Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.113756
Revised: October 25, 2025
Accepted: October 30, 2025
Published online: November 27, 2025
Processing time: 86 Days and 5.8 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease, affecting more than 30% of adults and 7%-14% of youths globally. MASLD and its advanced form of metabolic dysfunction-associated steatohepatitis (MASH) can progress to liver cirrhosis and hepatocellular carcinoma. Despite its growing burden, effective therapies for MASLD and MASH remain limited. Accumulating evidence indicates that short-chain fatty acids (SCFAs) modulate the activation of hepatic innate and adaptive imm
Core Tip: Short-chain fatty acids (SCFAs) produced by gut microbial fermentation of indigestible fiber play important roles in regulating liver inflammation, fibrosis, and energy metabolism. The common SCFAs in the gut and liver are acetate, butyrate, and propionate. Strategies that enhance SCFA production, such as probiotic or dietary fiber supplementation, bariatric surgery, ketohexokinase inhibition, or fecal microbiota transplantation, offer promising methods for preventing metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis.
- Citation: Zhang CY, Liu S, Sui YX, Yang M. Roles of short-chain fatty acids in metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis. World J Hepatol 2025; 17(11): 113756
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/113756.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.113756
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease, affecting more than 30% of adults and 7%-14% of youths globally[1,2]. An advanced stage of MASLD is metabolic dysfunction-associated steatohepatitis (MASH). About 12%-40% of patients with steatotic livers develop MASH, 15%-25% of MASH livers progress to liver cirrhosis, and 2%-7% of cirrhotic livers develop hepatocellular carcinoma (HCC). MASLD can directly develop HCC without cirrhosis[3,4]. Currently, rezdiffra (resmetirom), an agonist of thyroid hormone receptor-β (THR-β), is the only approved drug by the United States Food and Drug Administration for the treatment of patients with noncirrhotic MASH and moderate to advanced liver scarring (fibrosis), along with diet and exercise[5].
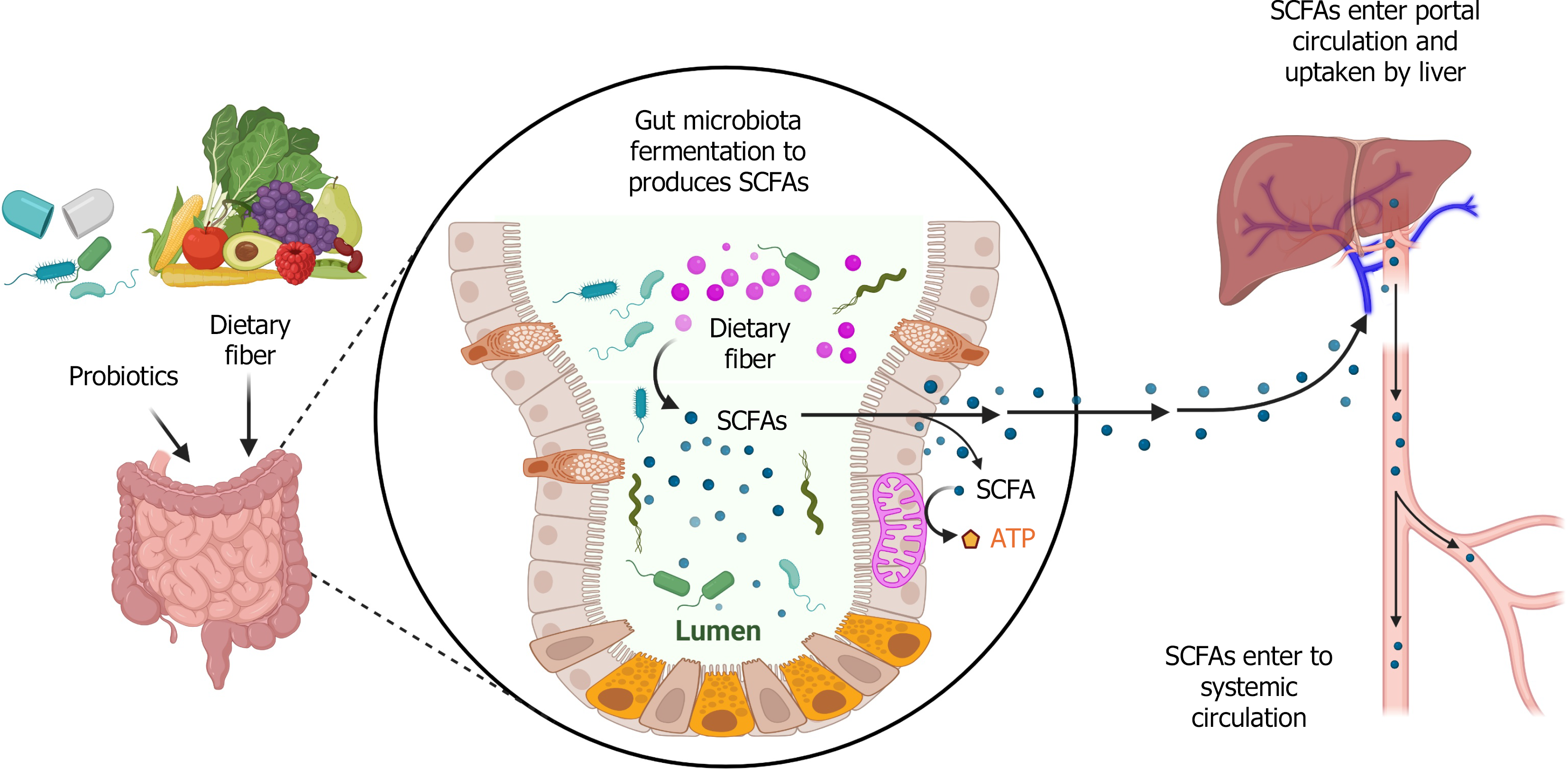
Accumulating evidence indicates that the gut-liver axis plays a pivotal role in liver health and the pathogenesis of MASLD[6,7]. Short-chain fatty acids (SCFAs) are one group of gut microbiota-derived metabolites. They are produced by the fermentation of indigestible fiber by gut microbiota[8-10] (Figure 1). SCFAs are fatty acids with less than six carbon atoms, primarily consisting of acetate (2 carbon atoms), propionate (3 carbon atoms), and butyrate (4 carbon atoms), which together account for 95% of SCFAs in the body. SCFAs exert their physiological effects by activating G-protein coupled receptors (GPCRs), such as GPR41, GPR43, and GPR109A[11-13]. SCFAs are extensively involved in the processes of anti-inflammatory responses, immune regulation, and metabolic modulation through various molecular signaling pathways[12]. Consequently, SCFAs have been implicated in numerous diseases, such as inflammatory bowel disease[14], cardiovascular disease[15], cancers[16], type 2 diabetes mellitus[17], obesity[18,19], MASLD or MASH[20,21], and other metabolic disorders[22]. This review will mainly focus on the roles of SCFAs in MASLD and MASH.
Gut microbiota dysbiosis in patients with MASLD is associated with intestinal inflammation and alteration of immune response[23]. In addition, alteration of gut microbiota can impact extraintestinal immune responses by regulating both innate and adaptive immune cell activation, including intrahepatic immune responses[24]. Macrophages play a pivotal role in MASLD, MASH, and other metabolic-associated diseases[25,26]. For example, a study showed that liver-resident macrophages, Kupffer cells, serve as a great promoter in liver inflammation, which is mediated by expression of vascular cell adhesion molecule-1 in MASLD[27]. The polarization of M1 and M2 macrophages is critically involved in hepatic inflammation, which drives the MASLD and MASH progression[28]. γδ T cells function as a bridge of innate and adaptive immune responses, playing an important role in promoting MASH progression. γδ T cells can produce IL-17 to promote liver inflammation. A study showed that Tcrd-deficient mice had less liver injury and lower severity of MASH compared to wild-type mice. In addition, Tcrd-deficient mice that received transfer of IL17a-/-γδ T cells had less liver injury compared with mice that received γδ T cells[29]. In a high-fat high-fructose diet (HFHFD)-induced MASH model, hepatic T helper (Th) 17 cells were significantly increased[30]. SCFAs are important microbial metabolic regulators in the pathogenesis of MASLD and MASH. In this section, we will review the functions of three SCFAs in immune responses during MASLD and MASH.
Patients with MASLD had elevated plasma levels of propionate, formate, valerate, and α-methylbutyrate, and a decreased level of acetate compared with the healthy controls[31]. The logistic regression analyses showed that the plasma level of acetate was inversely correlated with the odds of MASLD development, after adjusting for age and sex. However, there was a positive correlation between the plasma level of propionate and the severity of fibrosis[31]. Oral supplementation of branched-chain amino acids to high-fructose diet (HFD)-fed rats significantly decreased hepatic lipid accumulation and hepatic steatosis score by decreasing the expression of lipid biosynthetic enzymes in the liver, such as fatty acid synthase and acetyl-CoA carboxylase. Branched-chain amino acid-mediated reduction of hepatic steatosis was contr
Acetate receptor, free fatty acid receptor 2 (FFAR2 or GPR43), is expressed in neutrophils, monocytes, and regulatory T cells (Tregs)[34,35]. Inulin consumption can change gut microbial profile and the production of acetate, which inhibits MASLD progression. In germ-free mice, acetate can be produced from inulin by gut microbiota such as Blautia producta and Bacteroides acidifaciens. Depletion of FFAR2 abolished inulin-induced protective effects in liver inflammation, insulin resistance, and metabolic dysfunction[36]. Acetate selectively interacts with GPR43 to regulate neutrophil recruitment and activation[37,38]. These studies suggest that acetate can regulate immune responses to modulate the progression of MASLD and MASH.
Macrophages, as a major component of innate immunity, play multiple roles in the pathogenesis of MASLD or MASH. Sodium acetate has a dose-dependent effect on macrophage activation and polarization. A lower dose (0.1 mmol/L) of sodium acetate induced M1 polarization of macrophages (RAE264.7 cells and Kupffer cells) and increased their proinflammatory cytokine production [e.g., interleukin (IL)-1b, IL-6, and tumor necrosis factor-alpha (TNF-α)] by promoting the phosphorylation of NF-κB p65 and c-Jun (transcription factor Jun), whereas a higher dose (2 mmol/L) of sodium acetate reduced macrophage inflammation[39]. The different functions of sodium acetate at low and high doses were caused by the intracellular concentration of acetate in macrophages. Notably, the effect of sodium acetate on the gene expression of lipid synthesis and cellular levels of total cholesterol (TC) and triglycerides (TG) in both macrophages and hepatocytes was independent of its concentration[39]. The function of SCFAs may be influenced by disease stage, cell type, and microenvironment. For example, high-dose exogenous acetate inhibits macrophage glycolysis to protect mice from sepsis, which is not mediated by the activation of its receptors GPR41 and GPR43 but through its transient regulation of acetyl-coenzyme A production[40]. Another recent study used modified sodium acetate-loaded liposomes for targeted delivery to hepatocytes and Kupffer cells. The results showed that compared to free sodium acetate, sodium acetate-loaded liposomes effectively increased hepatic acetate accumulation and extension, to reduce hepatic TC and TG and lipid droplets, and to prevent macrophage infiltration and proinflammation[41]. In addition, the liposomes showed similar effects in inhibiting lipopolysaccharide-activated Kupffer cell proinflammation and fatty acid-induced lipid accumulation in hepatocytes. These findings demonstrate that sodium acetate has protective effects in MASLD and MASH by protecting against macrophage inflammation and lipid accumulation in hepatocytes at an appropriate dose.
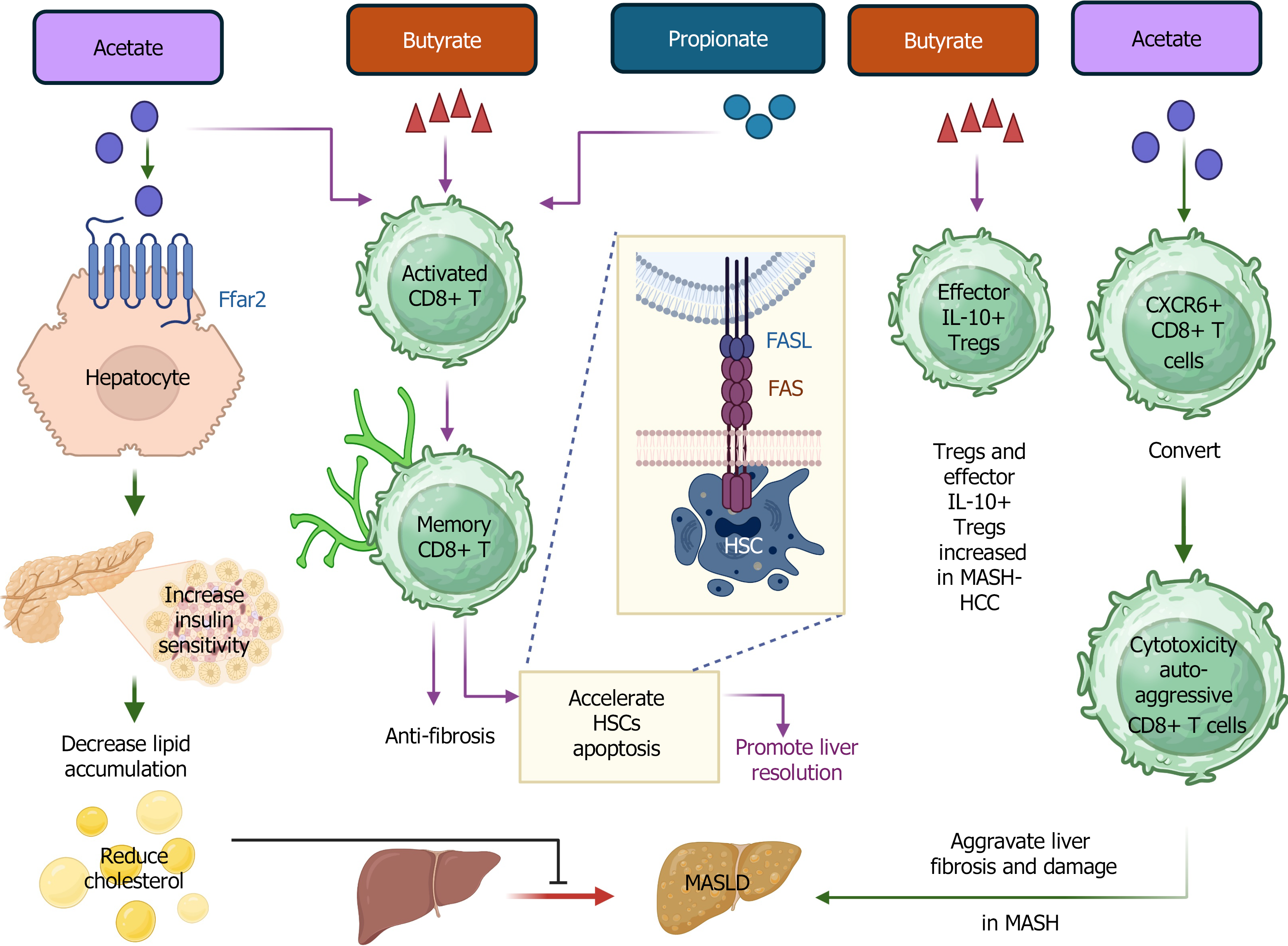
However, there is a deleterious role of acetate in CD8+ T cells in MASH liver. Acetate can promote the conversion of liver-resident CXCR6+ CD8+ T cells to cytotoxic, auto-aggressive CD8+ T cells in MASH (Figure 2), which aggravate liver fibrosis and injury. Mechanistically, metabolic stimulation of acetate and extracellular ATP triggers IL-15-modulated liver-resident CXCR6+ CD8+ T cell auto-aggression, with downregulation of FOXO1 and CXCR6. These auto-aggressive CD8+ T cells are cytotoxic to hepatocytes in an MHC-I-independent manner[42]. The ratio of Th17 to resting Tregs (CD4+CD45RA+CD25++ cells) in peripheral blood was positively associated with fecal concentrations of acetate and propionate[43]. This underscores the important association between the concentration of SCFAs and the levels of Th17 and Tregs in MASLD and MASH. However, more mechanistic studies are favored for further dissecting the roles of acetate in MASLD, to modulate its concentration to prevent MASLD or MASH.
Treatment with sodium butyrate can inhibit the infiltration of monocyte-derived macrophages into MASH liver and promote the apoptosis of proinflammatory M1 macrophages. In addition, sodium butyrate-treated macrophages develop towards M2-like macrophages to promote the healing process[44]. A study also showed that gut bacteria-derived butyrate can promote the maturation of mouse and human liver-resident natural killer (NK) cells by increasing the expression levels of IFN-γ and CD107a. Further studies revealed that butyrate-mediated maturation of NK cells was through IL-18 production in hepatocytes and Kupffer cells by activating the GPR109A signaling pathway[45].
A recent study found that an elevated level of SCFAs, such as both fecal and serum levels of acetate and butyrate, was detected in patients with MASLD-HCC compared to that in patients with MASLD-cirrhosis and non-MASLD controls. Additionally, further study revealed that an increased level of butyrate in MASLD-HCC was associated with increased amounts of Tregs and effector IL-10-expressing Tregs in peripheral blood, which attenuate the expansion of cytotoxic CD8+ T cells in MASLD-HCC[46]. This study highlights the important role of SCFAs in the progression from non-MASLD or MASLD-cirrhosis to MASLD-HCC. Most interestingly, gut microbiota-induced butyrate is responsible for the successful transition from activated CD8+ T cells to memory CD8+ T cells. It plays a crucial role in maintaining the survival of memory precursors of CD8+ T cells[47]. In MASH, a study found that tissue-resident memory CD8+ T cells showed direct anti-fibrotic function, and they promoted MASH liver resolution by accelerating hepatic stellate cells (HSCs) apoptosis mediated by Fas (tumor necrosis factor receptor superfamily member 6)/Fas ligand (FasL)-mediated signaling pathway[48].
CD8+ T cells play a substantial role in the activation of HSCs in obesity-associated mouse MASH models and human subjects with MASH and cirrhosis, aggravating hepatic inflammation and fibrosis[49]. They can directly activate HSCs by secreting IL-10, IL-2, IL-6, and TNF-α. Indirectly, CD8+ T cells promote the activation of macrophages to induce HSC activation via the transforming growth factor-beta (TGF-β) signaling pathway[49]. On the other hand, perforin produced by CD8+ T cells showed the function to constrain the liver inflammation in MASH, which highlights the role of perforin from CD8+ T cells in MASH management[50].
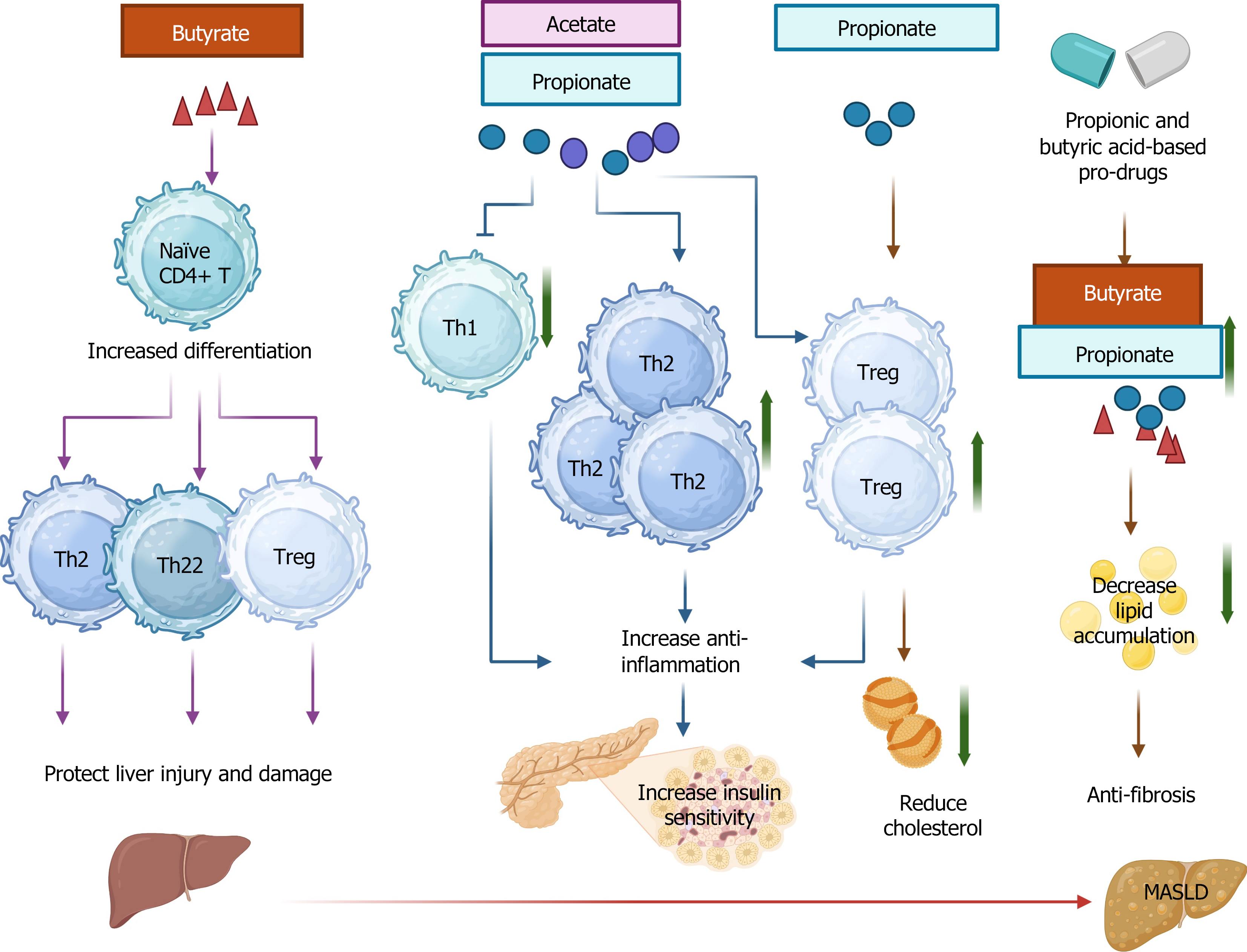
The alteration and dysregulation of CD4+ T cells serve as important indicators of pathogenesis in MASLD and MASH[51]. Some subsets of CD4+ T cells, such as Th1 and Th17 cells, can promote liver inflammation and fibrosis, whereas other subsets, such as Th2 and Th22 cells, have opposite effects[52,53]. A recent study reported that administration of butyrate-producing Clostridium butyricum B1 (CB) suppressed HFD-induced MASH by reducing the expression levels of monocyte chemotactic protein-1 (MCP-1, or CCL2) and TNF-α in the liver[54]. Mechanistically, CB administration induced an elevated level of butyrate in the cecal content and liver tissues. Further investigation revealed that sodium butyrate contributed to CD4+ T cell differentiation towards Th2, Th22, and Tregs, which collectively protect the liver from injury and damage[54].
Treatment of Lactobacillus johnsonii can increase the concentration of propionic acid. Propionic acid can suppress MAPK signaling pathway[55]. In addition to macrophages, propanoic acid derived from gut microbiota can suppress liver inflammation by decreasing the IL-17-producing γδ T cells[56]. HFD reduced the gut bacteria Akkermansia muciniphila (A. muciniphila), which induced liver inflammation featured by an elevated level of M1 macrophages and γδT cells along with a decreased level of M2 macrophages[56]. Supplementation of A. muciniphila decreased liver inflammation by improving gut barrier function, downregulating hepatic toll-like receptor 2 expression, reducing hepatic γδT cells, especially IL-17a-producing γδT cells, and promoting macrophage M2 polarization. Mechanistically, A. muciniphila supplementation increased intestinal concentration of SCFAs in HFD-fed mice, including propanoic acid, as well as the expression of SCFA receptor FFAR2. Activated hepatic γδT cells induce macrophage polarization from proinflammatory M1-like macrophages to M2-like macrophages by producing IL-17. These results underscore the important role of SCFAs in modulating the communication of immune cells in MASH liver, such as the γδT cells and macrophages.
Active neutrophil infiltration in the liver is a hallmark of MASH, contributing to the progression from MASLD to MASH[57]. Increased neutrophil infiltration is known to be positively correlated with the severity of MASLD. Recent studies have discovered that neutrophil extracellular traps (NETs) aggravated hepatic fibrosis in MASH by inducing HSC activation, proliferation, and migration[58]. Neutrophils collectively contribute to the formation of NETs and HSC activation-mediated fibrosis, resulting in the progression of macrophage-regulated inflammation and liver injury to induce progression of MASLD to MASH[57,59]. Propionate and butyrate serve as natural histone deacetylase (HDAC) inhibitors to induce apoptosis of neutrophils independent of their receptor activation (GPR41 and GPR43)[60]. Additionally, they can directly activate GPR43 to boost neutrophil chemotaxis[61].
The role of NKT cells in MASLD remains controversial. Mice fed a fast food diet (FFD) had mild to moderate MASH with a significant reduction of NKT cells, while the number of NKT cells was not dramatically changed in the methionine choline-deficient (MCD) diet-induced mouse MASH model[62]. However, CD1d-deficient mice without NKT cells had advanced liver fibrosis compared to wild-type mice when they were MCD, but not FFD. These findings demonstrate that NKT cells may have a preventive role in liver fibrosis at different stages based on the dietary MASLD models[62]. Dietary fatty acids can impair hepatocyte antigen CD1d presentation to impact NKT cell apoptosis and depletion to promote MASLD progression[63]. The specific role of propionic acid or propionate in NKT cells during MASLD progression remains to be investigated.
The numbers of Th1 and Th17 cells were increased while the number of Tregs was decreased in the spleens and mesenteric lymph nodes of HFD-fed mice[64]. Like butyrate, acetate, and propionate alone or their combination reverse HFD-induced changes of T cell profile in a healthy manner. Propionate coupled with acetate significantly reduced serum IL-6 levels in HFD-fed mice compared to the butyrate-treated group, which consequently promoted insulin sensitivity in HFD-fed mice[64]. In addition, propionate showed a beneficial effect in reducing cholesterol via increasing the number of Treg cells in Apoe−/− mice with HFD treatment[65]. MASH treated with propionic acid and butyric acid-based pro-drugs obtained an effective therapeutic effect with reduced lipid accumulation and fibrosis[66].
Overall, SCFAs play multiple roles in immune regulation in MASLD and MASH (Table 1, Figures 2 and 3).
| SCFAs | Immune cells | Function | Ref. |
| Acetate | Macrophages | A lower dose (0.1 mmol/L) of sodium acetate promotes macrophage inflammation (RAE264.7 cells and Kupffer cells) by promoting the phosphorylation of NF-κB p65 and c-Jun (transcription factor Jun), whereas a higher dose (2 mmol/L) of sodium acetate showed the opposite effect | [39] |
| Acetate | Macrophages | Sodium acetate-loaded liposomes effectively inhibited lipopolysaccharide-activated Kupffer cell proinflammation | [41] |
| Acetate | CD8+ T cells | Acetate can promote the conversion of liver-resident CXCR6+ CD8+ T cells to cytotoxic, auto-aggressive CD8+ T cells in MASH liver, promoting liver damage | [42] |
| Butyrate | Macrophages | Sodium butyrate can inhibit hepatic monocyte-derived macrophage infiltration and promote the apoptosis of proinflammatory M1 macrophages while activating the M2-like macrophages in MASH livers, thereby leading to the healing process | [44] |
| Butyrate | NK cells | Gut bacteria-derived butyrate can promote the maturation of mouse and human liver-resident NK cells by increasing the expression levels of IFN-γ and CD107a, through IL-18 production in hepatocytes and Kupffer cells | [45] |
| Butyrate | CD8+ T cells | Gut microbiota-induced butyrate is responsible for the successful transition from activated CD8+ T cells to memory CD8+ T cells and maintains the survival of memory precursors of CD8+ T cells | [47] |
| Butyrate | CD4+ T cells | Sodium butyrate can promote CD4+ T cell differentiation towards Th2, Th22, and Tregs | [54] |
| Propionate | Macrophages | Propionic acid can suppress M1 macrophage polarization by inhibiting MAPK signaling pathway | [55] |
| Propionate | γδ T cells | Propanoic acid derived from gut microbiota can suppress liver inflammation by decreasing the IL-17-producing γδ T cells | [56] |
| Propionate | T helper cells | Propionate alone or in combination with acetate reduced the numbers of Th1 and Th17 cells and increased the number of Tregs in the spleens and mesenteric lymph nodes of HFD-fed mice | [64] |
Altered energy metabolism in the liver is a major cause of insulin resistance, contributing to the MASLD progression[67,68]. AMPK is an important enzyme that serves as an intracellular energy sensor and regulator, which is responsible for maintaining energy homeostasis[69]. In adipocytes, SCFAs interact with their receptors, such as GPR41 and GPR43, to activate AMPK signaling, which is involved in lipid accumulation and thermogenesis. The activation of AMPK signaling also activates the PPARα, subsequently promotes fatty acid oxidation, and eventually reduces lipid storage in the normal liver[70]. In MASLD or MASH, the activation of the AMPK signaling pathway is decreased, which causes the upregulation of lipogenesis and downregulation of fatty acid oxidation, mitochondrial dysfunction, resulting in the exacerbation of hepatic steatosis[71,72]. A recent study revealed that sodium butyrate can promote the phosphorylation of liver kinase B1 to boost the activation of AMPK. Simultaneously, sodium butyrate shows the function to augment the activity of insulin-induced genes to suppress the lipogenic gene expression in an AMPK-dependent manner, eventually alleviating hepatic steatosis in HFD-induced MASLD[73]. These studies suggest that SCFAs can regulate lipid and sugar metabolism, as well as insulin resistance, to influence MASLD progression.
Lipid metabolism and lipogenesis play fundamental roles in the pathogenesis of MASLD and MASH[74]. Hepatic lipid profiles in patients could be applied to predict the progression of diseases, such as C14: 0, C16: 0, C16: 1n-7, C18: 1n-7, C18: 1n-9, and C18: 2n-6 fatty acids. These fatty acids belong to long-chain fatty acids, and their synthesis can be regulated by elongases and desaturases, such as elongase of very long chain fatty acid protein 6 and fatty acid desaturase 1[75]. SCFAs participate in hepatic lipid metabolism and lipogenesis. Studies have demonstrated that acetate and propionate have the function to reduce HFD-induced body weight, insulin resistance, and hepatic steatosis[76]. Acetate derived from HFD is responsible for promoting hepatic de novo lipogenesis (DNL)[77]. Moreover, gut microbiota-produced acetate can be used by hepatocytes to increase DNL. However, propionate may compete with acetate to suppress DNL by reducing hepatic expression of enzymes in lipid metabolism[78-80].
One study showed that Ganoderma meroterpene derivative treatment can confer an anti-fibrosis effect on the livers of fa/fa rats by altering gut microbial profiles, resulting in an increased production of butyrate and folate. These changes were associated with an enrichment in the abundances of butyrate-producing bacterium Kineothrix alysoides (K. alysoides) and folate-synthesis-deficient Bacteroides xylanisolvens[81]. Consistently, another study also revealed that treatment with K. alysoides attenuated HFHFD-induced bodyweight gain, high liver injury markers, and high serum levels of TC and TG in mice. The associated mechanism is that administration of K. alysoides can significantly reduce hepatic lipid accumulation by decreasing the mRNA expression levels of peroxisome proliferator-activated receptor γ (PPAR-γ) and CD36 in the liver tissues[82].
A probiotic mixture, known as Prohep composed of several gut bacteria species, was applied for the treatment of HFD-induced MASLD and MASH. The results suggest that Prohep can alleviate the MASLD progression by reducing liver steatosis, inflammation, and serum alanine aminotransferase (ALT) levels. Further investigation suggests that treatment-mediated reduction of hepatic lipid accumulation, DNL, and cholesterol biosynthesis is potentially modulated by the change of gut microbial components and elevated production of SCFAs[83]. Collectively, the aforementioned studies highlight the essential regulatory role of SCFAs in lipid metabolism and lipogenesis in MASLD and MASH.
Glucose metabolism is altered in the pathogenesis of chronic liver diseases, such as MASLD and MASH[84,85]. SCFAs mediated glucose metabolism through different mechanisms in the liver. For instance, SCFAs can reduce lipid storage and glucogenesis, which is beneficial to lower blood glucose levels and prevent hypoglycemia[86,87]. By interacting with its receptor GPR43, propionate can activate the AMPK signaling pathway to down-regulate glucose-6-phosphatase and phosphoenolpyruvate carboxykinase expression, eventually inhibiting hepatic gluconeogenesis. AMPK phosphorylation mediated by propionate was due to an increase in intracellular Ca2+ concentration and activation of the Ca2+/CaMKKβ signaling pathway[88].
Insulin resistance is one of the causative factors that is responsible for the progression of MASLD and MASH[89]. Multi-omics studies reveal that insulin resistance is associated with microbial carbohydrate metabolisms, which are caused by changes in gut microbial components and genetic functions[90]. The fecal levels of propionate were increased in patients with insulin resistance. This finding highlights the association between SCFAs and insulin resistance. Sulforaphane, a phytochemical in cruciferous vegetables, decreased blood glucose and improved insulin resistance in MASLD[91]. The associated mechanism is related to an increase in SCFAs-producing bacteria, Bacteroides and Lactobacillus. SCFAs can activate their receptors GPR41 and GPR43 to promote the secretion of glucagon-like peptide-1 (GLP-1)[91]. HFD-induced insulin resistance was reversed by administration of high-fiber basil seeds, which enhanced the production of acetic acid, butyric acid, and propionic acid to improve insulin sensitivity and increased the production of n-3 polyunsaturated fatty acids in the liver. In addition, this treatment decreased hepatic lipid accumulation, inflammation, and oxidative stress[92].
Inflammation serves as a great contributor to the development and progression of fatty liver from MASLD to advanced MASH. Hepatic inflammation, adipose tissue inflammation, and systemic inflammation collectively contribute to the disease progression[93,94]. Numerous discoveries have been made to reveal inflammation-mediated molecular signaling pathways in the pathogenesis of MASLD or MASH[95,96]. Here, we will discuss several updated discoveries that are associated with SCFAs.
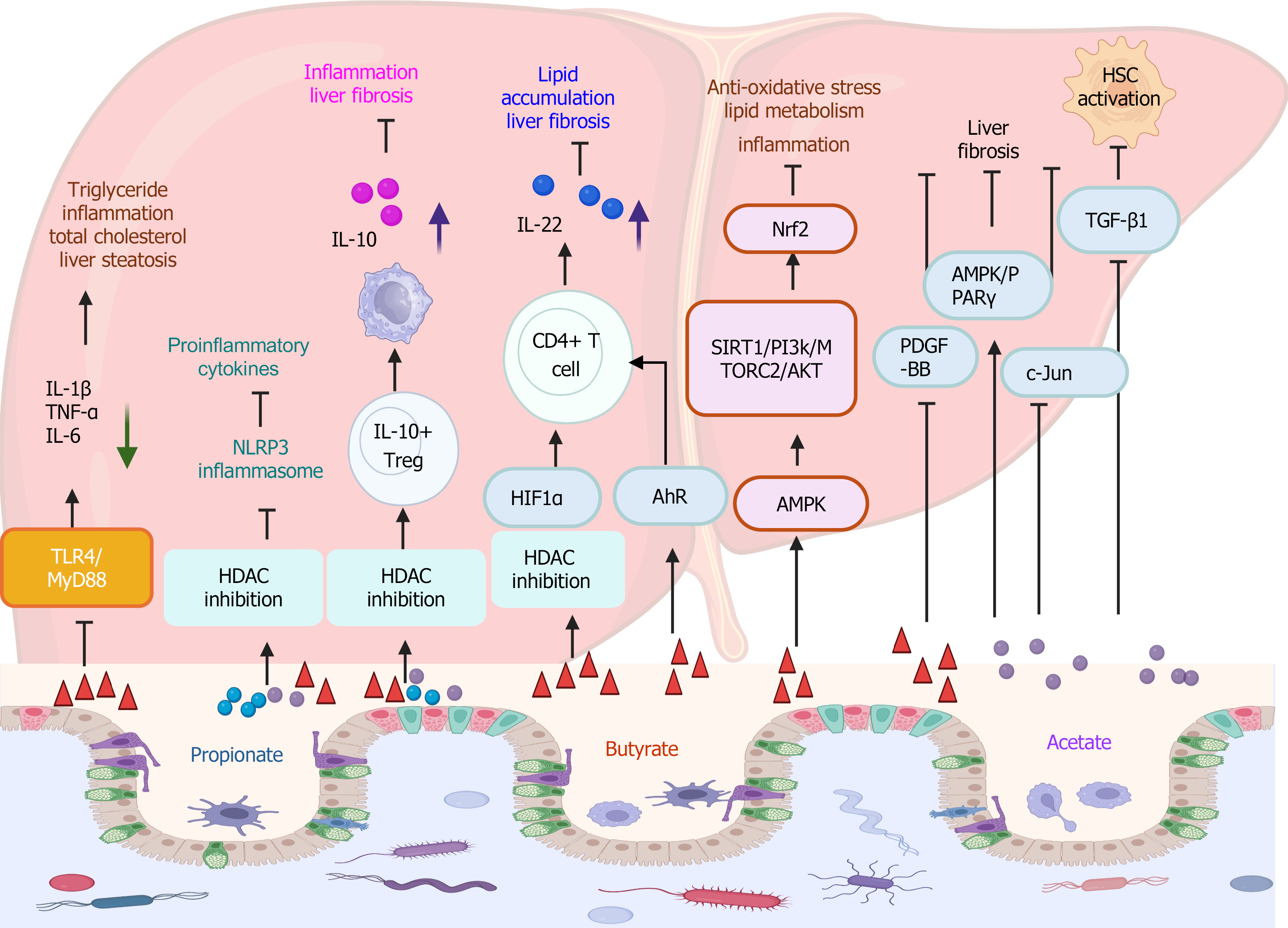
The expression of inflammatory cytokines, such as TNF-α and IL-6, was associated with the progression of hepatic steatosis to early MASH. Oral supplementation of sodium butyrate suppressed the TLR4/MyD88 signaling pathway in liver tissues to decrease liver inflammation and hepatic steatosis[97,98]. Most recently, a big data-based analysis of preclinical experimental studies suggests that the supplementation of sodium butyrate has a therapeutic effect in MASLD, by reducing inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α), decreasing TG and TC levels, and reducing hepatic steatosis[99].
SCFAs serve as inhibitors of HDACs. A recent study revealed that SCFAs, acetate (0.5 mmol/L), propionate (0.01 mmol/L), and butyrate (0.01 mmol/L) inhibited the activation of NLRP3 inflammasome due to their inhibitory property to HDAC[100]. NLRP3 plays an important role in the progression from MASLD to MASH[101,102]. Therefore, SCFAs might confer a beneficial effect on suppressing MASLD/MASH via regulating the activation of NLRP3 inflammasome.
SCFAs such as butyrate can increase the secretion of IL-10 and IL-4 in anti-CD3-stimulated peripheral blood mononuclear cells, but suppress the expression of IL-12 and IFN-γ[103]. The production of IL-10 in brown adipocytes displays an anti-fibrotic effect by suppressing HSC activation and α-smooth muscle actin expression[104]. Butyrate, but not acetate and propionate, can decrease the proportion of induced Tregs from naïve non-Treg cells (CD4+CD45RO−CD25−CD127hi) in adult peripheral mononuclear cells[105].
SCFAs can increase the secretion of IL-22 in CD4+ T cells and innate lymphoid cells through the activation of their receptor GPR41 and inhibiting HDAC[106]. The underlying mechanism is mediated by upregulation of hypoxia-inducible factor-1α (HIF-1α) and aryl hydrocarbon receptor, as HIF-1α can directly bind with the Il22 gene promoter, to protect against intestinal inflammation[106]. In MASLD and MASH, targeting IL-22 signaling can effectively inhibit liver inflammation and reverse hepatic steatosis and fibrosis in diet-induced MASLD[107,108]. Another recent study suggests that IL-22 is a promising therapeutic target in MASH management. Human IL-22-ScFv (single chain variable fragment) treatment targeting the liver and pancreas significantly decreased hepatic lipid accumulation and liver fibrosis in murine MASH model[109].
Sodium butyrate activates the AMPK signaling pathway to promote the activation of the downstream SIRT1/PI3K/mTORC2/protein kinase B (PKB, or AKT), followed by the activation of Nrf2[110]. Nrf2 activation plays a protective role in MASLD and MASH by reducing lipid metabolism, alleviating anti-oxidative stress, and decreasing inflammation[111,112].
In MASLD and MASH, liver fibrosis has mainly resulted from metabolic-related liver injury and inflammation. In MASH, liver fibrosis serves as a critical indicator associated with morbidity and mortality[113]. HSC activation is the major driver causing hepatic fibrosis[114], which is mainly driven by the TGF-β signaling pathway[115].
A recently published report revealed that lower levels of SCFAs, especially butyrate and acetate, were associated with a high degree of severity of liver fibrosis in patients with MASLD. Among patients with elevated levels of SCFAs, only 50% of them developed significant liver fibrosis (scored by liver stiffness value > 7.6 kPa); however, among patients with lower levels of SCFAs, about 92.3% of them developed significant liver fibrosis[116]. This result suggests that a higher level of SCFAs may have a significant protective effect against liver fibrosis.
Butyrate plays an anti-fibrotic function through different mechanisms. For instance, in a diet-induced MASH model, butyrate can suppress non-canonical TGF-β signaling pathways to decrease liver collagen production[117]. In the CCl4-induced liver fibrosis model, lactucin displayed an anti-fibrotic effect by elevating the products of acetic acid and butyric acid, which conferred anti-fibrotic function by inhibiting TGF-β1/STAT3 signaling pathway[118]. Sodium butyrate can reduce liver fibrosis in MASLD by decreasing the production of profibrotic mediator platelet-derived growth factor-BB, which is known as an important player in liver fibrosis and tissue repair[20]. Sodium acetate also showed anti-fibrotic effect through AMPK/PPAR-γ activation and inhibition of c-Jun signaling pathway[119]. Notably, acetate-mediated suppression of TGF-β1-induced activation of HSCs (LX2 cells) was GPR43-independent manner.
Overall, the function of SCFAs in liver inflammation and fibrosis is summarized in a graphic picture (Figure 4).
A study showed that MASH was the leading cause on the liver transplantation waitlist in 2016, among United States adults who were 50 to 70 years old[120]. Unfortunately, currently available treatments for MASLD and MASH are limited[121], even though many strategies have been evaluated in clinical trials. In addition to FDA-approved THR-β agonist Resmetirom, Farnesoid X receptor agonists, PPAR agonists, GLP-1 receptor agonists, and sodium-glucose co-transporter 2 inhibitors, and anti-inflammatory and anti-oxidative natural products, as well as lifestyle interventions like dietary changes, weight management, and exercise, or their combinations, are promising strategies to treat MASLD and MASH[4,122,123].
Supplementation of SCFAs has been applied in clinical trials to treat diseases, as seen in studies such as NCT07024238 (https://clinicaltrials.gov/, clinical trial number), NCT06951581, and NCT06951386. Here, we review clinical trials related to MASLD or MASH management or therapy (Table 2). Current clinical trials primarily focus on how various treatments, including dietary supplementation with fiber and probiotics, energy restriction, bariatric surgery, regulation of fructose metabolism (ketohexokinase inhibitors), and fecal microbiota transplantation, can modulate SCFA changes in the treatment of MASLD and MASH.
| Clinical trials | Phase | Disease | Treatment | Measurements |
| NCT05654805 | N/A | MASLD | Oat-fiber supplementation | Fermentation to short-chain fatty acids (SCFAs) |
| NCT05523024 | N/A | MASLD | Dietary supplementation of probiotics; Dietary supplementation of Berberine; Dietary supplementation of Probiotics and Berberine | The concentrations of SCFAs in stool |
| NCT05402449 | N/A | MASLD | Dietary supplementation of probiotics, including Lactobacillus reuteri GMNL-263 (heat-killed) and GMNL-89 (alive), and Lactobacillus rhamnosus GMNL-74 (alive) | Concentrations of SCFAs in blood |
| NCT04594954 | N/A | MASLD | Fecal microbiota transplantation | Production of SCFAs |
| NCT04415632 | N/A | MASLD | Low glycemic index diet; High glycemic index diet | Plasma concentrations of SCFAs |
| NCT04117802 | N/A | MASLD | Maple syrup | Change of fecal SCFAs |
| NCT01856465 | N/A | MASLD | Bariatric surgery | Change of fecal SCFAs |
| NCT05463575 | 2 | MASLD | Ketohexokinase inhibition | Change of fecal SCFAs, including acetate, propionate, and butyrate |
| NCT05821010 | 2 | MASH | Lyophilized fecal microbiota transplantation capsules Anaerobutyricum soehngenii; Pasteurized Akkermansia muciniphila; Bifidobacterium animalis subsp. Lactis; Fructo-oligosaccharides | Concentrations of SCFAs in blood (i.e., acetate, butyrate, and propionate) |
| NCT05647915 | 4 | Obesity MASLD | Berberine plus lifestyle intervention | Change of SCFAs |
| NCT04465032 | 4 | MASLD | Gut microbiome transplantation | Production of SCFAs |
Both MASLD and MASH are complicated and dynamic diseases. As illustrated above, SCFAs play an essential role in inflammation, immune response, fibrosis, lipid metabolism, glucose metabolism, and insulin resistance, collectively influencing the development and progression of MASLD or MASH. Since SCFAs are derived from gut fermentation of indigestible fiber or dietary supplementation, targeting SCFAs regulation can be harnessed as a strategy for managing MASLD and MASH. For example, dietary intervention or energy restriction[124] and probiotic supplementation[83] can be selected as treatment options to enhance levels of SCFAs. However, many challenges need to be addressed, such as targeted delivery methods[41,66], absorption of SCFAs[125], low bioavailability, hepatic first-pass metabolism[126], and a personalized treatment regimen[127]. For example, researchers have developed a strategy to conjugate L-serine with butyrate for improving the oral bioavailability of butyrate[128]. To overcome hepatic first-pass metabolism, microencapsulation technology has been applied to protect propionate and butyrate, enhancing their bioactivity. Additionally, tributyrin has been developed to increase its tolerability and biological effect[129].
In the era of artificial intelligence (AI), AI-based technology can be harnessed for the battle against many diseases. For instance, based on machine learning and clinical big data, abundant information on pathogenic factors can be analyzed, which can be applied for the prediction of disease progression and outcome prognosis[130]. The deep learning and AI-driven approach could be applied in histopathological diagnosis in MASLD/MASH[131]. In addition, AI-driven clinical decision-making can be a beneficial tool to facilitate the therapeutic strategy[132]. Using images attained by second harmonic generation/two photon excitation fluorescence microscopy, AI/machine learning analysis increases the accuracy of fibrosis quantification by identifying lobular and portal histopathological zones and calculating collagen distribution[133,134]. An explainable XGBoost algorithm has been applied to predict the high risk of MASH in patients based on the top 5 predictors[135], including alanine ALT, gamma glutamyl transpeptidase, platelet count, waist circumference, and age.
Moreover, in addition to mechanistic dissection, biomarker discovery, diagnostic and prognostic application, AI-based treatment and therapy have attracted extensive attention in the field. Many investigations can be applied in the management of MASLD and MASH, including AI-based lifestyle intervention[136], AI and multi-omics-based personalized prebiotic or dietary nutrition therapy[127,137], and AI-driven medicines, such as gut microbiome-specific nanoparticles[138]. In summary, rapidly emerging technologies such as AI, machine learning, deep learning, big data, and meta-omics could be harnessed to facilitate biomedicine discovery, particularly from the perspective of tackling uncommunicable and chronic diseases. A multifaceted approach holds great promise in accelerating translational and precision medicine.
| 1. | Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025;31:S32-S50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 331] [Article Influence: 331.0] [Reference Citation Analysis (3)] |
| 2. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 422] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 3. | Rao G, Peng X, Li X, An K, He H, Fu X, Li S, An Z. Unmasking the enigma of lipid metabolism in metabolic dysfunction-associated steatotic liver disease: from mechanism to the clinic. Front Med (Lausanne). 2023;10:1294267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Zhang CY, Liu S, Yang M. Antioxidant and anti-inflammatory agents in chronic liver diseases: Molecular mechanisms and therapy. World J Hepatol. 2023;15:180-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Keam SJ. Resmetirom: First Approval. Drugs. 2024;84:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 6. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1457] [Article Influence: 242.8] [Reference Citation Analysis (1)] |
| 7. | Choi J, Choi YR, Jeong MK, Song HH, Yu JS, Song SH, Park JH, Kim MJ, Park H, Ham YL, Han SH, Kim DJ, Lee DY, Suk KT. Phocaeicola dorei ameliorates progression of steatotic liver disease by regulating bile acid, lipid, inflammation and proliferation. Gut Microbes. 2025;17:2539448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Dyshlyuk LS, Milentyeva IS, Asyakina LK, Ostroumov LA, Osintsev AM, Pozdnyakova AV. Using bifidobacterium and propionibacterium strains in probiotic consortia to normalize the gastrointestinal tract. Braz J Biol. 2022;84:e256945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1754] [Article Influence: 116.9] [Reference Citation Analysis (1)] |
| 10. | Zou Y, Lin X, Xue W, Tuo L, Chen MS, Chen XH, Sun CH, Li F, Liu SW, Dai Y, Kristiansen K, Xiao L. Characterization and description of Faecalibacterium butyricigenerans sp. nov. and F. longum sp. nov., isolated from human faeces. Sci Rep. 2021;11:11340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 4640] [Article Influence: 464.0] [Reference Citation Analysis (0)] |
| 12. | Mukhopadhya I, Louis P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat Rev Microbiol. 2025;23:635-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 98] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 13. | Yang M, Zhang CY. G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 2021;27:677-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Ji W, Qin H, Chen Z, Zhou Y, Zhou Z, Wang J, Wang K. Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr Polym. 2025;349:122829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (1)] |
| 15. | Yang R, Pang J, Zhong X, Pang S, Hu X, Wei C, Yan W, Chen X, Zhao R, Xu B, Cao Z. Molecular mechanisms of aberrant fatty acids metabolism in driving cardiovascular diseases: key regulatory targets and dietary interventions. Food Funct. 2025;16:5961-5993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Li S, Duan Y, Luo S, Zhou F, Wu Q, Lu Z. Short-chain fatty acids and cancer. Trends Cancer. 2025;11:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 17. | Hsu CH, Tsai YC, Yu PS, Hung WW, Hung WC, Hwang SJ, Tsai HJ. Circulating short chain fatty acid levels and body composition in type 2 diabetes mellitus. Int J Med Sci. 2025;22:2289-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Li S, Ma X, Mei H, Chang X, He P, Sun L, Xiao H, Wang S, Li R. Association between gut microbiota and short-chain fatty acids in children with obesity. Sci Rep. 2025;15:483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Willis NB, Cannavale CN, Walk AM, Burd NA, Holscher HD, Khan NA. Inhibitory control is related to fecal short-chain fatty acid concentrations in adults with overweight and obesity. Nutr Res. 2025;138:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Huang LJ, Wang MY, Xin FZ, Yang RX, Zeng J, Ren TY, Fan JG. Sodium butyrate ameliorates liver fibrosis in metabolic dysfunction-associated steatohepatitis rats via miR-155-5p/SOCS1/PDGF signaling pathway. Hepatobiliary Pancreat Dis Int. 2025;24:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Wang SL, Liang S, Li SY, Fu JW, Wang ZY, Zhu DQ, Chen N. Lactobacillus rhamnosus GG attenuates MASLD/MASH progression by modulating gut microbiota and metabolic pathways. Front Microbiol. 2025;16:1586678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Tao Z, Wang Y. The health benefits of dietary short-chain fatty acids in metabolic diseases. Crit Rev Food Sci Nutr. 2025;65:1579-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 473] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 24. | Kim NH, Kim MY, Yang YM, Jeong WI, Lee HW, Kim W, Kang SG, Han YH. Bacterial components-driven intrahepatic CXCR5(hi) B cells are important population for MASH progression through inducing inflammation. FASEB J. 2025;39:e70322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Yang M, Liu S, Sui Y, Zhang C. Macrophage metabolism impacts metabolic dysfunction-associated steatotic liver disease and its progression. Immunometabolism. 2024;6:e00047. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Park JS, Lee J, Wang F, Ma H, Zhou Z, Lee YS, Oh K, Lee H, Sui G, Lee S, Yang YM, Lee JW, Ji YH, Park CW, Yoo HS, Hwang BY, Han SB, Song N, Oh S, Kim B, Seki E, Hong JT, Roh YS. A1AT dysregulation of metabolically stressed hepatocytes by Kupffer cells drives MASH and fibrosis. Exp Mol Med. 2025;57:450-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Lin SZ, Xie Y, Cheng YQ, Xue R, Su YS, Liu M, Chen YW, Fan JG. C/EBPβ-VCAM1 axis in Kupffer cells promotes hepatic inflammation in MASLD. JHEP Rep. 2025;7:101418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Ma C, Wang S, Dong B, Tian Y. Metabolic reprogramming of immune cells in MASH. Hepatology. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Li F, Hao X, Chen Y, Bai L, Gao X, Lian Z, Wei H, Sun R, Tian Z. The microbiota maintain homeostasis of liver-resident γδT-17 cells in a lipid antigen/CD1d-dependent manner. Nat Commun. 2017;7:13839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 30. | Van Herck MA, Vonghia L, Kwanten WJ, Julé Y, Vanwolleghem T, Ebo DG, Michielsen PP, De Man JG, Gama L, De Winter BY, Francque SM. Diet Reversal and Immune Modulation Show Key Role for Liver and Adipose Tissue T Cells in Murine Nonalcoholic Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2020;10:467-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Thing M, Werge MP, Kimer N, Hetland LE, Rashu EB, Nabilou P, Junker AE, Galsgaard ED, Bendtsen F, Laupsa-Borge J, McCann A, Gluud LL. Targeted metabolomics reveals plasma short-chain fatty acids are associated with metabolic dysfunction-associated steatotic liver disease. BMC Gastroenterol. 2024;24:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Iwao M, Gotoh K, Arakawa M, Endo M, Honda K, Seike M, Murakami K, Shibata H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci Rep. 2020;10:18768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Sahuri-Arisoylu M, Brody LP, Parkinson JR, Parkes H, Navaratnam N, Miller AD, Thomas EL, Frost G, Bell JD. Reprogramming of hepatic fat accumulation and 'browning' of adipose tissue by the short-chain fatty acid acetate. Int J Obes (Lond). 2016;40:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Kim S, Kim JH, Park BO, Kwak YS. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep. 2014;47:173-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger T, Renz H, Offermanns S, Steinhoff U, Visekruna A. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front Immunol. 2017;8:1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 36. | Aoki R, Onuki M, Hattori K, Ito M, Yamada T, Kamikado K, Kim YG, Nakamoto N, Kimura I, Clarke JM, Kanai T, Hase K. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome. 2021;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 37. | Altamura S, Lombardi F, Palumbo P, Cinque B, Ferri C, Del Pinto R, Pietropaoli D. The Evolving Role of Neutrophils and Neutrophil Extracellular Traps (NETs) in Obesity and Related Diseases: Recent Insights and Advances. Int J Mol Sci. 2024;25:13633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 38. | Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Häsler R, Nikolaus S, Fölsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514-7522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Li W, Deng M, Gong J, Hou Y, Zhao L. Bidirectional Regulation of Sodium Acetate on Macrophage Activity and Its Role in Lipid Metabolism of Hepatocytes. Int J Mol Sci. 2023;24:5536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 40. | Li N, Gong Y, Zhu Y, Li B, Wang C, Wang Z, Wang J, Huang J, Bian J, Zhang Y. Exogenous acetate attenuates inflammatory responses through HIF-1α-dependent glycolysis regulation in macrophage. Cell Mol Life Sci. 2024;82:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Hou Y, Gao X, Gong J, Dong X, Hao Y, Zhai Z, Zhang H, Zhang M, Liu R, Wang R, Zhao L. Targeted Sodium Acetate Liposomes for Hepatocytes and Kupffer Cells: An Oral Dual-Targeted Therapeutic Approach for Non-Alcoholic Fatty Liver Disease Alleviation. Nutrients. 2025;17:930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F, Inverso D, Wigger J, Sebode M, Öllinger R, Rad R, Hegenbarth S, Anton M, Guillot A, Bowman A, Heide D, Müller F, Ramadori P, Leone V, Garcia-Caceres C, Gruber T, Seifert G, Kabat AM, Mallm JP, Reider S, Effenberger M, Roth S, Billeter AT, Müller-Stich B, Pearce EJ, Koch-Nolte F, Käser R, Tilg H, Thimme R, Boettler T, Tacke F, Dufour JF, Haller D, Murray PJ, Heeren R, Zehn D, Böttcher JP, Heikenwälder M, Knolle PA. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 43. | Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J. 2018;6:1496-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 44. | Sarkar A, Mitra P, Lahiri A, Das T, Sarkar J, Paul S, Chakrabarti P. Butyrate limits inflammatory macrophage niche in NASH. Cell Death Dis. 2023;14:332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 45. | Tian P, Yang W, Guo X, Wang T, Tan S, Sun R, Xiao R, Wang Y, Jiao D, Xu Y, Wei Y, Wu Z, Li C, Gao L, Ma C, Liang X. Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-IL-18 axis. Nat Commun. 2023;14:1710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 46. | Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 47. | Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, Jönsson J, Gressier E, Lew AM, Perdomo C, Kupz A, Figgett W, Mackay F, Oleshansky M, Russ BE, Parish IA, Kallies A, McConville MJ, Turner SJ, Gebhardt T, Bedoui S. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity. 2019;51:285-297.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 513] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 48. | Koda Y, Teratani T, Chu PS, Hagihara Y, Mikami Y, Harada Y, Tsujikawa H, Miyamoto K, Suzuki T, Taniki N, Sujino T, Sakamoto M, Kanai T, Nakamoto N. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun. 2021;12:4474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 49. | Breuer DA, Pacheco MC, Washington MK, Montgomery SA, Hasty AH, Kennedy AJ. CD8(+) T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G211-G224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Wang T, Sun G, Wang Y, Li S, Zhao X, Zhang C, Jin H, Tian D, Liu K, Shi W, Tian Y, Zhang D. The immunoregulatory effects of CD8 T-cell-derived perforin on diet-induced nonalcoholic steatohepatitis. FASEB J. 2019;33:8490-8503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Her Z, Tan JHL, Lim YS, Tan SY, Chan XY, Tan WWS, Liu M, Yong KSM, Lai F, Ceccarello E, Zheng Z, Fan Y, Chang KTE, Sun L, Chang SC, Chin CL, Lee GH, Dan YY, Chan YS, Lim SG, Chan JKY, Chandy KG, Chen Q. CD4(+) T Cells Mediate the Development of Liver Fibrosis in High Fat Diet-Induced NAFLD in Humanized Mice. Front Immunol. 2020;11:580968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 52. | Ferreyra Solari NE, Inzaugarat ME, Baz P, De Matteo E, Lezama C, Galoppo M, Galoppo C, Cherñavsky AC. The role of innate cells is coupled to a Th1-polarized immune response in pediatric nonalcoholic steatohepatitis. J Clin Immunol. 2012;32:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 54. | Zhou D, Pan Q, Liu XL, Yang RX, Chen YW, Liu C, Fan JG. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol. 2017;32:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Wu Z, He J, Zhang Z, Li J, Zou H, Tan X, Wang Y, Yao Y, Xiong W. Propionic Acid Driven by the Lactobacillus johnsonii Culture Supernatant Alleviates Colitis by Inhibiting M1 Macrophage Polarization by Modulating the MAPK Pathway in Mice. J Agric Food Chem. 2023;71:14951-14966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 56. | Han Y, Ling Q, Wu L, Wang X, Wang Z, Chen J, Zheng Z, Zhou Z, Jia L, Li L, Wang B. Akkermansia muciniphila inhibits nonalcoholic steatohepatitis by orchestrating TLR2-activated γδT17 cell and macrophage polarization. Gut Microbes. 2023;15:2221485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 57. | Hwang S, Yun H, Moon S, Cho YE, Gao B. Role of Neutrophils in the Pathogenesis of Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne). 2021;12:751802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | Xia Y, Wang Y, Xiong Q, He J, Wang H, Islam M, Zhou X, Kim A, Zhang H, Huang H, Tsung A. Neutrophil extracellular traps promote MASH fibrosis by metabolic reprogramming of HSC. Hepatology. 2025;81:947-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 59. | Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 60. | Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition. 2010;26:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 61. | Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 2011;6:e21205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 62. | Zheng S, Yang W, Yao D, Tang S, Hou J, Chang X. A comparative study on roles of natural killer T cells in two diet-induced non-alcoholic steatohepatitis-related fibrosis in mice. Ann Med. 2022;54:2233-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Hua J, Ma X, Webb T, Potter JJ, Oelke M, Li Z. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J Lipid Res. 2010;51:1696-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Mandaliya DK, Patel S, Seshadri S. The Combinatorial Effect of Acetate and Propionate on High-Fat Diet Induced Diabetic Inflammation or Metaflammation and T Cell Polarization. Inflammation. 2021;44:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, Heinze P, Kaisler J, Nageswaran V, Aigner A, Ceglarek U, Cineus R, Hegazy AN, van der Vorst EPC, Döring Y, Strauch CM, Nemet I, Tremaroli V, Dwibedi C, Kränkel N, Leistner DM, Heimesaat MM, Bereswill S, Rauch G, Seeland U, Soehnlein O, Müller DN, Gold R, Bäckhed F, Hazen SL, Haghikia A, Landmesser U. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. 2022;43:518-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (2)] |
| 66. | Shashni B, Tajika Y, Ikeda Y, Nishikawa Y, Nagasaki Y. Self-assembling polymer-based short chain fatty acid prodrugs ameliorate non-alcoholic steatohepatitis and liver fibrosis. Biomaterials. 2023;295:122047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 67. | Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol. 2013;379:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 68. | Dewidar B, Mastrototaro L, Englisch C, Ress C, Granata C, Rohbeck E, Pesta D, Heilmann G, Wolkersdorfer M, Esposito I, Reina Do Fundo M, Zivehe F, Yavas A, Roden M. Alterations of hepatic energy metabolism in murine models of obesity, diabetes and fatty liver diseases. EBioMedicine. 2023;94:104714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 69. | Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017;66:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1489] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 70. | Lee WH, Kim SG. AMPK-Dependent Metabolic Regulation by PPAR Agonists. PPAR Res. 2010;2010:549101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Mottillo EP, Desjardins EM, Crane JD, Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A, Sakamoto K, Scheele C, Kemp BE, Hawke TJ, Ortega J, Granneman JG, Steinberg GR. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016;24:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 72. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 3043] [Article Influence: 338.1] [Reference Citation Analysis (0)] |
| 73. | Zhao ZH, Wang ZX, Zhou D, Han Y, Ma F, Hu Z, Xin FZ, Liu XL, Ren TY, Zhang F, Xue Y, Cui A, Liu Z, Bai J, Liu Y, Cai G, Su W, Dai X, Shen F, Pan Q, Li Y, Fan JG. Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene. Cell Mol Gastroenterol Hepatol. 2021;12:857-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 74. | Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. 2023;5:735-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 150] [Reference Citation Analysis (0)] |
| 75. | Chiappini F, Coilly A, Kadar H, Gual P, Tran A, Desterke C, Samuel D, Duclos-Vallée JC, Touboul D, Bertrand-Michel J, Brunelle A, Guettier C, Le Naour F. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci Rep. 2017;7:46658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 76. | Weitkunat K, Stuhlmann C, Postel A, Rumberger S, Fankhänel M, Woting A, Petzke KJ, Gohlke S, Schulz TJ, Blaut M, Klaus S, Schumann S. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci Rep. 2017;7:6109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 77. | Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 78. | Steensels S, Qiao J, Zhang Y, Maner-Smith KM, Kika N, Holman CD, Corey KE, Bracken WC, Ortlund EA, Ersoy BA. Acyl-Coenzyme A Thioesterase 9 Traffics Mitochondrial Short-Chain Fatty Acids Toward De Novo Lipogenesis and Glucose Production in the Liver. Hepatology. 2020;72:857-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Park G, Jung S, Wellen KE, Jang C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp Mol Med. 2021;53:809-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Weitkunat K, Schumann S, Nickel D, Kappo KA, Petzke KJ, Kipp AP, Blaut M, Klaus S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol Nutr Food Res. 2016;60:2611-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 81. | Qiao S, Bao L, Wang K, Sun S, Liao M, Liu C, Zhou N, Ma K, Zhang Y, Chen Y, Liu SJ, Liu H. Activation of a Specific Gut Bacteroides-Folate-Liver Axis Benefits for the Alleviation of Nonalcoholic Hepatic Steatosis. Cell Rep. 2020;32:108005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 82. | Choi KJ, Yoon MY, Kim JE, Yoon SS. Gut commensal Kineothrix alysoides mitigates liver dysfunction by restoring lipid metabolism and gut microbial balance. Sci Rep. 2023;13:14668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 83. | Zhang F, Lo EKK, Chen J, Wang K, Felicianna, Ismaiah MJ, Leung HKM, Zhao D, Lee JC, El-Nezami H. Probiotic Mixture Ameliorates a Diet-Induced MASLD/MASH Murine Model through the Regulation of Hepatic Lipid Metabolism and the Gut Microbiome. J Agric Food Chem. 2024;72:8536-8549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 84. | Scoditti E, Sabatini S, Carli F, Gastaldelli A. Hepatic glucose metabolism in the steatotic liver. Nat Rev Gastroenterol Hepatol. 2024;21:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 85. | Sabatini S, Sen P, Carli F, Pezzica S, Rosso C, Lembo E, Verrastro O, Daly A, Govaere O, Cockell S, Hyötyläinen T, Mingrone G, Bugianesi E, Anstee QM, Orešič M, Gastaldelli A. Hepatic glucose production rises with the histological severity of metabolic dysfunction-associated steatohepatitis. Cell Rep Med. 2024;5:101820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 86. | Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 2021;58:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 87. | Zheng Y, Qu H, Xiong X, Wang Y, Liu X, Zhang L, Liao X, Liao Q, Sun Z, Ouyang Q, Yang G, Zhu Z, Xu J, Zheng H. Deficiency of Mitochondrial Glycerol 3-Phosphate Dehydrogenase Contributes to Hepatic Steatosis. Hepatology. 2019;70:84-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Yoshida H, Ishii M, Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys. 2019;672:108057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 89. | Huang DQ, Wong VWS, Rinella ME, Boursier J, Lazarus JV, Yki-Järvinen H, Loomba R. Metabolic dysfunction-associated steatotic liver disease in adults. Nat Rev Dis Primers. 2025;11:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 102] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 90. | Takeuchi T, Kubota T, Nakanishi Y, Tsugawa H, Suda W, Kwon AT, Yazaki J, Ikeda K, Nemoto S, Mochizuki Y, Kitami T, Yugi K, Mizuno Y, Yamamichi N, Yamazaki T, Takamoto I, Kubota N, Kadowaki T, Arner E, Carninci P, Ohara O, Arita M, Hattori M, Koyasu S, Ohno H. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. 2023;621:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 244] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 91. | Tian S, Lei Y, Zhao F, Che J, Wu Y, Lei P, Kang YE, Shan Y. Improving insulin resistance by sulforaphane via activating the Bacteroides and Lactobacillus SCFAs-GPR-GLP1 signal axis. Food Funct. 2024;15:8644-8660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 92. | Farías C, Cisternas C, Caicedo A, Mercado L, Valenzuela R, Calderón H, Espinosa A, Videla LA, Muñoz LA. High-fiber basil seed flour reduces insulin resistance and hepatic steatosis in high-fat diet mice. NPJ Sci Food. 2024;8:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 93. | Qian S, Wang X, Chen Y, Zai Q, He Y. Inflammation in Steatotic Liver Diseases: Pathogenesis and Therapeutic Targets. Semin Liver Dis. 2024;44:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Liu Q, Han M, Li M, Huang X, Feng R, Li W, Chen J, He H, Zheng W, Hu Z, Du S, Ye W. Shift in prevalence and systemic inflammation levels from NAFLD to MAFLD: a population-based cross-sectional study. Lipids Health Dis. 2023;22:185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 95. | Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, Wei Q, Zhao C, Lin C, Yang J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. 2022;7:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 96. | Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL, Luo M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front Immunol. 2022;13:880298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 97. | Baumann A, Jin CJ, Brandt A, Sellmann C, Nier A, Burkard M, Venturelli S, Bergheim I. Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients. 2020;12:951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 99. | Xu H, Wang X, Song S, Zhang L. Efficacy of sodium butyrate in improving nonalcoholic fatty liver disease: A meta-analysis of preclinical studies. Medicine (Baltimore). 2025;104:e42101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 100. | Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol Biochem. 2018;49:190-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (5)] |
| 101. | Yu L, Hong W, Lu S, Li Y, Guan Y, Weng X, Feng Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front Pharmacol. 2022;13:780496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 102. | Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016;6:24399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 103. | Säemann MD, Böhmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C, Stöckl J, Hörl WH, Zlabinger GJ. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 356] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 104. | Nga HT, Moon JS, Tian J, Lee HY, Kim SH, Lee YS, Jeon JH, Yi HS. Interleukin-10 Attenuates Liver Fibrosis Exacerbated by Thermoneutrality. Front Med (Lausanne). 2021;8:672658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Hu M, Alashkar Alhamwe B, Santner-Nanan B, Miethe S, Harb H, Renz H, Potaczek DP, Nanan RK. Short-Chain Fatty Acids Augment Differentiation and Function of Human Induced Regulatory T Cells. Int J Mol Sci. 2022;23:5740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 106. | Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, Yao S, Maynard CL, Singh N, Dann SM, Liu Z, Cong Y. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 772] [Article Influence: 128.7] [Reference Citation Analysis (1)] |
| 107. | Zai W, Chen W, Liu H, Ju D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines. 2021;9:1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Zhang P, Liu J, Lee A, Tsaur I, Ohira M, Duong V, Vo N, Watari K, Su H, Kim JY, Gu L, Zhu M, Shalapour S, Hosseini M, Bandyopadhyay G, Zeng S, Llorente C, Zhao HN, Lamichhane S, Mohan S, Dorrestein PC, Olefsky JM, Schnabl B, Soroosh P, Karin M. IL-22 resolves MASLD via enterocyte STAT3 restoration of diet-perturbed intestinal homeostasis. Cell Metab. 2024;36:2341-2354.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 109. | Sajiir H, Keshvari S, Wong KY, Borg DJ, Steyn FJ, Fercher C, Taylor K, Taylor B, Barnard RT, Müller A, Moniruzzaman M, Miller G, Wang R, Fotheringham A, Schreiber V, Sheng YH, Hancock JL, Loo D, Burr L, Huynh T, Lockett J, Ramm GA, Macdonald GA, Prins JB, McGuckin MA, Hasnain SZ. Liver and pancreatic-targeted interleukin-22 as a therapeutic for metabolic dysfunction-associated steatohepatitis. Nat Commun. 2024;15:4528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 110. | Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 111. | Jiang H, Mao T, Sun Z, Shi L, Han X, Zhang Y, Zhang X, Wang J, Hu J, Zhang L, Li J, Han H. Yinchen Linggui Zhugan decoction ameliorates high fat diet-induced nonalcoholic fatty liver disease by modulation of SIRT1/Nrf2 signaling pathway and gut microbiota. Front Microbiol. 2022;13:1001778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 112. | Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, Tie J, Hu D. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 359] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 113. | Parola M, Pinzani M. Liver fibrosis in NAFLD/NASH: from pathophysiology towards diagnostic and therapeutic strategies. Mol Aspects Med. 2024;95:101231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 127] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 114. | De Smet V, Eysackers N, Merens V, Kazemzadeh Dastjerd M, Halder G, Verhulst S, Mannaerts I, van Grunsven LA. Initiation of hepatic stellate cell activation extends into chronic liver disease. Cell Death Dis. 2021;12:1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 115. | Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 2760] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 116. | Cao X, Zolnikova O, Maslennikov R, Reshetova M, Poluektova E, Bogacheva A, Zharkova M, Ivashkin V. Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointest Disord. 2023;5:464-473. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 117. | Gart E, van Duyvenvoorde W, Toet K, Caspers MPM, Verschuren L, Nielsen MJ, Leeming DJ, Souto Lima E, Menke A, Hanemaaijer R, Keijer J, Salic K, Kleemann R, Morrison MC. Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells. Biomedicines. 2021;9:1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Qin D, Han C, Gao Y, Li H, Zhu L. Lactucin reverses liver fibrosis by inhibiting TGF-β1/STAT3 signaling pathway and regulating short-chain fatty acids metabolism. Sci Rep. 2024;14:19323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 119. | Li W, Deng M, Gong J, Zhang X, Ge S, Zhao L. Sodium Acetate Inhibit TGF-β1-Induced Activation of Hepatic Stellate Cells by Restoring AMPK or c-Jun Signaling. Front Nutr. 2021;8:729583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 120. | Shirazi F, Wang J, Wong RJ. Nonalcoholic Steatohepatitis Becomes the Leading Indication for Liver Transplant Registrants Among US Adults Born Between 1945 and 1965. J Clin Exp Hepatol. 2020;10:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 121. | Zhang C, Yang M. Current Options and Future Directions for NAFLD and NASH Treatment. Int J Mol Sci. 2021;22:7571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 122. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 532] [Article Influence: 177.3] [Reference Citation Analysis (2)] |
| 123. | Jin Y, Wang X, Chen K, Chen Y, Zhou L, Zeng Y, Zhou Y, Pan Z, Wang D, Li Z, Liang Y, Ling W, Li D. Silymarin decreases liver stiffness associated with gut microbiota in patients with metabolic dysfunction-associated steatotic liver disease: a randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2024;23:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 124. | Koutoukidis DA, Yen S, Gomez Castro P, Misheva M, Jebb SA, Aveyard P, Tomlinson JW, Mozes FE, Cobbold JF, Johnson JS, Marchesi JR. Changes in intestinal permeability and gut microbiota following diet-induced weight loss in patients with metabolic dysfunction-associated steatohepatitis and liver fibrosis. Gut Microbes. 2024;16:2392864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 125. | Chen D, Chen C, Qu H, Ren C, Yan X, Huang Y, Guan C, Zhang C, Li Q, Gu R. Screening of Lactobacillus strains that enhance SCFA uptake in intestinal epithelial cells. Eur Food Res Technol. 2021;247:1049-1060. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 127. | Ghosh S, Zhao X, Alim M, Brudno M, Bhat M. Artificial intelligence applied to 'omics data in liver disease: towards a personalised approach for diagnosis, prognosis and treatment. Gut. 2025;74:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 128. | Cao S, Budina E, Raczy MM, Solanki A, Nguyen M, Beckman TN, Reda JW, Hultgren K, Ang PS, Slezak AJ, Hesser LA, Alpar AT, Refvik KC, Shores LS, Pillai I, Wallace RP, Dhar A, Watkins EA, Hubbell JA. A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice. Nat Biomed Eng. 2024;8:611-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 129. | Smith M, Lelah M, Goggans M, Tunio S, Naqib A, Burton-Freeman B, Edirisinghe I. Investigation of the tolerability and potential health benefits of a novel butyrate generating supplement in a pilot human study. Nutr Healthy Aging. 2024;9:133-144. [DOI] [Full Text] |
| 130. | Li Y, Wang X, Zhang J, Zhang S, Jiao J. Applications of artificial intelligence (AI) in researches on non-alcoholic fatty liver disease(NAFLD) : A systematic review. Rev Endocr Metab Disord. 2022;23:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 131. | Takahashi Y, Dungubat E, Kusano H, Fukusato T. Artificial intelligence and deep learning: New tools for histopathological diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Comput Struct Biotechnol J. 2023;21:2495-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 132. | Pugliese N, Bertazzoni A, Hassan C, Schattenberg JM, Aghemo A. Revolutionizing MASLD: How Artificial Intelligence Is Shaping the Future of Liver Care. Cancers (Basel). 2025;17:722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 133. | Akbary K, Noureddin M, Yayun R, Tai D, Boudes P. Development of AI Based Fibrosis Detection Algorithm by SHG/TPEF Microscopy for Fully Quantified Liver Fibrosis Assessment in MASH. Liver Int. 2025;45:e70258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 134. | Neuschwander-Tetri BA, Akbary K, Carpenter DH, Noureddin M, Alkhouri N. The emerging role of second harmonic generation/two photon excitation for precision digital analysis of liver fibrosis in MASH clinical trials. J Hepatol. 2025;83:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 135. | Njei B, Osta E, Njei N, Al-Ajlouni YA, Lim JK. An explainable machine learning model for prediction of high-risk nonalcoholic steatohepatitis. Sci Rep. 2024;14:8589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 136. | Jiménez-González C, Alonso-Peña M, Argos Vélez P, Crespo J, Iruzubieta P. Unraveling MASLD: The Role of Gut Microbiota, Dietary Modulation, and AI-Driven Lifestyle Interventions. Nutrients. 2025;17:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 137. | Pokushalov E, Ponomarenko A, Shrainer E, Kudlay D, Miller R. Biomarker-Guided Dietary Supplementation: A Narrative Review of Precision in Personalized Nutrition. Nutrients. 2024;16:4033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 138. | Khurana A, Hartmann P. Gut microbiome-specific nanoparticle-based therapeutics for liver diseases. World J Gastroenterol. 2025;31:109105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/