Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.115551
Revised: October 27, 2025
Accepted: November 24, 2025
Published online: December 27, 2025
Processing time: 68 Days and 1.4 Hours
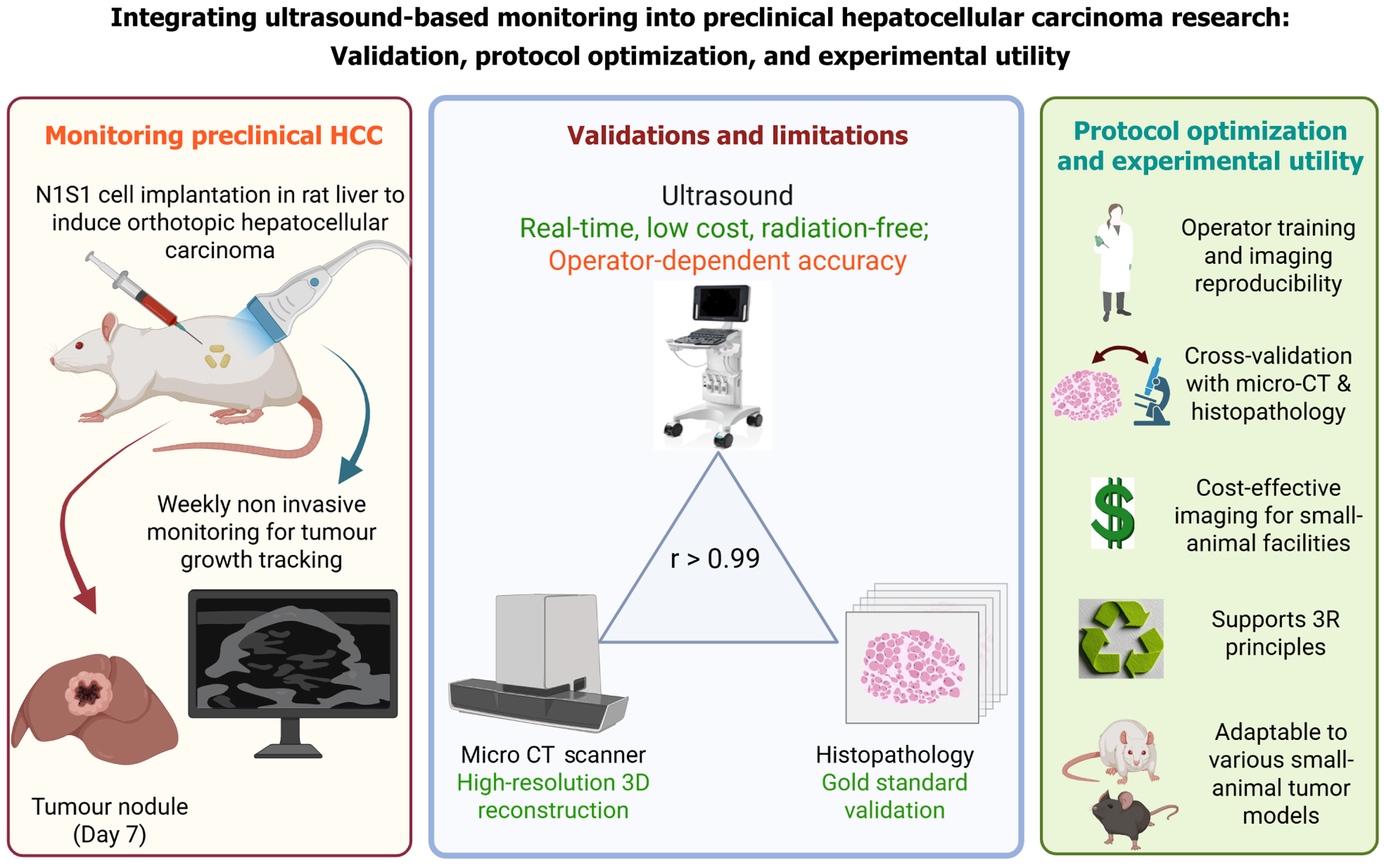
The study by Devan et al presents an ultrasound-based protocol for monitoring tumor growth in a syngeneic orthotopic rat model of hepatocellular carcinoma (HCC). This approach is commendable for its reproducibility, cost-effectiveness, and alignment with ethical imperatives, particularly in reducing the need for invasive assessments. The strong correlation of ultrasound-based volumes with histology and therapeutic response highlights its translational promise. However, certain considerations merit further discussion. Ultrasound imaging, while accessible, is inherently operator-dependent, and its accuracy may decline with irregular or heterogeneous tumor morphology. Moreover, the exclusive reliance on the rat hepatoma cell line (N1S1) cells raises questions about generalizability to other HCC models with differing immune interactions. Future refinements should standardize training protocols, incorporate multimodal validation, and explore diverse tumor settings. Despite these limitations, the study provides a useful app
Core Tip: High frequency ultrasound is a rapid, low-cost, minimally invasive tool for serial volumetric monitoring of orthotopic hepatocellular carcinoma that correlates well with terminal histology and can match micro computed tomography (CT)/magnetic resonance imaging (MRI) for gross tumor size, but it lacks cellular resolution and remains operator-dependent. To increase translational impact, future work should standardize acquisition and training, perform multimodal validation in subset cohorts (histology, micro CT/MRI), and adopt contrast-enhanced/photoacoustic imaging, along with automated radiomics/artificial intelligence segmentation to reduce bias and improve sensitivity.
- Citation: Ghosh D, Kumar A. Ultrasound imaging in orthotopic hepatocellular carcinoma models: Promise, practicality, and points for refinement. World J Hepatol 2025; 17(12): 115551
- URL: https://www.wjgnet.com/1948-5182/full/v17/i12/115551.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i12.115551
Devan et al[1] describe an ultrasound-based protocol for longitudinal monitoring of tumor growth in a syngeneic orthotopic rat model of hepatocellular carcinoma (HCC). The authors demonstrate that high-frequency ultrasound yields reproducible volumetric measurements that strongly correlate with terminal histology and with observed therapeutic responses, enabling serial, noninvasive assessment of tumor kinetics. The protocol is low-cost, scalable, and reduces reliance on invasive endpoints, aligning with 3R (replacement, reduction, and refinement) principles. By validating imaging against histopathology and treatment outcomes, the study advances preclinical HCC models and offers a pra
A syngeneic orthotopic HCC model was established in male Sprague Dawley rats by subcapsular implantation of the rat hepatoma cell line (N1S1) hepatoma cells into the left lateral liver lobe. Tumor-bearing rats were randomized into control and sorafenib-treated groups, and tumor progression was monitored longitudinally using high-frequency ultrasound. Volumetric estimates were validated against micro computed tomography (CT), gross, and histopathological mea
Devan et al[1] show that high-frequency ultrasound provides reproducible longitudinal volumetry in a syngeneic orthotopic rat HCC model, correlating strongly with terminal histopathology and detecting treatment-related changes in tumor kinetics. The technique is low-cost and minimally invasive, allowing serial assessments in the same subjects and reducing animal use and invasive procedures. Its scalability and high throughput make it suitable for repeated efficacy testing, even in resource-limited laboratories[2,3]. Key limitations of the study include operator dependence and reduced accuracy for very small, irregular, or highly heterogeneous lesions. In addition, reliance on a single N1S1 cell line con
| Imaging modality | Advantages | Limitations | Ref. |
| High-frequency ultrasound | Real-time, non-invasive, affordable; enables serial monitoring and correlates well with histology or MRI | Limited soft-tissue contrast for small or heterogeneous lesions; accuracy may decrease for deep or poorly defined tumors | Devan et al[1], Roth et al[2], Molière et al[3] |
| Histology | Gold standard for cellular detail; assessment of necrosis, vessels and immune cells | Requires tissue harvest; terminal procedure; not suitable for longitudinal studies | Herrero de la Parte et al[5], Choi et al[10] |
| Micro computed tomography | High-resolution three dimensional anatomy and vasculature; volume quantification | Involves radiation exposure and need for contrast agents; limited soft-tissue contrast | Singh et al[8], Cigliano et al[11] |
| MRI | Excellent soft-tissue contrast; quantitative; reproducible; translational | Expensive; long scan time; limited accessibility in small animal settings | Rojas et al[4], Renzulli and Giampalma[12] |
| Photoacoustic imaging | Combines optical and ultrasound contrast to visualize vascular oxygenation and perfusion | Restricted penetration depth; often relies on exogenous optical contrast agents; specialized setup required (operator dependent) | Nyayapathi et al[7] |
| Super-resolution ultrasound | Allows visualization of microvascular changes beyond conventional resolution | Requires contrast microbubbles and operator dependent complex data processing; limited availability and standardization | Hoyt[6], Riberdy et al[9] |
Devan et al[1] reinforce a pivotal shift: High-frequency ultrasound is evolving from a practical imaging tool into a versatile, translational platform for longitudinal HCC research. Their demonstration of reproducible, treatment-sensitive volumetry complements benchmark studies showing ultrasound outperforms caliper-based measures and closely approximates MRI for gross tumor size[2,4,5]. Practical workflow innovations, rapid, high-throughput scanning protocols, and ultrasound-based orthotopic implantation further reduce inter-sample variability and increase experimental efficiency[3,13]. At the technological frontier, super-resolution ultrasound and microvascular mapping enable detection of vascular remodeling and perfusion changes that are mechanistically linked to antiangiogenic and immu
Recent technological advances are improving methodological rigor. Studies comparing ultrasound findings with histopathology and other imaging techniques such as micro CT, positron emission tomography (PET)-CT, MRI, bioluminescence etc. strengthen external validity and support the use of ultrasound-based measures as reliable surrogate end
Overall, these advances offer a practical pathway to expand longitudinal HCC research: Low-cost high-frequency ultrasound enables widespread use in resource-limited settings, while contrast techniques, multimodal validation, and AI analytics improve sensitivity, objectivity, and translational potential[12]. Combining Devan et al’s reproducible protocol[1] with these technological and methodological improvements can accelerate reliable, ethical, and clinically informative preclinical oncology studies.
To realize ultrasound’s full translational potential for orthotopic HCC, coordinated efforts must prioritize standardization, multimodal validation, technological augmentation, and broad biological testing. Validated acquisition standard operating procedures for high-frequency ultrasound, operator training curricula, and quality assurance checklists should be developed and disseminated to reduce inter-operator variability and enable multicenter comparability[3]. Routine validation of ultrasound-derived volumetry and functional indices against histopathology and at least one orthogonal imaging modality (MRI, CT, PET-CT or bioluminescence) in representative subsets will establish external validity across tumor morphologies and growth kinetics[2,4,8]. Integrating super-resolution, contrast-enhanced, and photoacoustic imaging can enhance detection of microvascular changes vital for targeted therapies[6,7]. Open imaging datasets and AI/radiomics tools will improve automation and biomarker discovery[9]. Validating ultrasound workflows across diverse HCC models; including syngeneic, carcinogen-induced, and patient-derived xenograft systems, will ensure broader translational relevance[10,11].
Devan et al’s ultrasound-based protocol[1] establishes high-frequency ultrasonography as a practical, animal-sparing tool for longitudinal volumetric assessment in orthotopic HCC, demonstrating strong concordance with histopathology and treatment response. When combined with standardized training, multimodal validation (MRI/micro CT/histology), and emerging contrast and AI-driven analytics, ultrasound can deliver scalable, cost-effective imaging endpoints for pre
| 1. | Devan AR, Sasidharan SM, Sreekumar KP, Unni AKK, Mangalathillam S, Ansar A, Unni AR, Nath LR. Ultrasound imaging-guided protocol for monitoring tumor growth in orthotopic rat model of hepatocellular carcinoma. World J Hepatol. 2025;17:109517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Roth D, Safi M, Vilhelmsson Timmermand O, Sereti E, Molendowska M, Gottschalk M, Bjartell A, Ceberg C, Szczepankiewicz F, Strand J. Evaluation of superficial xenograft volume estimation by ultrasound and caliper against MRI in a longitudinal pre-clinical radiotherapeutic setting. PLoS One. 2024;19:e0307558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Molière S, Martinet A, Jaulin A, Lodi M, Chamaraux-Tran TN, Alpy F, Bierry G, Tomasetto C. Fast Ultrasound Scanning is a Rapid, Sensitive, Precise and Cost-Effective Method to Monitor Tumor Grafts in Mice. J Mammary Gland Biol Neoplasia. 2024;29:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Rojas JD, Joiner JB, Velasco B, Bautista KJB, Aji AM, Moore CJ, Beaumont NJ, Pylayeva-Gupta Y, Dayton PA, Gessner RC, Czernuszewicz TJ. Validation of a combined ultrasound and bioluminescence imaging system with magnetic resonance imaging in orthotopic pancreatic murine tumors. Sci Rep. 2022;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Herrero de la Parte B, García-Alonso I, Mar-Medina C, Iturrizaga S, Saiz-López A, Hernández-Farto L, Del Campo-Clemente C, Echevarría-Uraga JJ. Ultrasound Tumor Size Assessment, Histology and Serum Enzyme Analysis in a Rat Model of Colorectal Liver Cancer. Ultrasound Med Biol. 2020;46:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hoyt K. Super-Resolution Ultrasound Imaging for Monitoring the Therapeutic Efficacy of a Vascular Disrupting Agent in an Animal Model of Breast Cancer. J Ultrasound Med. 2024;43:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Nyayapathi N, Zheng E, Zhou Q, Doyley M, Xia J. Dual-modal Photoacoustic and Ultrasound Imaging: from preclinical to clinical applications. Front Photon. 2024;5:1359784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Singh G, Bendale K, Talwelkar S, Pawade S, Gera P, Patil A, Chavan P, Subramanian S, Chaudhari P. Establishment of an orthotopic syngeneic rat model of hepatocellular carcinoma and its validation with microPET-CT imaging. Integr Cancer Sci Therap. 2020;7. [DOI] [Full Text] |
| 9. | Riberdy V, Guida A, Rioux J, Brewer K. Radiomics in preclinical imaging research: methods, challenges and opportunities. Npj Imaging. 2025;3:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Choi B, Pe J, Yu B, Kim DH. Syngeneic N1-S1 Orthotopic Hepatocellular Carcinoma in Sprague Dawley Rat for the Development of Interventional Oncology-Based Immunotherapy: Survival Assay and Tumor Immune Microenvironment. Cancers (Basel). 2023;15:913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Cigliano A, Liao W, Deiana GA, Rizzo D, Chen X, Calvisi DF. Preclinical Models of Hepatocellular Carcinoma: Current Utility, Limitations, and Challenges. Biomedicines. 2024;12:1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 12. | Renzulli M, Giampalma E. Hepatocellular Carcinoma: Imaging Advances in 2024 with a Focus on Magnetic Resonance Imaging. Curr Oncol. 2025;32:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | McVeigh LE, Wijetunga I, Ingram N, Marston G, Prasad R, Markham AF, Coletta PL. Development of orthotopic tumour models using ultrasound-guided intrahepatic injection. Sci Rep. 2019;9:9904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Nittayacharn P, Abenojar E, Cooley MB, Berg FM, Counil C, Sojahrood AJ, Khan MS, Yang C, Berndl E, Golczak M, Kolios MC, Exner AA. Efficient ultrasound-mediated drug delivery to orthotopic liver tumors - Direct comparison of doxorubicin-loaded nanobubbles and microbubbles. J Control Release. 2024;367:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/