Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.112675
Revised: September 7, 2025
Accepted: October 27, 2025
Published online: November 27, 2025
Processing time: 117 Days and 3.4 Hours
Hepatocellular carcinoma (HCC) is imposing a growing global health burden, with China accounting for nearly half of incident cases and mortality worldwide. Early screening is critical to improving survival in high-burden regions. However, the global standardized screening rate for high-risk populations is less than 24%, and HCC screening currently faces severe challenges. We synthesize recent ad
Core Tip: Hepatocellular carcinoma imposes a significant disease burden globally, particularly in China, where early screening faces challenges of low screening rates and insufficient sensitivity of traditional methods. This review focuses on the potential of innovative strategies, including optimized combinations of serum biomarkers, advanced imaging techniques, and liquid biopsy, to enhance early diagnosis rates. We emphasize the need for multidisciplinary collaboration and risk stratification management to im
- Citation: Liu ZH, Wang WJ, Dang SS. Early screening for liver cancer must be performed. World J Hepatol 2025; 17(11): 112675
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/112675.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.112675
Primary liver cancer, especially hepatocellular carcinoma (HCC), is one of the leading causes of cancer-related deaths worldwide, with a regionally uneven disease burden. In 2022, more than 860000 new cases of liver cancer were diagnosed globally[1], with China accounting for 45.3% of these cases and 39.1% of global deaths[2]. The total age-standardized death rate in China is significantly higher than the worldwide age-standardized death rate[3]. Epidemiological models predict that without effective intervention, the number of new liver cancer cases worldwide will rise to 1.4 million annually by 2040, with 1.3 million deaths[4].
The prognosis of liver cancer is highly dependent on the stage at diagnosis. Early-stage lesions can be treated with curative therapy to extend survival. Over 60% of liver cancer patients diagnosed in Japan have early-stage HCC, and the 5-year overall survival rate for early-stage HCC patients who undergo curative treatment can be as high as 43%[5], which is significantly better than in China. The survival disparity between China and Japan directly reflects the gap in early diagnosis rates. Japan’s successful experience in early liver cancer screening offers valuable insights for adoption and adaptation[6]. HCC screening is crucial for early diagnosis, improving curative rates, extending patient survival, and enhancing long-term prognosis[7]. However, current liver cancer screening strategies face challenges in clinical application, such as low implementation rates and poor sensitivity in detecting early-stage HCC. Additionally, the rapid rise in metabolic-associated fatty liver disease has posed new challenges for traditional HCC screening methods[8-10]. To address these challenges, this paper comprehensively reviews the main technological advancements and challenges in early HCC screening, aiming to promote further development of early HCC screening efforts and improve survival rates and quality of life for HCC patients.
In the early screening of liver cancer, serological marker testing has been indispensable in clinical practice due to its non-invasiveness, convenience, and low cost. Traditional markers such as alpha-fetoprotein (AFP) have played an important role in liver cancer screening, but other potential novel markers have been discovered and applied to clinical practice as the molecular mechanisms of HCC have been studied more deeply[11].
As a conventional HCC screening marker, AFP maintains high diagnostic utility[12]. Persistent AFP elevation (≥ 10% over 3-6 months) or levels ≥ 20 ng/mL in benign liver disease significantly elevate 6-month HCC risk[13]. Some researchers have developed a biosensor based on droplet evaporation, which exhibits high selectivity, stability, and reproducibility in detecting AFP, demonstrating great potential for clinical application in early screening[14]. However, as the proportion of AFP-negative liver cancers increases, studies have found that the sensitivity of AFP detection for early-stage HCC is only 56.3%, limiting its clinical application[15]. Therefore, exploring more sensitive and specific biomarkers, and their combinations, for liver cancer screening is particularly important.
This vitamin K-deficient protein is a promising biomarker for HCC. Increasing research suggests that it outperforms traditional AFP[16-18]. Asia-Pacific consensus guidelines endorse des-gamma-carboxy prothrombin (DCP) for AFP-negative HCC detection, particularly when combined with AFP[19]. Despite superior accuracy, DCP requires complementary biomarkers for optimal screening efficacy.
Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) is a more liver cancer-specific biomarker, but it has relatively low sensitivity[20]. A phase III biomarker study showed that the sensitivity of AFP-L3% was only 35.7% in the 12 months prior to HCC diagnosis[21]. To improve the detection accuracy and clinical application value of AFP-L3%, researchers have developed an electrochemical aptamer sensor, which can precisely detect AFP-L3% and accurately diagnose liver cancer at an early stage[22].
The well-documented limitations of conventional markers in achieving high sensitivity without compromising specificity remains a significant diagnostic challenge. AFP remains the most accessible and inexpensive test, yet its utility is curtailed by suboptimal sensitivity, particularly in early-stage and non-viral HCC. DCP demonstrates superior sensitivity to AFP in many studies, though its performance can vary across etiologies. AFP-L3% offers high specificity for HCC but is hampered by low absolute sensitivity, limiting its standalone use. The insufficiency of existing biomarkers has driven extensive research into other novel biomarkers. Golgi protein 73 (GP73) participates in liver pathophysiology and may drive hepatocarcinogenesis via tumor microenvironment interactions[23]. Clinically, combining AFP, DCP, and GP73 enhances diagnostic accuracy for hepatitis B virus (HBV)-related HCC, correlating with histopathological findings[24]. Recently, researchers have developed a competitive electrochemical sensor for detecting GP73 with a detection limit of 0.15 pg/mL, demonstrating significant potential for clinical applications[25]. Due to its unique cell membrane loca
Abdominal ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) are commonly used screening methods for early detection of liver cancer[31]. Abdominal US is recommended as the standard monitoring method for liver cancer, while CT and MRI are often used as supplementary methods for early screening. Traditional US, with its non-invasive nature, widespread availability, and low cost, is recommended by mainstream international guidelines as the standard monitoring tool for liver cancer in high-risk populations[32,33]. However, due to factors such as operator experience variability, abdominal gas in patients, and obesity, the detection rate of US is low for liver cancer. As the global etiological spectrum of HCC rapidly shifts toward non-viral fatty liver disease, the limitations of US for liver cancer screening are further exacerbated. Approximately 20% of cirrhosis patients, particularly those with obesity-related, alcohol-associated, or non-alcoholic steatotic liver disease, demonstrate reduced ultrasonographic detectability of HCC nodules[34]. Even with adjunct AFP testing, early HCC detection sensitivity remains suboptimal (74.1%)[15]. Contrast-enhanced US (CEUS) addresses these constraints through dynamic visualization of tumor microvascular perfusion following microbubble contrast administration, achieving superior diagnostic accuracy[35]. A meta-analysis of 23 studies confirmed that CEUS has a sensitivity of 77.8% and specificity of 93.8% for diagnosing liver cancer[36], making it an important tool for resolving uncertainties when small liver nodules are detected by MRI but cannot be definitively diagnosed[37].
While multiphase CT allows hemodynamic assessment of HCC, radiation concerns preclude routine screening use. Studies have shown that when the liver cancer risk index is ≥ 2.33, low-dose CT has enhanced performance vs US[38]. While conventional MRI offers high HCC detection rates, its cost and time limitations hinder routine surveillance. Abbreviated MRI (AMRI), particularly non-contrast AMRI, addresses this by reducing scan time to < 10 minutes and eliminating contrast-related risks[39,40]. Studies demonstrate non-contrast-AMRI’s superior annual sensitivity over semi-annual US, while gadoxetic acid-enhanced MRI (EOB-MRI) provides cost-effective early detection in metabolic-related cirrhosis patients[41]. Overall, MRI outperforms US in HCC surveillance in terms of earlier staging and reduced false positives[42]. Collectively, these imaging modalities present a trade-off between accessibility, cost, and accuracy.
Several liver cancer risk assessment tools have been developed (Table 1). By implementing risk stratification for patients with chronic liver disease, these tools can accurately distinguish between low-, medium-, and high-risk populations for liver cancer, thereby enabling the development of tailored screening protocols for different risk levels. This approach quantifies the probability of HCC occurrence in patients over the next few years. A Chinese research team has proposed the first liver cancer risk assessment tool that spans multiple etiologies and ethnicities, and the aMAP score has gained widespread international recognition[43].
| Model | Region | Derivation cohort | Variables included | Risk stratification cutoffs | Ref. |
| aMAP | Global | Treated chronic liver disease patients | Age, sex, bilirubin, albumin, platelet count | Low: 0-50. Intermediate: 50-60. High: 60-100 | Fan et al[43], 2020 |
| GAG-HCC | Hong Kong, China | CHB patients | Age, sex, HBV DNA level, core promoter mutations, cirrhosis | Low: < 100. High: ≥ 100 | Yuen et al[72], 2009 |
| CU-HCC | Taiwan, China, South Korea, Hong Kong | Non-cirrhotic CHB patients | Age, sex, platelet count, ALT level, HBeAg status | Low: < 5. Intermediate: | Kim et al[73], 2024 |
| REACH-B | Taiwan, China | Untreated non-cirrhotic CHB patients | Age, albumin, bilirubin, HBV DNA, cirrhosis | Low: ≤ 5. Intermediate: | Wong et al[74], 2010 |
| LSM-HCC | Hong Kong, China | CHB patients | LSM, age, serum albumin, HBV DNA | Low: < 11. High: ≥ 11 | Wong et al[75], 2014 |
| mREACH-B | South Korea | CHB patients | Age, sex, HBeAg, LSM, ALT | Low: < 10. High: ≥ 10 | Jung et al[76], 2015 |
| HCC-RESCUE | South Korea | CHB patients on oral antivirals | Age, sex, cirrhosis | Low: < 5%. Intermediate: 5%-20%. High: ≥ 20% | Sohn et al[77], 2017 |
| AGED | China | HBsAg-positive CHB patients | Age, sex, HBeAg status, HBV DNA level | Low: 0-4. Intermediate: | Fan et al[78], 2019 |
| CAMD | Hong Kong, Taiwan | CHB patients on ETV/TDF | Age, sex, cirrhosis, diabetes | Low: 0-7. Intermediate: | Hsu et al[79], 2018 |
| PAGE-B | Europe | Caucasian CHB patients on antivirals | Age, sex, platelet count | Low: ≤ 9. Intermediate: 10-17. High: ≥ 18 | Papatheodoridis et al[80], 2016 |
| mPAGE-B | South Korea | Asian CHB patients on antivirals (≥ 12 months) | Age, sex, platelet count, albumin | Low: 0-8. Intermediate: | Kim et al[81], 2018 |
| REAL-B | Asia-Pacific, United States | CHB patients on antivirals | Sex, age, alcohol use, diabetes, cirrhosis, platelet count, AFP | Low: 0-3. Intermediate: | Yang et al[82], 2020 |
| RWS-HCC | Singapore | CHB patients | Age, sex, cirrhosis, AFP | Low: < 4.5. Significant: | Poh et al[83], 2016 |
| THRI | Canada | Cirrhosis patients | Age, sex, etiology, platelet count | Low: < 120. Intermediate: 120-240. High: > 240 | Sharma et al[84], 2017 |
| GES | Egypt | HCV cirrhosis or advanced fibrosis patients | Age, sex, albumin, AFP, pretreatment fibrosis stage | Low: ≤ 6. Intermediate: | Shiha et al[85], 2020 |
| George et al | United States | NAFLD or ALD cirrhosis patients | Age, sex, BMI, diabetes, platelet count, albumin, AST/ALT ratio | Low: 0%-1%. Intermediate: > 1%-3%. High: > 3% | Ioannou et al[86], 2019 |
| PAaM | United States | Cirrhosis (mixed etiology) | Prognostic liver secretome signature + AFP, age, male sex, ALBI, platelet count | Low: < 4.318. High: | Fujiwara et al[87], 2025 |
Risk stratification optimizes the selection of screening populations and timing but cannot predict the diagnosis of HCC. To overcome the limitations of single serum markers, researchers have developed predictive diagnostic models based on serum markers combined with clinical variables (Table 2), achieving a closed-loop management system from “risk warning” to “lesion identification”. Taking the GALAD model, which includes five variables (AFP, AFP-L3%, DCP, age, and gender), as an example, this model aims to significantly enhance the early diagnostic efficacy of HCC through com
| Model name | Components/equation | Ref. |
| GALAD | -10.08 + 0.09 × age + 1.67 × gender + 0.04 × AFP-L3% + 2.34 × log10 AFP + 1.33 × log10 DCP | Johnson et al[88], 2014 |
| GAAP | 9.134 + 0.892 × gender + 0.073 × age + 1.057 × log10 AFP + 1.015 × log10 DCP | Liu et al[89], 2020 |
| AALP | 7.245 + 0.056 × age + 0.431 × log10 AFP + 3.112 × AFP-L3 + 1.162 × log10 PIVKA-II | Ren et al[90], 2023 |
| ASAP | -7.577 + 0.047 × age - 0.576 × gender + 0.422 × lnAFP + 1.105 × lnDCP | Sun et al[91], 2025 |
| GAAD | Gender, age, PIVKA-II, AFP | Piratvisuth et al[92], 2023 |
| GAADPB | 0.176 + 0.162 × gender + 0.002 × age + 0.178 × log10 AFP + 0.164 × log10 DCP - 0.007 × TP - 0.002 × TB | Chen et al[93], 2023 |
| BALAD | Bilirubin, albumin, AFP-L3, AFP, DCP | Toyoda et al[94], 2006 |
| BALAD-2 | 0.02 × (AFP-2.57) + 0.012 × (AFP-L3 - 14.19) + 0.19 × (lnDCP - 1.93) + 0.17 × (Bilirubin1/2 - 4.5) - 0.09 × (albumin - 35.11) | Fox et al[95], 2014 |
| Doylestown | 1/{1 + exp[-(-10.307 + 0.097 × age + 1.645 × gender + 2.315 × log10 AFP + 0.011 × ALP - 0.008 × ALT)]} | Wang et al[96], 2016 |
| Doylestown Plus | Age, logAFP, PEG-precipitated IgG, fucosylated kininogen | Singal et al[97], 2022 |
| HES V1.0 | Age, current AFP level, AFP change rate, ALT, platelets | Tayob et al[98], 2021 |
| HES V2.0 | Age, current AFP level, AFP change rate, ALT, platelets, AFP-L3, DCP | El-Serag et al[99], 2025 |
With the advancement of liquid biopsy technology, novel biomarkers such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), microRNA (miRNA), and long non-coding RNA (lncRNA) have provided new options for screening and early diagnosis of liver cancer patients[45].
ctDNA primarily originates from the apoptosis and necrosis of tumor cells. Luo et al[46] developed the DELFI model based on changes in cfDNA fragments, and the model demonstrated good performance in HCC screening in healthy populations and early diagnosis of HCC in high-risk populations. A research team at the University of Oxford developed a multimodal ctDNA detection method capable of simultaneously analyzing genomic and methylomic data[47]. However, the high detection cost limits its clinical application.
CTCs are tumor cells that detach from the primary or metastatic site and enter the peripheral circulatory system. Currently, various CTC detection platforms based on immunological affinity and biophysical characteristics have been developed, significantly improving the efficiency of CTC identification and detection[48]. However, most studies are still limited to small-scale, single-center case-control studies, and there are significant demographic differences, making it difficult to validate and generalize the research results[49].
miRNAs are endogenous non-coding RNAs with gene regulatory functions. In hepatitis B-related HCC, serum miRNome profiling has identified 30 upregulated and 61 downregulated species[50]. Li et al[51] used next-generation sequencing to isolate fucosylated extracellular vesicles from serum and identified five miRNAs as biomarkers for HCC detection. Nevertheless, miRNA biomarker translation faces challenges due to molecular heterogeneity and individual sample limitations, necessitating further optimization of combinatorial detection strategies.
H19 is the earliest imprinted gene identified among lncRNAs[52]. It exerts oncogenic effects through multiple signaling pathways, including regulation of epigenetic modifications and the H19/miR-675 axis, and may serve as a potential biomarker for liver cancer. Lnc-MyD88 shows diagnostic promise in HBV-HCC, achieving 80.95% sensitivity for AFP-negative cases[53]. Research on lncRNAs remains fragmented and lacks an established framework, with many gaps yet to be filled in this field of study.
Liquid biopsy technologies show promise for clinical implementation potential but lack randomized trial validation for early intervention guidance. Current protocols recommend shortened follow-up intervals for positive cases. While broader adoption requires further evidence, these high-sensitivity/specificity assays represent transformative tools for HCC diagnosis and management.
Artificial intelligence (AI) has demonstrated significant potential in the early screening of liver cancer[54]. Machine learning (ML) is a computational model inspired by the biological structure and function of the human brain. In deep learning technology, convolutional neural networks have proven to be particularly efficient in processing visual data[55]. Radiological features from CT and MRI scans, when combined with ML models, can aid in the differential diagnosis of HCC[56]. A meta-analysis indicated that models based on convolutional neural networks and those combining CEUS with AI exhibit good sensitivity[57]. Cheng et al[58] developed a model using ML methods based on personalized biological pathway analysis and regularized regression, achieving high-precision diagnosis of HCC. Wang et al[59] proposed a bioinformatics strategy named TopMarker, which calculates and screens biomarkers for HCC based on differential network topological parameters. Additionally, AI systems outperform clinicians in interpreting imaging data, minimizing diagnostic discrepancies. As technology continues to advance, the application of AI in liver cancer screening will provide more precise and efficient solutions for clinical diagnosis.
Although tissue biopsy is not a first-line routine method for early screening of liver cancer, it can provide clear histological diagnosis and molecular typing evidence in patients with atypical imaging findings or the absence of cirrhosis. The American Association for the Study of Liver Diseases guidelines support the use of confirmatory liver biopsy in HCC, particularly for patients with liver nodules without cirrhosis or hepatitis B infection[60]. The advantage of liver biopsy lies in its ability to directly obtain tumor tissue structure, enabling precise pathological subtype classification and molecular target identification[61]. In recent years, tissue biopsy has been deeply integrated with molecular pathology. Based on multi-omics analysis, molecular markers for precancerous lesions and early-stage liver cancer can be identified, significantly improving diagnostic sensitivity and prognostic prediction capabilities[62].
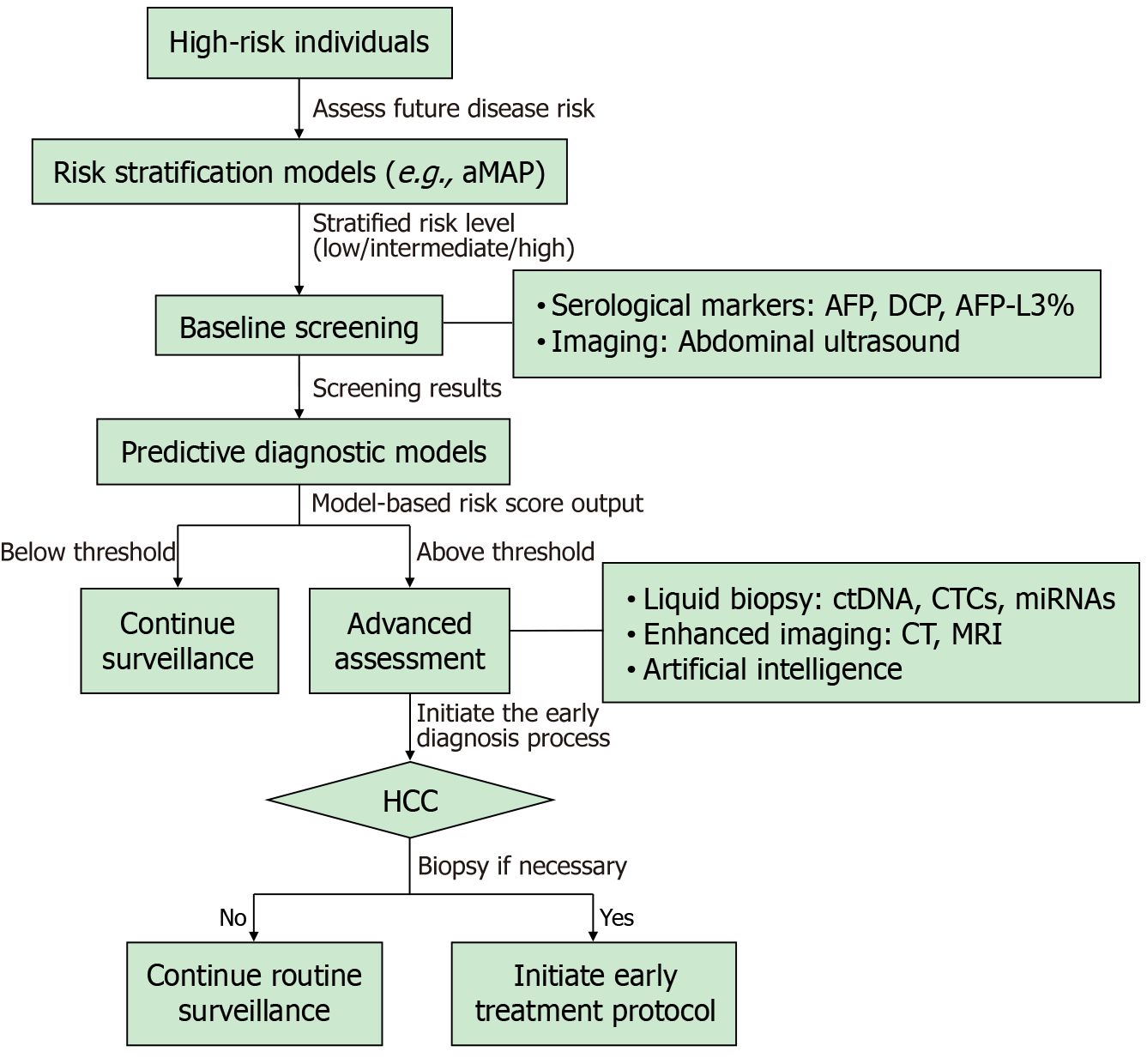
Figure 1 outlines a proposed clinical pathway for screening and diagnosis in high-risk populations. However, despite the availability of comprehensive guidelines, various risk scoring systems, and screening technologies, the current state of liver cancer screening is not encouraging. Globally, less than a quarter (24%) of cirrhosis patients undergo HCC moni
The low rate of liver cancer screening can be attributed to several factors. From the patient’s perspective, many residents lack sufficient health awareness and medical knowledge, leading to an insufficient understanding of the importance of liver cancer screening. Additionally, individuals who require follow-up after an abnormal initial screening often overlook the importance of regular follow-up visits. In a cohort study of cirrhosis patients, only 37.2% underwent routine outpatient follow-up in the year prior to liver cancer diagnosis. Even among those who did receive follow-up, 32.6% failed to undergo regular screening[69]. A study in Argentina involving 301 high-risk HCC patients found that 25% of patients were unaware of their chronic liver disease history at the time of liver cancer diagnosis[70]. Additionally, factors such as misunderstandings about HCC screening, time and financial costs, and difficulties in scheduling US examinations also affect screening compliance. Improving the screening rate for liver cancer requires not only the cooperation of patients, but also the efforts of multiple sectors, including medical institutions at all levels, the govern
Liver cancer screening currently faces multiple challenges, including insufficient screening coverage, limited sensitivity in detecting early-stage lesions, and heterogeneous risk profiles across populations. Future efforts should actively promote screening strategies that integrate stratified, combined, and intelligent approaches. We recommend adopting risk-scoring model-based stratified management in clinical practice and promoting multimodal screening strategies combining imaging and serological markers to enhance the detection rate of early-stage liver cancer. Concurrently, governments and healthcare institutions must strengthen health education for the public and primary care providers to comprehensively improve screening adherence. Achieving early liver cancer screening and enhancing patient long-term survival ultimately requires collaborative and sustained efforts from medical institutions, research teams, public health systems, and societal forces.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6098] [Article Influence: 3049.0] [Reference Citation Analysis (4)] |
| 2. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 249] [Reference Citation Analysis (0)] |
| 3. | Cao M, Xia C, Cao M, Yang F, Yan X, He S, Zhang S, Teng Y, Li Q, Tan N, Wang J, Chen W. Attributable liver cancer deaths and disability-adjusted life years in China and worldwide: profiles and changing trends. Cancer Biol Med. 2024;21:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1419] [Article Influence: 354.8] [Reference Citation Analysis (1)] |
| 5. | Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 525] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 7. | Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology. 2023;78:319-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Rich NE. Changing Epidemiology of Hepatocellular Carcinoma Within the United States and Worldwide. Surg Oncol Clin N Am. 2024;33:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Narro GEC, Díaz LA, Ortega EK, Garín MFB, Reyes EC, Delfin PSM, Arab JP, Bataller R. Alcohol-related liver disease: A global perspective. Ann Hepatol. 2024;29:101499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Danpanichkul P, Duangsonk K, Diaz LA, Arab JP, Liangpunsakul S, Wijarnpreecha K. Editorial: Sounding the alarm-The rising global burden of adolescent and young adult alcohol-related liver disease. Author's reply. Aliment Pharmacol Ther. 2024;60:521-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Shahini E, Pasculli G, Solimando AG, Tiribelli C, Cozzolongo R, Giannelli G. Updating the Clinical Application of Blood Biomarkers and Their Algorithms in the Diagnosis and Surveillance of Hepatocellular Carcinoma: A Critical Review. Int J Mol Sci. 2023;24:4286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Yeo YH, Lee YT, Tseng HR, Zhu Y, You S, Agopian VG, Yang JD. Alpha-fetoprotein: Past, present, and future. Hepatol Commun. 2024;8:e0422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 13. | Su TH, Chang SH, Chen CL, Liao SH, Tseng TC, Hsu SJ, Hong CM, Liu CH, Yang HC, Liu CJ, Chen PJ, Kao JH. Serial increase and high alpha-fetoprotein levels predict the development of hepatocellular carcinoma in 6 months. Hepatol Res. 2023;53:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Jia Y, Zhao H, Huang S, Yin F, Wang W, Hu Q, Wang Y, Feng B. Rapid and portable detection of hepatocellular carcinoma marker alpha-fetoprotein using a droplet evaporation-based biosensor. Talanta. 2025;294:128189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Singal AG, Haaland B, Parikh ND, Ozbay AB, Kirshner C, Chakankar S, Porter K, Chhatwal J, Ayer T. Comparison of a multitarget blood test to ultrasound and alpha-fetoprotein for hepatocellular carcinoma surveillance: Results of a network meta-analysis. Hepatol Commun. 2022;6:2925-2936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Yang X, Shao Y, Zhao H, Jiang J, Huang P, Lu Y, Xuan Z. Comparison of diagnostic performance of AFP, DCP and two diagnostic models in hepatocellular carcinoma: a retrospective study. Ann Hepatol. 2023;28:101099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Kobeissy A, Merza N, Al-Hillan A, Boujemaa S, Ahmed Z, Nawras M, Albaaj M, Dahiya DS, Alastal Y, Hassan M. Protein Induced by Vitamin K Absence or Antagonist-II Versus Alpha-Fetoprotein in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review With Meta-Analysis. J Clin Med Res. 2023;15:343-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 18. | Zhou Z, Liu Q, Liu J, Li W, Cao S, Xu J, Chen J, Xu X, Chen C. Research progress of protein induced by vitamin K absence or antagonist II in liver transplantation for hepatocellular carcinoma. Heliyon. 2024;10:e30622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Kim DY, Toan BN, Tan CK, Hasan I, Setiawan L, Yu ML, Izumi N, Huyen NN, Chow PK, Mohamed R, Chan SL, Tanwandee T, Lee TY, Hai TTN, Yang T, Lee WC, Chan HLY. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29:277-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 20. | Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2021;100:e27673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. 2023;21:415-423.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Liu Q, Qin Y, Cao Y, Zhao J, Zhang K, Cao Y. Ordered Labeling-Facilitated Electrochemical Assay of Alpha-Fetoprotein-L3 Ratio for Diagnosing Hepatocellular Carcinoma. ACS Appl Mater Interfaces. 2023;15:6411-6419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wang Q, Cui H, Zhu Y, Zhu Y, He C. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of hepatocellular carcinoma. Front Med (Lausanne). 2025;12:1571761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Rui H, Yueqin N, Wei W, Bangtao L, Li X. Combining AFP, PIVKA-II, and GP73 has diagnostic utility for hepatitis B-associated hepatocellular carcinoma and is consistent with liver pathology results. Sci Rep. 2025;15:14869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Li G, Wang B, Li S, Li X, Yan R, Tan X, Liang J, Zhou Z. Competitive electrochemical aptasensor for high sensitivity detection of liver cancer marker GP73 based on rGO-Fc-PANi nanocomposites. Bioelectrochemistry. 2024;160:108767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Couzinet A, Suzuki T, Nakatsura T. Progress and challenges in glypican-3 targeting for hepatocellular carcinoma therapy. Expert Opin Ther Targets. 2024;28:895-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Zhao S, Long M, Zhang X, Lei S, Dou W, Hu J, Du X, Liu L. The diagnostic value of the combination of Golgi protein 73, glypican-3 and alpha-fetoprotein in hepatocellular carcinoma: a diagnostic meta-analysis. Ann Transl Med. 2020;8:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Poot AJ, Lapa C, Weber WA, Lam MGEH, Eiber M, Dierks A, Bundschuh RA, Braat AJAT. [(68)Ga]Ga-RAYZ-8009: A Glypican-3-Targeted Diagnostic Radiopharmaceutical for Hepatocellular Carcinoma Molecular Imaging-A First-in-Human Case Series. J Nucl Med. 2024;65:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 29. | Liu B, Qian D. Hsp90α and cell death in cancers: a review. Discov Oncol. 2024;15:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Han Y, Zhang Y, Cui L, Li Z, Feng H, Zhang Y, Sun D, Ren L. Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: in patients with different clinicopathologic characteristics. World J Surg Oncol. 2021;19:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Candita G, Rossi S, Cwiklinska K, Fanni SC, Cioni D, Lencioni R, Neri E. Imaging Diagnosis of Hepatocellular Carcinoma: A State-of-the-Art Review. Diagnostics (Basel). 2023;13:625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 32. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82:315-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 350] [Article Influence: 350.0] [Reference Citation Analysis (6)] |
| 33. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 1171] [Article Influence: 390.3] [Reference Citation Analysis (23)] |
| 34. | Schoenberger H, Chong N, Fetzer DT, Rich NE, Yokoo T, Khatri G, Olivares J, Parikh ND, Yopp AC, Marrero JA, Singal AG. Dynamic Changes in Ultrasound Quality for Hepatocellular Carcinoma Screening in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1561-1569.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 35. | Eisenbrey JR, Gabriel H, Savsani E, Lyshchik A. Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom Radiol (NY). 2021;46:3579-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Fraquelli M, Nadarevic T, Colli A, Manzotti C, Giljaca V, Miletic D, Štimac D, Casazza G. Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev. 2022;9:CD013483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Saborido B, Darnell A, Forner A, García-Criado Á, Díaz A, Ayuso C, Vilana R, Bruix J, Reig M, Rimola J. Diagnostic performance of contrast-enhanced US in small liver nodules not conclusively characterized after MRI in cirrhotic patients. Eur Radiol. 2025;35:5771-5780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, Kim ST, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH. A Comparison of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance in a Group at High Risk of HCC Development. Liver Cancer. 2020;9:503-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Ronot M. Improving HCC surveillance with abbreviated MRI: A call to integrate and innovate? J Hepatol. 2024;81:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Vietti Violi N, Taouli B. Abbreviated MRI for HCC surveillance: is it ready for clinical use? Eur Radiol. 2020;30:4147-4149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Kim DH, Yoon JH, Choi MH, Lee CH, Kang TW, Kim HA, Ku YM, Lee JM, Kim SH, Kim KA, Lee SL, Choi JI. Comparison of non-contrast abbreviated MRI and ultrasound as surveillance modalities for HCC. J Hepatol. 2024;81:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Kowada A. Risk-stratified hepatocellular carcinoma screening according to the degree of obesity and progression to cirrhosis for diabetic patients with metabolic dysfunction-associated steatotic liver disease (MASLD) in Japan: a cost-effectiveness study. BMJ Open. 2024;14:e080549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, Mo S, Sypsa V, Guha IN, Kumada T, Niu J, Dalekos G, Yasuda S, Barnes E, Lian J, Suri V, Idilman R, Barclay ST, Dou X, Berg T, Hayes PC, Flaherty JF, Zhou Y, Zhang Z, Buti M, Hutchinson SJ, Guo Y, Calleja JL, Lin L, Zhao L, Chen Y, Janssen HLA, Zhu C, Shi L, Tang X, Gaggar A, Wei L, Jia J, Irving WL, Johnson PJ, Lampertico P, Hou J. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 44. | Nartey YA, Yang JD, Zemla TJ, Ayawin J, Asibey SO, El-Kassas M, Bampoh SA, Duah A, Agyei-Nkansah A, Awuku YA, Afihene MY, Yamada H, Yin J, Plymoth A, Roberts LR. GALAD Score for the Diagnosis of Hepatocellular Carcinoma in Sub-Saharan Africa: A Validation Study in Ghanaian Patients. Cancer Res Commun. 2024;4:2653-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Mayerhoefer ME, Kienzle A, Woo S, Vargas HA. Update on Liquid Biopsy. Radiology. 2025;315:e241030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Luo B, Ma F, Liu H, Hu J, Rao L, Liu C, Jiang Y, Kuangzeng S, Lin X, Wang C, Lei Y, Si Z, Chen G, Zhou N, Liang C, Jiang F, Liu F, Dai W, Liu W, Gao Y, Li Z, Li X, Zhou G, Li B, Zhang Z, Nian W, Luo L, Liu X. Cell-free DNA methylation markers for differential diagnosis of hepatocellular carcinoma. BMC Med. 2022;20:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Vavoulis DV, Cutts A, Thota N, Brown J, Sugar R, Rueda A, Ardalan A, Howard K, Matos Santo F, Sannasiddappa T, Miller B, Ash S, Liu Y, Song CX, Nicholson BD, Dreau H, Tregidgo C, Schuh A. Multimodal cell-free DNA whole-genome TAPS is sensitive and reveals specific cancer signals. Nat Commun. 2025;16:430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 48. | Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, Yang JD. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology. 2021;73:422-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 49. | Ring A, Nguyen-Sträuli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023;23:95-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 226] [Article Influence: 75.3] [Reference Citation Analysis (10)] |
| 50. | Sartorius K, Sartorius B, Winkler C, Chuturgoon A, Shen TW, Zhao Y, An P. Serum microRNA Profiles and Pathways in Hepatitis B-Associated Hepatocellular Carcinoma: A South African Study. Int J Mol Sci. 2024;25:975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Li B, Hao K, Li M, Wang A, Tang H, Xu L, Ma C, Du W, Sun L, Hou X, Jia T, Liu A, Gao Q, Zhao Z, Jin R, Yang R. Five miRNAs identified in fucosylated extracellular vesicles as non-invasive diagnostic signatures for hepatocellular carcinoma. Cell Rep Med. 2024;5:101716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 52. | Wang Y, Zeng J, Chen W, Fan J, Hylemon PB, Zhou H. Long Noncoding RNA H19: A Novel Oncogene in Liver Cancer. Noncoding RNA. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Wang Z, Gao P, Sun W, Rehman AU, Jiang J, Xu S, Xue C, Zhu C, Qin X. Long noncoding RNA MyD88 functions as a promising diagnostic biomarker in hepatocellular carcinoma. Front Endocrinol (Lausanne). 2023;14:938102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Chatzipanagiotou OP, Loukas C, Vailas M, Machairas N, Kykalos S, Charalampopoulos G, Filippiadis D, Felekouras E, Schizas D. Artificial intelligence in hepatocellular carcinoma diagnosis: a comprehensive review of current literature. J Gastroenterol Hepatol. 2024;39:1994-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 55. | Salehi MA, Harandi H, Mohammadi S, Shahrabi Farahani M, Shojaei S, Saleh RR. Diagnostic Performance of Artificial Intelligence in Detection of Hepatocellular Carcinoma: A Meta-analysis. J Imaging Inform Med. 2024;37:1297-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Ma Y, Gong Y, Qiu Q, Ma C, Yu S. Research on multi-model imaging machine learning for distinguishing early hepatocellular carcinoma. BMC Cancer. 2024;24:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 57. | Al-Obeidat F, Hafez W, Gador M, Ahmed N, Abdeljawad MM, Yadav A, Rashed A. Diagnostic performance of AI-based models versus physicians among patients with hepatocellular carcinoma: a systematic review and meta-analysis. Front Artif Intell. 2024;7:1398205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 58. | Cheng B, Zhou P, Chen Y. Machine-learning algorithms based on personalized pathways for a novel predictive model for the diagnosis of hepatocellular carcinoma. BMC Bioinformatics. 2022;23:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Wang Y, Wang T, Cao Y, Qiao X, Han X, Liu ZP. TopMarker: Computational screening biomarkers of hepatocellular carcinoma from transcriptome and interactome based on differential network topological parameters. Comput Biol Chem. 2024;112:108166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3145] [Article Influence: 393.1] [Reference Citation Analysis (3)] |
| 61. | Gopal P, Hu X, Robert ME, Zhang X. The evolving role of liver biopsy: Current applications and future prospects. Hepatol Commun. 2025;9:e0628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 62. | Lehrich BM, Zhang J, Monga SP, Dhanasekaran R. Battle of the biopsies: Role of tissue and liquid biopsy in hepatocellular carcinoma. J Hepatol. 2024;80:515-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 69] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 63. | Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (2)] |
| 64. | Nguyen MH, Roberts LR, Engel-Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr Med Res Opin. 2022;38:2163-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Yeo YH, Hwang J, Jeong D, Dang N, Kam LY, Henry L, Park H, Cheung R, Nguyen MH. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol. 2021;75:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Hwang SY, Danpanichkul P, Agopian V, Mehta N, Parikh ND, Abou-Alfa GK, Singal AG, Yang JD. Hepatocellular carcinoma: updates on epidemiology, surveillance, diagnosis and treatment. Clin Mol Hepatol. 2025;31:S228-S254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 104] [Article Influence: 104.0] [Reference Citation Analysis (1)] |
| 67. | Lim RY, Koh B, Ng CH, Kulkarni AV, Liu K, Wijarnpreecha K, Kim BK, Muthiah MD, Lee SW, Zheng MH, Kawaguchi T, Takahashi H, Huang DQ; Liver Cancer Research Network. Hepatocellular Carcinoma Surveillance and Survival in a Contemporary Asia-Pacific Cohort. JAMA Netw Open. 2025;8:e2520294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 68. | Fang K, Li S, Lin Y, Zhang Y, Wu J. Economic evaluation of hepatocellular carcinoma surveillance in chronic hepatitis B patients with virological remission. BMC Public Health. 2024;24:2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Marquardt P, Liu PH, Immergluck J, Olivares J, Arroyo A, Rich NE, Parikh ND, Yopp AC, Singal AG. Hepatocellular Carcinoma Screening Process Failures in Patients with Cirrhosis. Hepatol Commun. 2021;5:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 70. | Dirchwolf M, Marciano S, Ruf AE, Singal AG, D'Ercole V, Coisson P, Zerega A, Orozco F, Palazzo A, Fassio E, Arufe D, Anders M, D'Amico C, Gaite L, Thompson M, Perez D, Haddad L, Demirdjian E, Zunino M, Gadano A, Murga MD, Bermudez C, Tomatis J, Grigera N, Antinucci F, Baravalle M, Gazari MMR, Ferreiro M, Barbero M, Curia A, Demonte M, Gualano G. Failure in all steps of hepatocellular carcinoma surveillance process is frequent in daily practice. Ann Hepatol. 2021;25:100344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Singal AG, Reddy S, Radadiya Aka Patel H, Villarreal D, Khan A, Liu Y, Cerda V, Rich NE, Murphy CC, Tiro JA, Kramer JR, Hernaez R. Multicenter Randomized Clinical Trial of a Mailed Outreach Strategy for Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2022;20:2818-2825.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 72. | Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M, Lai CL. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 497] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 73. | Kim GA, Lim YS, Han S, Choi GH, Choi WM, Choi J, Sinn DH, Paik YH, Lee JH, Lee YB, Cho JY, Heo NY, Yuen MF, Wong VW, Chan SL, Yang HI, Chen CJ. Viral Load-Based Prediction of Hepatocellular Carcinoma Risk in Noncirrhotic Patients With Chronic Hepatitis B: A Multinational Study for the Development and External Validation of a New Prognostic Model. Ann Intern Med. 2024;177:1308-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, Chan HL, Mok TS. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 75. | Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, Chung VC, Chan ZC, Tse YK, Chim AM, Lau TK, Wong VW. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 76. | Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Kim BK, Han KH. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology. 2015;62:1757-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Sohn W, Cho JY, Kim JH, Lee JI, Kim HJ, Woo MA, Jung SH, Paik YH. Risk score model for the development of hepatocellular carcinoma in treatment-naïve patients receiving oral antiviral treatment for chronic hepatitis B. Clin Mol Hepatol. 2017;23:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Fan C, Li M, Gan Y, Chen T, Sun Y, Lu J, Wang J, Jin Y, Lu J, Qian G, Gu J, Chen J, Tu H. A simple AGED score for risk classification of primary liver cancer: development and validation with long-term prospective HBsAg-positive cohorts in Qidong, China. Gut. 2019;68:948-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, Lee TY, Wu MS, Lin JT, Wong GL, Wu CY. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol. 2018;69:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 425] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 81. | Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, Kang SH, Kim MY, Cheon GJ, Kim DJ, Baik SK, Choi DH. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 82. | Yang HI, Yeh ML, Wong GL, Peng CY, Chen CH, Trinh HN, Cheung KS, Xie Q, Su TH, Kozuka R, Lee DH, Ogawa E, Zhao C, Ning HB, Huang R, Li J, Zhang JQ, Ide T, Xing H, Iwane S, Takahashi H, Wong C, Wong C, Lin CH, Hoang J, Le A, Henry L, Toyoda H, Ueno Y, Gane EJ, Eguchi Y, Kurosaki M, Wu C, Liu C, Shang J, Furusyo N, Enomoto M, Kao JH, Yuen MF, Yu ML, Nguyen MH. Real-World Effectiveness From the Asia Pacific Rim Liver Consortium for HBV Risk Score for the Prediction of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Treated With Oral Antiviral Therapy. J Infect Dis. 2020;221:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 83. | Poh Z, Shen L, Yang HI, Seto WK, Wong VW, Lin CY, Goh BB, Chang PE, Chan HL, Yuen MF, Chen CJ, Tan CK. Real-world risk score for hepatocellular carcinoma (RWS-HCC): a clinically practical risk predictor for HCC in chronic hepatitis B. Gut. 2016;65:887-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, Shah H, Khalili K, Yim C, Heathcote EJ, Janssen HLA, Sherman M, Hirschfield GM, Feld JJ. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;S0168-8278(17)32248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 85. | Shiha G, Waked I, Soliman R, Elbasiony M, Gomaa A, Mikhail NNH, Eslam M. GES: A validated simple score to predict the risk of HCC in patients with HCV-GT4-associated advanced liver fibrosis after oral antivirals. Liver Int. 2020;40:2828-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 87. | Fujiwara N, Lopez C, Marsh TL, Raman I, Marquez CA, Paul S, Mishra SK, Kubota N, Katz C, Kanzaki H, Gonzalez M, Quirk L, Deodhar S, Selvakumar P, Raj P, Parikh ND, Roberts LR, Schwartz ME, Nguyen MH, Befeler AS, Page-Lester S, Srivastava S, Feng Z, Reddy KR, Khaderi S, Asrani SK, Kanwal F, El-Serag HB, Marrero JA, Singal AG, Hoshida Y. Phase 3 Validation of PAaM for Hepatocellular Carcinoma Risk Stratification in Cirrhosis. Gastroenterology. 2025;168:556-567.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 88. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 89. | Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, Zhan M, Wang B, Peng F, Gao X, Shi Y, Wen X, Ji Y, Jin Q, Niu J. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J Hepatocell Carcinoma. 2020;7:219-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Ren T, Hou X, Zhang X, Chen D, Li J, Zhu Y, Liu Z, Yang D. Validation of combined AFP, AFP-L3, and PIVKA II for diagnosis and monitoring of hepatocellular carcinoma in Chinese patients. Heliyon. 2023;9:e21906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 91. | Sun LY, Wang NY, Diao YK, Yan CL, Fan ZP, Wei LH, Li HJ, Guan MC, Wang MD, Pawlik TM, Lau WY, Shen F, Lv GY, Yang T. Comparison between models for detecting hepatocellular carcinoma in patients with chronic liver diseases of various etiologies: ASAP score versus GALAD score. Hepatobiliary Pancreat Dis Int. 2025;24:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 92. | Piratvisuth T, Hou J, Tanwandee T, Berg T, Vogel A, Trojan J, De Toni EN, Kudo M, Eiblmaier A, Klein HG, Hegel JK, Madin K, Kroeniger K, Sharma A, Chan HLY. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol Commun. 2023;7:e0317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 93. | Chen P, Song H, Xu W, Guo J, Wang J, Zhou J, Kang X, Jin C, Cai Y, Feng Z, Gao H, Lu F, Li L. Corrigendum: Validation of the GALAD model and establishment of a new model for HCC detection in Chinese patients. Front Oncol. 2023;13:1170066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Toyoda H, Kumada T, Osaki Y, Oka H, Urano F, Kudo M, Matsunaga T. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol. 2006;4:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Fox R, Berhane S, Teng M, Cox T, Tada T, Toyoda H, Kumada T, Kagebayashi C, Satomura S, Johnson PJ. Biomarker-based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br J Cancer. 2014;110:2090-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Wang M, Devarajan K, Singal AG, Marrero JA, Dai J, Feng Z, Rinaudo JA, Srivastava S, Evans A, Hann HW, Lai Y, Yang H, Block TM, Mehta A. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev Res (Phila). 2016;9:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 97. | Singal AG, Tayob N, Mehta A, Marrero JA, Jin Q, Lau J, Parikh ND. Doylestown Plus and GALAD Demonstrate High Sensitivity for HCC Detection in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:953-955.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, White DL, Davila J, Kanwal F, El-Serag HB. Validation of the Updated Hepatocellular Carcinoma Early Detection Screening Algorithm in a Community-Based Cohort of Patients With Cirrhosis of Multiple Etiologies. Clin Gastroenterol Hepatol. 2021;19:1443-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 99. | El-Serag HB, Jin Q, Tayob N, Salem E, Luster M, Alsarraj A, Khaderi S, Singal AG, Marrero JA, Asrani SK, Kanwal F. HES V2.0 outperforms GALAD for detection of HCC: A phase 3 biomarker study in the United States. Hepatology. 2025;81:465-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/