Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.101691
Revised: December 3, 2024
Accepted: January 21, 2025
Published online: February 27, 2025
Processing time: 149 Days and 19.4 Hours
Non-alcoholic fatty liver disease (NAFLD) is a disease of increasing global prevalence and an important risk factor for the development of insulin resistance, type 2 diabetes, non-alcoholic steatohepatitis and hepatocellular carcinoma, but the pathogenesis is not clear. The aim of this study was to explore the role of ILF3 in NAFLD.
To investigate the molecular processes through which ILF3 facilitates the advancement of NAFLD by inhibiting the expression of p-AMPK. This explo
In vitro and in vivo experiments were conducted using HepG2 cells and NAFLD animal models. The effects of ILF3 knockdown on lipid synthesis and triglyceride (TG) secretion were examined by analyzing the expression levels of p-AMPK. Additionally, the roles of ILF3 and the AMPK signaling pathway were verified using techniques such as Western blotting, quantitative reverse transcription PCR, Oil Red O staining, and immunohistochemistry.
Investigations revealed an increase in ILF3 Levels within both HepG2 cells and animal models of NAFLD, concurrently with a decrease in p-AMPK expression. Knocking down ILF3 activated the AMPK pathway, reducing lipid production and TG secretion in hepatocytes, thereby mitigating the advancement of NAFLD.
ILF3 promotes the evolution of NAFLD by inhibiting the expression of p-AMPK. The knockdown of ILF3 activates the AMPK signaling pathway, alleviating the severity of NAFLD. These findings underscore the function of ILF3 in the pathogenesis of NAFLD and demonstrate its viability as a treatment focus and diagnostic indicator.
Core Tip: First, a correlation is found between ILF3 expression and non-alcoholic fatty liver disease (NAFLD). Second, high ILF3 expression in NAFLD patients suggests its involvement in disease progression. Third, inhibiting ILF3 expression reduces lipid deposition and triglyceride secretion in NAFLD, regulating lipid metabolism. Fourth, suppressing ILF3 stimulates the AMPK pathway, which governs the hepatic energy equilibrium and lipid processing. Fifth, ILF3 modulates the AMPK pathway as a viable therapeutic candidate for NAFLD, providing new perspectives on diagnosis and treatment.
- Citation: Zhan T, Liu JX, Huang M, Chen MT, Tian XR, Yang XL, Tan J, Zou YL, Han Z, Chen W, Tian X, Huang XD. ILF3 inhibits p-AMPK expression to drive non-alcoholic fatty liver disease progression. World J Hepatol 2025; 17(2): 101691
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/101691.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.101691
Non-alcoholic fatty liver disease (NAFLD) represents a persistent condition of the liver, characterized by the accumulation of fats within the liver cells, unrelated to the intake of alcohol or other identifiable factors contributing to liver harm[1]. It encompasses two main conditions: Non-alcoholic fatty liver and non-alcoholic steatohepatitis[2]. NAFLD progression may result in fibrosis, cirrhosis, and even hepatocellular carcinoma, posing a significant economic and health burden globally[3]. NAFLD has become a significant factor contributing to the rising number of liver transplantations[4]. Recent research efforts have focused on unraveling the molecular mechanisms underlying the development of NAFLD, which could enhance diagnostic accuracy and guide personalized treatment approaches[2,5,6]. However, the specific pathogenesis of NAFLD continues to be enigmatic.
ILF3, alternatively referred to as NF90/NF110, is a member of the double-stranded RNA (dsRNA)-binding protein group that holds a pivotal function in various cellular processes. It has been implicated in mRNA stability enhancement[7], translation regulation (inhibition or activation)[8], transcription factor activation[9], and the regulation of miRNA biology[8]. Recent research has concentrated on the association between ILF3 and cancer[10-13]. For instance, ILF3 has been shown to activate the transcription of calcineurin 11, thereby promoting breast cancer cell proliferation and metastasis[14]. Besides cancer, ILF3 has additionally been recognized as a contributory element in the development of atherosclerosis and stroke[15]. ILF3 has previously been found to be overexpressed in vascular smooth muscle tissue and macrophages, promoting atherosclerotic calcification through the activation of BMP2/STAT1[16]. The RNA-seq analysis has indicated a strong association between ILF3 and energy metabolism, particularly lipid metabolism (unpublished data). Furthermore, analysis of multiple Gene Expression Omnibus datasets revealed significant upregulation of hepatic ILF3 expression in individuals diagnosed with NAFLD and mouse primary liver cells exposed to oleic acid[17]. Therefore, exploring the function of ILF3 in the advancement of NAFLD presents significant interest.
AMPK is a heterotrimeric complex that consists of α catalytic subunits and β and γ regulatory subunits. It is encoded by various genes, leading to several subtypes (α1/α2; β1/β2; γ1/γ2/γ3)[18]. This complex plays a vital role in preserving energy equilibrium and is involved in various systemic metabolic pathways, including the regulation of lipid metabolism in hepatocytes[19]. A recent investigation has demonstrated that the AMPK activator PXL770 has a positive impact on reducing hepatic steatosis in NAFLD patients[20]. Furthermore, research has shown that activating AMPK improves insulin resistance and liver lipids in HepG2 cells[21] while also inducing liver autophagy through phosphorylation to reduce fatty acid synthesis[22]. ILF3 has been documented to participate in energy metabolism, including the AMPK signaling pathway, in colon cancer[11]. However, the relationship between ILF3 and AMPK in NAFLD is still not well understood.
This study was conducted in vitro and in vivo to explore the role of ILF3 within lipid metabolism during the development of NAFLD, specifically through the AMPK pathway. The study observed that ILF3 was markedly upregulated in both HepG2 cells and a NAFLD animal model. Conversely, the expression of p-AMPK was downregulated. By knocking down ILF3, the study activated the AMPK pathway, inhibiting the synthesis of fats and decreasing the release of triglycerides (TG) in liver cells. The results provide new perspectives on the development of NAFLD and propose ILF3 as a potential indicator and treatment target for NAFLD in the future.
This study included a collective of 60 individuals diagnosed with NAFLD. The NAFLD group comprised 28 females and 32 males who were received at Wuhan Third Hospital between May 2020 and May 2022. A control group consisting of 20 healthy subjects, comprising 10 women and 10 men, was incorporated. The age range of the NAFLD patients was 18 to 80 years, and their inclusion was determined by specific criteria, which excluded viral liver disease, cancer, past alcohol consumption, autoimmune hepatic conditions, medication-induced liver disorders, and other conditions linked to fatty liver. The healthy individuals were aged between 22 and 78 years and had no underlying diseases. Ethical standards were adhered to, and the Wuhan Third Hospital Ethics Committee approved the study.
HepG2 cells, a human liver cell line procured from Shanghai Aussie Biology (China), were maintained in Dulbecco's Modified Eagle Medium (DMEM) sourced from Sevier (China), enriched with 1% penicillin-streptomycin from Invitrogen (United States), and 10% fetal bovine serum (FBS) also from Sevier (China). These cells were incubated in a moisture-controlled environment at 37 °C and 5% CO2.
To establish an in vitro model of NAFLD, HepG2 cells were cultured with or without an Oleic acid mixture (OA) at concentrations of 20/40/60 μM/mmol for 48 hours. These treated cells were then used for subsequent analysis.
Thirty-two male C57/BL6 mice (six weeks old) were acquired from Hunan Slrek da Experimental Animal Co, Ltd (Hunan, China) and housed in specific pathogen-free conditions in groups of five per cage. The housing conditions were set to a temperature of 24 ± 1 °C and a relative humidity of 40%-80%. The mice were assigned randomly to five distinct groups as follows:
Normal group (n = 12): Mice were assigned to a standard chow diet and administered PBS intravenously (iv).
NAFLD model group (n = 20): Mice were subjected to a high-fat diet (HFD) for the induction of NAFLD.
NAFLD model control group (n = 12): Mice were provided with a HFD and received PBS iv.
shLF3 group (n = 4): Mice received a HFD and were given specific small hairpin RNA (shILF3) targeting LF3 iv.
shILF3 + CompoudC group (n = 4): Mice were fed a HFD and administered shILF3 + CompoudC iv.
The mice in the control group and NAFLD model group received equivalent volumes of solutions after four weeks. At the 4th, 8th, and 12th weeks of the experiment, four mice from every group were sacrificed, and blood samples were obtained for analysis. Liver samples were also obtained for further analysis. These procedures adhered to the "Principles of Laboratory Animal Care" outlined by the National Institutes of Health, and the Ethics Committee of Wuhan Third Hospital approved the study.
The ILF3 virus and negative control (NC) were procured from GenePharma (Beijing, China). Cell infection was carried out using either HiTranceA or HiTranceB, adhering to the guidelines provided by the manufacturer. Briefly, 40 μL of HiTranceA was diluted with 1 mL of DMEM medium in a six-well plate containing OA-treated HepG2 cells. Then, 20 μL of shILF3 or NC was introduced into the plate and gently blended to ensure even distribution. After incubating for 6 hours, the medium was substituted with a new DMEM medium augmented with 10% FBS. The cells expressing the desired protein were isolated by treating them with 2 µg/mL puromycin and were further cultured for 72 hours before subsequent experiments.
Total RNA was isolated from HepG2 cells, mice liver tissues, and serum samples, including patients' serum, utilizing the TRIzol solution (by Invitrogen, United States) following the guidelines provided by the manufacturer (TOYOBO, Japan). To assess the levels of gene expression, quantitative real-time-PCR was performed utilizing the ABI StepOne Plus qPCR System by Applied Biosystems, based in the United States. The PCR conditions consisted of an initial denaturation step at 95 °C for 10 minutes, succeeded by 40 cycles of denaturation at 95 °C for 15 seconds, annealing at 60 °C for 20 seconds, and extension at 72 °C for 40 seconds. The cycle threshold (Ct) values were determined, and the expression levels were assessed relatively using the 2-△△Ct approach. GAPDH was utilized as the standard for mRNA normalization. Each sample underwent triplicate testing for statistical analysis. The PCR primer sequences employed were as stated below:
Human GAPDH: F 5'-GGGGCTCTCCAGAACATC-3', R 5'-TGACACGTTGGCAGTGG-3' Mice GAPDH: F 5'-AGGTCGGTGTGAACGGATTTG-3', R 5'-TGTAGACCATGTAGTTGAGGTCA-3'
Human ILF3: F 5'-TTCGGCCAGCTCCATAA-3', R 5'-GCGTTTCATGGGCGTAA-3'
Mice ILF3: F 5'-GGGAACACCGGCAAGATAGT-3', R 5'-CTCATGGGACGCATTTTCCC-3'
Protein extraction was performed from cultured hepatocytes and mice liver tissues to examine the levels of specific proteins. The samples were lysed and homogenized using RIPA buffers (Beyotime, Shanghai, China), and the protein concentrations in the cell lysates were determined using a PierceTM Rapid Gold BCA Protein Assay Kit (Thermo Fisher, China). Subsequently, they were segregated by SDS-PAGE and transferred to a PAVD membrane (MILLIPORE, China). Following an overnight blockade with 5% skim milk, the ILF3, AMPK, pAMPK, and GAPDH proteins were detected using specific monoclonal primary and secondary antibodies.
By the instructions provided by the reagent vendors, HepG2 cells and liver tissue underwent Oil Red O staining. Initially, the HepG2 cells were rinsed with PBS and immersed in 10% formalin for 5 minutes after being cultured in flasks. After fixation, the cells were rinsed with isopropyl alcohol and stained with Oil Red O. Following a 30-minute incubation, the cells were rinsed with distilled water and subsequently subjected to hematoxylin counterstaining for 1 minute.
In the case of liver tissues, the liver samples from mice were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned into 6 μm-thick slices. These sections were then dewaxed and rehydrated using xylene, followed by rinsing with PBS. Subsequently, the tissues were subjected to Oil Red O staining for a duration of 30 minutes, followed by counterstaining with hematoxylin for 1 minute.
Following the cleaning and dehydration of the dyed sections, they were prepared for observation with a fluorescence microscope (IX-51, Olympus). For each sample, five images were randomly selected. Data collection was conducted from at least three independent experiments.
Immunohistochemistry (IHC) analysis was conducted to assess the expression of ILF3, AMPK and pAMPK in mouse liver tissue. The liver tissue specimens were preserved in 4% PFA, embedded in paraffin, and cut into 6 μm sections. After mounting the sections on slides, the endogenous peroxidase activity was suppressed by treating them with a wash solution containing 3% hydrogen peroxide in methanol. Subsequently, the tissue samples were subjected to overnight incubation at 4 °C with a primary antibody targeting Sirt1 (dilution: 1:500, Abcam). Following a PBS wash, the sections were exposed to secondary antibodies and incubated at room temperature for 1 hour. The sections were rewashed and treated with DAB substrate to visualize the staining. Upon completion of the washes and dehydration process, the sections were mounted with a coverslip and examined under fluorescence microscopy (IX-51, Olympus).
After culturing the transfected cells in six-well plates for 48 hours, the study performed cell staining using the Annexin V-PE assay kit provided by BD Biosciences, United States, following the manufacturer's instructions. Afterward, the study examined the samples using the FACS Caliber II sorter and the Cell Task FACS system manufactured by BD Biosciences, United States.
The TG levels were determined following the instructions provided with the TG colorimetry kit from Shanghai Rongbai Biological Technology Co., Ltd., China. The study used the Boehringer Mannheim biochemical analyzer to measure the concentrations of aspartate transaminase (AST) and alanine transaminase (ALT).
Statistical analyses were conducted utilizing SPSS software, and the data were depicted using GraphPad Prism. The significance of the results was assessed using Student's t-test or one-way ANOVA. Statistical significance was evaluated using data displayed in the format of mean ± SD, with significance denoted by a P value below 0.05.
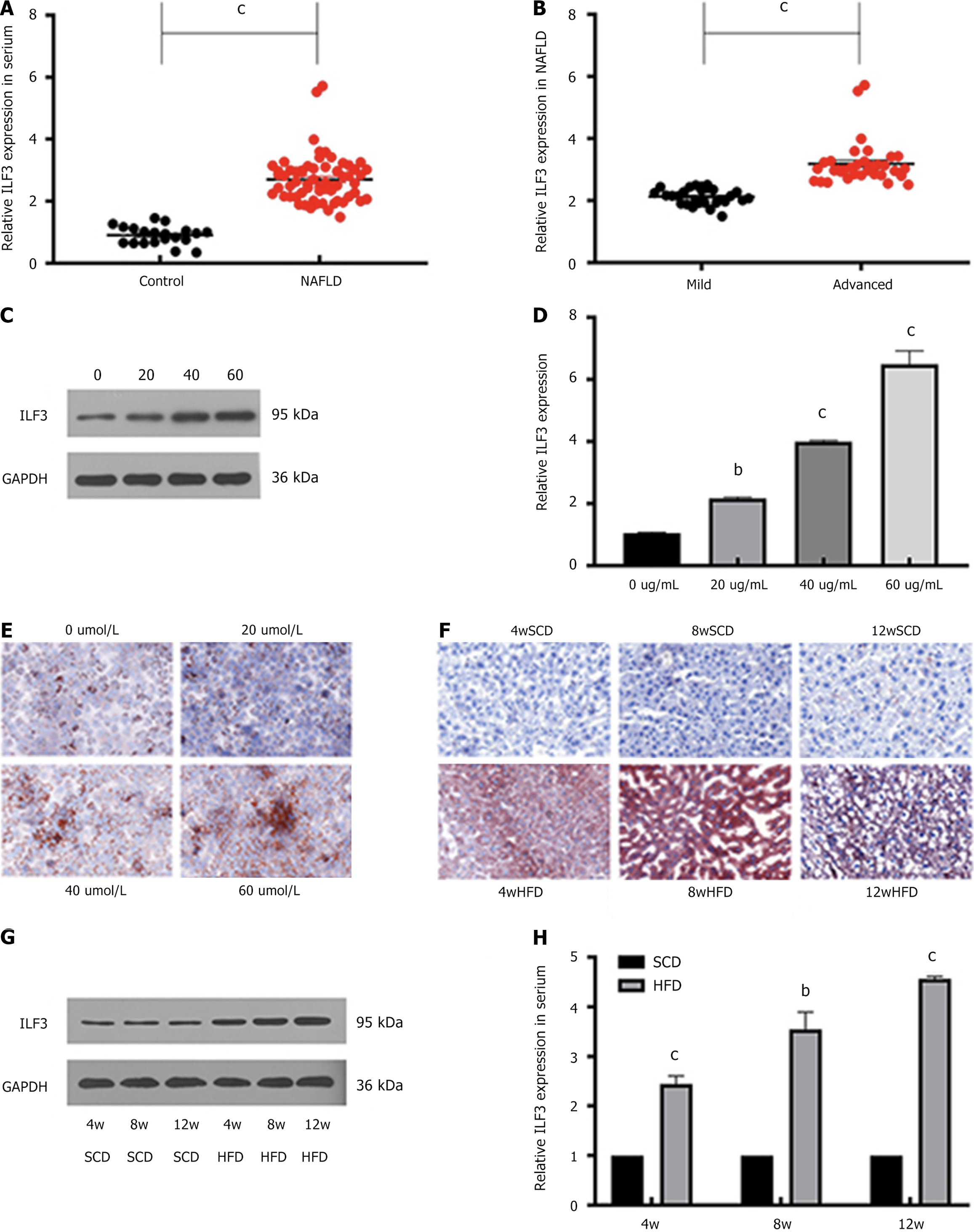
ILF3, a potential regulator of lipogenesis, was found to be upregulated in the serum of NAFLD patients (Figure 1A and B) and in HepG2 cells treated with OA to mimic NAFLD phenotype (Figure 1C and D). The expression levels of ILF3 exhibited a positive correlation with the severity of NAFLD and OA concentration in both human serum and HepG2 cells. Lipid deposition, indicated by Oil Red O staining, was observed in both OA-treated HepG2 cells (Figure 1E) and NAFLD mouse liver tissue (Figure 1F). Western blotting and qPCR analyses confirmed the heightened expression of ILF3 observed in the liver tissue of mice with NAFLD and further demonstrated its upregulation in serum (Figure 1G and H). Notably, ILF3 expression increased with the duration of HFD feeding in mice. These findings imply that ILF3 may contribute to the growth and advancement of NAFLD through the regulation of lipogenesis.
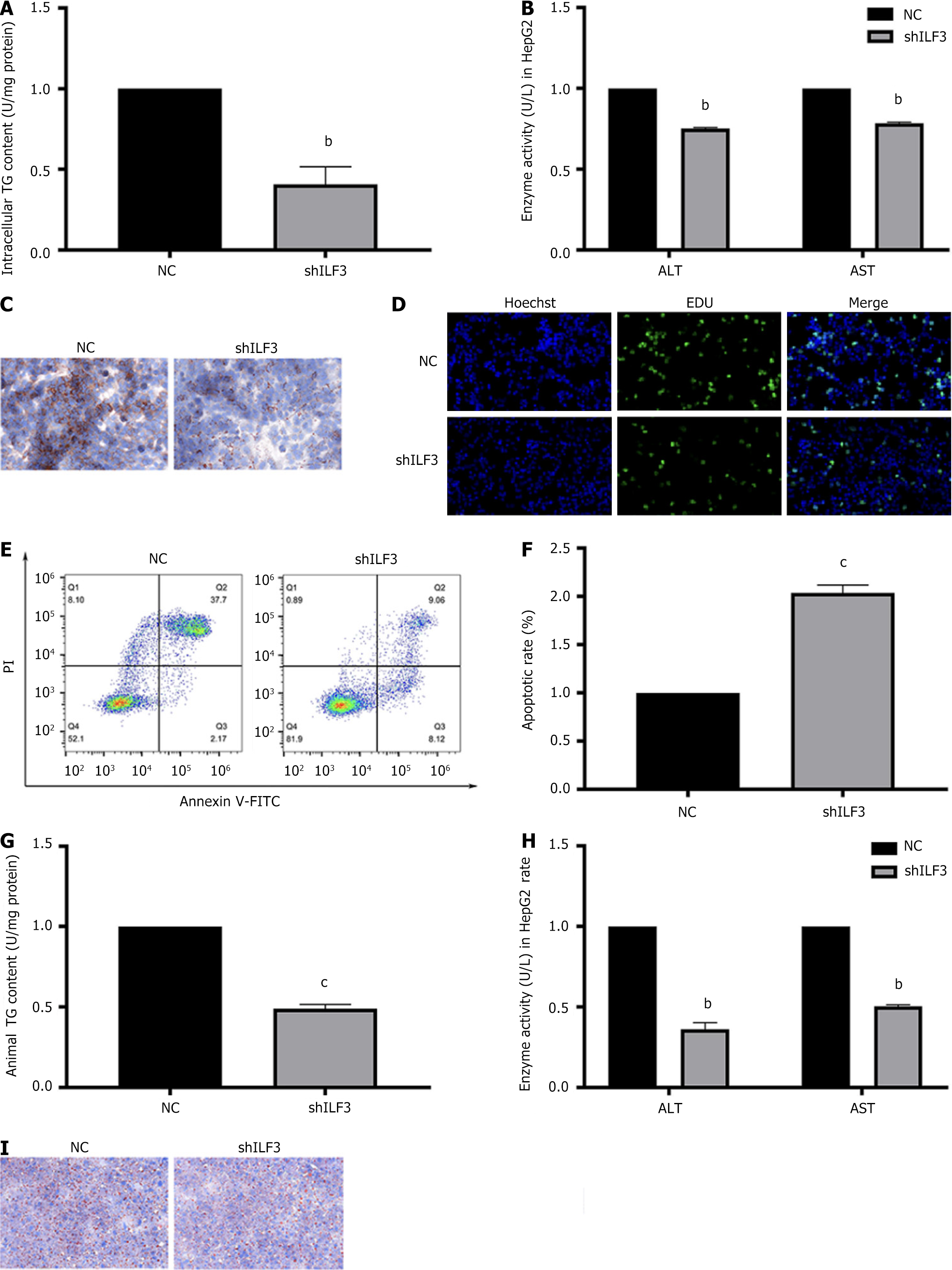
To investigate the association between ILF3 and NAFLD, the experiment initially infected HepG2 cells with shILF3 or a NC. The study observed that shILF3-infected HepG2 cells displayed reduced levels of ALT, AST, and TG secretion (Figure 2A and B). Additionally, Oil Red O staining revealed a reduction in lipid deposition following ILF3 knockdown (Figure 2C). Moreover, the study found that knockdown of ILF3 promoted proliferation and inhibited hepatocyte apoptosis of OA-treated HepG2 cells (Figure 2D-F).
Subsequently, to downregulate ILF3 expression, the study injected shILF3 or NC into the tail vein of mice fed a HFD. In line with the findings from HepG2 cells, the shILF3 group exhibited suppressed levels of ALT, AST, and TG secretion and reduced lipid deposition (Figure 2G-I). These findings provide evidence that ILF3 inhibition could protect hepatocytes from lipid deposition.
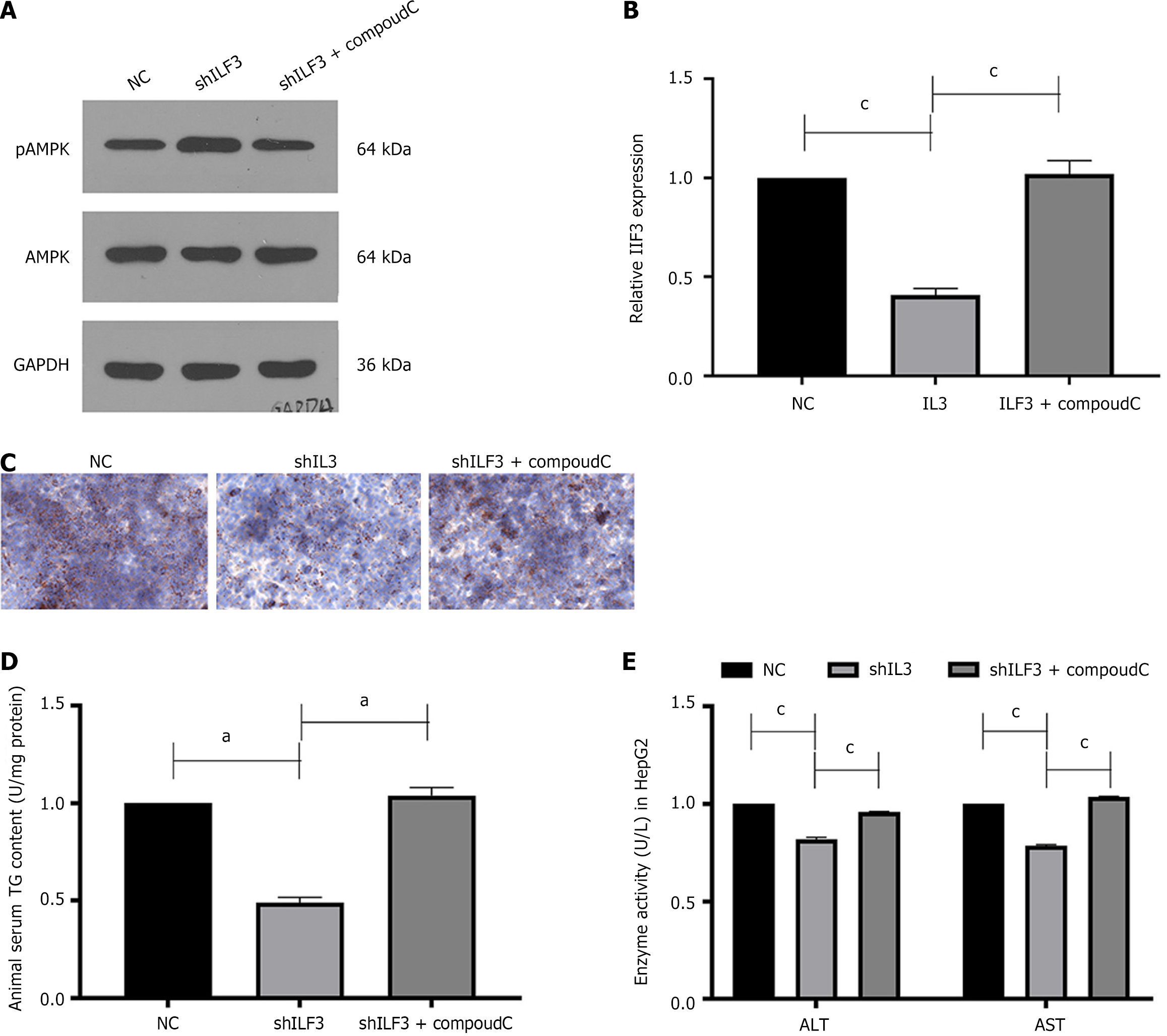
The stimulation of the AMPK signaling pathway has been demonstrated to contribute to the advancement of NAFLD in previous studies[23]. To investigate whether ILF3 activates the AMPK pathway to regulate lipogenesis, the study infected HepG2 cells with shILF3 and conducted Western blotting and qPCR, which showed an increase in the expression of pAMPK and no change in AMPK, while CompoudC partially counteracted this effect (Figure 3A and B). Furthermore, Oil Red O staining results revealed lower lipid deposition in HepG2 cells infected with shILF3, accompanied by reduced levels of ALT, AST, and TG secretion (Figure 3C-E). However, the addition of the AMPK inhibitor, CompoudC, partially counteracted this effect.
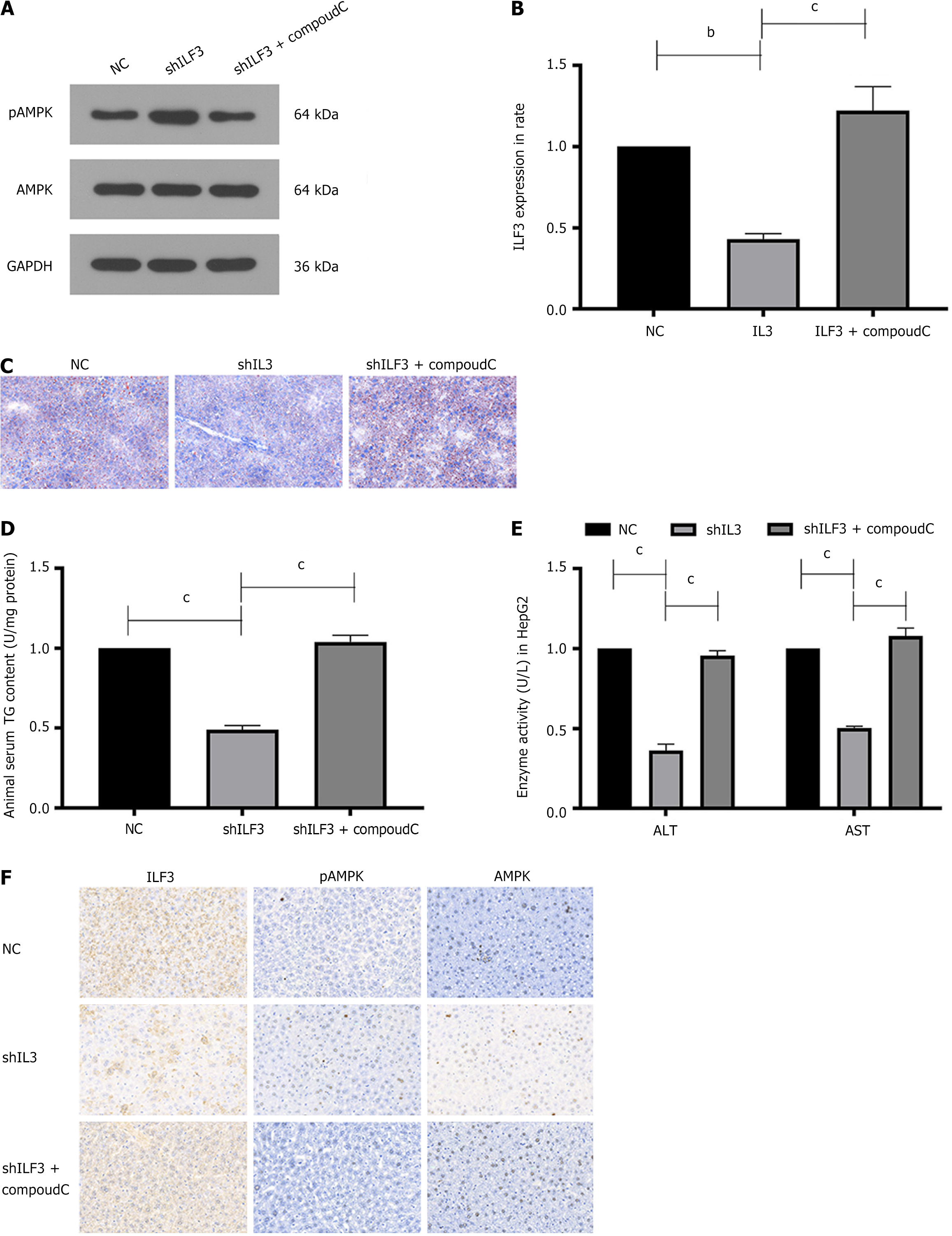
Additionally, the study treated NAFLD mice with shILF3, NC, or the AMPK inhibitor CompoudC. Western blotting and qPCR showed an elevation in pAMPK expression (Figure 4A and B). Oil Red staining and the levels of TG and liver enzymes were in line with the outcomes observed in the hepatocyte experiments (Figure 4C-E). Furthermore, IHC results demonstrated a significant upregulation of pAMPK and notable downregulation of ILF3 expression in mice tissue injected with shILF3 compared to the NC group, with no significant change observed in AMPK expression (Figure 4F).
These findings suggest that the knockdown of ILF3 activates the AMPK signaling pathway to mediate the progression of NAFLD.
NAFLD is a common liver disorder encountered in clinical settings, impacting around 25% of the worldwide populace[24]. Moreover, NAFLD shows a strong association with type 2 diabetes, atherosclerosis, cardiovascular disease, and chronic kidney disease[25]. In this research, the study initially observed an elevation in ILF3 expression in the serum samples obtained from patients diagnosed with NAFLD. Subsequently, the study established NAFLD models both in vitro and in vivo settings and confirmed that ILF3 expression was upregulated in both HepG2 cells and a mouse model of NAFLD, while pAMPK expression was downregulated. Further investigation involved the knockdown of ILF3 in both in vitro and in vivo models, resulting in a significant decrease in lipid accumulation. This reduction can be ascribed to the activation of the AMPK signaling cascade and the downregulation of lipid synthesis genes. Consequently, these findings offer novel insights and possible therapeutic strategies for addressing NAFLD.
ILF3 is generated through the selective splicing of genes and features two dsRNA-binding motifs that facilitate its interaction with proteins, mRNAs, sncRNAs, and dsRNAs, thereby regulating gene expression and stabilizing RNAs[8]. Given its role in gene regulation[26,27], ILF3 has been linked with tumor proliferation, invasion, and metastasis. For instance, ILF3 promotes cervical cancer by enhancing angiogenesis via the PI3K/AKT signaling pathway. A recent study has demonstrated that increased ILF3 expression stimulates cell proliferation and accelerates hepatocellular carcinoma cell cycle progression[28]. Additionally, the RNA-seq findings suggest a robust correlation between ILF3 and energy metabolism, particularly in lipid metabolism (unpublished data).
Furthermore, ILF3 has been implicated in serum LDL cholesterol levels and identified as a prospective gene of interest for myocardial infarction in Japanese individuals[29,30]. It has been reported that ILF3 can induce dyslipidemia in the cardiovascular system[15]. Hence, the study postulates that ILF3 may contribute to the advancement of NAFLD.
In the research, it was found that the level of ILF3 expression in HepG2 cells was notably elevated following the administration of OA, as well as in mice and patients with NAFLD, as determined by qPCR. This observation aligns with prior research that documented elevated ILF3 expression in primary hepatocytes of mice treated with PA[16]. Additionally, the study noted that the knockdown of ILF3 resulted in a notable reduction in lipid deposition and TG secretion in both cellular and mouse models of NAFLD. Furthermore, low ILF3 expression promotes hepatocyte apoptosis and inhibits proliferation. Based on these findings, ILF3 potentially has implications for identifying and assessing NAFLD, although the precise molecular mechanisms governing its function require further investigation.
To delve deeper into understanding the significance of ILF3 in the advancement of NAFLD, the study initially focused on AMPK, a potential therapeutic target identified for treating NAFLD[31]. AMPK, a serine/threonine protein kinase, is critical in maintaining intracellular energy balance. When ATP levels are reduced, AMPK phosphorylates target proteins, resulting in decreased ATP utilization or increased ATP production[32]. Due to its crucial involvement in energy homeostasis, AMPK has become an appealing target for developing drugs to prevent and treat metabolic disorders, including NAFLD[33]. Clinical trials have shown the effectiveness of AMPK activation in NAFLD management. For instance, Vanillic acid, as an anti-inflammatory, has been found to reduce liver enzymes and the accumulation of collagen in the liver through the activation of AMPK[34]. Another study utilizing the direct AMPK activator PXL770 also demonstrated a decrease in TG levels and hepatic steatosis in patients diagnosed with NAFLD[19,35]. These findings collectively support the beneficial effect of AMPK activation in NAFLD patients.
Additionally, prior research has indicated that people suffering from NAFLD experience significantly decreased levels of phosphorylated AMPK in comparison to individuals who are in good health[35]. Moreover, recent studies have proposed that ILF3 might contribute to energy metabolism by interacting with AMPK[11]. Accordingly, the study aimed to investigate the involvement of ILF3 in mediating AMPK activity. The results, obtained through Western blotting and IHC, demonstrated that the downregulation of ILF3 Led to an upregulation of phosphorylated AMPK expression. Interestingly, when the study utilized CompoudC, an AMPK inhibitor, it found that it counteracted the repressive effect of ILF3 knockdown on lipid deposition. This suggests that ILF3 may exert its influence on lipid metabolism through mediatory regulation of AMPK.
In summary, the research presented in this study offers proof that ILF3 can block the AMPK signaling pathway, which consequently facilitates the advancement of NAFLD. Conversely, when ILF3 is silenced, AMPK is activated, resulting in its phosphorylation and subsequent suppression of excessive lipid synthesis, ultimately mitigating the progression of NAFLD. These research findings illuminate the potential of ILF3 as a valuable indicator for diagnosing NAFLD and as a promising candidate for prospective therapeutic interventions against the disease. However, there are some limitations in this paper during the study. First, we diagnosed different severities of NAFLD by abdominal CT (liver-spleen density ratio) rather than by gold standard liver biopsy. Second, our study suggests that ILF3 plays a positive role in NAFLD progression and has a detrimental effect on pAMPK expression, but the exact mechanism needs to be further investigated.
| 1. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3283] [Article Influence: 328.3] [Reference Citation Analysis (7)] |
| 2. | Chen JYS, Chua D, Lim CO, Ho WX, Tan NS. Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. Int J Mol Sci. 2022;24:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 2007] [Article Influence: 669.0] [Reference Citation Analysis (3)] |
| 4. | Albhaisi S, McClish D, Kang L, Gal T, Sanyal AJ. Nonalcoholic fatty liver disease is specifically related to the risk of hepatocellular cancer but not extrahepatic malignancies. Front Endocrinol (Lausanne). 2022;13:1037211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, Proikas-Cezanne T, Reggiori F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 6. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2306] [Article Influence: 230.6] [Reference Citation Analysis (1)] |
| 7. | Liu Y, Wang JX, Nie ZY, Wen Y, Jia XJ, Zhang LN, Duan HJ, Shi YH. Upregulation of ERp57 promotes clear cell renal cell carcinoma progression by initiating a STAT3/ILF3 feedback loop. J Exp Clin Cancer Res. 2019;38:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Castella S, Bernard R, Corno M, Fradin A, Larcher JC. Ilf3 and NF90 functions in RNA biology. Wiley Interdiscip Rev RNA. 2015;6:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Agnoletto C, Brunelli L, Melloni E, Pastorelli R, Casciano F, Rimondi E, Rigolin GM, Cuneo A, Secchiero P, Zauli G. The anti-leukemic activity of sodium dichloroacetate in p53mutated/null cells is mediated by a p53-independent ILF3/p21 pathway. Oncotarget. 2015;6:2385-2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Jiang F, Tang X, Tang C, Hua Z, Ke M, Wang C, Zhao J, Gao S, Jurczyszyn A, Janz S, Beksac M, Zhan F, Gu C, Yang Y. HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A-dependent stabilization of ILF3 mRNA. J Hematol Oncol. 2021;14:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 11. | Li K, Wu JL, Qin B, Fan Z, Tang Q, Lu W, Zhang H, Xing F, Meng M, Zou S, Wei W, Chen H, Cai J, Wang H, Zhang H, Cai J, Fang L, Bian X, Chen C, Lan P, Ghesquière B, Fang L, Lee MH. ILF3 is a substrate of SPOP for regulating serine biosynthesis in colorectal cancer. Cell Res. 2020;30:163-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Sun D, Zhang M, Wei M, Wang Z, Qiao W, Liu P, Zhong X, Liang Y, Chen Y, Huang Y, Yu W. Ox-LDL-mediated ILF3 overexpression in gastric cancer progression by activating the PI3K/AKT/mTOR signaling pathway. Aging (Albany NY). 2022;14:3887-3909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Zhang J, Ma Q, Yu Q, Xiao F, Zhang Z, Feng H, Liang C. PSMD3-ILF3 signaling cascade drives lung cancer cell proliferation and migration. Biol Direct. 2023;18:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Yang C, Zhang M, Liu H, Gong C, Zhang J, Xu S, Zou J, Kai Y, Li Y. Interleukin enhancer-binding factor 3 and HOXC8 co-activate cadherin 11 transcription to promote breast cancer cells proliferation and migration. Oncotarget. 2017;8:107477-107491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Xie F, Cui QK, Wang ZY, Liu B, Qiao W, Li N, Cheng J, Hou YM, Dong XY, Wang Y, Zhang MX. ILF3 is responsible for hyperlipidemia-induced arteriosclerotic calcification by mediating BMP2 and STAT1 transcription. J Mol Cell Cardiol. 2021;161:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Zhang JJ, Shen Y, Chen XY, Jiang ML, Yuan FH, Xie SL, Zhang J, Xu F. Integrative network-based analysis on multiple Gene Expression Omnibus datasets identifies novel immune molecular markers implicated in non-alcoholic steatohepatitis. Front Endocrinol (Lausanne). 2023;14:1115890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Chen Y, He X, Chen X, Li Y, Ke Y. SeP is elevated in NAFLD and participates in NAFLD pathogenesis through AMPK/ACC pathway. J Cell Physiol. 2021;236:3800-3807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Yong Z, Ruiqi W, Hongji Y, Ning M, Chenzuo J, Yu Z, Zhixuan X, Qiang L, Qibing L, Weiying L, Xiaopo Z. Mangiferin Ameliorates HFD-Induced NAFLD through Regulation of the AMPK and NLRP3 Inflammasome Signal Pathways. J Immunol Res. 2021;2021:4084566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Fouqueray P, Bolze S, Dubourg J, Hallakou-Bozec S, Theurey P, Grouin JM, Chevalier C, Gluais-Dagorn P, Moller DE, Cusi K. Pharmacodynamic effects of direct AMP kinase activation in humans with insulin resistance and non-alcoholic fatty liver disease: A phase 1b study. Cell Rep Med. 2021;2:100474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 20. | Li J, Ding X, Jian T, Lü H, Zhao L, Li J, Liu Y, Ren B, Chen J. Four sesquiterpene glycosides from loquat (Eriobotrya japonica) leaf ameliorates palmitic acid-induced insulin resistance and lipid accumulation in HepG2 Cells via AMPK signaling pathway. PeerJ. 2020;8:e10413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Ke R, Xu Q, Li C, Luo L, Huang D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol Int. 2018;42:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 22. | Zhai M, Zhang C, Cui J, Liu J, Li Y, Xie K, Luo E, Tang C. Electromagnetic fields ameliorate hepatic lipid accumulation and oxidative stress: potential role of CaMKKβ/AMPK/SREBP-1c and Nrf2 pathways. Biomed Eng Online. 2023;22:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2652] [Article Influence: 189.4] [Reference Citation Analysis (3)] |
| 24. | Muzurović E, Mikhailidis DP, Mantzoros C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism. 2021;119:154770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 25. | Chen G, Yang Y, Wu QJ, Cao L, Ruan W, Shao C, Jiang L, Tang P, Ma S, Jiang A, Wang Z, Wu K, Zhang QC, Fu XD, Zhou Y. ILF3 represses repeat-derived microRNAs targeting RIG-I mediated type I interferon response. J Mol Biol. 2022;434:167469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 26. | Yan G, Yang J, Li W, Guo A, Guan J, Liu Y. Genome-wide CRISPR screens identify ILF3 as a mediator of mTORC1-dependent amino acid sensing. Nat Cell Biol. 2023;25:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Zhang W, Xiong Z, Wei T, Li Q, Tan Y, Ling L, Feng X. Nuclear factor 90 promotes angiogenesis by regulating HIF-1α/VEGF-A expression through the PI3K/Akt signaling pathway in human cervical cancer. Cell Death Dis. 2018;9:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Bo C, Li N, He L, Zhang S, An Y. Long non-coding RNA ILF3-AS1 facilitates hepatocellular carcinoma progression by stabilizing ILF3 mRNA in an m(6)A-dependent manner. Hum Cell. 2021;34:1843-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Yoshida T, Kato K, Oguri M, Horibe H, Kawamiya T, Yokoi K, Fujimaki T, Watanabe S, Satoh K, Aoyagi Y, Tanaka M, Yoshida H, Shinkai S, Nozawa Y, Yamada Y. Association of polymorphisms of BTN2A1 and ILF3 with myocardial infarction in Japanese individuals with different lipid profiles. Mol Med Rep. 2011;4:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab. 2017;28:545-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 31. | Marcondes-de-Castro IA, Reis-Barbosa PH, Marinho TS, Aguila MB, Mandarim-de-Lacerda CA. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J Gastroenterol Hepatol. 2023;38:1868-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Wu S, Zou MH. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int J Mol Sci. 2020;21:4987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 33. | Hsu CC, Peng D, Cai Z, Lin HK. AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. 2022;85:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 34. | Shekari S, Khonsha F, Rahmati-Yamchi M, Nejabati HR, Mota A. Vanillic Acid and Non-Alcoholic Fatty Liver Disease: A Focus on AMPK in Adipose and Liver Tissues. Curr Pharm Des. 2021;27:4686-4692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Sheng D, Zhao S, Gao L, Zheng H, Liu W, Hou J, Jin Y, Ye F, Zhao Q, Li R, Zhao N, Zhang L, Han Z, Wei L. BabaoDan attenuates high-fat diet-induced non-alcoholic fatty liver disease via activation of AMPK signaling. Cell Biosci. 2019;9:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/