhang Y, Zhou F, Meng X, Yu Z, Li G, Zheng L, Zhang K, Li Y, Guo B, Liu Q, Zhang M, Du X, Hong W, Han T. LncRNA-Airn alleviates acute liver injury by inhibiting hepatocyte apoptosis via the NF-κB signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2022;54:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 147. | Zhou J, Li Y, Liu X, Long Y, Chen J. LncRNA-Regulated Autophagy and its Potential Role in Drug-Induced Liver Injury. Ann Hepatol. 2018;17:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 709] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 149. | Zhang X, Wang J, Qian W, Zhao J, Sun L, Qian Y, Xiao H. Dexmedetomidine inhibits tumor necrosis factor-alpha and interleukin 6 in lipopolysaccharide-stimulated astrocytes by suppression of c-Jun N-terminal kinases. Inflammation. 2014;37:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 150. | Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ, Pan BB. Dexmedetomidine alleviates hepatic injury via the inhibition of oxidative stress and activation of the Nrf2/HO-1 signaling pathway. Eur Cytokine Netw. 2019;30:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 151. | Nakamura K, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, Datta N, Zarrinpar A, Busuttil RW, Kupiec-Weglinski JW. Sirtuin 1 attenuates inflammation and hepatocellular damage in liver transplant ischemia/Reperfusion: From mouse to human. Liver Transpl. 2017;23:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Li F, Zhang L, Xue H, Xuan J, Rong S, Wang K. SIRT1 alleviates hepatic ischemia-reperfusion injury via the miR-182-mediated XBP1/NLRP3 pathway. Mol Ther Nucleic Acids. 2021;23:1066-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 153. | Tiegs G, Horst AK. TNF in the liver: targeting a central player in inflammation. Semin Immunopathol. 2022;44:445-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 154. | Rossi RE, Parisi I, Despott EJ, Burroughs AK, O'Beirne J, Conte D, Hamilton MI, Murray CD. Anti-tumour necrosis factor agent and liver injury: literature review, recommendations for management. World J Gastroenterol. 2014;20:17352-17359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 155. | Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 715] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 156. | Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9:875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 861] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 157. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1391] [Article Influence: 278.2] [Reference Citation Analysis (0)] |
| 158. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (10)] |
| 159. | Froese AR, Shimbori C, Bellaye PS, Inman M, Obex S, Fatima S, Jenkins G, Gauldie J, Ask K, Kolb M. Stretch-induced Activation of Transforming Growth Factor-β1 in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;194:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 160. | Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 2760] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 161. | Kim J, Kang W, Kang SH, Park SH, Kim JY, Yang S, Ha SY, Paik YH. Proline-rich tyrosine kinase 2 mediates transforming growth factor-beta-induced hepatic stellate cell activation and liver fibrosis. Sci Rep. 2020;10:21018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 162. | Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 163. | Liu G, Wei C, Yuan S, Zhang Z, Li J, Zhang L, Wang G, Fang L. Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother Res. 2022;36:4230-4243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 164. | Yuan S, Wei C, Liu G, Zhang L, Li J, Li L, Cai S, Fang L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022;55:e13158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 304] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 165. | Song X, Liu J, Kuang F, Chen X, Zeh HJ 3rd, Kang R, Kroemer G, Xie Y, Tang D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021;34:108767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 166. | Qi F, Lv ZD, Huang WD, Wei SC, Liu XM, Song WD. LncRNA TUG1 promotes pulmonary fibrosis progression via up-regulating CDC27 and activating PI3K/Akt/mTOR pathway. Epigenetics. 2023;18:2195305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 167. | Wang T, Cui S, Liu X, Han L, Duan X, Feng S, Zhang S, Li G. LncTUG1 ameliorates renal tubular fibrosis in experimental diabetic nephropathy through the miR-145-5p/dual-specificity phosphatase 6 axis. Ren Fail. 2023;45:2173950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 168. | Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA. 2023;329:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 222] [Article Influence: 74.0] [Reference Citation Analysis (33)] |
| 169. | Baiocchini A, Del Nonno F, Taibi C, Visco-Comandini U, D'Offizi G, Piacentini M, Falasca L. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci Rep. 2019;9:8760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 170. | An L, Wirth U, Koch D, Schirren M, Drefs M, Koliogiannis D, Nieß H, Andrassy J, Guba M, Bazhin AV, Werner J, Kühn F. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J Gastrointest Surg. 2022;26:671-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 171. | Ohtani N, Kamiya T, Kawada N. Recent updates on the role of the gut-liver axis in the pathogenesis of NAFLD/NASH, HCC, and beyond. Hepatol Commun. 2023;7:e0241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 172. | Kouroumalis E, Voumvouraki A, Augoustaki A, Samonakis DN. Autophagy in liver diseases. World J Hepatol. 2021;13:6-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 173. | Ruan B, Duan JL, Xu H, Tao KS, Han H, Dou GR, Wang L. Capillarized Liver Sinusoidal Endothelial Cells Undergo Partial Endothelial-Mesenchymal Transition to Actively Deposit Sinusoidal ECM in Liver Fibrosis. Front Cell Dev Biol. 2021;9:671081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 174. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 451] [Article Influence: 150.3] [Reference Citation Analysis (1)] |
| 175. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 957] [Article Influence: 478.5] [Reference Citation Analysis (1)] |
| 176. | Zhang DG, Zhao T, Xu XJ, Xu YH, Wei XL, Jiang M, Luo Z. Selenoprotein F (SELENOF)-mediated AKT1-FOXO3a-PYGL axis contributes to selenium supranutrition-induced glycogenolysis and lipogenesis. Biochim Biophys Acta Gene Regul Mech. 2022;1865:194814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 177. | Zheng X, Ren B, Wang H, Huang R, Zhou J, Liu H, Tian J, Huang K. Hepatic proteomic analysis of selenoprotein F knockout mice by iTRAQ: An implication for the roles of selenoprotein F in metabolism and diseases. J Proteomics. 2020;215:103653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 178. | Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024;59 Suppl 1:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 179. | Zhang Y, Gu M, Ma Y, Peng Y. LncRNA TUG1 reduces inflammation and enhances insulin sensitivity in white adipose tissue by regulating miR-204/SIRT1 axis in obesity mice. Mol Cell Biochem. 2020;475:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 180. | Tang X, Qin Q, Xu W, Zhang X. Long Non-Coding RNA TUG1 Attenuates Insulin Resistance in Mice with Gestational Diabetes Mellitus via Regulation of the MicroRNA-328-3p/SREBP-2/ERK Axis. Diabetes Metab J. 2023;47:267-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 181. | Ebrahimi R, Toolabi K, Jannat Ali Pour N, Mohassel Azadi S, Bahiraee A, Zamani-Garmsiri F, Emamgholipour S. Adipose tissue gene expression of long non-coding RNAs; MALAT1, TUG1 in obesity: is it associated with metabolic profile and lipid homeostasis-related genes expression? Diabetol Metab Syndr. 2020;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 182. | Rasaei N, Gholami F, Samadi M, Shiraseb F, Khadem A, Yekaninejad MS, Emamgholipour S, Mirzaei K. The interaction between MALAT1 and TUG1 with dietary fatty acid quality indices on visceral adiposity index and body adiposity index. Sci Rep. 2024;14:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 183. | Rasaei N, Samadi M, Daneshzad E, Hassan-Zadeh M, Gholami F, SaeedYekaninejad M, Clark CCT, Emamgholipour S, Mirzaei K. The transcript level of long non-coding RNAs; MALAT1 and TUG1, and the association with metabolic syndrome-related parameters in women with overweight and obesity. J Diabetes Metab Disord. 2024;23:917-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 184. | Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2305] [Cited by in RCA: 3187] [Article Influence: 265.6] [Reference Citation Analysis (0)] |
| 185. | Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1116] [Cited by in RCA: 1637] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 186. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 187. | Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70:784-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 188. | Zeng X, Yuan X, Cai Q, Tang C, Gao J. Circular RNA as An Epigenetic Regulator in Chronic Liver Diseases. Cells. 2021;10:1945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 189. | Wang P, Zhang Y, Deng L, Qu Z, Guo P, Liu L, Yu Z, Wang P, Liu N. The function and regulation network mechanism of circRNA in liver diseases. Cancer Cell Int. 2022;22:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 190. | Nokkeaw A, Thamjamrassri P, Tangkijvanich P, Ariyachet C. Regulatory Functions and Mechanisms of Circular RNAs in Hepatic Stellate Cell Activation and Liver Fibrosis. Cells. 2023;12:378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 191. | Zhang P, Wu W, Chen Q, Chen M. Non-Coding RNAs and their Integrated Networks. J Integr Bioinform. 2019;16:20190027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 192. | Chew CL, Conos SA, Unal B, Tergaonkar V. Noncoding RNAs: Master Regulators of Inflammatory Signaling. Trends Mol Med. 2018;24:66-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 193. | Ling H, Wang XC, Liu ZY, Mao S, Yang JJ, Sha JM, Tao H. Noncoding RNA network crosstalk in organ fibrosis. Cell Signal. 2024;124:111430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 194. | Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 1215] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 195. | Shang R, Lee S, Senavirathne G, Lai EC. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023;24:816-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 551] [Article Influence: 183.7] [Reference Citation Analysis (0)] |
| 196. | Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3273] [Cited by in RCA: 3347] [Article Influence: 478.1] [Reference Citation Analysis (0)] |
| 197. | Yan S, Fu P, Li H, Huang Z, Shan R, Gong B. Comprehensive Analysis of circRNA, lncRNA, miRNA and mRNA Expression Profiles and Their Competing Endogenous RNA Networks in Hepatitis B Virus-Related Hepatocellular Carcinoma. Mol Biotechnol. 2025;67:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 198. | Szymanowska A, Rodriguez-Aguayo C, Lopez-Berestein G, Amero P. Non-Coding RNAs: Foes or Friends for Targeting Tumor Microenvironment. Noncoding RNA. 2023;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 199. | Xue C, Gu X, Bao Z, Su Y, Lu J, Li L. The Mechanism Underlying the ncRNA Dysregulation Pattern in Hepatocellular Carcinoma and Its Tumor Microenvironment. Front Immunol. 2022;13:847728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 200. | Moldogazieva NT, Zavadskiy SP, Astakhov DV, Sologova SS, Margaryan AG, Safrygina AA, Smolyarchuk EA. Differentially expressed non-coding RNAs and their regulatory networks in liver cancer. Heliyon. 2023;9:e19223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 201. | Li C, Ni YQ, Xu H, Xiang QY, Zhao Y, Zhan JK, He JY, Li S, Liu YS. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct Target Ther. 2021;6:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 202. | Guo C, Liu J, Zhou Q, Song J, Zhang Z, Li Z, Wang G, Yuan W, Sun Z. Exosomal Noncoding RNAs and Tumor Drug Resistance. Cancer Res. 2020;80:4307-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 203. | Goñi E, Mas AM, Gonzalez J, Abad A, Santisteban M, Fortes P, Huarte M, Hernaez M. Uncovering functional lncRNAs by scRNA-seq with ELATUS. Nat Commun. 2024;15:9709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 204. | Yang LT, Du Y, Yu PF, Fan J, Wang XB, Wu YL. Long non-coding RNA TUG1 regulates the development of multidrug resistance in hepatocellular carcinoma via P-gp and MDR1. Int J Clin Exp Med. 2016;9:21388-21396. |
| 205. | Gu L, Li Q, Liu H, Lu X, Zhu M. Long Noncoding RNA TUG1 Promotes Autophagy-Associated Paclitaxel Resistance by Sponging miR-29b-3p in Ovarian Cancer Cells. Onco Targets Ther. 2020;13:2007-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 206. | Wang M, Hu H, Wang Y, Huang Q, Huang R, Chen Y, Ma T, Qiao T, Zhang Q, Wu H, Chen Q, Han D, Wang G, Wang X. Long non-coding RNA TUG1 mediates 5-fluorouracil resistance by acting as a ceRNA of miR-197-3p in colorectal cancer. J Cancer. 2019;10:4603-4613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 207. | Niu Y, Ma F, Huang W, Fang S, Li M, Wei T, Guo L. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 208. | Wei X, Zhou Y, Qiu J, Wang X, Xia Y, Sui L. Low expression of TUG1 promotes cisplatin sensitivity in cervical cancer by activating the MAPK pathway. J BUON. 2019;24:1020-1026. [PubMed] |
| 209. | Cao Y, Chai W, Wang Y, Tang D, Shao D, Song H, Long J. lncRNA TUG1 inhibits the cancer stem celllike properties of temozolomideresistant glioma cells by interacting with EZH2. Mol Med Rep. 2021;24:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 210. | Ntellas P, Chau I. Updates on Systemic Therapy for Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2024;44:e430028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 211. | Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 1149] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 212. | Coan M, Haefliger S, Ounzain S, Johnson R. Targeting and engineering long non-coding RNAs for cancer therapy. Nat Rev Genet. 2024;25:578-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 213. | Sidi AA, Ohana P, Benjamin S, Shalev M, Ransom JH, Lamm D, Hochberg A, Leibovitch I. Phase I/II marker lesion study of intravesical BC-819 DNA plasmid in H19 over expressing superficial bladder cancer refractory to bacillus Calmette-Guerin. J Urol. 2008;180:2379-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/