Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.109517
Revised: June 20, 2025
Accepted: September 25, 2025
Published online: October 27, 2025
Processing time: 167 Days and 1.6 Hours
Syngeneic orthotopic tumor models offer an optimal functional tumor–immune interface for hepatocellular carcinoma research. Yet, unpredictable growth ki
To optimize the orthotopic hepatocellular carcinoma model and evaluate the potential of US imaging for accurate and cost-effective tumor monitoring.
Hepatocellular carcinoma was induced in 28 Sprague Dawley rats by implanting 5 × 106 N1S1 cells into the left lateral hepatic lobe. Tumor progression was monitored weekly via US. Upon reaching 100-150 mm³, an experimental group (n = 14) received Sorafenib (40 mg/kg) orally on alternate days for 28 days; efficacy was compared to untreated controls. US accuracy was validated against micro-computed tomography, gross caliper measurements and histopathological analysis. Reliability and operator proficiency in US assessment were also evaluated.
US images procured 7-day post-surgery revealed a well-defined hypoechoic nodule at the left liver lobe tip, confirming successful tumor induction (mean volume 130 ± 39 mm³). Only three animals exhibited spontaneous regression by week 2, underscoring the model’s stability. Sorafenib treatment elicited a marked tumor reduction (678 ± 103 mm³) vs untreated control (6005 ± 1760 mm³). US assessment demonstrated robust intra and interobserver reproducibility with high sensitivity and specificity for tumor detection. Moreover, US derived volumes correlated strongly with gross caliper measurements, histopathological analysis, and microcomputed tomography imaging, validating its reliability as a non-invasive monitoring tool in preclinical hepatocellular carcinoma studies.
The results demonstrate that US imaging is a reliable, cost-effective, and animal sparing approach with an easy to-master protocol, enabling monitoring of tumor progression and therapeutic response in orthotopic liver tumor models.
Core Tip: Orthotopic rat tumor models are valuable for replicating human tumor microenvironments, yet tumor regression complicates consistent growth assessment, underscoring the need for reliable imaging modalities. While advanced techniques like magnetic resonance imaging and micro-computed tomography deliver high-resolution data, their expense, effects and demands limit routine implementation. We present an ultrasound (US) imaging protocol that achieves accuracy and sensitivity for regular tumor growth monitoring. Our results demonstrate US’s potential for early detection of orthotopic liver tumors and robust evaluation of antitumor drug efficacy, offering a cost-effective, accessible alternative strategy in preclinical research.
- Citation: Devan AR, Sasidharan SM, Sreekumar KP, Unni AKK, Mangalathillam S, Ansar A, Unni AR, Nath LR. Ultrasound imaging-guided protocol for monitoring tumor growth in orthotopic rat model of hepatocellular carcinoma. World J Hepatol 2025; 17(10): 109517
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/109517.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.109517
Hepatocellular carcinoma (HCC) accounts for more than 80% of liver cancer cases and is the third leading cause of cancer-related mortality[1]. The global burden of liver cancer is rising alarmingly, particularly due to a recent epidemiological shift in incidence from viral to non-viral aetiologies, such as alcohol consumption and metabolic syndrome. In the majority of patients, HCC is diagnosed at an advanced and often incurable stage, with more than 50% of patients receiving systemic drug therapy[2]. An appropriate animal model that accurately recapitulates the pathogenesis of HCC is essential for the preclinical evaluation of anti-HCC drugs.
Preclinical animal models play a critical role in cancer research, encompassing a wide range of applications from understanding disease progression mechanisms to the development of effective anticancer therapies. Given the extensive effort, time, and cost involved in establishing tumor models, it is crucial to align experimental protocols with the 3Rs principles—Reduction, Replacement, and Refinement—to improve model accuracy and reliability[3]. Animal models of HCC primarily include chemically induced models (such as DEN, TAA, CCl4), genetically engineered models, and graft models. In engrafted HCC models, tumors are generated by implanting human xenografts (tumor fragments or cultured HCC cells) either under the skin (ectopic) or into the liver (orthotopic) of the host animal[4]. Orthotopic syngeneic models serve as valuable tools for the preclinical assessment of anti-HCC therapies. Unlike xenograft models, orthotopic rat models offer the advantage of intact murine immunity and a comprehensive stromal environment, facilitating the evaluation of drug-immune cell interactions[4]. Therefore, syngeneic HCC rat models offer tumor development analogous to human HCC and are particularly valuable for preclinical evaluation of targeted and immune-based anti-HCC therapies[5]. However, performing repeated exploratory surgeries to monitor tumor growth is both impractical and ethically questionable. As such, the implementation of non-invasive imaging tools for accurate measurement is essential for orthotopic HCC models. These tools enable longitudinal studies, reduce animal numbers, and provide statistically robust data, observing the ethical principles.
Imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT)/micro-CT, positron emission tomography (PET), and ultrasound (US) are commonly used for tumor imaging in small animals. In addition to key characteristics such as sensitivity, rapidity, and imaging quality, these modalities should also be user-friendly, affordable, and safe. Since repeated assessments of tumor response to therapy are required during treatment, limitations such as increased radiation exposure, regular use of intravenous or oral contrast agents (CT/PET), long imaging times, the need for high technical support (MRI), and high costs of equipment and imaging procedures hinder the routine use of MRI and CT in animal tumor analysis[6-8]. In this context, US imaging presents a promising alternative, as it can be used repeatedly without radiation exposure, less time consumption with precision. Additionally, it is relatively inexpensive, portable, and accessible for many research groups[9,10]. In the current study, we induced Orthotopic HCC using N1S1 cells in Sprague-Dawley (SD) rats and monitored tumor progression and treatment responses via US imaging. The aim of this study was to evaluate the feasibility of diagnostic US imaging for assessing tumor growth and the response to drug treatment in a cost-effective, efficient and accurate manner.
The rat hepatoma cell line, N1S1, was received as a gift from Dr. Manzoor K, Professor at Amrita Centre for Nanoscience and Molecular Medicine. This cell line was originally derived from a hepatoma induced by feeding 4-dimethylamino-benzene (DMAB) through the diet to a male SD rat. For the experiments, HCC cells (up to PN 35) were grown in Dulbecco’s Modified Eagle Medium (DMEM, Himedia) supplemented with 5% fetal bovine serum and 1% antibiotic-antimycotic solution (100 IU/mL penicillin, 100 µg/mL streptomycin, 0.25 µg/mL amphotericin, Himedia). The cells were maintained at 37 °C in a 5% CO2 humidified incubator. Upon reaching adequate confluency, cell suspensions were centrifuged at 1500 rpm for 3 minutes. The pellet was then resuspended in DMEM, and the cell count was determined. Sorafenib (purity > 98.0% by HPLC) is purchased from TCI chemicals.
A cohort of 28 male Sprague–Dawley rats (7-8 weeks old, 200-250 g; n = 14 per experimental group) was obtained from the Central Laboratory Animal Facility, Amrita Viswa Vidyapeetham. Group sizes were determined by G*Power analysis (effect size f = 0.45, α = 0.05, power = 0.80), which indicated a minimum of 12 animals per group; two additional rats were included per group to offset potential tumor regression in accordance with precedent N1S1 orthotopic HCC studies reporting group sizes of 12-15 animals[11,12]. The Institutional Animal Ethics Committee approved all protocols (IAEC/2023/1/10), and experiments conformed to the Committee for the Control and Supervision of Animal Experiments (CCSEA) guidelines.
Rats were housed individually in cages within an environmentally controlled room (21-23 °C, 60%-70% relative humidity) on a 12 hours light/12 hours dark cycle. Standard laboratory chow and filtered water were provided ad libitum, and nesting materials plus shelters were supplied as environmental enrichment. Animals were acclimatized for one week prior to any procedures. After physical examination, healthy animals were selected for the study and subjected to fasting 3 hours before surgery, with free access to water.
N1S1 hepatoma cells (5 × 106 cells in 30 µL DMEM) were prepared on the day of surgery, increasing the inoculum from previous studies (2.5 × 106 cells) to reduce spontaneous regression and improve tumor take rate[13]. Rats were anesthetized by intramuscular injection of anesthetic cocktail containing ketamine HCl (50 mg/mL) and xylazine HCl (20 mg/mL) at a 3:2 ratio (2.5 µL/g body weight), then maintained under 2% isoflurane in 100% O2 (1 L/minute) via nose cone. Core body temperature was monitored and maintained at 37 ± 0.5 °C using a heating pad. The ventral abdomen, from sternum to umbilicus, was shaved and aseptically prepped with povidone iodine and 70% ethanol.
A 1.5-2 cm midline incision was made through skin, subcutaneous tissue, and linea alba to expose the peritoneal cavity. The left lateral liver lobe was gently exteriorized using a sterile cotton swab. Cell suspension was injected subcapsularly into the distal liver lobe using a 30 gauge needle; successful delivery was confirmed by formation of a pale bleb. Gentle compression was applied for six minutes to prevent backflow. The liver was repositioned, and the muscular and fascial layers were closed with continuous 40 Vicryl sutures; the skin was closed with interrupted 40 Vicryl sutures. Postoperatively, injections meloxicam (0.25 mg/kg) and ceftriaxone (20 mg/kg) were administered intramuscular once daily for five days. Animals were observed twice daily for signs of pain, infection, or distress; body weight and surgical sites were recorded. All personnel were trained in rodent anesthesia, aseptic technique, and humane endpoints to minimize pain and distress throughout the study.
US imaging of orthotopic liver tumors was carried out using a Samsung MySono U6 portable system fitted with a linear multifrequency transducer (5-12 MHz) and the focal zone optimized at 8 MHz for maximal resolution in soft tissue. Prior to each imaging session, rats were gently anesthetized in an induction chamber with 2% isoflurane in 100% medical oxygen (flow rate 3 L/minute), then maintained under anesthesia via a nose cone to ensure stability of respiration and minimal motion artifact. Core body temperature was monitored with a rectal probe and maintained at 37 ± 0.5 °C using a warming pad, to prevent hypothermia and physiological alterations that could affect tumor perfusion and echogenicity.
The abdominal fur overlying the liver region was shaved, followed by cleansing with sterile saline to ensure optimal acoustic coupling. A warmed, sterile US gel was applied liberally to eliminate air gaps between the transducer and skin, providing clear delineation of liver parenchyma and implanted tumor nodules.
Baseline and longitudinal imaging: Baseline measurements of the left liver lobe thickness were acquired 0.5-1.5 cm from the lobe tip (the cell injection site) prior to tumor induction, establishing each animal’s reference anatomy. Follow up imaging was performed on Days 7, 14, 21, 28, and 35 postimplantation. In each session, tumors were visualized in both the sagittal (longitudinal) and axial (transverse) planes. In the sagittal plane—dividing the body into left (sinister) and right (dexter) halves—the maximum axis (L1) and thickness (T1) of the tumor was measured. In the axial plane—dividing into cranial and caudal sections—the maximal axis (L2) and thickness (T2) was recorded. Targeting the lobe tip for both injection and imaging minimized overlap with adjacent lobes and adjacent vascular structures, thereby reducing measurement variability.
Image acquisition settings: To standardize image quality, the following 2Dmode parameters were held constant across all animals and time points: Frame averaging frequency: 10, harmonic imaging: Enabled, dynamic range: 115 dB, reject (TGC) level: 10, Edge enhancement: Level 3, 2D/Cine live focus: Zone 4. These settings balanced spatial resolution with tissue penetration, enhancing tumor boundary detection against healthy parenchyma.
Tumor volume calculation and treatment initiation: Tumor volume (V) was estimated using the ellipsoid approximation: V = (l × s2)/2 where both l and s are recorded in millimeters (mm). Once a tumor reached 100–150 mm³, systemic therapy with Sorafenib—administered orally at 40 mg/kg, thrice weekly for four weeks—was initiated. On day 35, animals were euthanized by CO2 asphyxiation and ex vivo tumor dimensions were measured with digital vernier caliper (VC) for comparison against US derived volumes.
To assess reproducibility, interobserver variability was quantified using tumor dimension data (n = 25) measured independently by four examiners: Two US trained examiners (1 & 3), one novice examiner (2) and one US trained radiologist (4). Linear regression analysis provided the coefficient of determination (R²) between nonexpert and expert measurements, while Bland–Altman plots evaluated the mean difference and 95% limits of agreement. Intraobserver variability was determined by repeat imaging of the same tumor on two occasions separated by one week; intraclass correlation coefficients were calculated to assess measurement consistency over time.
To investigate the impact of targeted training, the novice examiner received a structured 1 hour session covering probe handling, optimal imaging planes, and measurement protocols. This examiner then performed daily tumor mea
Receiver operating characteristic (ROC) curves were constructed by defining tumor status categories (e.g., malignant vs benign appearance) based on combined visual inspection and histopathological confirmation. US Volumetric mea
Collectively, this comprehensive US imaging protocol—combining rigorous standardization of acquisition parameters, careful physiological monitoring, and robust statistical analysis—ensures reliable, reproducible assessment of orthotopic liver tumor growth and treatment response in the rat model[14,15].
Micro-computed tomography (µCT) imaging was conducted using the MILabs Micro CT system (MILabs, Netherlands), a high-resolution platform designed for non-invasive anatomical imaging in small animals. The system operates on a voltage range of 110–240 V AC, at a frequency of 50-60 Hz, with a maximum power capacity of 400 W, allowing stable and high-fidelity imaging performance.
Prior to imaging, animals were subjected to a standard pre-anesthetic evaluation, including assessment of respiratory pattern, weight, behavior, and general health. Anesthesia was induced using an inhalation chamber delivering 4%-5% isoflurane mixed with 100% medical-grade oxygen at a flow rate of 2 L/minute for approximately 2-3 minutes. Once a stable anesthetic depth was achieved—characterized by reduced response to external stimuli and steady respiration—rats were gently positioned on the heated scanner bed of the µCT system.
The scanner bed was remotely moved into the gantry using an external computer workstation to prevent unnecessary personnel exposure to radiation. Animals were maintained under anesthesia throughout the imaging session using 1%-2% isoflurane in 100% oxygen at a reduced flow rate of 1 L/minute, administered via a nose cone. Physiological parameters including respiratory rate and heart rate were continuously monitored using integrated sensors embedded in the scanner platform. Body temperature was also maintained using a heated platform to avoid hypothermia, which can compromise animal welfare and data accuracy.
High-resolution imaging protocols were selected based on the anatomical region of interest, slice thickness, and reconstruction matrix size. Raw projection data were processed and reconstructed into 3D volumetric datasets using the proprietary MILabs µCT reconstruction software. The resulting DICOM images were appropriately labeled with animal ID, timepoint, and treatment group and stored in a secure server for future morphometric and density-based quantitative analysis[16].
All twenty-eight rats were humanely euthanized on day 35 post-surgery, following imaging and final assessments. The method of euthanasia used was carbon dioxide (CO2) inhalation, in accordance with guidelines recommended by institutional animal ethics committees (IAEC). Animals were placed individually in a transparent euthanasia chamber without pre-filling. 100% CO2 was then introduced into the chamber at a controlled rate of 30%-70% of the chamber volume per minute, ensuring gradual displacement of ambient air to minimize distress. Unconsciousness was typically observed within 2-3 minutes, confirmed by the absence of voluntary movements and a blank or faded ocular response. The CO2 flow was continued for at least 2 minutes after visible respiration had ceased. Final confirmation of death was established by verifying the absence of heartbeat, respiration, and fixed dilated pupils.
Following euthanasia, a thorough gross necropsy was performed on each animal. Abdominal and thoracic cavities were opened using sterile dissection tools. Visceral organs, particularly the liver, lungs, and gastrointestinal tract, were examined for gross abnormalities, tumor nodules, or metastases. The liver was excised and inspected for tumor location, vascular invasion, and surface irregularities.
Tumor nodules were measured using a digital VC caliper with measurement range of 0-150 mm resolution 0.1 ± 0.2 mm accuracy. The long (l), short (s), and height (h) dimensions were recorded, and tumor volume was calculated using the standard ellipsoid formula: V = (l × s²)/2.
Representative tissue samples from both control and treated groups were collected, fixed in 10% neutral buffered formalin for up to 14 days, and processed using a routine paraffin-embedding protocol. After dehydration in graded alcohols and clearing in xylene, tissues were embedded in paraffin blocks, sectioned at 5 µm thickness, and stained with hematoxylin and eosin (H&E). Microscopic analysis was performed using a binocular light microscope and images were captured using a digital photomicrography system.
All data are expressed as mean ± SD. Statistical analysis was performed using GraphPad Prism software (version 8.0.2). A two-way analysis of variance (ANOVA) was employed to examine the effects of treatment, time, and their interaction on tumor volume and body weight. Where significant interactions were detected, post hoc comparisons were made using Tukey’s Honest Significant Difference test. Adjusted mean differences, q-statistics, and corrected P values were computed, with significance set at P value < 0.05.
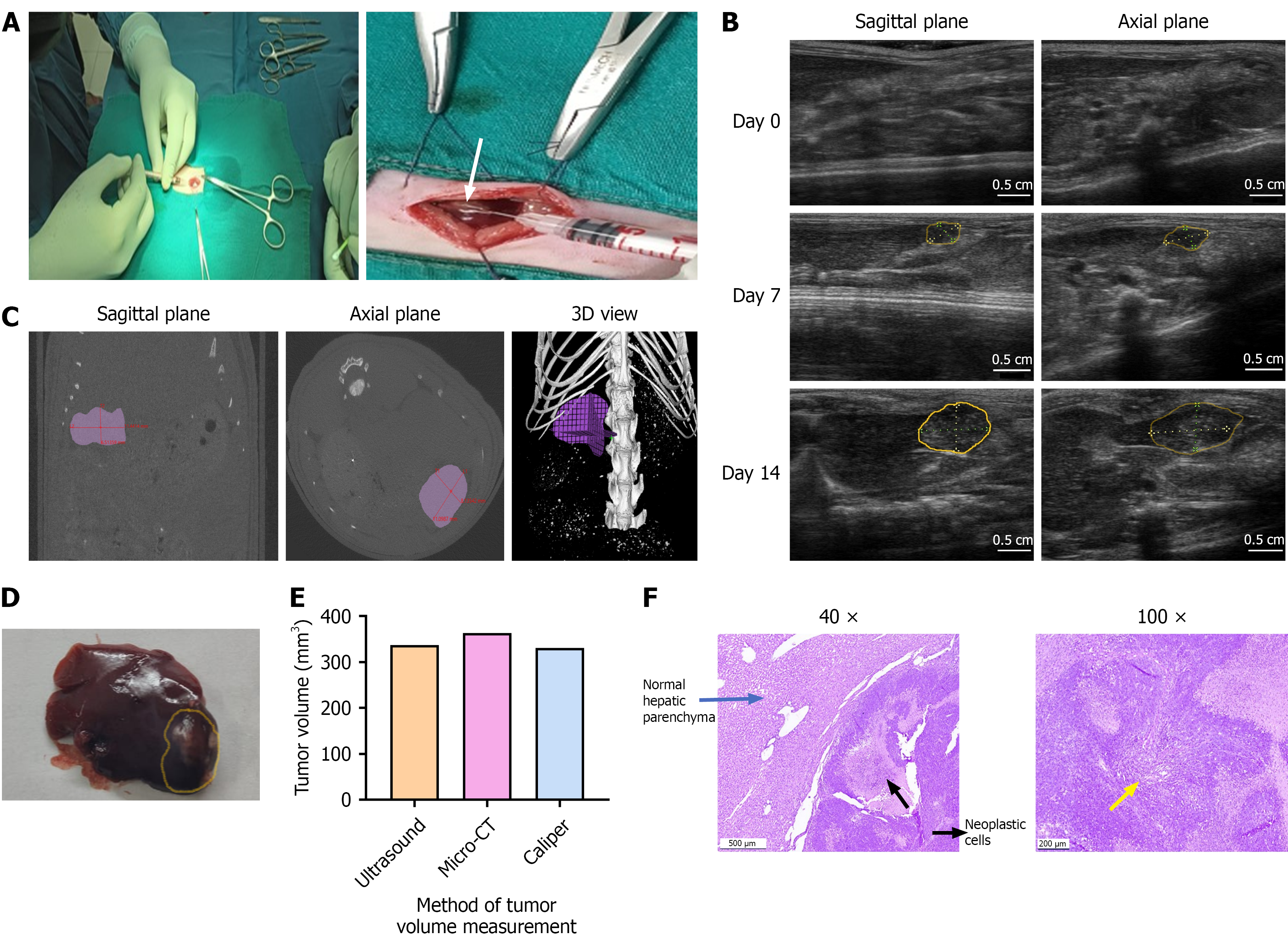
Orthotopic implantation of rat HCC cells (N1S1) into the liver were successfully performed using a laparotomy procedure. Tumor induction was achieved by the slow injection of N1S1 cells suspended in 30 µL DMEM into the subcapsular space at the tip of the left liver lobe (Figure 1A). All animals tolerated the surgery well, with no postoperative complications such as bleeding, infection, weight loss, or mortality.
Baseline lobe thickness measured on day 0 ranged from 0.30 ± 0.05 cm (0.5 cm from the tip) to 0.38 ± 0.02 cm (from 1.5 cm from the tip), confirms reproducible injection depth and minimal anatomical variability. By day 7, US imaging revealed a single hypoechoic nodule with a mean volume of 130 ± 39 mm³ at the tip of the left lobe, indicating consistent tumor establishment across all animals. No extrahepatic spread or distant metastases were observed. The tumor growth with well-defined margin was evident on US imaging by day 14 (Figure 1B). To validate the measurement accuracy of US imaging, a conventional micro-CT imaging was also performed on day 14. Both imaging modalities and Vernier Caliper measurements were conducted in a blinded manner to minimize observer bias. Tumor was imaged in both sagittal and axial planes, and 3D reconstructions were generated (Figure 1C). In one representative animal on day 14, volumetric estimates were 364 mm3 by micro-CT, 337.6 mm3 by US and 330.4 mm3 by VC, demonstrating close agreement (Figure 1D and E). These results demonstrate the comparable accuracy of US imaging in assessing tumor volume when compared with advanced imaging modalities such as micro-CT. Histopathological examination also confirmed the establishment of well-differentiated hepatocellular carcinoma in the liver (Figure 1F).
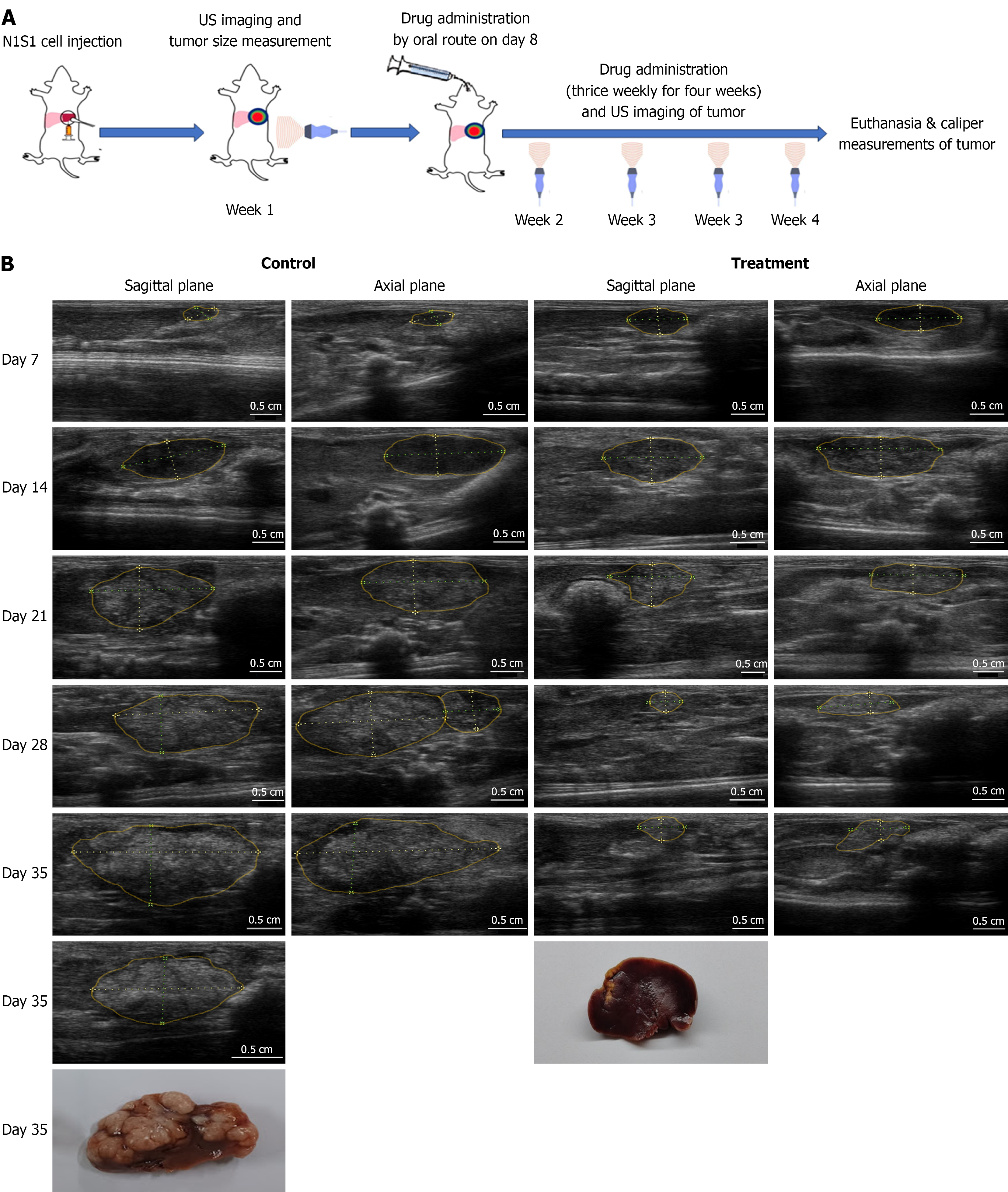
Sorafenib is the globally accepted standard care for HCC. In this study, Sorafenib was administered orally at a dose of 40 mg/kg, equivalent to the human therapeutic dose of 400 mg, three times per week for four weeks. An experimental plan is schematically represented (Figure 2A). In most animals, drug treatment was initiated following day 7 imaging, when the tumor volume ranged between 100-150 mm³. However, tumor regression was observed in three animals (10%) by the second week post-implantation of N1S1 cells. To minimize the confounding effects of spontaneous tumor regression, US imaging was performed on Days 7 and 10, and only animals exhibiting active tumor growth were selected for treatment evaluation.
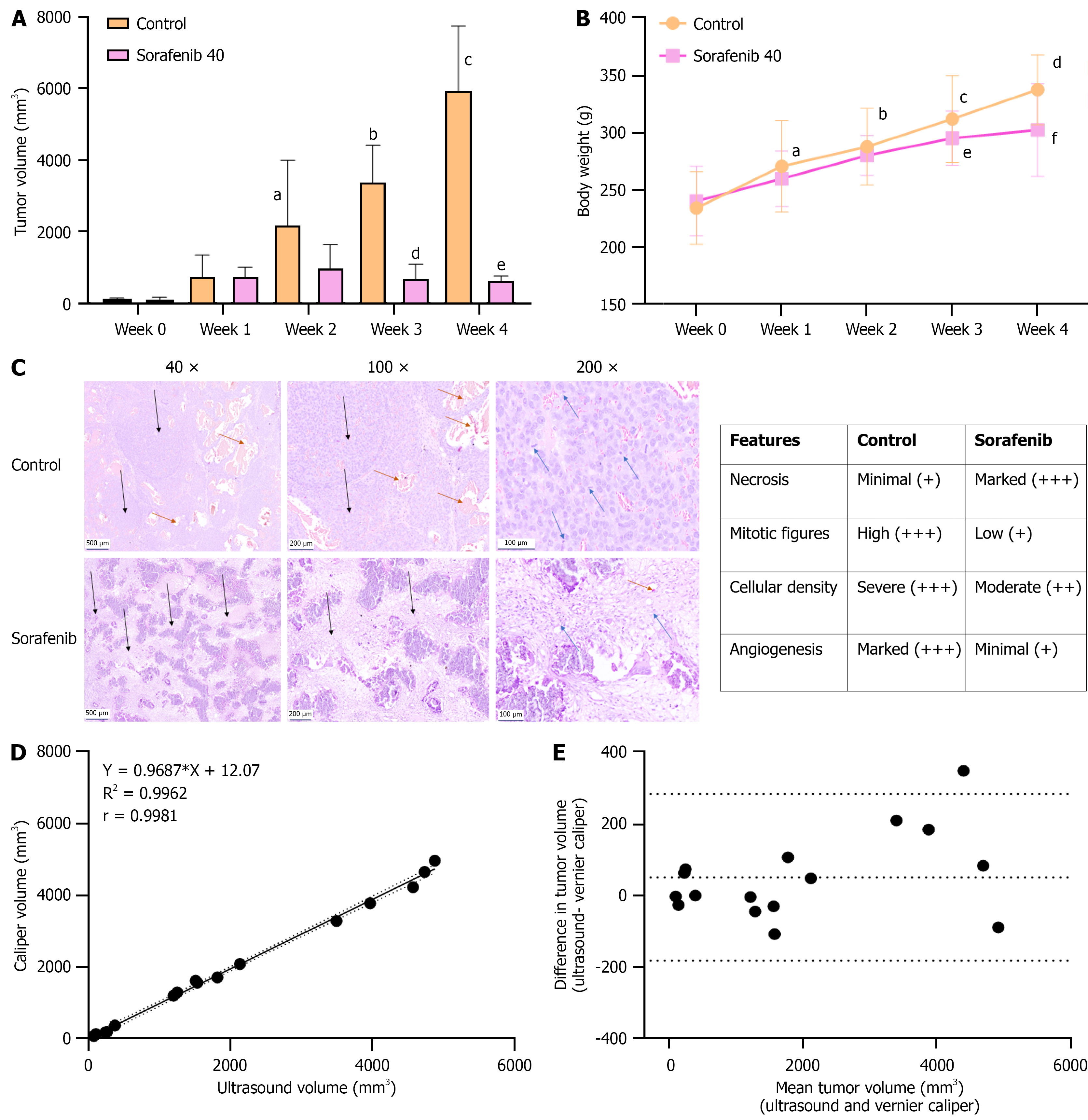
Tumor growth in Sorafenib-treated animals was monitored weekly using US imaging and compared with that of untreated control animals. Following the initiation of treatment, control group demonstrated a change in tumor echogenicity from hypoechoic to hyperechoic paralleled the aggressiveness, whereas Sorafenib-treated group showed a marked reduction in tumor growth, and remained uniformly hypoechoic throughout the treatment period. A hypoechoic tumor appears darker on US due to lower tissue density, while a hyperechoic tumor appears brighter, indicating increased fibrous content and tissue density (Figure 2B). According to US measurements, mean tumor volume in control animals increased exponentially from 139.87 ± 41 to 3435.2 ± 1000 by week 3. In contrast, Sorafenib-treated animals exhibited a significant inhibition of tumor growth, with limited tumor growth from 126.06 ± 76.6 to 750.44 ± 366 over the same period (Figure 3A). Both groups didn’t exert any weight loss during the treatment period and treatment was well tolerated (Figure 3B). At the end of the treatment period (day 35), US measurements were compared with tumor volumes obtained via Vernier Caliper following necropsy. US imaging confirmed an aggressive, multi-nodular tumor spread throughout the left liver lobe in control animals, whereas Sorafenib-treated animals showed a limited tumor growth, localized to the tip of the liver lobe and the result was also evident in tumor photograph (Figure 2B). US-based tumor features were further correlated with histopathological findings. Control tumors were characterized by dense sheets of neoplastic cells, numerous mitotic figures, and extensive neovascularization, indicative of an aggressive tumor phe
Correlation between US and VC measurements was analysed using 15 tumor samples, with tumor volumes ranging from 81 mm³ to 4800 mm³. A strong correlation was observed, with a Pearson correlation coefficient of r = 0.998 (P < 0.001) (Figure 3D). Vernier Caliper measurements were conducted in a blinded manner to minimize observer error. Agreement between the two methods was further assessed using a Bland-Altman plot, where the difference in tumor volumes (US-VC) was plotted against the mean tumor volume of both methods. The analysis revealed a bias of 51 mm³, which lies within the limits of agreement (-184 to +284 mm³) with a 95% confidence interval. Notably, the largest discrepancies were observed in samples with tumor volumes exceeding 2000 mm³, while small to medium-sized tumors demonstrated minimal variation between the two measurement methods (Figure 3E).
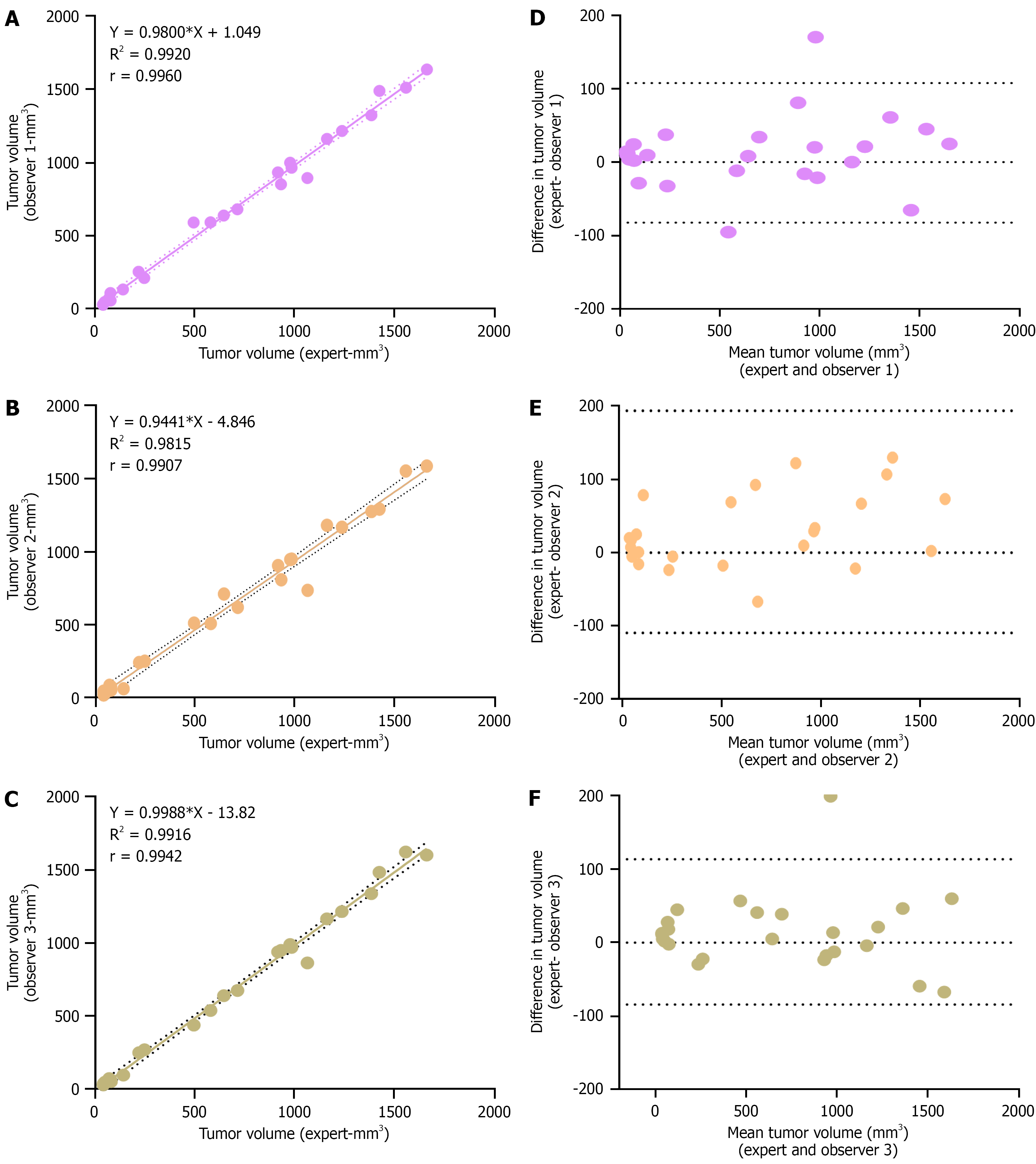
To validate the US-based tumor volume measurements, 25 distinct tumor samples were analysed in a blinded manner by three different observers. Observers 1 and 3 directly involved in the project and received basic training under the supervision of an expert radiologist during imaging sessions. Observer 2, while familiar with the theoretical principles, had no prior hands-on experience in US imaging. A brief demonstration is provided for all observers prior to mea
Bland-Altman analysis further confirmed the reliability of measurements. For Observers 1 and 3, the plots demonstrated a tight clustering of differences around zero, suggesting low variability relative to expert measurements. The limits of agreement were narrow—−82 to +108 mm³ for Observer 1 and −84 to +113 mm³ for Observer 3—indicating high measurement consistency (Figure 4D-F). In contrast, Observer 2 exhibited a broader range of agreement (-110 to 194 mm3), suggesting moderate variability likely due to limited practical exposure (Figure 4E). The Bland-Altman plot also reveals that measurement discrepancies may slightly increase for tumors larger than 500 mm³, with a chance of measuring tumor volume as higher than the actual volume.
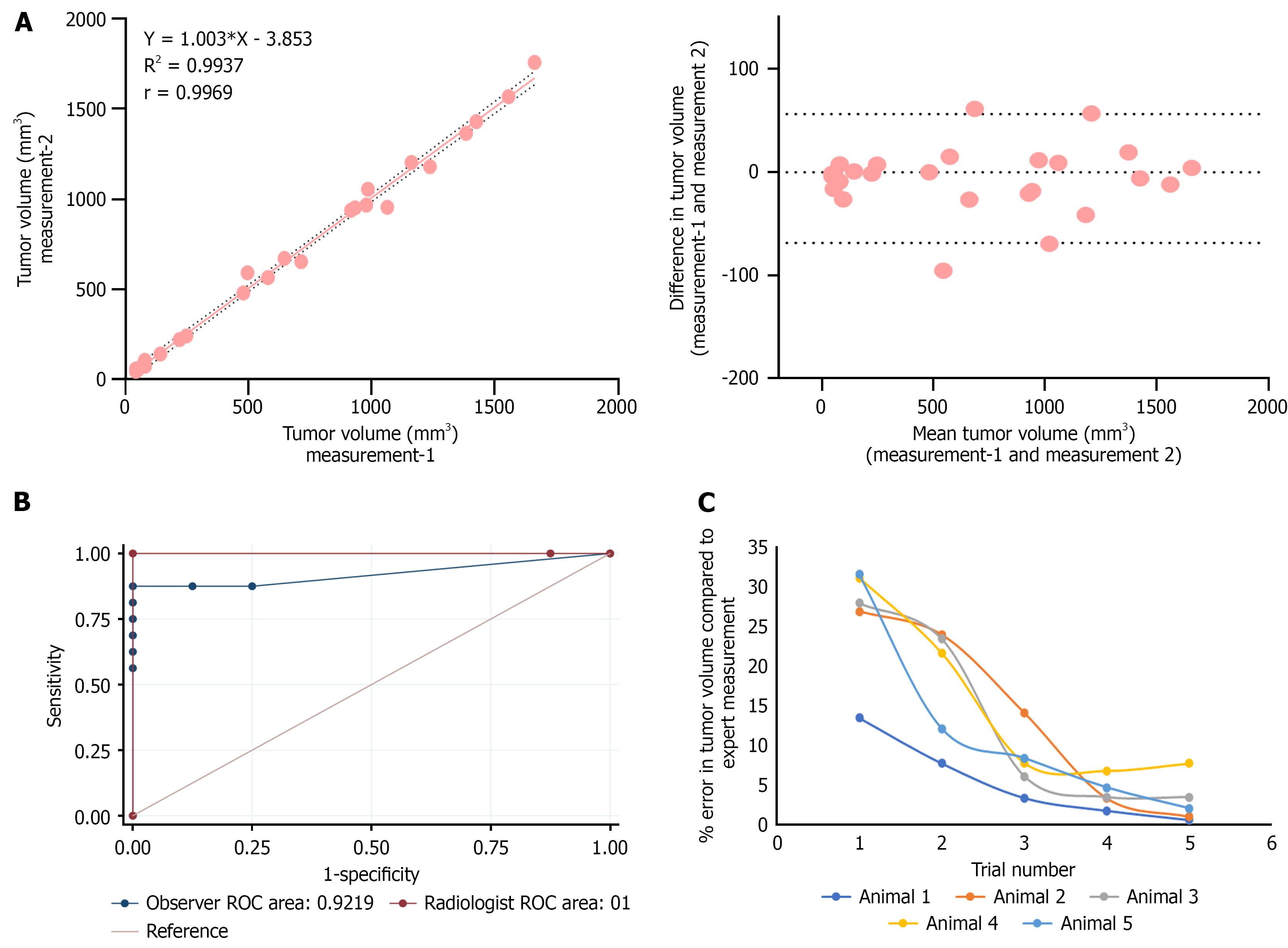
To evaluate the reliability of tumor volume measurements using US imaging, tumor samples previously analysed by an expert radiologist were re-analysed, and further intra-observer variability was assessed. A linear correlation plot demonstrated a strong association between the two measurements, with an r-value of 0.9969. The Bland–Altman plot indicated minimal deviation and excellent agreement, confirming high repeatability of the measurements (Figure 5A). ROC analysis was performed to assess the diagnostic accuracy of both the expert radiologist and a non-expert observer in differentiating tumor and non-tumor cases based on US imaging. Visual inspection and histopathological evaluation were used as the reference standards. The radiologist achieved 100% sensitivity and specificity, indicating perfect classification of tumor status. In contrast, the non-expert observer misclassified two tumor and two non-tumor samples, resulting in a sensitivity of 87.5% and specificity of 75%. This variation is indicated as lower AUC for the observer compared to the radiologist (Figure 5B).
To assess the learning curve that visualizes how a model's error rate changes as the amount of training data increases in US imaging, a researcher without prior experience underwent a five-day training program. Tumor volumes were estimated in five samples each day, and percentage error was calculated relative to expert measurements. Over the training period, the cumulative measurement error was significantly reduced from 148% to 16%, highlighting a substantial improvement in accuracy (Figure 5C). These findings support the user-friendly and accessible nature of US imaging as a reliable tool for tumor monitoring in preclinical research settings.
Hepatocellular carcinoma is the sixth most commonly diagnosed cancer worldwide and ranks as the third leading cause of cancer-related mortality[17]. Unlike many other solid tumors, HCC is more commonly treated with immunotherapy and targeted therapies rather than conventional cytotoxic chemotherapy[2]. In this context, orthotopic liver tumor models offer significant advantages over subcutaneous models, as they allow evaluation of therapeutic efficacy within the native hepatic microenvironment. These models more accurately reflect the tissue architecture, immune contexture, and tumor-host interactions, all of which are critical for understanding treatment response. Importantly, orthotopic models closely mimic the clinical presentation of HCC, making them a valuable platform for biomarker discovery and preclinical assessment of anti-HCC therapies[18].
Our study utilised syngeneic N1S1 orthotopic model of HCC in SD rat for evaluation of drug efficacy. Inducing tumor growth in immunocompetent animals poses significant challenges, primarily due to the low rate of tumor induction and the spontaneous regression of tumors even in the absence of therapeutic intervention. Several mechanisms have been proposed to explain the spontaneous regression, most of which are related to the healthy cellular microenvironment within the animals. The innate immune response, triggered by granulocytes, elicits an inflammatory reaction that helps eliminate cancer cells. Additionally, oxidative stress-induced free radicals exhibit selective cytotoxicity toward cancer cells, contributing to spontaneous tumor regression[12,19]. Lee et al[20] established orthotopic HCC rat models using two different cell lines, N1S1 and McARH7777. In the N1S1 model, the tumor induction rate was 10%, while in the McARH7777 model, all tumors underwent spontaneous regression within the first week. However, the administration of cyclosporin reduced tumor regression and increased the incidence rate of tumors in the McARH7777 model. Similarly, Buijs et al[21] assessed the regression pattern in orthotopic HCC models using N1S1 and McARH7777 cells. They implanted 2.5 × 106 cells mixed with Matrigel subcapsularly into the left liver lobe. Visible tumors were detected on US imaging by day 7 post-implantation. Tumor volume was significantly higher in the N1S1 model (231 mm3) compared to the McARH7777 model (82 mm3). From week 3 onwards, they observed approximately 50% tumor regression in the McARH7777 model and about 10% tumor regression in the N1S1 group. Complete tumor regression was seen in both models by week 5-6. Choi et al[12] compared the incidence rate and regression pattern in the N1S1 HCC model using two different cell numbers: 2.5 × 106 and 5 × 106 cells. Both groups exhibited 100% tumor incidence without regression up to week 3 post-implantation. However, after week 3, the tumor size decreased in the 2.5 × 106 cell group, while consistent tumor growth continued in the 5 × 106 group. Another study assessed tumor induction, size, progression, and mortality rates in the N1S1 rat HCC model using three different cell line compositions. One group of 15 animals was injected with 4 × 106 N1S1 cells in 100 µL DMEM. A second group of 15 animals received 2 × 106 cells in 30 µL DMEM, and a third group of 30 animals received 3 × 106 cells in 100 µL DMEM. The first group showed 100% tumor incidence, but also a high rate of intrahepatic and peritoneal metastasis, as well as mortality. The second group showed no tumor formation until week 4 post-implantation. In the third group, 100% tumor incidence was observed on day 7, with consistent tumor growth thereafter[22]
Methodological modifications implemented in this study ensured effective tumor development, promoting sci
As the tumor develops inside the body, it necessitates the adoption of a cost-effective imaging technique to obtain longitudinal data throughout the study period. Otherwise, sacrificing animals at various time points would have been required, leading to increased animal usage. The implementation of US imaging allowed for longitudinal monitoring with a reduced number of animals, adhering to the principles of the 3Rs (Replacement, Reduction, and Refinement). While advanced imaging modalities such as MRI, micro-CT, and PET are available for tumor imaging, these methods are often complex, hazardous, time-consuming, and costly. In contrast, an enhanced US system equipped with a high-frequency probe offers high-resolution images capable of detecting small tumor implants in a short time frame. This system is user-friendly, portable, safe for both the operator and the animal, and more affordable to purchase and maintain[22,24]. Herrero de la Parte et al[10] reported the utility of US imaging in an animal model of liver metastasis to monitor tumor progression and natural necrosis occurring in the tumor. Lin et al[24] reported the use of ultrasonic spectrum analysis to precisely evaluate changes in the tumor environment after Adriamycin therapy for breast cancer in nude mice. Laschke et al[14], reported high resolution US images as a reliable an easy method for evaluation endometriosis in small animal models and for evaluation of treatment response.
Our study demonstrates the utility of US imaging for tumor monitoring in a rodent model, specifically for assessing tumor induction by N1S1 cell implantation and evaluating the effects of Sorafenib, a first-line therapy for HCC, on N1S1 tumor growth in SD rats. A scan head with a 5-12 MHz frequency range provided adequate spatial resolution to clearly visualize liver tumors, as well as sufficient depth penetration to image the abdominal cavity without the need for contrast agents. US scanning was initiated on day 7 post-implantation and continued at weekly intervals throughout the treatment period up to day 35. Our imaging protocol confirmed a 100% tumor induction rate, with a hypoechoic nodule at the liver tip consistently visualized by day 7. Given the high risk of spontaneous tumor regression in this model, we performed frequent early-phase monitoring (three scans per week) to track tumor growth dynamics. Tumors in the exponential growth phase were selected for evaluating treatment response.
To validate the measurement accuracy of US imaging, we compared tumor volumes with micro-CT imaging. Micro-CT allowed precise visualization of liver tumors, and tumor dimensions were found to be comparable to those obtained via US. Histopathological confirmation of tumor presence further validated the imaging findings. Previous studies have also highlighted the utility of advanced imaging techniques in similar models. For example, Singh et al[22] used micro-PET-CT to validate the orthotopic N1S1 HCC model, while Nota et al[25] evaluated the feasibility of ExiTron nano 12000-enhanced CT imaging in a diethylnitrosamine-induced rat HCC model. Although these studies demonsated the accuracy and safety of CT imaging, the high cost of ExiTron nano 12000 limits its routine use in preclinical research. In our evaluation, US imaging required only approximately 10 minutes for both examination and analysis, compared to micro-CT imaging without contrast, which took approximately 10 minutes for scanning and an additional 20 minutes for image reconstruction. Linxweiler et al[26] reported comparable effectiveness of US with MRI and CT for monitoring orthotopic renal tumors in athymic nude mice, showing a high correlation (r > 0.96) among the three modalities. In contrast, tumor volume measurements using calipers showed slightly lower correlation.
US imaging effectively differentiated the antitumor efficacy of Sorafenib in the N1S1 HCC model. It clearly distinguished between the aggressive tumor progression in untreated animals and the significant tumor growth inhibition in the Sorafenib-treated group. Tumor volumes estimated by US imaging corresponded closely with histopathological evaluation, confirming the accuracy of the imaging method. After completion of treatment, animals were euthanized and tumor dimensions were measured using a VC. A strong agreement was observed between US and VC measurements. However, for larger tumors, the agreement was slightly reduced—likely due to challenges in defining tumor boundaries in large or irregularly shaped masses using calipers. Consistent with findings by Laschke et al[14], we recommend using VC measurements to validate US imaging results, rather than relying on caliper measurements alone for tumor volume assessment.
US imaging conducted by an expert radiologist demonstrated high sensitivity, specificity, and minimal intra-observer variability. In contrast, measurements by non-experts showed reduced specificity, particularly in detecting small or early-stage tumors and regressive tumors. Notably, trained observers demonstrated excellent correlation with expert measurements, maintaining strong sensitivity and specificity in tumor detection. However, novice observers exhibited substantial inter-observer variability, underscoring the importance of proper training and experience for accurate and consistent tumor diagnosis and monitoring. Learning curve analysis revealed a rapid improvement in performance, with significant error reduction over time—highlighting the user-friendliness and operational ease of US imaging systems. Singh et al[27] also reported good intra- and inter-observer reproducibility of US in the serial imaging of mouse prostate tumors, supporting our findings.
Roth et al[15] demonstrated that US imaging offers accurate volumetric assessments, reporting a median bias of −21% compared to MRI in the evaluation of superficial xenograft model. Similarly, a study evaluating robotic US systems integrating bioluminescence imaging and high-resolution US showed strong correlations with MRI for orthotopic tumor models[28]. Although US may not fully replace advanced imaging modalities (MRI, micro-CT, or PET)—especially in functional imaging or detailed anatomical assessment—it offers significant advantages in preclinical research. MRI and CT require long scan times, costly infrastructure, and specialized personnel. In contrast, US systems are portable, cost-effective, and easier to operate within standard animal facilities. Importantly, our study confirms that US imaging provides tumor volume measurements comparable to advanced imaging techniques, while offering substantial reductions in time and cost. This highlights the potential of US imaging in the development of cost-efficient and scalable tumor models with high induction rates, low regression rates, and effective monitoring of anti-tumor therapeutic responses—facilitating progress in translational oncology research
We have established a reproducible orthotopic N1S1 hepatocellular carcinoma model in SD rats, achieving tumor induction by day 7, without perioperative complications. High frequency US imaging emerges as a promising and cost-effective technique for evaluating the effects of various drugs in the treatment of HCC. In our study, we utilized a portable US system equipped with a high-frequency transducer to obtain high-resolution images of N1S1 HCC tumors and assess the impact of Sorafenib on tumor growth. The resolution and accuracy of US imaging were comparable to those of more complex and costly techniques, such as CT scans, which require specialized expertise. Our findings validate that US imaging in this animal model offers a reliable, affordable, and user-friendly approach for accurate tumor growth monitoring. In summary, US imaging offers a robust platform for serial volumetric evaluation in orthotopic HCC model, suitable for therapeutic screening and translational research, while upholding ethical and economic imperatives.
We would like to acknowledge Dr. Deepthy Menon, Professor, Amrita Centre for Nanoscience and Molecular Medicine for her kind support in providing US imaging system for the work. We would like to acknowledge Dr. Siddaramana Gowd, Assistant Professor, Amrita Centre for Nanoscience and Molecular Medicine for performing micro-CT analysis. We would like to acknowledge, Ms. Bhagyalakshmi G. Nair and Ms. M. Divyadarshini for their support during US imaging evaluation. We would like to acknowledge the language editing support by Ms. Devi.S, faculty, Verbal and communication skills, Amrita School of Pharmacy. We would like to acknowledge Dr. Shanti Kumar V. Nair, Associate Provost, Amrita Vishwa Vidyapeetham for the facilities provided.
| 1. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 533] [Article Influence: 177.7] [Reference Citation Analysis (2)] |
| 2. | Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens VEPP, Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer. 2022;161:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 349] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 3. | Guo H, Xu X, Zhang J, Du Y, Yang X, He Z, Zhao L, Liang T, Guo L. The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies. Pharmaceuticals (Basel). 2024;17:1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 4. | Zhang HE, Henderson JM, Gorrell MD. Animal models for hepatocellular carcinoma. Biochim Biophys Acta Mol Basis Dis. 2019;1865:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Macek Jilkova Z, Kurma K, Decaens T. Animal Models of Hepatocellular Carcinoma: The Role of Immune System and Tumor Microenvironment. Cancers (Basel). 2019;11:1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Puaux AL, Ong LC, Jin Y, Teh I, Hong M, Chow PK, Golay X, Abastado JP. A comparison of imaging techniques to monitor tumor growth and cancer progression in living animals. Int J Mol Imaging. 2011;2011:321538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Li J, Wang X, Ren M, He S, Zhao Y. Advances in experimental animal models of hepatocellular carcinoma. Cancer Med. 2023;12:15261-15276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Baker MA. Technical note – Considerations for MR imaging of small animals. Radiography. 2011;17:171-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Coatney RW. Ultrasound imaging: principles and applications in rodent research. ILAR J. 2001;42:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Herrero de la Parte B, García-Alonso I, Mar-Medina C, Iturrizaga S, Saiz-López A, Hernández-Farto L, Del Campo-Clemente C, Echevarría-Uraga JJ. Ultrasound Tumor Size Assessment, Histology and Serum Enzyme Analysis in a Rat Model of Colorectal Liver Cancer. Ultrasound Med Biol. 2020;46:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Charles L, Sekar S, Osooly M, Javed S, Williams KC, Welch I, Barta I, Saatchi K, Häfeli UO. Development of an immunosuppressed orthotopic hepatocellular carcinoma rat model for the evaluation of chemo- and radioembolization therapies. Eur J Pharm Biopharm. 2024;196:114180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Choi B, Pe J, Yu B, Kim DH. Syngeneic N1-S1 Orthotopic Hepatocellular Carcinoma in Sprague Dawley Rat for the Development of Interventional Oncology-Based Immunotherapy: Survival Assay and Tumor Immune Microenvironment. Cancers (Basel). 2023;15:913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chandrababu G, Varkey M, Devan AR, Anjaly MV, Unni AR, Nath LR. Kaempferide exhibits an anticancer effect against hepatocellular carcinoma in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:2461-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Laschke MW, Körbel C, Rudzitis-Auth J, Gashaw I, Reinhardt M, Hauff P, Zollner TM, Menger MD. High-resolution ultrasound imaging: a novel technique for the noninvasive in vivo analysis of endometriotic lesion and cyst formation in small animal models. Am J Pathol. 2010;176:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Roth D, Safi M, Vilhelmsson Timmermand O, Sereti E, Molendowska M, Gottschalk M, Bjartell A, Ceberg C, Szczepankiewicz F, Strand J. Evaluation of superficial xenograft volume estimation by ultrasound and caliper against MRI in a longitudinal pre-clinical radiotherapeutic setting. PLoS One. 2024;19:e0307558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Liu CN, Morin J, Dokmanovich M, Bluette CT, Goldstein R, Manickam B, Bagi CM. Nanoparticle contrast-enhanced micro-CT: A preclinical tool for the 3D imaging of liver and spleen in longitudinal mouse studies. J Pharmacol Toxicol Methods. 2019;96:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12727] [Article Influence: 6363.5] [Reference Citation Analysis (8)] |
| 18. | Stribbling SM, Beach C, Ryan AJ. Orthotopic and metastatic tumour models in preclinical cancer research. Pharmacol Ther. 2024;257:108631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 19. | Žarković N, Jaganjac M, Žarković K, Gęgotek A, Skrzydlewska E. Spontaneous Regression of Cancer: Revealing Granulocytes and Oxidative Stress as the Crucial Double-edge Sword. Front Biosci (Landmark Ed). 2022;27:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lee TK, Na KS, Kim J, Jeong HJ. Establishment of animal models with orthotopic hepatocellular carcinoma. Nucl Med Mol Imaging. 2014;48:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Buijs M, Geschwind JF, Syed LH, Ganapathy-Kanniappan S, Kunjithapatham R, Wijlemans JW, Kook Kwak B, Ota S, Vali M. Spontaneous tumor regression in a syngeneic rat model of liver cancer: implications for survival studies. J Vasc Interv Radiol. 2012;23:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Singh G, Bendale K, Talwelkar S, Pawade S, Gera P, Patil A, Chavan P, Subramanian S, Chaudhari P. Establishment of an orthotopic syngeneic rat model of hepatocellular carcinoma and its validation with microPET-CT imaging. Integr Cancer Sci Therap. 2020;7. [DOI] [Full Text] |
| 23. | Bernsen MR, McDougald W, Mezzanotte L, Moran CM, Tavares A, van der Weerd L. Editorial: Small animal imaging: Technological and methodological advances to improve the translational power. Front Med (Lausanne). 2022;9:1099233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Lin CY, Cao LH, Wang JW, Zheng W, Chen Y, Feng ZZ, Li AH, Zhou JH. Ultrasonic spectrum analysis for in vivo characterization of tumor microstructural changes in the evaluation of tumor response to chemotherapy using diagnostic ultrasound. BMC Cancer. 2013;13:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Nota T, Kageyama K, Yamamoto A, Kakehashi A, Yonezawa H, Jogo A, Sohgawa E, Murai K, Ogawa S, Miki Y. Safety and Feasibility of Contrast-Enhanced Computed Tomography with a Nanoparticle Contrast Agent for Evaluation of Diethylnitrosamine-Induced Liver Tumors in a Rat Model. Acad Radiol. 2023;30:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Linxweiler J, Körbel C, Müller A, Jüngel E, Blaheta R, Heinzelmann J, Stöckle M, Junker K, Menger MD, Saar M. Experimental imaging in orthotopic renal cell carcinoma xenograft models: comparative evaluation of high-resolution 3D ultrasonography, in-vivo micro-CT and 9.4T MRI. Sci Rep. 2017;7:14249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Singh S, Pan C, Wood R, Yeh CR, Yeh S, Sha K, Krolewski JJ, Nastiuk KL. Quantitative volumetric imaging of normal, neoplastic and hyperplastic mouse prostate using ultrasound. BMC Urol. 2015;15:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Rojas JD, Joiner JB, Velasco B, Bautista KJB, Aji AM, Moore CJ, Beaumont NJ, Pylayeva-Gupta Y, Dayton PA, Gessner RC, Czernuszewicz TJ. Validation of a combined ultrasound and bioluminescence imaging system with magnetic resonance imaging in orthotopic pancreatic murine tumors. Sci Rep. 2022;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/