Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.107735
Revised: May 28, 2025
Accepted: September 11, 2025
Published online: October 27, 2025
Processing time: 213 Days and 12.3 Hours
Chronic hepatitis B (CHB) remains a significant global health challenge. The natural course of CHB is traditionally divided into four phases: (1) Immune tolerance; (2) Immune activation; (3) Immune control; and (4) Immune escape. However, approximately 20%-30% of patients referred to as the "gray zone" (GZ) do not fit neatly into these categories. These patients often exhibit elevated hepatitis B virus DNA levels alongside normal or mildly elevated alanine aminotransferase levels, placing them at significant risk for liver fibrosis, cirrhosis, and hepatocellular carcinoma. However, current clinical guidelines generally do not recommend antiviral therapy for GZ patients, increasing their vulnerability to adverse outcomes. This mini-review explores the challenges and gaps in CHB management, focusing on GZ patients. It also highlights recent advancements in therapeutic strategies and updates in clinical guidelines, emphasizing the need for a more inclusive, risk-adapted approach to treatment. By leveraging novel biomarkers, noninvasive fibrosis assessment tools, and artificial intelligence-driven predictive models, this article advocates for early intervention to mitigate disease progression and improve clinical outcomes in this overlooked population.
Core Tip: Gray zone patients with chronic hepatitis B represent a critical subgroup that is often overlooked by traditional treatment guidelines. This mini-review highlights the need for a paradigm shift toward a more inclusive and risk-adapted therapeutic approach. By utilizing novel biomarkers, advanced predictive models, and noninvasive tools, clinicians can better identify high-risk patients and implement early antiviral therapy, ultimately reducing the risks of liver fibrosis, cirrhosis, and hepatocellular carcinoma in this vulnerable population.
- Citation: Viet Luong T, Phan Hong Nguyen N, Nguyen TV, Tran DH, Dinh Nguyen T, Nguyen Ngoc Dang H. Gray zone and the need for expansion in chronic hepatitis B: From theory to clinical practice. World J Hepatol 2025; 17(10): 107735
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/107735.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.107735
Chronic hepatitis B (CHB) remains a major global health challenge, and is a leading cause of chronic hepatitis, liver fibrosis, and hepatocellular carcinoma (HCC)[1].
Timely and effective antiviral therapy can suppress hepatitis B virus (HBV) replication, reduce hepatic inflammation and necrosis, and prevent or even reverse liver fibrosis, including early-stage cirrhosis. As a result, antiviral treatment significantly lowers the risk of disease progression to cirrhosis and HCC, ultimately reducing mortality rates[2]. Notably, a study by Kim et al[3] demonstrated that nucleos(t)ide analog (NAs) therapy significantly reduced the incidence of HCC, highlighting the long-term benefits of early antiviral intervention in CHB patients.
However, most major international clinical practice guidelines, including those from the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Asian Pacific Association for the Study of the Liver (APASL), recommend antiviral treatment primarily for patients in the immune-active and immune clearance phases[1,4,5].
Nevertheless, some patients who fall outside the current treatment window remain at a considerably elevated risk of hepatic inflammation, liver fibrosis, and HCC. In particular, these individuals do not fit clearly into any of the recognized natural history phases of HBV infection and are classified as being in the Gray zone (GZ). Current clinical guidelines do not provide specific recommendations for antiviral therapy in this patient subgroup[6].
Given these uncertainties, there is an urgent need to reassess risk stratification and expand treatment indications for GZ CHB patients. Recent advancements in noninvasive fibrosis assessment [FibroScan, magnetic resonance elastography (MRE)], novel biomarkers [quantitative hepatitis B surface antigen (qHBsAg), HBV RNA, hepatitis B core-related antigen (HBcrAg)], and artificial intelligence (AI)-driven predictive models have provided new insights into disease progression and individualized treatment strategies[7-9]. Furthermore, accumulating evidence suggests that early antiviral therapy may help reduce long-term complications and improve patient outcomes[10].
This mini-review aims to (1) Define GZ in CHB patients; (2) Summarize recent advancements in risk assessment; (3) Explore the rationale for expanding antiviral treatment indications; and (4) Discuss future perspectives on improving CHB management. By addressing these aspects, this review highlights the need for a more inclusive approach to treating CHB, ensuring that patients in the GZ receive timely and appropriate care to prevent disease progression.
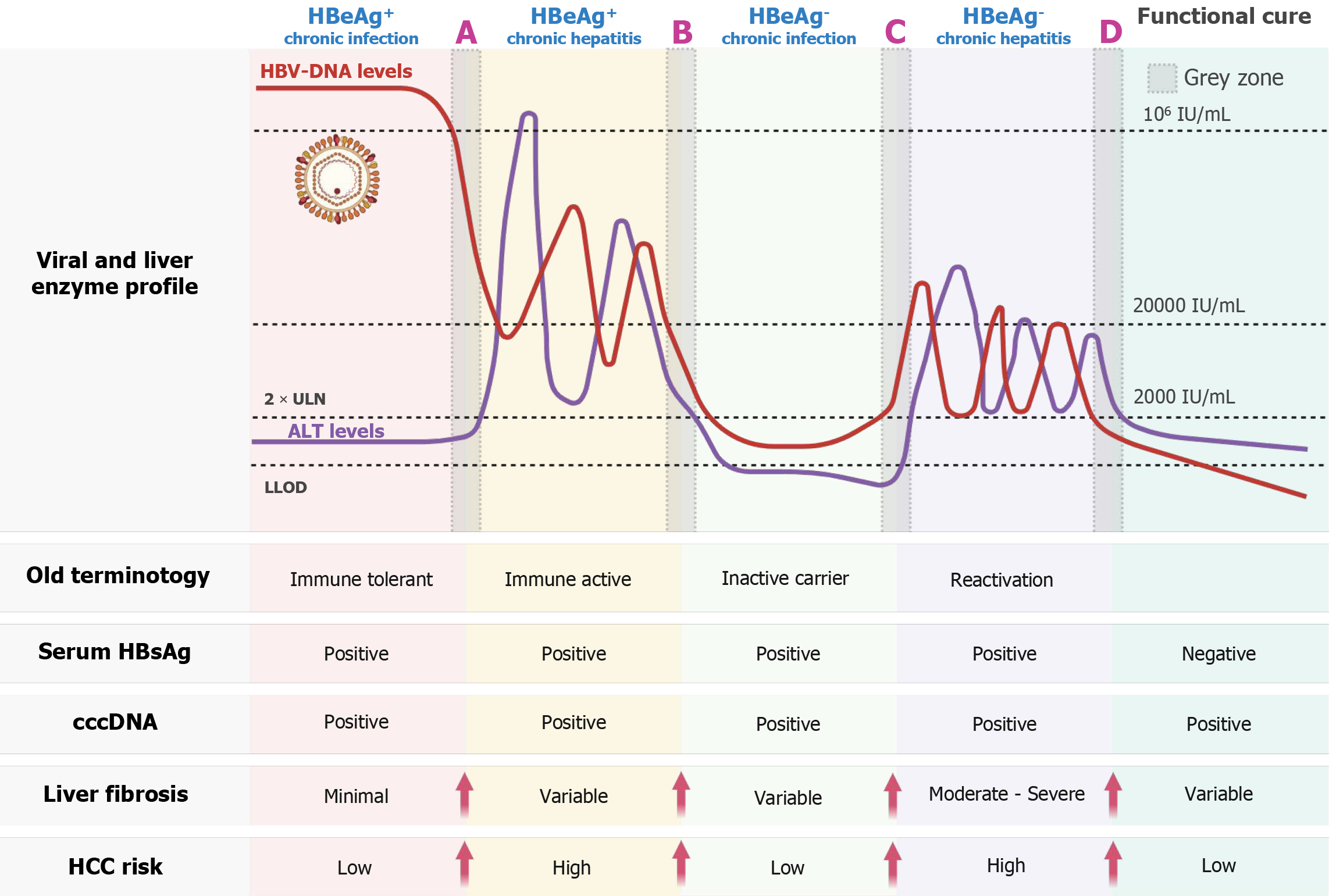
The natural course of CHB is conventionally divided into four distinct phases-immune-tolerant, immune-active, inactive carrier, and reactivation-on the basis of hepatitis B e antigen (HBeAg) status, HBV DNA levels, ALT levels, and his
In numerous instances, fluctuations in the serum HBV DNA level occur before ALT levels rise. In HBeAg-positive CHB patients, the viral load at the early stage of infection typically exceeds 109 IU/mL and may gradually decline to below 106 IU/mL before any ALT elevation is observed. Similarly, in HBeAg-negative individuals during the inactive phase, some patients experience a gradual increase in viral load, followed by ALT elevation months or even years later. These groups are characterized by HBV DNA levels ≥ 2000 IU/mL, normal or minimally elevated ALT levels [2 × upper limit of normal (ULN)][6]. These two examples of transitional or indeterminate presentations do not fit into any of the traditional phases and are collectively referred to as the “gray zone”. Specifically, they are considered two of the four GZ subgroups.
GZ patients are often overlooked or inconsistently managed. A representative cohort study by Spradling et al[12] in the United States involving 1598 CHB patients revealed that more than 50% could not be definitively assigned to a conventional disease phase.
To better understand this group, GZ patients have been subclassified into four categories on the basis of HBeAg status and ALT and HBV DNA levels: GZ-A: HBeAg-positive, normal ALT, and HBV DNA ≤ 106 IU/mL; GZ-B: HBeAg-positive, elevated ALT, and HBV DNA ≤ 2 × 104 IU/mL; GZ-C: HBeAg-negative, normal ALT, and HBV DNA ≥ 2 × 10³ IU/mL; and GZ-D: HBeAg-negative, elevated ALT, and HBV DNA ≤ 2 × 10³ IU/mL[13,14].
To complement this classification, Figure 1 maps the GZ subgroups (GZ-A to GZ-D) onto the updated natural history framework of CHB, integrating serologic profiles, HBV DNA levels, ALT activity, fibrosis stage, and estimated HCC risk. This schematic provides a comprehensive visual summary of the biological heterogeneity and prognostic relevance of each subgroup.
Importantly, GZ patients constitute a heterogeneous population. While some remain stable for years, others may progress to advanced fibrosis or HCC[13]. A cohort study by Yao et al[13] of 4759 CHB patients revealed that 27.78% fell into the GZ, with GZ-D being the most common subgroup (53.56%), and the majority of patients in the GZ stage were HBeAg (-) (78.3%). Among those in classified within the indeterminate phases GZ-B and GZ-D of chronic hepatitis B, both the Aspartate Aminotransferase to Platelet Ratio Index (APRI) and Fibrosis-4 (FIB-4) scores were significantly elevated compared to other groups (P < 0.001). Notably, a substantial proportion of GZ-B patients exhibited advanced fibrosis, with 33.3% having FIB-4 scores > 3.25, and cirrhosis, with 25.8% having APRI scores > 2.0. In contrast, the prevalence of advanced fibrosis and cirrhosis was significantly lower in the GZ-A, GZ-C, and GZ-D subgroups.
A study by Zhang et al[14] further revealed that the risk of HCC progression in GZ patients was comparable to that observed in immune-tolerant, immune-active HBeAg-positive, and inactive carrier phases. Specifically, GZ-B patients, similar to immune-active individuals, carry a particularly high risk of progression to HCC. GZ-D also represents a high-risk subgroup due to ALT elevation. In contrast, GZ-A and GZ-C, although seemingly inactive, are not necessarily benign and warrant close surveillance owing to the potential for silent disease progression. These findings highlight the necessity of subgroup-specific management strategies.
Furthermore, several risk factors, including older age, HBeAg positivity, and elevated ALT levels, have been identified as predictors of disease progression in GZ CHB patients. Age plays a pivotal role in the natural history and prognosis of CHB, with the risk of adverse outcomes increasing with increasing age[15]. The HBeAg-positive state typically reflects active viral replication and hepatic inflammation and has been linked to increased rates of fibrosis and cirrhosis[16]. Serum ALT remains a cornerstone biomarker for hepatitis activity[17]. Accordingly, older patients who are HBeAg positive with elevated ALT warrant particularly close monitoring, given their heightened risk of developing advanced liver disease[13].
Patients in the GZ of CHB often fall outside the scope of conventional antiviral treatment criteria. Nevertheless, accumulating evidence suggests that this population remains at significant risk for disease progression, including liver fibrosis, cirrhosis, and HCC, thereby warranting reconsideration of current treatment thresholds.
A retrospective cohort study[18] followed 3366 untreated, noncirrhotic CHB patients over a mean of 12.5 years. At baseline, 38.7% were classified as having indeterminate disease, and by year 10, 52.7% of these patients remained in the indeterminate phase, whereas 21.7% had transitioned to the immune-active phase. Notably, persistently indeterminate patients had a 14-fold increased risk of developing HCC compared with inactive carriers (P < 0.05), even after adjusting for age and HBV DNA levels.
A retrospective study conducted between 2013 and 2023 by Zhang et al[14] examined 2906 GZ patients and reported 5-year and 10-year HCC incidences of 3.26% and 6.91%, respectively, which are comparable to rates reported in immune-active CHB patients. Within the GZ classification, the GZ-B subgroup had the highest HCC risk, with a 10-year incidence reaching 26.98%, paralleling that of the HBeAg-positive and HBeAg-negative immune-active groups. Furthermore, significant histological disease (SHD) was prevalent across all GZ subtypes, particularly GZ-B (100%), followed by GZ-A (84.0%), GZ-D (69.9%), and GZ-C (67.0%), reflecting substantial underlying liver pathology despite the absence of classical treatment indications.
Zhang et al[14] reported that a substantial proportion of CHB patients who ultimately developed HCC were initially ineligible for antiviral therapy according to the AASLD, EASL, or APASL criteria. This gap underscores the limitations of traditional ALT-based thresholds in identifying at-risk individuals.
While serum ALT is widely used as a marker of hepatic inflammation, it is prone to fluctuations and lacks sensitivity in detecting ongoing necroinflammation. Several studies have reported progressive liver disease even among patients with normal or mildly elevated ALT levels. Wang et al[19] reported that 72.7% of GZ patients had significant fibrosis (≥ F2), with the highest prevalence observed in HBeAg-positive GZ-B individuals. Based on the findings, the study recommends initiating antiviral therapy for GZ CHB patients, including both HBeAg-positive and HBeAg-negative individuals.
Concerns have also been raised regarding the accuracy of the current ALT, which is traditionally set at 40 U/L. Lower ALT thresholds, 30 U/L for men and 19 U/L for women, have demonstrated better predictive value for liver fibrosis and disease progression[20].
Collectively, these findings argue against a narrow, ALT-centric approach and support a more individualized strategy for antiviral initiation in GZ patients. Incorporating HBV DNA levels, fibrosis staging, HBeAg status, and age into treatment decision-making may improve long-term outcomes and help reduce the burden of cirrhosis and HCC in this overlooked but high-risk population.
Diverse global studies, ranging from prospective cohorts to meta-analyses, consistently demonstrate that early antiviral therapy in 'GZ' patients reduces the risk of fibrosis, cirrhosis, and HCC, as summarized in Table 1[21-25].
| Publication year | Ref. | Country | Study sample characteristics | Key findings |
| 2007 | Chen et al[21] | Taiwan | 3653 chronic hepatitis B patients without cirrhosis, followed for 11.1 years, mostly HBeAg (+), HBV DNA ≥ 2000 IU/mL | HBV DNA ≥ 2000 IU/mL increases cirrhosis risk (RR: 2.5) and HCC risk (RR: 9.9), independent of ALT |
| 2023 | Lee et al[22] | South Korea | 2978 "GZ" patients, treated vs untreated, HBV DNA ≥ 2000 IU/mL, nationwide data | Untreated patients have higher fibrosis risk (HR: 1.8) and HCC risk (HR: 2.1) compared to the treated group |
| 2023 | Zhou et al[23] | China | 194 HBeAg (-) patients, HBV DNA positive, normal ALT, treated vs untreated, followed for 54 months | Treatment reduces cirrhosis (2.3% vs 13.4%; P = 0.011) compared to the untreated group |
| 2024 | Zhang et al[24] | United States | 324 "GZ" untreated patients, HBV DNA ≥ 2000 IU/mL, normal/Low ALT, liver biopsy performed | 37% have significant histological disease: 19.2% severe fibrosis (F3-F6), 9% severe inflammation (G7-G18), despite normal ALT |
| 2025 | Lai et al[25] | International | 103 studies (70 case-control: 18739 patients; 32 cohort: 15118 patients; 1 randomised controlled trial: 160 patients) with HBV cirrhosis | NAs improve survival (HR: 0.65; 95%CI: 0.56-0.76) and reduce HCC risk (RR: 0.78; 95%CI: 0.66-0.92) |
Since 2016, the APASL, EASL, and AASLD have introduced several changes compared with previous guidelines. However, significant limitations remain for patients in the GZ (Table 2)[1,4,5].
| Guideline | Recommendation for GZ | Limitations |
| Asian Pacific Association for the Study of the Liver (2016, updated 2021) | Therapy if HBV DNA > 2000 IU/mL and histological evidence of damage; normal ALT often delays treatment | Requires invasive biopsy; limited access to advanced diagnostics in low-resource settings; no clear GZ protocol |
| American Association for the Study of Liver Diseases (2018) | Antiviral therapy if HBV DNA > 2000 IU/mL, ALT > 2 × upper limit of normal, or fibrosis ≥ F2. GZ patients often excluded unless biopsy-confirmed damage | Heavy reliance on ALT thresholds; no specific GZ criteria; limited guidance for normal ALT with high HBV DNA |
| European Association for the Study of the Liver (2017, updated 2022) | Therapy considered for HBV DNA > 2000 IU/mL, age > 30, or family history of hepatocellular carcinoma, even with normal ALT | Inconsistent ALT thresholds; lack of GZ-specific trial data; variable adoption across regions |
While the above classical guidelines have long provided a foundation for the management of CHB-particularly in GZ patients-recent years have seen the emergence of new evidence that further informs and refines treatment strategies for this challenging population.
The 2022 Chinese Guidelines recommend treatment for patients with normal ALT and detectable HBV DNA if aged > 30 years or with a family history of HCC[26]. A 2024 update from the Chinese Society of Hepatology proposed a "Treat-all" strategy, advocating therapy for approximately 95% of patients with detectable HBV DNA to align with the WHO’s 2030 hepatitis elimination goal[27].
The WHO 2024 guidelines endorse early therapy to reduce the risk of HCC and cirrhosis, particularly in high-prevalence regions, including GZ patients with HBV DNA ≥ 2000 IU/mL and risk factors such as age or fibrosis[28].
The EASL 2025 guidelines also support early intervention for GZ patients, recommending treatment when HBV DNA > 2000 IU/mL with ALT > ULN and/or fibrosis ≥ F2 (assessed via noninvasive tests such as APRI or FibroScan), and considering therapy in individuals > 30 years old or family history of HCC, using a simplified algorithm independent of HBeAg status to enhance global HBV elimination efforts[29].
Together, this evidence and guidance underscore that HBV DNA, rather than ALT alone, should drive treatment decisions, especially for GZ patients with risk factors such as age > 30 years, a family history of HCC, or metabolic syndrome.
Accurate risk stratification is pivotal in managing CHB, particularly for GZ patients who often evade timely intervention under traditional guidelines. In recent years, advancements in noninvasive fibrosis assessment, novel biomarkers, and AI-driven predictive models have revolutionized the ability to identify high-risk individuals in this elusive subgroup, shifting the focus from ALT-centric thresholds to a more nuanced, risk-adapted approach.
Liver fibrosis is a key determinant of CHB progression, yet many GZ patients may already have significant fibrosis despite normal ALT levels. While liver biopsy remains the gold standard for fibrosis staging, it is an invasive diagnostic method, and its use is not practical, especially for follow-up or screening. For this reason, noninvasive techniques are used to estimate liver fibrosis.
FibroScan, which works with the principles of transient elastography, is widely used to detect fibrosis in clinical practice. A correlation has been found between biopsy-detected fibrosis and FibroScan measurements. Liver stiffness is a surrogate for fibrosis, with high diagnostic accuracy for significant fibrosis (≥ F2) and cirrhosis (F4), but this method is not universally applicable and is contraindicated in patients with ascites, significant obesity, or substantial chest wall adipose tissue, as these conditions can compromise the accuracy of the measurements. In the context of GZ CHB, studies have demonstrated that a substantial proportion of GZ patients have FibroScan values that are consistent with advanced fibrosis. FibroScan can help identify patients with significant fibrosis who may benefit from early antiviral treatment, even if their ALT levels are normal. This is crucial because untreated GZ patients with advanced fibrosis are at greater risk of disease progression and complications such as HCC[30].
Serum fibrosis scores are noninvasive tests that use a combination of blood test results to estimate the degree of liver fibrosis in individuals with CHB. These scores can be helpful in risk stratification, particularly when FibroScan results fall within the GZ and a liver biopsy is not readily available. Two commonly used serum fibrosis scoring systems are the APRI and FIB-4[31].
+ APRI: The APRI utilizes aspartate aminotransferase (AST) and platelet counts from a standard blood test. However, the APRI has limitations in accuracy, especially in the GZ, and a significant proportion of patients may have indeterminate scores. One study analyzed 324 CHB patients in the GZ and reported that the APRI differed significantly among various GZ subgroups (GZ-A to GZ-D). Specifically, the proportion of patients with an APRI ≥ 2 (a threshold suggestive of cirrhosis) was 15.4% in the GZ-B group, which was characterized by higher ALT levels and lower HBV DNA. These findings suggest that the APRI may have the potential to differentiate varying risks of fibrosis within GZ patient subgroups[24].
+ FIB-4: FIB-4 incorporates age, AST, ALT, and platelet count. It is another readily available score with relatively good performance in identifying advanced fibrosis. However, similar to the APRI, it may have limitations in accurately staging fibrosis in the GZ[24].
Although these scores are simple, cost-effective tools that combine biochemical markers to estimate fibrosis severity, it is important to note that serum fibrosis scores are not a replacement for liver biopsy, which remains the gold standard for assessing liver fibrosis. However, they can be useful for initial risk stratification and to help guide decisions about further investigations, such as liver biopsy or repeat Fibroscan, in CHB patients with GZ Fibroscan results.
MRE is a noninvasive imaging technique that assesses the stiffness of liver tissue by measuring the velocity of shear waves passing through it. It is similar to an advanced form of palpation, providing a visual map (elastogram) of tissue stiffness. MRE assesses the entire liver, providing a more complete picture of fibrosis than a localized biopsy sample does. MRE can help differentiate between different stages of fibrosis when FibroScan results are inconclusive. This is particularly important in GZ, where treatment decisions may be challenging. Compared with conventional imaging techniques, MRE can also detect fibrosis at earlier stages but is limited by cost and availability[32].
HBV DNA and ALT provide limited insight into CHB activity, often underestimating risk in GZ patients. Novel biomarkers address this gap, enhancing stratification.
Reflects intrahepatic HBV replication activity and immune control. The qHBsAg levels can also be used to distinguish between different phases of CHB infection. In addition, qHBsAg can be used to monitor disease activity and predict the likelihood of HBsAg sero-clearance (loss of HBsAg from the blood), which is associated with a reduced risk of liver complications. The level of HBsAg, such as HBeAg positivity, HBV DNA elevation, and genotype, is a factor affecting the prognosis of patients with this disease. Previous studies have shown that a qHBsAg level > 1000 IU/mL in HBeAg-negative CHB patients is associated with disease progression and HCC development[33]. However, the precise magnitude of HBsAg reduction or the threshold for treatment success remains uncertain. Low qHBsAg levels before treatment (< 1000 IU/mL) and a decrease in the early stages of treatment (e.g., 10-100 IU/mL) were indicative of the development of HBsAg loss, but the limit value was not clearly determined. While qHBsAg is a promising tool for managing CHB, it is important to note that it is not a perfect test. The qHBsAg levels can be affected by factors such as the stage of liver disease and the presence of other liver conditions. Therefore, qHBsAg results should be interpreted in the context of a patient's overall clinical picture[34].
HBcrAg is a relatively new marker in CHB that reflects the activity of intrahepatic covalently closed circular DNA (cccDNA). HBcrAg testing presents a cost-effective alternative to HBV DNA quantification for determining treatment eligibility, particularly in settings with limited resources. It combines three viral proteins: (1) Hepatitis B core antigen; (2) HBeAg; and (3) A 22-kDa precore protein (p22cr). HBcrAg can be detected even when serum HBV DNA or HBsAg is undetectable. This makes it a potentially useful marker for monitoring patients on antiviral therapy and for predicting the risk of HCC. Higher HBcrAg levels generally correlate with increased HBV replication and cccDNA activity. In the GZ, an elevated HBcrAg may suggest a greater risk of disease progression and could influence the decision to initiate antiviral therapy[35].
HBV RNA is primarily pregenomic RNA that is transcribed from covalently closed cccDNA in liver cells. This cccDNA is responsible for the persistence of HBV infection, and measuring its activity is crucial for managing CHB. HBV RNA is a relatively new marker in CHB that reflects the activity of HBV inside the liver. It is a promising marker for monitoring disease activity and treatment response, even when other markers, such as HBV DNA, are undetectable[36].
The integration of HBV RNA, HBcrAg, and qHBsAg into clinical practice is still under investigation, but preliminary data suggest that these markers could play crucial roles in identifying high-risk GZ patients before irreversible liver damage occurs.
Advancements in machine learning (ML) and AI have enabled the development of predictive models that integrate multiple clinical variables to improve CHB risk stratification. AI-based algorithms can analyze large datasets, incor
ML models are being increasingly explored for their ability to predict liver fibrosis and HCC in patients with CHB, particularly those in the GZ, where traditional diagnostic methods may be inconclusive. These models often utilize routinely available clinical and laboratory data to identify complex patterns and predict disease progression[38].
A study developed a decision tree model using five readily available serological biomarkers, including HBV-DNA, platelet count, thrombin time, international normalized ratio (INR), and albumin, to predict liver fibrosis stages in CHB patients. This model demonstrated high diagnostic accuracy in both training and external validation cohorts for differentiating fibrosis stages. These findings demonstrate the potential of ML to accurately diagnose liver fibrosis stages via simple blood tests, which can be beneficial for the clinical monitoring and treatment of CHB patients[39].
AI and ML models offer powerful approaches for enhancing the detection of significant liver disease in CHB patients who fall within the GZ. By analyzing routinely collected clinical data, including demographics, laboratory results, and even lifestyle factors extracted from electronic health records, these technologies can identify individuals with underlying liver pathology, such as fibrosis or inflammation, potentially earlier than traditional methods relying solely on markers such as ALT levels. This early identification is crucial for timely intervention and management.
The GZ in CHB diagnosis represents a particularly profound challenge for AI. The lack of clear diagnostic criteria for these patients makes it exceptionally difficult for AI to learn distinct boundaries for diagnosis and treatment initiation. AI models trained primarily on data from patients in well-defined phases might misclassify or fail to provide clinically useful insights for individuals in this GZ, where nuanced clinical judgment is often required to determine the appropriate course of action. The absence of definitive diagnostic criteria for the GZ further complicates the development and, crucially, validation of AI models specifically designed for this patient subgroup. If there is no universally agreed-upon "ground truth" for these indeterminate cases, training an AI to predict the need for intervention accurately and assess the model's performance becomes exceedingly challenging[40,41].
The limitations of ALT-based treatment decisions underscore the need for a more comprehensive, risk-adapted approach to managing CHB, particularly in the GZ population. By integrating noninvasive fibrosis assessment, novel biomarkers, and AI-driven predictive models, clinicians could tailor personalized care to actual risk.
Combining FibroScan, biomarkers, and AI sharpens the lens on GZ risk. An XGBoost model integrating these markers achieved AUCs of 0.88-0.95 for fibrosis and the best calibration curve for the prediction of HBeAg seroconversion[42].
AI shifts treatment focus from ALT to virological and histological drivers, enabling earlier and more precise intervention. AI-driven risk assessment tailor treatment, improving outcomes and optimizing resource use.
Guidelines anchored to ALT and HBV DNA cutoffs fail to reflect these advances. Integrating AI-driven risk scores and biomarkers could redefine GZ management and reduce HCC and cirrhosis rates. Calls for multicenter trials to validate these tools in real-world settings signal a path to updated standards that match technological leaps
The push to expand treatment for GZ CHB patients faces formidable hurdles, yet it also opens doors to transformative future strategies. Bridging evidence with action requires dismantling barriers and charting a bold path toward a cure.
Despite mounting evidence favoring early intervention, entrenched obstacles hinder the adoption of broader treatment criteria for GZ patients, delaying care for those at risk of silent progression.
Divergent treatment thresholds across major guidelines create confusion among clinicians. The AASLD advocates therapy only when ALT exceeds twice the ULN or when significant fibrosis is evident, whereas the EASL adopts a more nuanced stance, considering HBV DNA > 2000 IU/mL as potential triggers. Similarly, the APASL varies, often requiring histological proof of damage. This lack of consensus complicates a unified approach, leaving GZ patients in limbo[1,4,5].
Long-term antiviral therapy demands substantial resources, increasing the burden in low- and middle-income regions. Although effective, NAs, such as entecavir and tenofovir, require sustained use, with costs often outweighing healthcare budgets. In many systems, reimbursement hinges on strict criteria - e.g., ALT > 2 × ULN or cirrhosis - excluding GZ patients despite their risk[28]. A study in Asia-Pacific highlighted that only 30% of eligible CHB patients access treatment due to financial and logistical barriers[4], underscoring global inequity.
Outdated reliance on ALT as a treatment trigger persists among clinicians, overshadowing the risks of ongoing HBV replication. Many physicians view normal ALT as a green light to delay, unaware that a substantial proportion of patients in the GZ harbor SHD despite having ALT values within the normal range[14]. Patients also misinterpret normal ALT levels as safe, underestimating the risks of HCC and fibrosis - a perception gap that stalls proactive care[5].
There are many challenges in terms of starting trials in the GZ CHB group. First, the lack of a universally accepted definition complicates the design and comparison of clinical trials. In addition, defining how patients could benefit from an intervention can be difficult without a surrogate endpoint.
To overcome the GZ challenge, CHB management must evolve beyond static thresholds, embracing precision tools and bold cures pursuits that promise lasting impact.
Advanced biomarkers and risk models are key to identifying GZ patients for early intervention. In addition to HBV DNA and ALT, liver stiffness (via FibroScan), qHBsAg, and HBcrAg offer deeper insights into disease activity and progression risk. A nomogram integrating age, ALT, lymphocyte percentage, platelet count, and the INR achieved AUCs of 0.755 (development) and 0.707 (validation) for predicting treatment need, providing a practical tool for GZ triage[43]. Updating guidelines to reflect these markers, alongside broader insurance coverage and lower drug costs, could dismantle access barriers[1,28].
Novel therapies targeting HBV RNA, such as siRNAs or antisense oligonucleotides, aim to silence viral replication at its source, reducing cccDNA activity. Early trials have shown promise, with a decrease in HBV RNA correlated with HBsAg loss[28]. Immune-based strategies, including therapeutic vaccines, checkpoint inhibitors (e.g., anti-programmed cell death 1), and toll-like receptor agonists, seek to restore HBV-specific immunity, offering a path to functional cure-defined as sustained HBsAg loss and undetectable HBV DNA[4,44]. Combining these strategies with NAs could increase treatment efficacy, reducing the need for lifelong therapy.
AI-driven predictive models enhance decision-making by synthesizing multiparametric data. The nomogram from Yang et al[43] exemplifies this, guiding timely treatment in GZ patients with high discriminative power. Future iterations could incorporate qHBsAg, HBcrAg, and imaging, refining risk stratification and personalizing care plans[28]. This data-driven shift promises to align therapy with individual trajectories, optimizing outcomes.
Melding expanded NA access with curative innovations that could redefine CHB management. By targeting GZ patients early through the use of biomarkers and AI, and by advancing toward a functional cure, the hepatology community can move beyond symptom control to disease eradication. This integrated vision not only elevates care for GZ patients but also sets a new standard for CHB, reducing long-term morbidity and mortality[28,44].
The diverse progression of GZ patients with CHB poses a significant challenge, exposing the limits of ALT-driven treatment guidelines. Emerging evidence demands a broader, risk-based approach involving the use of HBV DNA, fibrosis assessments, and novel biomarkers to curb fibrosis and HCC risk through early intervention. Evolving guidelines to embrace advanced risk tools and biomarkers is essential for precise, effective care. Expanding access to antivirals and pursuing functional cures further promises to transform management. This shift to personalized, proactive strategies will enhance outcomes and ease the global burden of hepatitis B.
| 1. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4011] [Article Influence: 445.7] [Reference Citation Analysis (1)] |
| 2. | Fan P, Li LQ, Chen EQ. The urgency to expand the antiviral indications of general chronic hepatitis B patients. Front Med (Lausanne). 2023;10:1165891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kim S, Lee Y, Bang SM, Bak H, Yim SY, Lee YS, Yoo YJ, Jung YK, Kim JH, Seo YS, Yim HJ, Um SH, Byun KS, Yeon JE. Early Normalization of Alanine Aminotransferase during Antiviral Therapy Reduces Risk of Hepatocellular Carcinoma in HBV Patients. J Clin Med. 2021;10:1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2058] [Article Influence: 205.8] [Reference Citation Analysis (12)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3097] [Article Influence: 387.1] [Reference Citation Analysis (1)] |
| 6. | Lim YS. Gray zone of hepatitis B virus infection. Saudi J Gastroenterol. 2024;30:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Huang W, Peng Y, Kang L. Advancements of non-invasive imaging technologies for the diagnosis and staging of liver fibrosis: Present and future. VIEW. 2024;5:20240010. [RCA] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Lim YS. New biomarkers of hepatitis B virus (HBV) infection: HBV RNA and HBV core-related antigen, new kids on the block? Clin Mol Hepatol. 2023;29:118-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Nishida N. Advancements in Artificial Intelligence-Enhanced Imaging Diagnostics for the Management of Liver Disease-Applications and Challenges in Personalized Care. Bioengineering (Basel). 2024;11:1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (2)] |
| 10. | Lim YS, Kim WR, Dieterich D, Kao JH, Flaherty JF, Yee LJ, Roberts LR, Razavi H, Kennedy PTF. Evidence for Benefits of Early Treatment Initiation for Chronic Hepatitis B. Viruses. 2023;15:997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Yang HC. Revisiting the natural history of chronic hepatitis B infection. Clin Liver Dis (Hoboken). 2024;23:e0195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, Lu M, Boscarino JA, Schmidt MA, Trinacty CM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006-2013. Aliment Pharmacol Ther. 2016;44:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 13. | Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, Wu W, Liu Y, Chen Y, Zhang Z, Li J, Huang R, Wu C. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Zhang J, Yu S, Zhu K, Li S, Huang Y. Probability analysis of hepatocellular carcinoma in hepatitis patients in the gray zone. Front Med (Lausanne). 2024;11:1464981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 454] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF, Zhou XQ. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 611] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 18. | Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, Zhang J, Li J, Wong C, Wong C, Cheung RC, Yang HI, Nguyen MH. Natural History and Hepatocellular Carcinoma Risk in Untreated Chronic Hepatitis B Patients With Indeterminate Phase. Clin Gastroenterol Hepatol. 2022;20:1803-1812.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 19. | Wang J, Yan X, Zhu L, Liu J, Qiu Y, Li Y, Liu Y, Xue R, Zhan J, Jiang S, Geng Y, Wan Y, Li M, Mao M, Gao D, Yin S, Tong X, Xia J, Ding W, Chen Y, Li J, Zhu C, Huang R, Wu C. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone. Aliment Pharmacol Ther. 2023;57:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 20. | Yu HS, Jiang H, Li MK, Yang BL, Smayi A, Chen JN, Wu B, Yang YD. Lowering the threshold of alanine aminotransferase for enhanced identification of significant hepatic injury in chronic hepatitis B patients. World J Gastroenterol. 2023;29:5166-5177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797-816, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Lee SK, Kwon JH. HBeAg-positive grey-zone patients: Treatment beyond guideline recommendations? Clin Mol Hepatol. 2023;29:825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Zhou J, Wang FD, Li LQ, Li YJ, Wang SY, Chen EQ. Antiviral Therapy Favors a Lower Risk of Liver Cirrhosis in HBeAg-negative Chronic Hepatitis B with Normal Alanine Transaminase and HBV DNA Positivity. J Clin Transl Hepatol. 2023;11:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Zhang J, Yu S, Zhu K, Li S, Huang Y. Necessity of antiviral treatment for patients with chronic hepatitis B in the grey zone based on liver pathology analysis. Ann Med. 2024;56:2399757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Lai JC, Wong GL, Tse YK, Hui VW, Lai MS, Chan HL, Wong VW, Yip TC. Histological severity, clinical outcomes and impact of antiviral treatment in indeterminate phase of chronic hepatitis B: A systematic review and meta-analysis. J Hepatol. 2025;82:992-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | You H, Wang F, Li T, Xu X, Sun Y, Nan Y, Wang G, Hou J, Duan Z, Wei L, Jia J, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J Clin Transl Hepatol. 2023;11:1425-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 27. | Zhang M, Kong Y, Xu X, Sun Y, Jia J, You H. "Treat-all" Strategy for Patients with Chronic Hepatitis B Virus Infection in China: Are We There Yet? J Clin Transl Hepatol. 2024;12:589-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wong GL, Lemoine M. The 2024 updated WHO guidelines for the prevention and management of chronic hepatitis B: Main changes and potential implications for the next major liver society clinical practice guidelines. J Hepatol. 2025;82:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 29. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2025;83:502-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 132] [Article Influence: 132.0] [Reference Citation Analysis (1)] |
| 30. | Bera C, Hamdan-Perez N, Patel K. Non-Invasive Assessment of Liver Fibrosis in Hepatitis B Patients. J Clin Med. 2024;13:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Xu X, Wang H, Shan S, Sun Y, Xu X, You H, Jia J, Zhuang H, Kong Y; On Behalf Of The China Registry Of Hepatitis B Cr-HepB Group. The Impact of the Definitions of Clinical Phases on the Profiles of Grey-Zone Patients with Chronic Hepatitis B Virus Infection. Viruses. 2023;15:1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 570] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 33. | Gan Q, Huang Y, Zhu C, Zhao S, Fu H, Cai M, Wang J, Zhang C, Guo S, Cao Z, Xie Q. qHBsAg for the Identification of Liver Histological Abnormalities in HBeAg-Negative Chronic Hepatitis B Patients with Normal and Mildly Elevated ALT Levels. Can J Gastroenterol Hepatol. 2022;2022:8695196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Yıldız Kaya S, Mete B, Kaya A, Balkan II, Saltoglu N, Tabak ÖF. The role of quantitative HBsAg in patients with HBV DNA between 2000-20,000 IU/ml. Wien Klin Wochenschr. 2021;133:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Inoue T, Tanaka Y. The Role of Hepatitis B Core-Related Antigen. Genes (Basel). 2019;10:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Liu S, Zhou B, Valdes JD, Sun J, Guo H. Serum Hepatitis B Virus RNA: A New Potential Biomarker for Chronic Hepatitis B Virus Infection. Hepatology. 2019;69:1816-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 37. | Hou Y, Yan J, Shi K, Liu X, Gao F, Wu T, Meng P, Zhang M, Jiang Y, Wang X. Development and Validation of a Machine Learning-Based Model Used for Predicting Hepatocellular Carcinoma Risk in Patients with Hepatitis B-Related Cirrhosis: A Retrospective Study. Onco Targets Ther. 2024;17:215-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Su TH, Kao JH. Role of artificial intelligence in the management of chronic hepatitis B infection. Clin Liver Dis (Hoboken). 2024;23:e0164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Zhang C, Shu Z, Chen S, Peng J, Zhao Y, Dai X, Li J, Zou X, Hu J, Huang H. A machine learning-based model analysis for serum markers of liver fibrosis in chronic hepatitis B patients. Sci Rep. 2024;14:12081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Kim HY, Lampertico P, Nam JY, Lee HC, Kim SU, Sinn DH, Seo YS, Lee HA, Park SY, Lim YS, Jang ES, Yoon EL, Kim HS, Kim SE, Ahn SB, Shim JJ, Jeong SW, Jung YJ, Sohn JH, Cho YK, Jun DW, Dalekos GN, Idilman R, Sypsa V, Berg T, Buti M, Calleja JL, Goulis J, Manolakopoulos S, Janssen HLA, Jang MJ, Lee YB, Kim YJ, Yoon JH, Papatheodoridis GV, Lee JH. An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J Hepatol. 2022;76:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 41. | Rui F, Yeo YH, Xu L, Zheng Q, Xu X, Ni W, Tan Y, Zeng QL, He Z, Tian X, Xue Q, Qiu Y, Zhu C, Ding W, Wang J, Huang R, Xu Y, Chen Y, Fan J, Fan Z, Qi X, Huang DQ, Xie Q, Shi J, Wu C, Li J. Development of a machine learning-based model to predict hepatic inflammation in chronic hepatitis B patients with concurrent hepatic steatosis: a cohort study. EClinicalMedicine. 2024;68:102419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 42. | Shang H, Hu Y, Guo H, Lai R, Fu Y, Xu S, Zeng Y, Xun Z, Liu C, Wu W, Guo J, Ou Q, Chen T. Using machine learning models to predict HBeAg seroconversion in CHB patients receiving pegylated interferon-α monotherapy. J Clin Lab Anal. 2022;36:e24667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 43. | Yang XY, Li XD, Wu BY, Yang Q, Zheng YB, Zheng MH, Wu YP, Ma HY, Zuo J, Jia RX, Yu Y, Xu LY, Tian YX, An Q, Zhang T, He YL, Shi Y, Fan YC. A Model to Identify Gray Zone Patients With Chronic Hepatitis B Requiring Antiviral Therapy: A Multicenter Retrospective Study. J Infect Dis. 2025;232:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, Piratvisuth T, Jia JD, Mizokami M, Cheng G, Chen GF, Liu ZW, Baatarkhuu O, Cheng AL, Ng WL, Lau P, Mok T, Chang JM, Hamid S, Dokmeci AK, Gani RA, Payawal DA, Chow P, Park JW, Strasser SI, Mohamed R, Win KM, Tawesak T, Sarin SK, Omata M. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15:1031-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/