Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.100923
Revised: January 10, 2025
Accepted: January 23, 2025
Published online: February 27, 2025
Processing time: 174 Days and 1.1 Hours
Idiopathic portal hypertension (IPH) is a subtype of portal hypertension that arises in the absence of cirrhosis. IPH frequently manifests with clinical features typical of portal hypertension, including splenomegaly and esophagogastric fundal varices, along with other associated symptoms. Imaging studies may in
A patient previously diagnosed with “hepatitis B cirrhosis” at an external hospital presented to our facility with gastrointestinal bleeding. Initial assessment revealed minor liver injury, splenomegaly, esophagogastric varices, and portal hyperten
Currently, there are no standardized diagnostic criteria for IPH, and its diagnosis is generally established by excluding other conditions. Liver biopsy remains the most reliable method for IPH diagnosis.
Core Tip: Idiopathic portal hypertension (IPH) is challenging to diagnose due to its rarity and non-specific clinical pre
- Citation: Liu XC, Yan HH, Wei W, Du Q. Idiopathic portal hypertension misdiagnosed as hepatitis B cirrhosis: A case report and review of the literature. World J Hepatol 2025; 17(2): 100923
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/100923.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.100923
Idiopathic portal hypertension (IPH) is a rare but clinically significant disorder characterized by portal hypertension in the absence of cirrhosis or other identifiable causes[1]. Despite being identified over five decades ago, its pathogenesis, diagnostic criteria, and optimal management remain subjects of ongoing discussion and research. This condition poses distinct challenges in clinical practice, as it often resembles cirrhosis or other hepatic diseases due to its diverse clinical manifestations and the absence of specific diagnostic markers[1]. Misdiagnosis may lead to inappropriate treatment strategies and delays in initiating targeted therapy, thereby affecting patient outcomes. Although advancements in imaging techniques and serological assays have improved diagnostic accuracy, liver biopsy remains the gold standard for definitive diagnosis[2]. Despite its rarity, IPH prevalence may be underestimated due to diagnostic challenges and limited awareness among clinicians. This report describes a case of misdiagnosed IPH and analyses the underlying reasons for this diagnostic error.
The 69-year-old female patient was admitted to hospital due to recurrent hematemesis over three months.
The patient experienced three episodes of hematemesis, with each episode involving approximately 50 mL of brown blood. No associated symptoms were observed, such as melena, dizziness, or amaurosis. Before admission to our hospital, the patient was diagnosed with cirrhosis secondary to viral hepatitis B, confirmed by a positive hepatitis B core antibody (HBcAb) result from external medical facilities.
The patient denied a history of alcohol consumption or toxic drug use. There was also no history of parasitic or gallbladder disease. No underlying conditions, including hypertension or heart disease, were observed. Consequently, illness, including alcoholic cirrhosis, drug toxicity-induced cirrhosis, and cirrhosis resulting from circulatory congestion were excluded.
There was no family history of liver disease, tumors, or bleeding disorders.
On hospitalization, an abdominal examination revealed no significant abnormalities except mild tenderness in the upper abdomen. The liver and spleen were non-palpable, and abdominal percussions revealed tympanitic sounds. No shifting of dullness was detected.
On admission, extensive blood testing revealed a hemoglobin level of 99 g/L and platelet count of 91 × 109/L, indicating mild anemia and hypersplenism. Bone marrow aspiration demonstrated no significant abnormalities, excluding thrombocytopenia and erythrocytopenia, from various hematologic disorders. Both direct and indirect bilirubin levels were slightly elevated (direct: 6.4 μmol/L, indirect: 32.3 μmol/L). The alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, and alkaline phosphatase levels were marginally above normal ranges. Tests for hepatitis series markers, hepatitis B DNA, autoimmune hepatitis antibodies, antinuclear antibodies, and copper blue protein yielded negative results. However, the patient had previously tested positive for HBcAbs at another facility, and the specific testing methods and values were unknown. Multiple qualitative assays for HBcAbs conducted at our hospital yielded negative results, thus excluding cirrhosis due to viral hepatitis B.
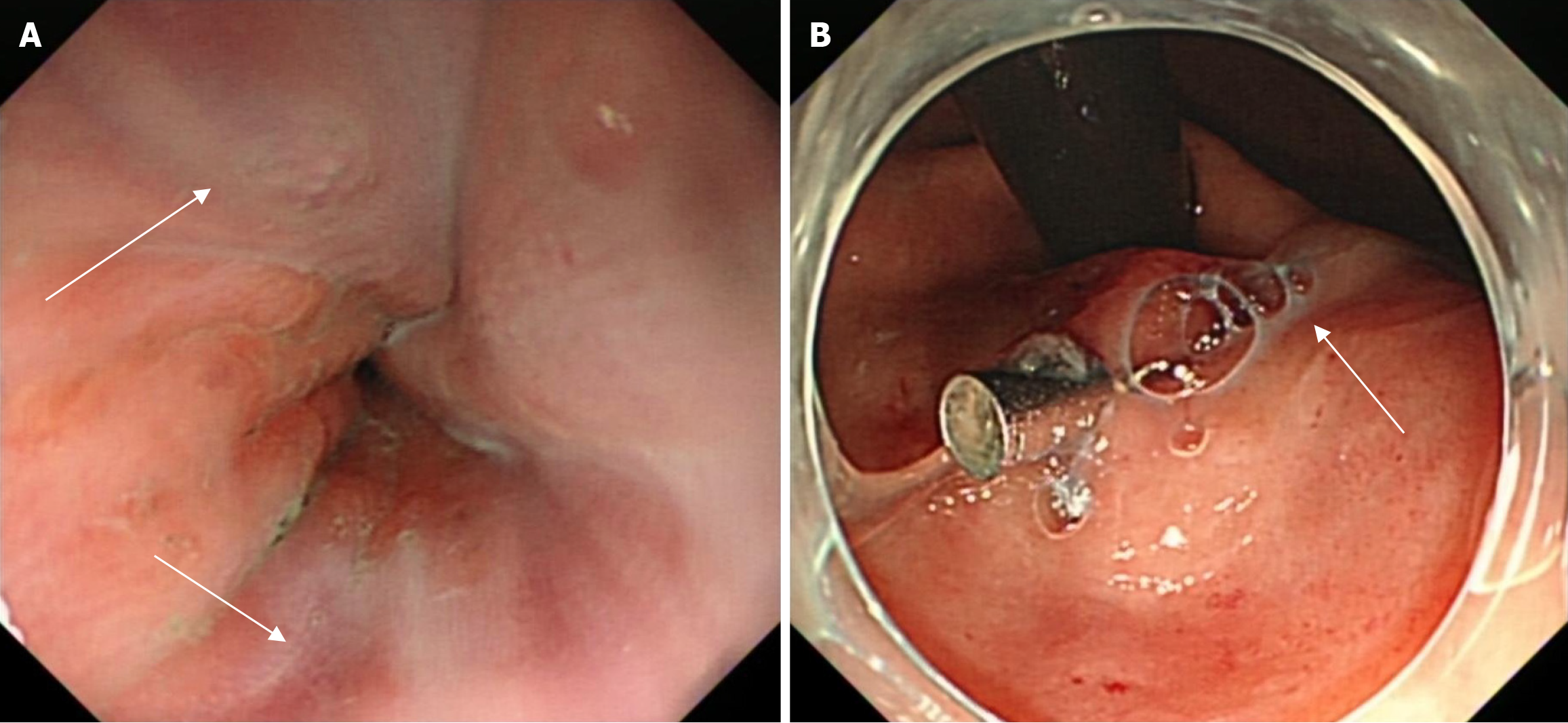
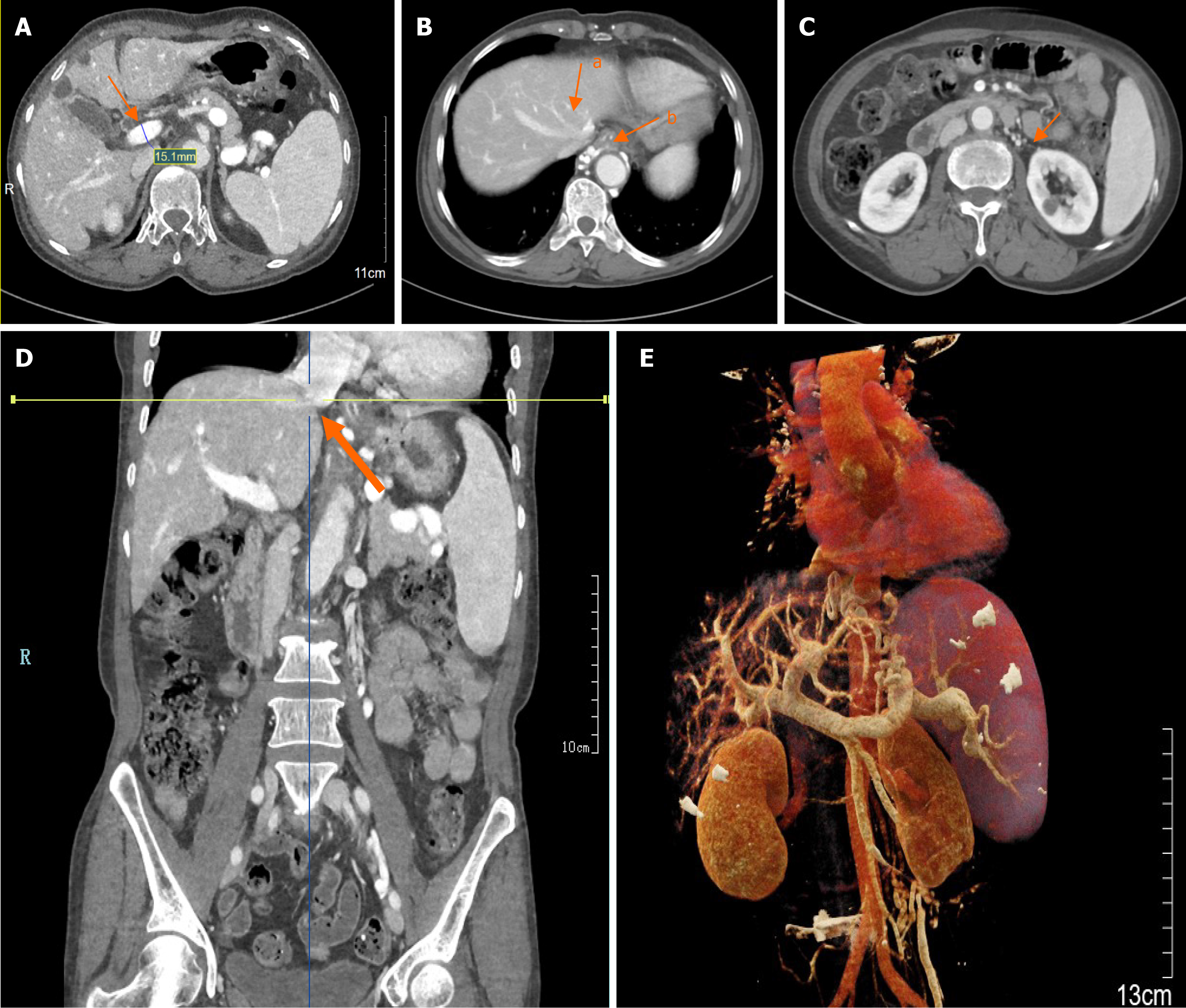
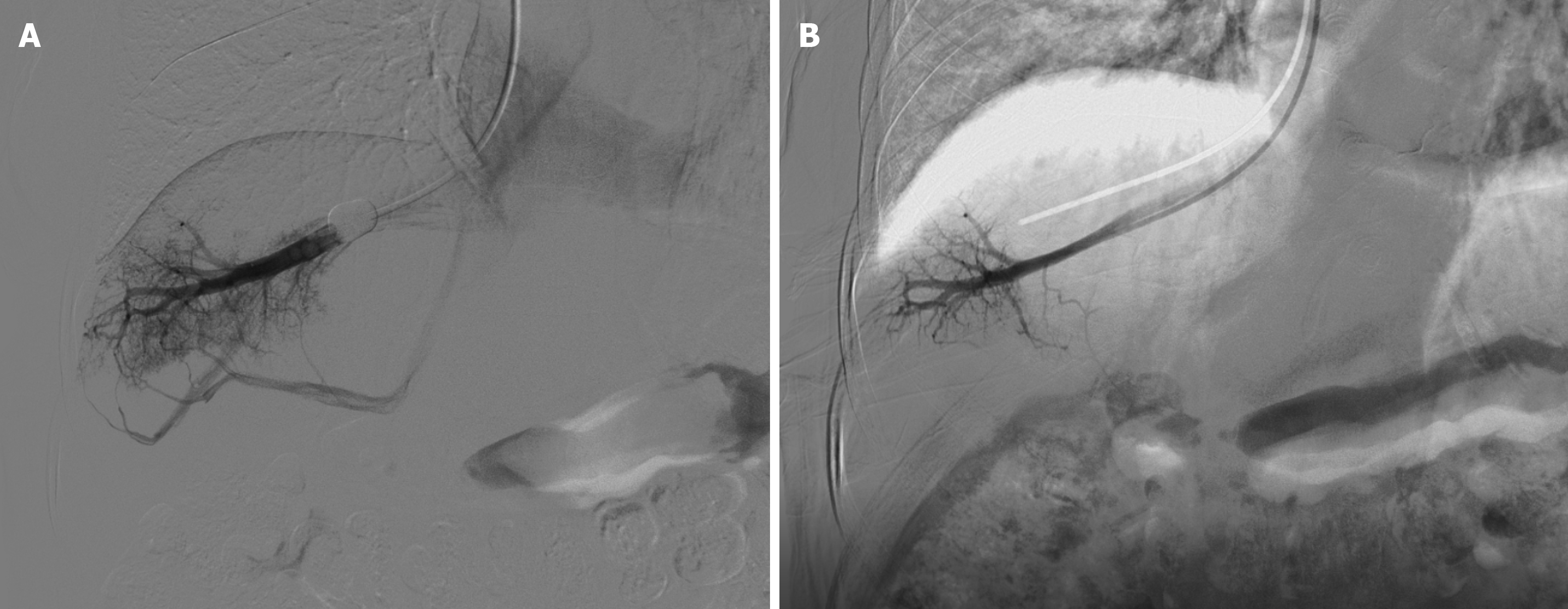
The patient underwent gastroscopy in the emergency clinic, which revealed multiple linear varicose veins approximately 30 cm from the incisors, along with diffuse hemorrhagic manifestations in the gastric body mucosa, indicative of portal hypertensive gastropathy (Figure 1). Subsequently, the patient was admitted for further evaluation. Elastography, utilizing the Myeri Resona R9T, and sound touch elastography of the liver were conducted, yielding six measurements with an EMedian of 9.22 kPa, which did not satisfy the criteria for cirrhosis. Abdominal enhanced computed tomography (CT) demonstrated multiple non-enhancing hypointense foci in the liver, interpreted as liver cysts, and a slightly enlarged spleen with no abnormal enhancement foci (Figure 2A). No significant alteration in liver size was observed; the liver lobe proportions and hepatic fissure width were normal, and the liver edges appeared flat, suggesting the absence of cirrhosis. The diameter of the portal vein was measured at 15.3 mm (Figure 2A). CT portal venography demonstrated dilated portal systemic vasculature, along with dilation and tortuosity of the esophageal-fundus vein (Figure 2B). CT reconstruction of the hepatic vein and inferior vena cava revealed no signs of obstruction (Figure 2C-E). Deep vein manometry indicated a hepatic vein pressure gradient (HVPG) of 5 mmHg (Figure 3). Due to compensatory visualization of the subhepatic vein during balloon occlusion, the measured wedged hepatic venous pressure of the horizontal branch of the right hepatic vein was lower than the actual value, suggesting that the true HVPG was likely greater than 5 mmHg.
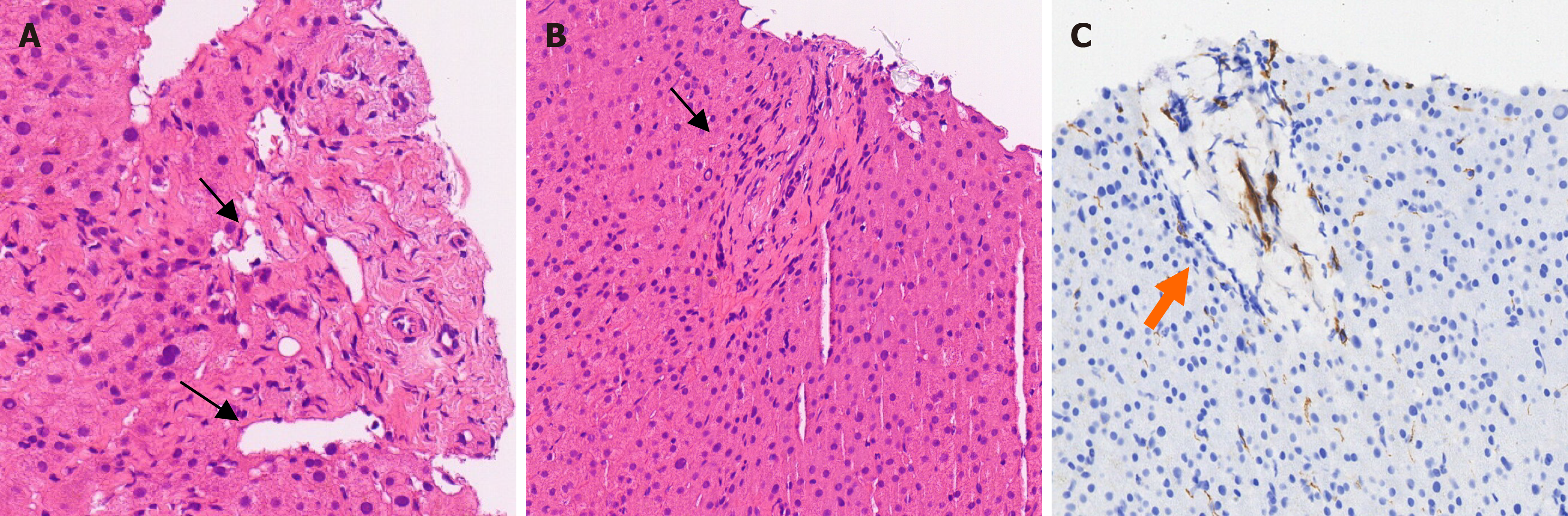
Despite comprehensive tests and examinations, the presence of cirrhosis and its specific etiology remained unclear. After obtaining informed consent, an ultrasound-guided hepatic puncture was performed. Histological analysis revealed multiple small dilated veins in the confluent area and small portal vein occlusions. Cirrhotic features, including stem cell necrosis, nodular regeneration, and pseudo-follicular formation, were not observed (Figure 4).
Eventually, the patient was diagnosed with IPH with esophageal and gastric varices.
Symptomatic treatment, including gastric protection and rehydration, was administered during the patient’s hospitalization. After excluding contraindications, endoscopic venous sclerotherapy was performed for esophageal varices. Upon discharge, the patient was prescribed 25 mg carvedilol daily. Vital signs, including blood pressure and heart rate, and routine blood and thyroid function tests, were monitored regularly, and no significant abnormalities were observed.
Eight months later, the patient returned to the hospital for scheduled esophageal varices venous sclerotherapy, which included endoscopic lauromacrogol injection and hydroxyl acrylate treatment. Her hemoglobin level had significantly increased compared to the previous measurements, and she remained free from gastrointestinal bleeding for the following eight months.
IPH is a rare disorder characterized by clinical portal hypertension without an identifiable cause. Despite unclear pa
The clinical presentation of IPH varies significantly and can be categorized into three distinct scenarios: (1) Patients who lack clinical signs of portal hypertension but exhibit histological findings consistent with IPH[8]; (2) Patients with definitive signs of portal hypertension, including thrombocytopenia, splenomegaly, and/or esophageal varices, but without associated symptoms[6]; and (3) Patients who present with complications related to portal hypertension, primarily bleeding from esophageal varices. Approximately 70% of patients are diagnosed with esophageal varices, and about one-third of these cases exhibit esophageal variceal bleeding as the initial manifestation. Less frequently, portal hypertensive gastropathy or gastric varices may be a source of bleeding[6]. Laboratory tests generally reveal normal to mildly impaired liver function, and disorders, including anemia, leukopenia, and thrombocytopenia due to hypersplenism are uncommon[6]. Severe complications, including hepatic encephalopathy and hepato-pulmonary syndrome, are exceedingly rare[6]. Due to the non-specific nature of the symptoms and the potential overlap with alternative causes of portal hypertension, a diagnosis of IPH cannot be made based solely on clinical symptoms and laboratory findings.
IPH poses significant diagnostic challenges due to its lack of distinctive imaging features, often presenting similarities to cirrhosis in imaging studies[9]. The primary imaging modalities used to diagnose IPH include ultrasound, CT, and magnetic resonance imaging (MRI)[10]. Ultrasound typically reveals non-specific alterations in liver morphology and echostructure, displaying signs of portal hypertension that are common in various chronic liver diseases[1]. CT imaging can identify vascular abnormalities, requiring meticulous evaluation of intrahepatic portal vein branches, acquisition of thin sections, and post-processing reconstruction. Certain atypical morphological findings may suggest a diagnosis of IPH, including subperitoneal parenchymal atrophy, which leads to the convergence of medium-caliber portal veins towards the end-hepatic vein[11,12]. These structural changes often result in perfusion deficits, which are observed in approximately 45% of cases. These deficits are characterized by increased hepatic artery inflow and decreased portal venous perfusion, particularly in the peripheral regions. Additionally, benign hypervascular nodules, resulting from these hemodynamic abnormalities, are observed in about 15% of cases[11]. The clinical significance and diagnostic contribution of these alterations specific to IPH remain unclear. In rare instances, particularly in drug-induced IPH cases such as those related to azathioprine, CT imaging may reveal nodular regenerative hyperplasia (NRH), complicating differentiation from cirrhotic nodules and necessitating detailed pathological examination for accurate diagnosis[11,13]. MRI is infrequently employed to differentiate cirrhosis from IPH due to its lower utility in this context[1,14].
Liver biopsy is considered the gold standard for diagnosing IPH[15]. However, IPH may exhibit only subtle his
NRH and incomplete septal cirrhosis are characteristic manifestations. NRH results from central hyperplasia in the liver’s most perfused areas, surrounded by atrophy and reduced portal blood supply. The histological criteria and classification of NRH were established in 1990, encompassing the presence of hepatocellular nodules measuring < 3 mm in diameter that were not surrounded by fibrosis (the nodules were graded from 0 to 3 + based on the extent of nodules at different biopsy sites), as well as the presence of fibrous septa (graded from 0-3)[18]. Histological features of NRH can be observed in various conditions, including systemic diseases and subsequent exposure to certain drugs[20,21]. NRH is not invariably associated with portal hypertension, and its clinical significance in the absence of portal hypertension remains unclear. However, when NRH is discovered without clear clinical evidence of portal hypertension, the possibility of IPH should be considered, and closer follow-up is warranted. Besides, NRH may be observed in patients undergoing liver transplantation[22]. Incomplete septal cirrhosis is characterized by blind-ended, elongated, and incomplete fibrotic bundles culminating in parenchymal nodules[23]. These features may represent disease regression and can be observed in patients with cirrhosis. Additional vascular alternations associated with IPH, including increased portal channels and vasodilatation within or near the portal bundle, are non-pathognomonic[24]. Consequently, although liver biopsy is crucial for diagnosing IPH, it entails the risk of misdiagnosis. To enhance diagnostic accuracy, it is essential to perform image-guided biopsies with a sufficient sample size from multiple sites. If specific nodules or other manifestations are present in the liver, targeting these areas for a biopsy is advisable. A biopsy must be analyzed by proficient pathologists who integrate clinical and imaging data to identify the essential features of IPH.
IPH diagnosis relies on excluding other liver diseases, as no specific diagnostic criteria exist for IPH[1]. Based on the available information, the diagnostic approach for IPH can be summarized as follows: (1) Identifying clinical manifestations: Clinical signs of portal hypertension, including esophagogastric varices (with or without bleeding), splenomegaly, thrombocytopenia, and occasionally ascites, must be evident; (2) Excluding cirrhosis of various causes: Comprehensive testing is essential to exclude cirrhosis caused by viral hepatitis, alcohol, biliary obstruction, and autoimmune me
Previous guidelines have characterized IPH as a condition manifesting in the presence of portal hypertension[7]. Several studies have highlighted the existence of histopathological findings, including non-cirrhotic portal fibrosis (NCPF), even without portal hypertension[18,25]. This has increased the complexity of IPH diagnosis. In the absence of overt portal hypertension, early diagnosis of IPH may be suggested by identifying the risk factors and histological characteristics[19].
The focus of IPH treatment is to manage portal hypertension and its associated complications[1]. Preventing variceal bleeding is a fundamental aspect of NCPF/IPH treatment, as the absence of such bleeding correlates with improved long-term outcomes. Consequently, patients diagnosed with IPH should undergo upper gastrointestinal endoscopy to assess esophageal or gastric varices[19]. Although clinical trials investigating the combination of propranolol and endoscopic variceal ligation (EVL) with pharmacological therapy have been conducted, the small sample sizes of these studies limit their ability to draw significant conclusions[26]. Relevant studies indicated that although portal shunt surgery is generally considered safe, it may lead to several delayed postoperative complications. Consequently, primary prevention of IPH is not currently recommended. In cases of acute bleeding, therapeutic strategies resemble those employed for patients with cirrhosis, with vasoactive drugs combined with endoscopic procedures to address acute variceal hemorrhage[27]. EVL is the preferred approach for managing esophageal variceal bleeding in patients with NCPF/IPH exhibiting esophageal varices. If endoscopic and pharmacological treatments are ineffective, transjugular intrahepatic portosystemic shunt (TIPS) may be considered a salvage option. The prospective function of early TIPS in NCPF/IPH requires additional investigation[19].
The 2024 Asian Pacific Association for the study of liver guidelines for NCPF/IPH suggest that a combination of non-selective β-blockers and EVL is recommended for secondary prevention of esophageal variceal bleeding[19,28]. Moreover, balloon-occluded retrograde transvenous obliteration, plug-assisted retrograde transvenous obliteration, or coil-assisted retrograde transvenous obliteration may be advantageous in mitigating gastric variceal bleeding, particularly in the presence of a gastro-renal shunt[29]. Despite the application of combined endoscopic and pharmacological therapy, TIPS may be contemplated if variceal bleeding recurs, although existing evidence is limited. Current guidelines indicate that treatment for patients with splenomegaly should only be considered if significant symptoms are present[19]. The treatment options include splenectomy or partial splenic artery embolization. For patients with symptomatic splenomegaly and variceal bleeding, shunt surgery may be an alternative; however, careful monitoring of the associated complication risks is crucial[30]. Liver transplantation is generally not indicated in patients with IPH; however, it may be considered a beneficial treatment option for those experiencing liver failure or refractory complications of portal hypertension[31].
In this case, the patient’s clinical manifestations, including hematemesis and splenomegaly, along with laboratory results indicating mild liver damage, and endoscopic and CT findings, suggested portal hypertension. Comprehensive testing and patient history inquiries, including serological assays for hepatitis and autoimmune antibodies, excluded the various causes of cirrhosis. Furthermore, alternative etiologies of non-cirrhotic portal hypertension, including Budd-Chiari syndrome and extrahepatic portal vein obstruction, were excluded using CT, portal venography, and other pertinent diagnostic assessments, verifying the absence of stenosis or thrombus in both intrahepatic and extrahepatic portal veins. Contrast investigations after balloon obstruction indicated subhepatic vein compensation, leading to lower HVPG measurements using the indirect method compared to the actual portal venous pressure. Consequently, direct portal venous pressure measurement was essential for a more precise value. IPH diagnosis was confirmed by the liver biopsy, which revealed occlusive portal veins.
During her treatment at a previous hospital, the patient was diagnosed with “viral hepatitis B cirrhosis”. This diagnosis was derived from a CT scan that indirectly suggested portal hypertension instead of definitive cirrhosis, as evidenced by splenomegaly and esophagogastric fundal varices, endoscopic findings of esophageal varices, and a positive anti-hepatitis B core (HBc) test result. However, the patient exhibited no clear evidence of chronic hepatitis B virus infection history, and subsequent tests at our institution yielded negative results for the anti-HBc antibody. The initial diagnostic error was primarily due to abnormal imaging findings and hepatitis B-related test outcomes.
Distinguishing IPH from cirrhosis on CT can be challenging because the CT report may indicate cirrhotic nodules. Chronic perihepatic hypoperfusion may present on CT as irregular portal vein perfusion and diminished enhancement of the perihepatic wall[32]. Over time, the liver contour may become “nodular”, rendering it indistinguishable from cirrhosis[1]. In advanced stages, differentiation may be challenging based exclusively on morphological evaluation, necessitating invasive diagnostic techniques, including biopsy, for a conclusive diagnosis. This is one reason for the misdiagnosis in CT scans conducted at external facilities. Diverse methodologies exist for measuring anti-HBc antibodies, utilizing distinct instruments, measurement units, and positive result ranges[33,34]. Our hospital performed multiple qualitative tests for hepatitis B using an enzyme-linked immunosorbent assay recognized for its reliable sensitivity and specificity. The specific anti-HBc test method employed at the external hospital is unidentified, potentially explaining the discrepancy in results - positive findings at the external hospital and negative outcomes at our institution - owing to variations in testing methods and parameter configurations. Errors in the inspection procedure or measurement inaccuracies cannot be excluded. This case review concluded that IPH should be considered when the clinical signs of portal hypertension are evident. However, careful exclusion of cirrhotic and non-cirrhotic causes of portal hypertension is essential. CT imaging is beneficial but must be supplemented with biopsy, particularly when imaging errors or misinterpretation of hepatitis B virus serology may occur. In regions with a significant prevalence of viral hepatitis, special attention should be paid to hepatitis markers to prevent misdiagnoses.
We report the case of a patient initially diagnosed with “viral hepatitis B cirrhosis” at an external institution, who was subsequently identified as having IPH with esophagogastric fundal varices at our facility. IPH is a rare disorder characterized by the absence of specific findings in blood tests or imaging studies. When imaging does not reveal cirrhosis, including in cases involving NRH or in the absence of cirrhotic features, liver biopsy becomes the most crucial diagnostic tool. This procedure is essential for accurately determining the disease’s underlying cause and ensuring the reliability of the pathological diagnosis.
| 1. | Hernández-Gea V, Baiges A, Turon F, Garcia-Pagán JC. Idiopathic Portal Hypertension. Hepatology. 2018;68:2413-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Zhao ZL, Wei Y, Wang TL, Peng LL, Li Y, Yu MA. Imaging and Pathological Features of Idiopathic Portal Hypertension and Differential Diagnosis from Liver Cirrhosis. Sci Rep. 2020;10:2473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Pulvirenti F, Pentassuglio I, Milito C, Valente M, De Santis A, Conti V, d'Amati G, Riggio O, Quinti I. Idiopathic non cirrhotic portal hypertension and spleno-portal axis abnormalities in patients with severe primary antibody deficiencies. J Immunol Res. 2014;2014:672458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Eapen CE, Nightingale P, Hubscher SG, Lane PJ, Plant T, Velissaris D, Elias E. Non-cirrhotic intrahepatic portal hypertension: associated gut diseases and prognostic factors. Dig Dis Sci. 2011;56:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC. Idiopathic portal hypertension: natural history and long-term outcome. Hepatology. 2014;59:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, Lesmana LA, Guha Mazumder D, Omata M, Qureshi H, Raza RM, Sahni P, Sakhuja P, Salih M, Santra A, Sharma BC, Sharma P, Shiha G, Sollano J; Members of the APASL Working Party on Portal Hypertension. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Cazals-Hatem D, Hillaire S, Rudler M, Plessier A, Paradis V, Condat B, Francoz C, Denninger MH, Durand F, Bedossa P, Valla DC. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (3)] |
| 10. | Rajesh S, Mukund A, Sureka B, Bansal K, Ronot M, Arora A. Non-cirrhotic portal hypertension: an imaging review. Abdom Radiol (NY). 2018;43:1991-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Glatard AS, Hillaire S, d'Assignies G, Cazals-Hatem D, Plessier A, Valla DC, Vilgrain V. Obliterative portal venopathy: findings at CT imaging. Radiology. 2012;263:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, Galambos MR, Rubin RA. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol. 2008;6:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Vilgrain V, Lagadec M, Ronot M. Pitfalls in Liver Imaging. Radiology. 2016;278:34-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Krishnan P, Fiel MI, Rosenkrantz AB, Hajdu CH, Schiano TD, Oyfe I, Taouli B. Hepatoportal sclerosis: CT and MRI appearance with histopathologic correlation. AJR Am J Roentgenol. 2012;198:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Da BL, Koh C, Heller T. Noncirrhotic portal hypertension. Curr Opin Gastroenterol. 2018;34:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 353] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 17. | Nayak NC, Ramalingaswami V. Obliterative portal venopathy of the liver. Associated with so-called idiopathic portal hypertension or tropical splenomegaly. Arch Pathol. 1969;87:359-369. [PubMed] |
| 18. | Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 409] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Shukla A, Rockey DC, Kamath PS, Kleiner DE, Singh A, Vaidya A, Koshy A, Goel A, Dökmeci AK, Meena B, Philips CA, Sharma CB, Payawal DA, Kim DJ, Lo GH, Han G, Qureshi H, Wanless IR, Jia J, Sollano JD, Al Mahtab M, Muthiah MD, Sonderup MW, Nahum MS, Merican MIB, Ormeci N, Kawada N, Reddy R, Dhiman RK, Gani R, Hameed SS, Harindranath S, Jafri W, Qi X, Chawla YK, Furuichi Y, Zheng MH, Sarin SK. Non-cirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and management. Hepatol Int. 2024;18:1684-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Breen DP, Marinaki AM, Arenas M, Hayes PC. Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transpl. 2005;11:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Chen AK, Lunow-Luke T, Yamaguchi S, Praglin C, Agudelo E, Mehta N, Dirks R, Braun HJ, Gardner JM, Roberts JP, Syed SM, Roll GR. Nodular Regenerative Hyperplasia After Liver Transplant; It's All in the Presentation. Front Surg. 2022;9:876818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 237] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Schouten JN, Garcia-Pagan JC, Valla DC, Janssen HL. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Wanless IR, Bernier V, Seger M. Intrahepatic portal vein sclerosis in patients without a history of liver disease. An autopsy study. Am J Pathol. 1982;106:63-70. [PubMed] |
| 26. | Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol. 2005;100:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Bañares R, Albillos A, Rincón D, Alonso S, González M, Ruiz-del-Arbol L, Salcedo M, Molinero LM. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | He TJ, Zhao ZD, Luo ZT, Jia W, Zhang JT, Zhao Y, Xiao WC, Ming ZZ, Chen K. Advances in microbial decorations and its applications in drug delivery. Acta Materia Medica. 2023;2:466-479. [DOI] [Full Text] |
| 29. | Henry Z, Patel K, Patton H, Saad W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1098-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 30. | Pal S, Radhakrishna P, Sahni P, Pande GK, Nundy S, Chattopadhyay TK. Prophylactic surgery in non-cirrhotic portal fibrosis:is it worthwhile? Indian J Gastroenterol. 2005;24:239-242. [PubMed] |
| 31. | Saigal S, Nayak NC, Jain D, Kumaran V, Mohanka R, Saraf N, Rastogi A, Mehta N, Nundy S, Soin A. Non-cirrhotic portal fibrosis related end stage liver disease in adults: evaluation from a study on living donor liver transplant recipients. Hepatol Int. 2011;5:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Waguri N, Suda T, Kamura T, Aoyagi Y. Heterogeneous hepatic enhancement on CT angiography in idiopathic portal hypertension. Liver. 2002;22:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Lazarevic I, Banko A, Miljanovic D, Cupic M. Clinical Utility of Quantitative HBV Core Antibodies for Solving Diagnostic Dilemmas. Viruses. 2023;15:373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 34. | Juhl D, Knobloch JK, Görg S, Hennig H. Comparison of Two Test Strategies for Clarification of Reactive Results for Anti-HBc in Blood Donors. Transfus Med Hemother. 2016;43:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/