Published online Apr 27, 2025. doi: 10.4254/wjh.v17.i4.104056
Revised: February 21, 2025
Accepted: April 3, 2025
Published online: April 27, 2025
Processing time: 137 Days and 22.2 Hours
Sarcopaenia is associated with a two-fold higher mortality rate in patients with cirrhosis independent of liver disease severity. Few treatments for cirrhosis related sarcopaenia exist beyond optimal nutritional management.
To assess if rifaximin-α, a minimally absorbed antimicrobial used to manage hepatic encephalopathy (HE), may improve sarcopaenia in cirrhosis through its ammonia lowering and anti-inflammatory properties.
This single-centre retrospective cohort study of patients with prior HE compared patients treated with lactulose alone to those on combination therapy with rifaximin-α. The primary outcome was a change in skeletal muscle area (SMA) as measured by computed tomography over two time points. Secondary outcomes included episodes of spontaneous bacterial peritonitis, variceal bleeding, and gastrointestinal Clostridium difficile infection.
Of the 142 patients included, 63 were on rifaximin-α [35% female, median age 57 (51, 62)], and 79 were on lactulose without rifaximin-α [20% female, median age 55 (51, 60)]. Univariate analysis for SMA found that male sex (P < 0.001), hepatocellular carcinoma presence (P = 0.024), and greater baseline body mass index (P = 0.001) were associated with improvement of SMA. Multivariate analysis that adjusted for baseline SMA was performed and found only use of rifaximin-α (P = 0.029) to be associated with improvement of SMA.
This study demonstrates a significant independent association between rifaximin-α therapy and muscle mass in patients with cirrhosis and HE. Prospective studies of rifaximin-α therapy examining its impact on sarcopenia are required to assess its potential therapeutic role in this cohort.
Core Tip: Sarcopaenia is associated with poorer outcomes in cirrhotic patients. In our study Rifaximin use was found to be independently associated with improvement of muscle mass in patients with cirrhosis. Rifaximin's potential as a therapeutic agent directed against cirrhosis associated sarcopaenia will require prospective research.
- Citation: Worland T, Hey P, Wong D, Apostolov R, Chan RK, Sinclair M, Gow P. Rifaximin-α use is associated with improved muscle mass in patients with cirrhosis. World J Hepatol 2025; 17(4): 104056
- URL: https://www.wjgnet.com/1948-5182/full/v17/i4/104056.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i4.104056
Sarcopaenia, defined as the loss of skeletal muscle mass, strength and physical function, is an important prognostic factor in cirrhosis[1] and is estimated to occur in up to 70% of patients with advanced liver disease[2]. Muscle depletion is associated with a two-fold higher mortality rate in patients with cirrhosis, independent of liver disease severity[3]. The mechanisms underlying sarcopaenia in liver disease are multifactorial with major contributors being malnutrition, systemic inflammation, hormonal dysregulation and hyperammonemia[1].
Ammonia is a key neurotoxin central to the pathophysiology of hepatic encephalopathy (HE) in cirrhosis. Ammonia is produced primarily by metabolism of dietary proteins and endogenous nitrogenous compounds by intestinal bacteria. Hyperammonemia in cirrhosis results from reduced hepatocyte clearance and portosystemic shunting. Increased ammonia production by gut organisms in cirrhosis associated dysbiosis may also contribute. In health, ammonia is detoxified to urea within the liver, but in states of hepatocyte dysfunction or portosystemic shunting, skeletal muscle acts as the major reservoir for ammonia clearance via the tricarboxylic acid (TCA) cycle. Ammonia has several negative effects on skeletal muscle; it upregulates myostatin, a potent negative regulator of muscle mass and function; depletes branched-chain amino acids within skeletal muscle and increases oxidative stress leading to muscle autophagy; and the process of ammonia detoxification depletes skeletal muscle of key intermediates of the TCA cycle required for energy production[4]. Given the key role that skeletal muscle plays in extra-hepatic ammonia clearance, muscle wasting in cirrhosis can further reduce ammonia clearance, resulting in a self-perpetuating cycle of muscle depletion.
Because of the integral role of muscle in ammonia clearance, there is a strong relationship between sarcopenia and the severity of HE in cirrhosis[5]. Rifaximin-α has a broad antimicrobial activity on ammonia-producing enteric organisms and thus has been increasingly used to treat HE.
This study aims to explore whether the use of rifaximin-α is associated with improvements in muscle mass by comparing two cohorts of patients with cirrhosis treated for HE using either rifaximin-α in combination with lactulose, vs lactulose alone.
This was a single-centre retrospective cohort study of patients treated with lactulose or rifaximin-α in combination with lactulose, under the care of the liver transplant service in Melbourne, Australia, between 2009 and 2022. The primary outcome measure was a change in muscle mass over time between the rifaximin-α vs non-rifaximin-α treated cohort. Secondary outcomes included development of spontaneous bacterial peritonitis (SBP), clostridium difficile infection, variceal haemorrhage, and mortality.
All adult patients (age > 18 years) with cirrhosis and documented clinically apparent HE known to the Victorian liver transplant service were considered for analysis. Inclusion criteria required patients to have had serial abdominal computed tomography (CT) scans between three and nine months apart during their time on the liver transplant waitlist to allow for comparison of muscle mass over time. Rifaximin-α became widely available as a treatment of HE on the Pharmaceutical Benefits Scheme in Australia in January 2014. Therefore, patients on lactulose monotherapy (January 2009 to December 2013) were compared to those receiving rifaximin-α with lactulose (January 2014 to July 2022). Patients otherwise received standard of care management of cirrhosis including nutritional advice from an experienced liver dietitian.
Initial patient identification was achieved through interrogation of the liver transplant database, a prospectively maintained database comprising all patients referred to the liver transplant unit. All patients prescribed lactulose or rifaximin-α during the study period were included. All cases were reviewed and censored. Any further required data was subsequently collected from electronic medical records, electronic pathology systems, and radiology reporting software.
Included data comprised baseline demographics [age, gender, aetiology of liver disease, body mass index (BMI)]; disease assessment at workup [sodium model of end-stage liver disease (NaMELD), child-Pugh-Turcotte score]; Sarcopaenia assessment [Skeletal muscle area (SMA; cm2)] from single slice CT scan, sepsis (hospitalisation data for infective complications, episodes of SBP); Rifaximin-α complications (Clostridium difficile infections); and mortality and transplantation outcome data.
Muscle mass data was calculated from CT scans performed at two time-points in the patient journey to determine progress and change of outcomes. The first time-point for data collection, including for demographics and blood parameters and baseline CT. The second time-point for data collection was collected between three to nine months following the first time point at a time closest to when follow up CT was performed.
In patients in whom more than two CT scans were performed, the two scans that had an intervening time closest to six months apart were chosen. SMA was measured using the cross-sectional area of the abdominal muscles at the third lumbar vertebrae (L3) using CoreSlicer (© 2018), a web-based program for accurate measurement of body composition, verified for this purpose[6].
Patients were followed up until end outcomes occurred; delisting from service, rejected prior to formal transplant workup, death prior to transplant, or liver transplantation.
Statistical analysis was performed using R version 4.1.1. Subgroups were compared by χ2 test for categorical variables and Student’s t-test or Wilcoxon rank sum test for parametric and non-parametric continuous variables, respectively. Linear regression models (analysis of covariance) were used to assess the impact of clinical variables on muscle endpoints. Univariate and multivariate analyses were performed to determine factors that influence differences seen between groups. Age, gender, and NaMELD score were included a priori in the multivariate model as they were deemed likely to influence muscle endpoints. Additionally, a pre-specified statistical cut-off of P ≤ 0.20 was used to select additional predictors for inclusion in the multivariate model. Predictors with P < 0.05 in the multivariate model were considered to be statistically significant and therefore significantly associated with the outcome. Predictors with the lowest association were sequentially removed from the model, with the likelihood ratio test performed with each iteration before arriving at the final model.
Averages were expressed as mean ± SD for normally distributed variables, and as medians ± interquartile range (IQR) for non-normally distributed variables.
This research project was approved by the local office of research and ethics, Audit/20/Austin/121.
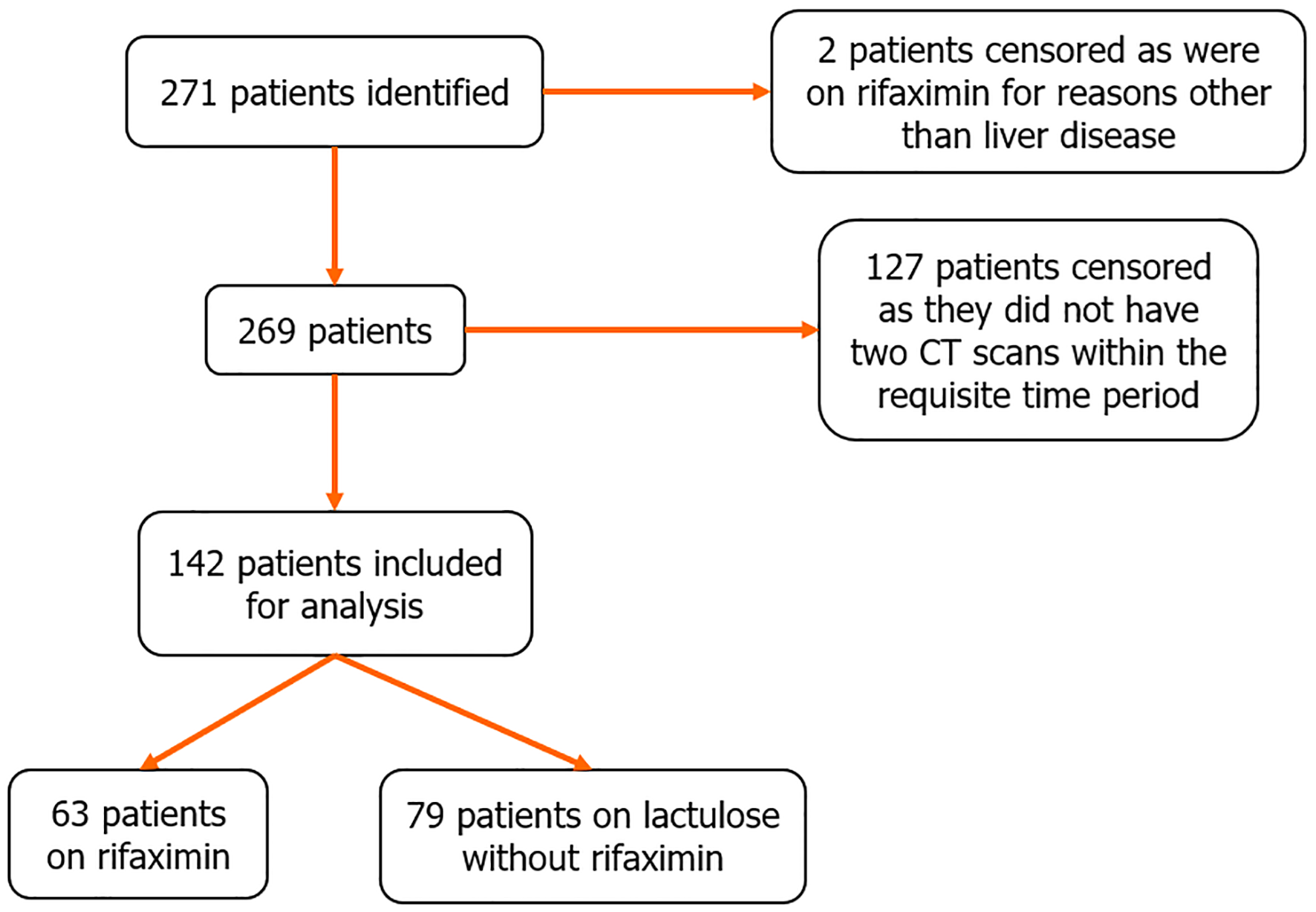
Two hundred and sixty-nine patients with cirrhosis and HE were screened for this study, 127 patients were excluded due to lack of serial CT imaging within the required timeframe for analysis. One hundred and forty-two patients met inclusion criteria and were included in this study (Figure 1).
Of the 142 patients, 63 were on rifaximin-α with lactulose [35% female, median age 57 (51, 62)], and 79 were on lactulose without rifaximin-α [20% female, median age 55 (51, 60)]. Baseline demographics are described in Table 1.
| Characteristic | Overall, n = 1421 | Lactulose alone, n = 791 | Rifaximin-α-α, n = 631 | P value2 |
| Follow-up skeletal muscle area (cm2) | 160 (137, 178) | 159 (137, 174) | 162 (134, 190) | 0.3 |
| Baseline skeletal muscle area (cm2) | 159 (139, 179) | 158 (145, 177) | 160 (131, 186) | > 0.9 |
| Gender | 0.050 | |||
| Female | 38 (27) | 16 (20) | 22 (35) | |
| Male | 104 (73) | 63 (80) | 41 (65) | |
| Age (years) | 56 (51, 61) | 55 (51, 60) | 57 (51, 62) | 0.4 |
| Viral hepatitis | 71 (50) | 44 (56) | 27 (44) | 0.2 |
| Alcohol | 46 (33) | 33 (42) | 13 (21) | 0.009 |
| Non-alcoholic fatty liver disease | 25 (18) | 15 (19) | 10 (16) | 0.7 |
| Other | 37 (26) | 15 (19) | 22 (35) | 0.027 |
| HCV eradication occurred during follow up | 0.3 | |||
| No | 138 (97) | 78 (99) | 60 (95) | |
| Yes | 4 (2.8) | 1 (1.3) | 3 (4.8) | |
| Hepatocellular carcinoma | > 0.9 | |||
| No | 75 (53) | 42 (53) | 33 (53) | |
| Yes | 66 (47) | 37 (47) | 29 (47) | |
| MELD score | 16 (12, 19) | 15 (12, 18) | 16 (13, 19) | 0.3 |
| Ascites | > 0.9 | |||
| None | 44 (31) | 24 (30) | 20 (33) | |
| Diuretic-controlled | 39 (28) | 23 (29) | 16 (26) | |
| Paracentesis | 57 (41) | 32 (41) | 25 (41) | |
| Charlson co-morbidity score | 5.00 (4.00, 6.00) | 5.00 (4.00, 6.00) | 5.00 (4.00, 6.00) | 0.3 |
| Baseline height (cm) | 173 (166, 178) | 173 (166, 179) | 172 (165, 178) | 0.5 |
| Baseline weight (kg) | 81 (72, 92) | 84 (73, 94) | 80 (70, 90) | 0.2 |
| Baseline BMI (kg/m2) | 27.8 (24.1, 31.1) | 28.0 (24.2, 30.9) | 26.8 (24.0, 31.2) | 0.8 |
| Testosterone therapy during follow up | > 0.9 | |||
| No | 118 (94) | 71 (93) | 47 (94) | |
| Yes | 8 (6.3) | 5 (6.6) | 3 (6.0) | |
| Duration of therapy before follow up SMA (days) | 161 (101, 267) | 150 (87, 227) | 179 (111, 301) | 0.3 |
| Days between scans | 218 (172, 296) | 227 (173, 343) | 216 (174, 271) | 0.5 |
The most common cause of liver disease in both groups was viral hepatitis, followed by alcohol, then metabolic associated fatty liver disease. The lactulose alone group contained a greater proportion of patients with alcohol induced cirrhosis compared to the rifaximin-α group (42% vs 21%, P = 0.009). Hepatocellular carcinoma (HCC) was present in 47% of lactulose and 47% of rifaximin-α patients (P > 0.9).
Median NaMELD scores were similar between the lactulose and rifaximin-α groups; 15 (12, 18) and 16 (13, 19) respectively, P = 0.3. The groups did not significantly differ in rates of paracentesis-dependent ascites, BMI, Charlson co-morbidity scores or presence of HCC.
There was no significant difference between lactulose vs rifaximin-α groups for successful elimination of hepatitis C (1.3% vs 4.8%, P = 0.3), or testosterone therapy (6.6% vs 6.0%, P > 0.9) between the two CT scans.
The median time between the two CT scans was 216 (IQR = 119) days in the rifaximin-α group and 227 (IQR = 144.5) days in the lactulose group, P = 0.5.
Using consensus gender-stratified definitions for sarcopaenia for skeletal muscle index in cirrhotic cohorts[7,8], baseline sarcopaenia was present at baseline in 59.7% of the rifaximin-α group and 41.8% of the lactulose group (P = 0.87).
Between the first and the second abdominal CT scan, the median change in SMA (cm2) in the rifaximin-α group was + 0.74 cm2 (IQR = 19.7), median change of SMA in lactulose group = - 6.9 cm2 (IQR = 23.95).
Change in SMA was analysed by regression modelling to determine factors associated with improvement of muscle mass. Univariate analysis for SMA found that male sex (P < 0.001), HCC presence at workup (P = 0.024), and greater baseline BMI (P = 0.001) to be associated with an increase in SMA between scans (Table 2). Multivariate analysis including severity of liver disease that adjusted for baseline SMA was performed and found only use of rifaximin-α (P = 0.029) to be associated with improvement of SMA (Table 3).
| Characteristic | n | 95%CI | P value |
| Gender | 142 | ||
| Female | - | ||
| Male | 26, 49 | < 0.001 | |
| Age (years) | 142 | -0.72, 0.73 | > 0.9 |
| Treatment | 142 | ||
| Lactulose alone | - | ||
| Rifaximin-α | -3.4, 20 | 0.2 | |
| Viral hepatitis | 141 | -5.0, 18 | 0.3 |
| Alcohol | 141 | -3.9, 21 | 0.2 |
| Non-alcoholic fatty liver disease | 141 | -18, 13 | 0.8 |
| Other | 141 | -17, 9.7 | 0.6 |
| HCV eradication occurred during follow up | 142 | ||
| No | - | ||
| Yes | -19, 52 | 0.4 | |
| Hepatocellular carcinoma | 141 | ||
| No | - | ||
| Yes | 1.8, 25 | 0.024 | |
| MELD score | 141 | -1.5, 0.69 | 0.5 |
| Ascites | 140 | ||
| None | - | ||
| Diuretic-controlled | -14, 17 | 0.8 | |
| Paracentesis | -20, 8.5 | 0.4 | |
| Charlson co-morbidity score | 141 | -2.2, 4.9 | 0.4 |
| Baseline BMI (kg/sq m) | 132 | 0.55, 2.1 | 0.001 |
| Baseline subjective global assessment | 16 | ||
| A | - | ||
| B | -55, 25 | 0.4 | |
| C | -78, 39 | 0.5 | |
| Testosterone therapy during follow up | 126 | ||
| No | - | ||
| Yes | -9.5, 40 | 0.2 | |
| Duration of rifaximin-α before follow up SMI (days) | 64 | -0.05, 0.12 | 0.4 |
| Days between scans | 142 | -0.06, 0.03 | 0.6 |
| Characteristic | 95%CI | P value |
| Male gender | -4.2, 23 | 0.2 |
| Age (years) | -0.38, 0.55 | 0.7 |
| Treatment with rifaximin-α | 0.95, 17 | 0.029 |
| Aetiology: Alcohol | -3.7, 13 | 0.3 |
| Hepatocellular carcinoma | -8.7, 8.2 | > 0.9 |
| MELD score | -1.0, 0.78 | 0.8 |
| Baseline height (cm) | -2.5, 2.2 | > 0.9 |
| Baseline weight (kg) | -2.2, 2.6 | 0.9 |
| Baseline BMI (kg/sq m) | -7.0, 7.4 | > 0.9 |
| Testosterone therapy during follow up | -1.6, 29 | 0.078 |
Of the lactulose group, 57 (74%) were transplanted, and 5 (6.5 %) died awaiting transplantation. In the rifaximin-α group, 51 (88%) were transplanted, and 2 (3.4%) died awaiting transplantation (Table 4). Median time-to-transplant was 353 days (IQR = 268 days) in the rifaximin-α group, and 312.5 days (IQR = 403.75 days) in the lactulose group. For patients who died awaiting transplant, the median time to death from listing was 265.5 days (IQR = 0 days) in the rifaximin-α group, and 282 days (IQR = 252 days) in the lactulose group. There was no significant difference in rates of outcomes between the two groups (P = 0.07).
To account for differing follow-up times on the liver transplant waitlist after study inclusion, secondary outcomes were calculated per patient year of follow-up. Six patients (9.7%) in the rifaximin-α group had at least one episode of variceal haemorrhage compared to 5 patients (6.4%) in the lactulose group (P = 0.50). Episodes of SBP occurred more frequently in the rifaximin-α group vs the lactulose group (231 vs 102 episodes per 1000 patient years, P < 0.001). There was only one episode of Clostridium difficile infection in the rifaximin-α group, and none in the lactulose group.
This retrospective study describes a positive association between the use of rifaximin-α and improvement in muscle mass over time as compared to lactulose monotherapy in a cohort of liver transplant candidates with HE. This finding was independent of baseline NaMELD score and gender, and was not influenced by potential confounders such as hepatitis C therapy or testosterone use.
Previous work assessing the impact of rifaximin-α on sarcopenia measures has been limited predominately to animal models. The anabolic effect of L-carnitine on cirrhotic rat myocytes was found to be attenuated when combined with oral rifaximin-α[9]. In addition, rifaximin-α prevented muscle atrophy by decreasing myostatin and other pro-inflammatory cytokine levels. In rat models, the use of combination rifaximin-α and L-ornithine L-aspartate, an amino acid mixture that enhances ammonia clearance, for a 4-week period increased muscle mass, muscle diameter, grip strength, and biomarkers of muscle autophagy[10]. Human data examining the impact of rifamycin/rifaxmin on muscle mass is limited to two published manuscripts. A single arm observational study of 10 cirrhotic patients found an increase in psoas muscle area was observed following at least 3 months treatment with rifaximin-α[11]. Recently, a phase two placebo controlled randomised controlled trial investigated the stool cirrhosis dysbiosis ratio in 15 subjects treated with rifamycin SV MMX vs 15 subjects who were not. Although the primary endpoint of difference of stool cirrhosis dysbiosis ratio was not met, the secondary outcomes that were met are mechanistically informative, including that in subjects treated with rifamycin there was lower microbial diversity but higher proportions of short-chain fatty acids, improved bile acid profile, and improved markers of sarcopaenia including greater handgrip strength, lean muscle mass, and patient reported outcomes[12]. Ammonia lowering therapies that included rifaximin-α have shown promise in reversing sarcopenia in animal models of hyperammonaemia[10].
Despite the acknowledged poor prognostic impact of sarcopenia in cirrhosis, there are few evidence-based interventions that attenuate muscle loss and dysfunction. Nutritional recommendations include a high energy high protein diet are advised by society guidelines[13] but this alone is insufficient to reverse the anabolic resistance observed in cirrhosis[14]. While exercise interventions and nutraceuticals have demonstrated some benefits in small studies[15] and testosterone therapy has been shown to increase muscle mass in hypogonadal men with cirrhosis[16], there remains a large unmet need for safe and effective therapies to mitigate the loss of muscle mass in this population.
Ammonia upregulates myostatin, resulting in inhibition of muscle cell growth and proliferation[17]. Elevated myostatin is seen in patients with cirrhosis related hyperammonemia[18,19] and is thought to be key to the pathogenesis of muscle atrophy. In health, ammonia is detoxified to urea within the liver via the urea cycle. However, in states of hepatocyte dysfunction or portosystemic shunting, skeletal muscle acts as the major reservoir for ammonia clearance via the TCA cycle. The process of ammonia detoxification by muscle also depletes skeletal muscle of branched-chain amino acids[20], and also releases reactive oxygen species causing mitochondrial toxicity and increased skeletal muscle cell death[21]. Due to the shared pathogenesis driven by hyperammonaemia, sarcopenia is closely linked to the presence of HE[5], and a vicious cycle can result in which progressive sarcopenia leads to further impairment of ammonia clearance[22].
Ammonia-lowering therapy in cirrhosis is commonly prescribed for primary or secondary prevention of HE. Lactulose is considered to be first-line therapy, acting as both an aperient and also lowers colonic pH, trapping ammonia in the colonic lumen as ammonium and subsequently lowering blood ammonia concentration. Rifaximin-α is a poorly absorbed semi-synthetic antibiotic that lowers serum ammonia via its activity against ammonia producing bacteria and is well tolerated with minimal side effects[23]. The comparative ammonia lowering efficacy of rifaximin-α and lactulose is controversial. Patients with cirrhosis and minimal HE were randomised to treatment with probiotics, lactulose (7 patients), or rifaximin-α (11 patients) for 4 weeks. Patients in the rifaximin-α group had a greater reduction of mean serum ammonia (24.8 μmol/L to 20.7 μmol/L) compared with the lactulose group (17.4 μmol/L to 18.8 μmol/L)[24]. However, a meta-analysis of 4 studies for 177 rifaximin-α patients and 175 lactulose or lactitol patients found no statistical difference between ammonia lowering effects of these agents (standard mean difference = -0.02, 95%CI = -0.40 to 0.02, P = 0.08), although numerically rifaximin-α was superior[25].
The positive association between rifaximin-α use and improvement in muscle parameters may relate to its additional ammonia-lowering effect compared to lactulose monotherapy, or rifaximin-α may have benefits via alternative mechanisms. Rifaximin-α use is associated with alterations in gut microbiota without significant changes in microbial abundance, and results in decreased endotoxaemia[26]. Endotoxins, particularly interleukin-6 (IL-6) and tumour necrosis factor alpha (TNF-α), mediate cirrhosis associated immune dysfunction[27]. Rifaximin-α use lowers systemic levels of IL-6 and TNF-α, subsequently improving systemic haemodynamics and hepatovenous pressure gradient[28]. Improved overall survival[29] and decreased complication rates including variceal haemorrhage, SBP, and ascites have been reported with rifaximin-α use compared to lactulose alone[30-32] suggesting additional clinical benefit to rifaximin-α use over lactulose alone for parameters other than HE. In this study we identified an unexpectedly higher rate of SBP in the rifaximin-α cohort compared to the lactulose group, however overall numbers were low and therefore a type 1 error is possible.
Strengths of this study include the serial measurements of muscle mass in a well characterised population with excellent follow-up. Despite our cohorts being recruited from different time periods, there were no significant differences with regards to baseline variables and potential confounders, including transplant status, use of testosterone, or hepatitis C therapy. All variables were adjusted for in the analysis by using multiple regression methods. Following this, only baseline rifaximin-α use (P = 0.029) was associated with improvement of SMA. We used a robust, objective measure of muscle mass, being CT SMA, which is the most commonly employed measure of muscle mass in cirrhosis that has been well validated as a predictor of poor outcome[33]. Rifaximin-α appeared to be safe with only one documented case of Clostridium difficile infection.
Limitations of this study include its retrospective nature, which limits our ability to confirm a potential causal relationship between rifaximin-α use and changes in muscle mass. This also meant that serial measures of muscle function and frailty were not available. Our rifaximin-α and control populations were recruited over different time periods based on the availability of rifaximin-α on the pharmaceutical benefits scheme as a therapy for HE in Australia. Although there were no significant differences between the baseline characteristics of the two populations, between group differences in other parameters such as oral nutritional intake, protein supplementation, medication or other clinical practices that may influence muscle changes were not captured. Finally, we did not have adequate data to explore the reasons behind the elevated rate of SBP in the rifaximin-α-treated group in our study, which is in contrast to prior studies suggesting the rifaximin-α can reduce SBP risk[30,34]. We hypothesise that more frequent episodes of SBP in the rifaximin-α group may represent less frequent use of other SBP prophylactic antibiotics in this group, due to patient or clinician belief that they are not required for patients taking rifaximin-α.
This study demonstrates a significant association between the use of rifaximin-α and increased muscle mass over time in a cohort of patients with cirrhosis and HE. Given the known deleterious impact of circulating ammonia on muscle metabolism, and the ammonia-lowering properties of rifaximin-α, we propose that rifaximin-α has the potential to mitigate sarcopenia in cirrhosis with HE. Its effects on altering systemic inflammation warrant attention as a possible additional mechanism for ameliorating muscle atrophy. This may offer promise for a broader population of cirrhotic patients with sarcopenia outside those with HE. Prospective interventional clinical trials with robust measures of muscle mass and function are required to better evaluate its potential role as a therapy for sarcopenia.
| 1. | Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 2. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 3. | Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 283] [Article Influence: 70.8] [Reference Citation Analysis (2)] |
| 4. | Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018;21:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Lattanzi B, D'Ambrosio D, Merli M. Hepatic Encephalopathy and Sarcopenia: Two Faces of the Same Metabolic Alteration. J Clin Exp Hepatol. 2019;9:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Mullie L, Afilalo J. CoreSlicer: a web toolkit for analytic morphomics. BMC Med Imaging. 2019;19:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 8. | Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD; Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 931] [Cited by in RCA: 810] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 9. | Murata K, Kaji K, Nishimura N, Enomoto M, Fujimoto Y, Takeda S, Tsuji Y, Fujinaga Y, Takaya H, Kawaratani H, Namisaki T, Akahane T, Yoshiji H. Rifaximin enhances the L‑carnitine‑mediated preventive effects on skeletal muscle atrophy in cirrhotic rats by modulating the gut‑liver‑muscle axis. Int J Mol Med. 2022;50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Kumar A, Davuluri G, Silva RNE, Engelen MPKJ, Ten Have GAM, Prayson R, Deutz NEP, Dasarathy S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017;65:2045-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | Borentain P, Rouabah K, Allard G, Ressiot E, Gerolami R. Letter: nutritional benefits of rifaximin in cirrhotic patients. Aliment Pharmacol Ther. 2018;47:699-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Bajaj JS, Fagan A, Gavis EA, Mousel T, Gallagher ML, Puri P, Fuchs M, Davis BC, Hylemon PB, Zhou H, Ahluwalia V, Cadrain R, Sikaroodi M, Gillevet PM. The RIVET RCT: Rifamycin SV MMX improves muscle mass, physical function, and ammonia in cirrhosis and minimal encephalopathy. Hepatol Commun. 2024;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (3)] |
| 14. | Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 15. | West J, Gow PJ, Testro A, Chapman B, Sinclair M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: A review. J Gastroenterol Hepatol. 2021;36:2687-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Geladari E, Alexopoulos T, Kontogianni MD, Vasilieva L, Mani I, Alexopoulou A. Mechanisms of sarcopenia in liver cirrhosis and the role of myokines. Ann Gastroenterol. 2023;36:392-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Han JW, Kim DI, Nam HC, Chang UI, Yang JM, Song DS. Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis. Clin Mol Hepatol. 2022;28:219-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 20. | Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, Hatzoglou M, Dasarathy S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Wijarnpreecha K, Werlang M, Panjawatanan P, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Ungprasert P. Association between sarcopenia and hepatic encephalopathy: A systematic review and meta-analysis. Ann Hepatol. 2020;19:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Iadevaia MD, Prete AD, Cesaro C, Gaeta L, Zulli C, Loguercio C. Rifaximin in the treatment of hepatic encephalopathy. Hepat Med. 2011;3:109-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Wang MW, Ma WJ, Wang Y, Ma XH, Xue YF, Guan J, Chen X. Comparison of the effects of probiotics, rifaximin, and lactulose in the treatment of minimal hepatic encephalopathy and gut microbiota. Front Microbiol. 2023;14:1091167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 25. | Cheng J, Chen Y, Cao W, Zuo G. Is rifaximin better than nonabsorbable disaccharides in hepatic encephalopathy?: A meta-analysis. Medicine (Baltimore). 2021;100:e28232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 27. | Jain L, Sharma BC, Sharma P, Srivastava S, Agrawal A, Sarin SK. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig Liver Dis. 2012;44:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, Yoo JJ, Cho YY, Cho EJ, Yu SJ, Kim MY, Kim YJ, Baik SK, Yoon JH. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Salehi S, Tranah TH, Lim S, Heaton N, Heneghan M, Aluvihare V, Patel VC, Shawcross DL. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol Ther. 2019;50:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol. 2018;11:1756284818800307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Zeng X, Sheng X, Wang PQ, Xin HG, Guo YB, Lin Y, Zhong JW, He CZ, Yin J, Liu TT, Ma WJ, Xiao X, Shi PM, Yuan ZL, Yang L, Ma X, Xu JM, Shen XZ, Yang CQ, Zhu X, Lv NH, Xie WF. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatol Int. 2021;15:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 34. | Praharaj DL, Premkumar M, Roy A, Verma N, Taneja S, Duseja A, Dhiman RK. Rifaximin Vs. Norfloxacin for Spontaneous Bacterial Peritonitis Prophylaxis: A Randomized Controlled Trial. J Clin Exp Hepatol. 2022;12:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/