Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.101165
Revised: December 9, 2024
Accepted: December 17, 2024
Published online: February 27, 2025
Processing time: 164 Days and 2.4 Hours
The etiology, risk factors, and management of sarcopenia and metabolic dys
Core Tip: Sarcopenia and metabolic dysfunction-associated steatotic liver disease (MASLD) share similar risk factors, pathophysiology, and cause (physical inactivity). In both conditions, physical inactivity is a remarkable clinical finding. Determination of irisin in the biological fluids serves as a good marker for physical activity as it elevates during muscle contraction. In sarcopenia, serum irisin declined, while in MASLD it increased. Therefore, a transition of serum irisin from higher to lower levels in MASLD could indicate the occurrence of sarcopenia.
- Citation: Al-Nimer MS. Sarcopenia and metabolic dysfunction-associated steatotic liver disease: The role of exercise-related biomarkers. World J Hepatol 2025; 17(2): 101165
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/101165.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.101165
I read with great interest an elegant review published recently by Wong and Yuan[1], who highlighted the similarities of the risk factors, physiopathology, and physical inactivity in both pathological conditions. I will add to this article the role of exercise-related hormones or myokines as biomarkers of physical activity, which are involved in the bidirectional relationship between sarcopenia and metabolic dysfunction-associated steatotic liver disease (MASLD). The measurement of circulating myokines could help us determine whether sarcopenia is associated with MASLD or vice versa. Therefore, determining the circulating myokine levels in MASLD patients could be a useful additive diagnostic tool for sarcopenia. The interrelationship between obesity, MASLD, and sarcopenia is linked with physical inactivity.
Physical activity could delay the progression but does not prevent sarcopenia by overcoming the dysfunction of myokines, which aggravates or causes sarcopenia[2]. Myokines, also known as exerkines, are exercise-related hormones and are defined as proteins or peptides produced and released from contracted muscles that exert autocrine, paracrine, and endocrine function. For that reason, their names are linked to the exercise-related hormones. These myokines have different effects on muscle growth or repair and are generally classified as positive or negative regulators of muscle size (Table 1).
| Positive regulators | Negative regulators |
| Irisin | Growth differentiation factor 8 (myostatin) |
| Insulin growth factor 1 | Transforming growth factor β |
| Follistatin | Activins |
| Bone-morphogenic proteins | - |
| Brain-derived neurotrophic factor | - |
| Meteorin like factor (metrnl) | - |
| Fibroblast growth factor 21 | - |
| Β-aminoisobutyric acid | - |
| Apelin | - |
| Interleukin 10 | Interleukin 6 |
| Interleukin 15 | - |
From the physiological point of view, physical activity regulates the expression of myokines, and their circulating levels will alter in diseases associated with physical inactivity such as sarcopenia or MASLD. Therefore, their determination in the circulation could be applied as physical activity indices. In both MASLD and sarcopenia, obesity and diabetes mellitus with or without complications were associated risk factors and interrelated with physical inactivity[3].
During muscle contraction, the proliferator-activated receptor gamma, coactivator 1 alpha protein is activated, leading to the upregulation of the expression of the fibronectin-III domain (domain-containing protein 5), which is cleaved at the N-terminal to release irisin through activation of different pathways, e.g., adenosine monophosphate-activated protein kinase. During physical activity, myocytes secrete irisin, which converts the white fat to brown fat, increasing exercise energy expenditure.
In the normal population, serum irisin levels are significantly associated with females, markers of inflammation, and high-density lipoprotein[4]. Lower levels are associated with aging, hypertension, obesity, diabetes mellitus, etc[5]. The peak level of irisin is observed 3-60 min after acute exercise and gradually declines to the baseline after 6 h. In chronic exercise ranging from 6 weeks to 1 year, there are no significant changes in the irisin level[6,7]. In addition, resistance exercises rather than endurance exercises are associated with higher irisin release[8].
Low serum irisin (< 118.0 ng/mL) is a predictor of sarcopenia, and it could be a useful diagnostic marker of sarcopenia[9]. Another study found higher circulating levels of irisin disease-related malnutrition without sarcopenia (651.3 ± 221.3 pg/mL), while the myostatin levels do not show a significant difference between non-sarcopenia and sarcopenia[10]. Few studies demonstrated the role of myostatin in causing muscle atrophy in obese and insulin-resistant patients[11].
In type 2 diabetes mellitus (T2DM), the circulating level of irisin is significantly lower in sarcopenia (10.7 ± 6.17 ng/mL) and sarcopenic obesity (SO) (7.66 ± 5.27 ng/mL) in sarcopenia compared with non-sarcopenia (13.67 ± 9.07 ng/mL). It is significantly and inversely correlated with the ratio of fat mass to fat-free mass (an index of SO). A cutoff value of 9.49 ng/mL is a predictor of SO in T2DM[12]. The prevalence of sarcopenia in nonalcoholic fatty liver disease (NAFLD) ranges from 1.6%-63, and it is associated with a higher risk of steatohepatitis, insulin resistance, progression of liver fibrosis, and cardiovascular events[13].
Patients with NAFLD have significantly higher serum irisin levels compared with the corresponding subjects without NAFLD (5.89 ± 3.53 μg/mL vs 4.53 ± 2.62 μg/mL, P < 0.01). The odds for NAFLD are increased by 1.17 times for each 1 μg/mL increment of irisin concentration[14]. Another study found that the median (interquartile) serum irisin level is significantly higher in NAFLD compared with the control: 5.7 (4.6-6.5) vs 7.5 (6.5-9.08) μg/mL[15]. Despite the technology used in the determination of serum irisin, it is significantly higher in NAFLD than in normal controls (63.4 ± 32.6 vs 43.0 ± 29.7 ng/mL, P < 0.001)[16].
Sarcopenia is a risk factor for NAFLD or its progression, regardless of the presence of obesity, insulin resistance, or metabolic syndrome[17,18]. Several mechanisms are shared in sarcopenia and NAFLD, including physical inactivity, insulin resistance, inflammation, dysregulation of myokines, hormonal imbalance, and nutritional deficiency[19]. T2DM accelerates the development of sarcopenia in aged subjects, and it is a risk factor for SO[20,21]. Irisin is a positive regulator of muscle growth and insulin sensitivity[22,23]. Therefore, the serum irisin levels are decreased in T2DM with poor glucose control or metabolic derangement, as with metabolic syndrome[24].
Patients with low irisin levels are at risk for insulin resistance[12], and physical inactivity is the main cause of the low irisin level, which is a cause of the loss of muscle mass and the occurrence of sarcopenia[25]. In addition, it has been reported that sarcopenia is an independent risk factor of new-onset T2DM in non-obese elderly subjects[26]. The mean ± SD of serum irisin levels in elderly patients with T2DM is significantly lower than in healthy subjects (703.37 ± 241.51 ng/mL vs 800.22 ± 275.59 ng/mL)[27].
Irisin is involved in the pathogenesis of MASLD by improving the progression of liver fibrosis via different me
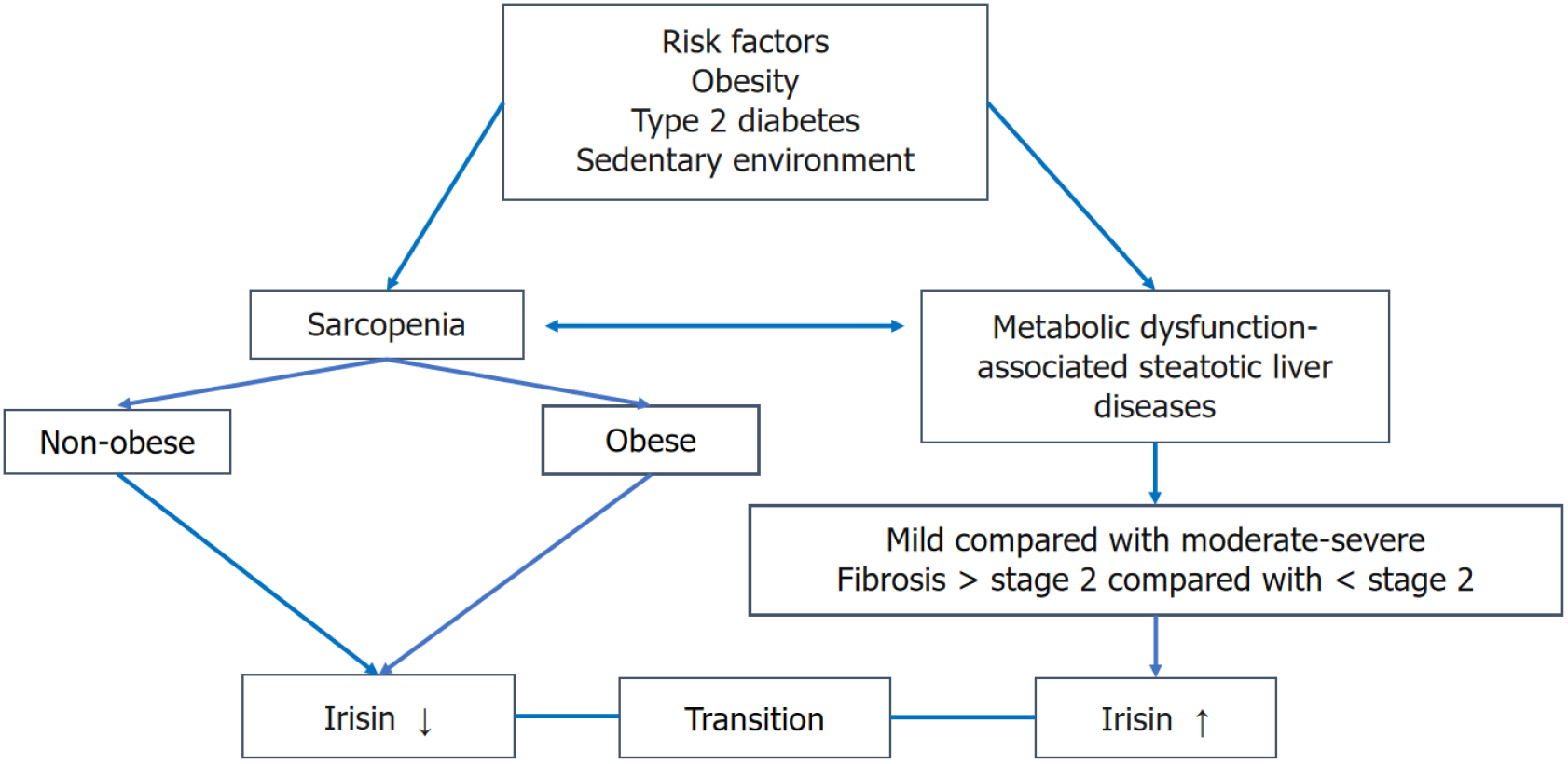
Therefore, to elucidate the importance of determining the irisin level in sarcopenia associated with MASLD, the serial determination of serum irisin showed a transition from higher levels of irisin in MASLD to decreasing levels in sarcopenia-associated MASLD in elderly patients with or without risk factors including T2DM and obesity (Figure 1). In addition, it is necessary to adjust the confounder variables and the disease-related sarcopenia when calculating the irisin cutoff value in MASLD and sarcopenia, as there are wide variations in the circulating irisin levels in the reported studies. The clinical implementation of the determination of serum irisin can be utilized in managing people with a low serum irisin level, e.g., sarcopenia. Experimental studies showed that using exogenous irisin increases muscle weight and strength and improves abnormalities in the skeletal muscle in pathological conditions[31,32].
The time of addressing MASLD and sarcopenia could be supplemented by serial determination of serum irisin levels in MASLD, and a transition from higher to lower levels of irisin could predict the occurrence of sarcopenia despite the presence of risk factors like T2DM or obesity that could act as confounding factors in the interpretation of serum irisin levels. Confounding factors (e.g., age, gender, obesity, smoking, etc.) and the progression of MASLD to liver fibrosis should be considered in the interpretation of the transition of serum irisin levels between MASLD and sarcopenia to eliminate the limitations of the study.
| 1. | Wong R, Yuan LY. Sarcopenia and metabolic dysfunction associated steatotic liver disease: Time to address both. World J Hepatol. 2024;16:871-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 2. | Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells. 2022;11:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Yang YJ, Kim DJ. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int J Mol Sci. 2021;22:2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 4. | Buscemi S, Corleo D, Vasto S, Buscemi C, Massenti MF, Nuzzo D, Lucisano G, Barile AM, Rosafio G, Maniaci V, Giordano C. Factors associated with circulating concentrations of irisin in the general population cohort of the ABCD study. Int J Obes (Lond). 2018;42:398-404. [PubMed] [DOI] [Full Text] |
| 5. | Maciorkowska M, Musiałowska D, Małyszko J. Adropin and irisin in arterial hypertension, diabetes mellitus and chronic kidney disease. Adv Clin Exp Med. 2019;28:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Huh JY, Mougios V, Skraparlis A, Kabasakalis A, Mantzoros CS. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism. 2014;63:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Nygaard H, Slettaløkken G, Vegge G, Hollan I, Whist JE, Strand T, Rønnestad BR, Ellefsen S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS One. 2015;10:e0121367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B, Li J, Chen E, Mulholland M. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther. 2015;29:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Yen CH, Chang PS, Chang YH, Lin PT. Identification of Coenzyme Q10 and Skeletal Muscle Protein Biomarkers as Potential Factors to Assist in the Diagnosis of Sarcopenia. Antioxidants (Basel). 2022;11:725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | de Luis D, Primo D, Izaola O, Gómez JJL. Role of irisin and myostatin on sarcopenia in malnourished patients diagnosed with GLIM criteria. Nutrition. 2024;120:112348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Esposito P, Picciotto D, Battaglia Y, Costigliolo F, Viazzi F, Verzola D. Myostatin: Basic biology to clinical application. Adv Clin Chem. 2022;106:181-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Oguz A, Sahin M, Tuzun D, Kurutas EB, Ulgen C, Bozkus O, Gul K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Medicine (Baltimore). 2021;100:e26529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Giri S, Anirvan P, Angadi S, Singh A, Lavekar A. Prevalence and outcome of sarcopenia in non-alcoholic fatty liver disease. World J Gastrointest Pathophysiol. 2024;15:91100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 14. | Kosmalski M, Drzewoski J, Szymczak-Pajor I, Zieleniak A, Mikołajczyk-Solińska M, Kasznicki J, Śliwińska A. Irisin Is Related to Non-Alcoholic Fatty Liver Disease (NAFLD). Biomedicines. 2022;10:2253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Ulualan G, Kiraz ZK, Kırel B. Relation of serum irisin levels to obesity and non-alcoholic fatty liver disease. Turk J Pediatr. 2022;64:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Choi ES, Kim MK, Song MK, Kim JM, Kim ES, Chung WJ, Park KS, Cho KB, Hwang JS, Jang BK. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PLoS One. 2014;9:e110680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 18. | Gan D, Wang L, Jia M, Ru Y, Ma Y, Zheng W, Zhao X, Yang F, Wang T, Mu Y, Zhu S. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. 2020;39:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Joo SK, Kim W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S68-S78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Qiao YS, Chai YH, Gong HJ, Zhuldyz Z, Stehouwer CDA, Zhou JB, Simó R. The Association Between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences From Observational Studies. Front Endocrinol (Lausanne). 2021;12:782391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol (Lausanne). 2020;11:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 22. | Eckel J. Myokines in metabolic homeostasis and diabetes. Diabetologia. 2019;62:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Garneau L, Aguer C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019;45:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Lisco G, Disoteo OE, De Tullio A, De Geronimo V, Giagulli VA, Monzani F, Jirillo E, Cozzi R, Guastamacchia E, De Pergola G, Triggiani V. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients. 2023;16:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Minniti G, Pescinini-Salzedas LM, Minniti GADS, Laurindo LF, Barbalho SM, Vargas Sinatora R, Sloan LA, Haber RSA, Araújo AC, Quesada K, Haber JFDS, Bechara MD, Sloan KP. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int J Mol Sci. 2022;23:13452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Luo C, Liu RY, Zhang GW, Hu F, Jin YH, Liu BY. Possible sarcopenia and risk of new-onset type 2 diabetes mellitus in older adults in China: a 7-year longitudinal cohort study. BMC Geriatr. 2023;23:404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Xuan X, Lin J, Zhang Y, Zhou L, Xu L, Jia J, Zhao B, Lin Z, Zhu Q, Li L, Wu T, Zhang S, Jiang H, Wang Y. Serum Irisin Levels and Clinical Implication in Elderly Patients With Type 2 Diabetes Mellitus. J Clin Med Res. 2020;12:612-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2420] [Article Influence: 403.3] [Reference Citation Analysis (4)] |
| 29. | Dong HN, Park SY, Le CT, Choi DH, Cho EH. Irisin Regulates the Functions of Hepatic Stellate Cells. Endocrinol Metab (Seoul). 2020;35:647-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Armandi A, Rosso C, Nicolosi A, Caviglia GP, Abate ML, Olivero A, D'Amato D, Vernero M, Gaggini M, Saracco GM, Ribaldone DG, Leeming DJ, Gastaldelli A, Bugianesi E. Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease. J Clin Med. 2022;11:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Reza MM, Sim CM, Subramaniyam N, Ge X, Sharma M, Kambadur R, McFarlane C. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget. 2017;8:98553-98566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Pan S, Ren W, Zhao Y, Cai M, Tian Z. Role of Irisin in exercise training-regulated endoplasmic reticulum stress, autophagy and myogenesis in the skeletal muscle after myocardial infarction. J Physiol Biochem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/