Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.102034
Revised: November 9, 2024
Accepted: December 6, 2024
Published online: January 27, 2025
Processing time: 91 Days and 18.7 Hours
Recent research indicates that the intestinal microbial community, known as the gut microbiota, may play a crucial role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). To understand this relationship, this study used a comprehensive bibliometric analysis to explore and analyze the currently little-known connection between gut microbiota and NAFLD, as well as new findings and possible future pathways in this field.
To provide an in-depth analysis of the current focus issues and research deve
In this study, all data were collected from the Web of Science Core Collection, and the related searches were completed on one day (February 21, 2024). The data were stored in plain text format to facilitate subsequent analysis. VOSviewer 1.6.20 and CiteSpace 6.1R6 Basic were used for knowledge graph construction and bibliometric analysis.
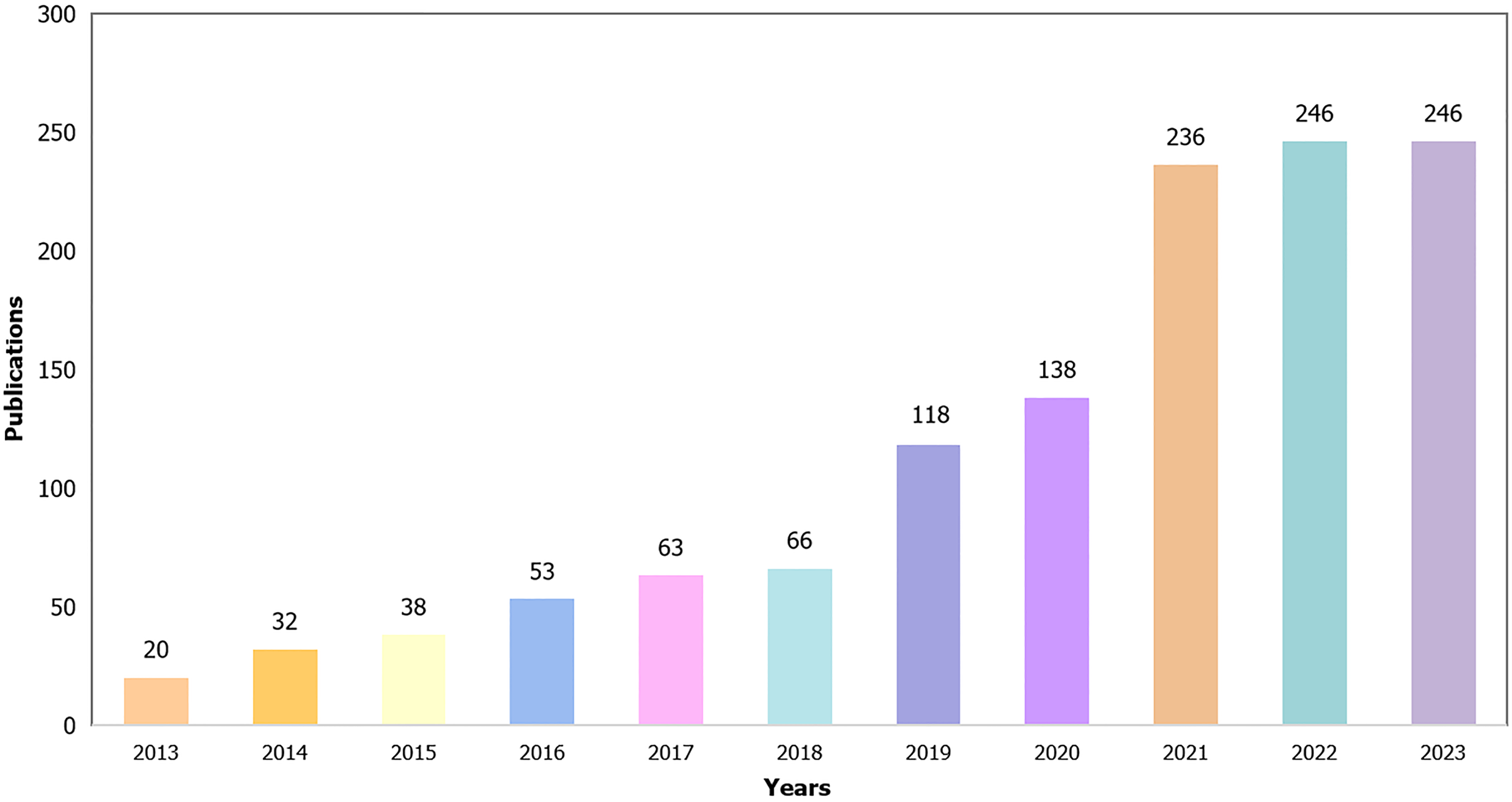
The study included a total of 1256 articles published from 2013 to 2023, and the number of published papers demonstrated an upward trend, reaching a peak in the last two years. The University of California, San Diego held the highest citation count, while Shanghai University of Traditional Chinese Medicine in China led in the number of published works. The journal "Nutrients" had the highest publication count, while "Hepatology" was the most frequently cited. South Korean author Suk Ki Tae was the most prolific researcher. The co-cited keyword cluster labels revealed ten major clusters, namely cortisol, endothelial dysfunction, carbohydrate metabolism, myocardial infarction, non-alcoholic steatohepatitis, lipotoxicity, glucagon-like peptide-1, non-islet dependent, ethnicity, and microRNA. Keyword outbreak analysis highlighted metabolic syndrome, hepatic steatosis, insulin resistance, hepatocellular carcinoma, cardiovascular disease, intestinal permeability, and intestinal bacterial overgrowth as prominent areas of intense research.
Through the quantitative analysis of relevant literature, the current research focus and direction of gut microbiota and NAFLD can be more clearly understood, which helps us better understand the pathogenesis of NAFLD, and also opens up innovative solutions and strategies for the treatment of NAFLD.
Core Tip: Gut microbiota and nonalcoholic fatty liver disease (NAFLD) have been increasingly linked through mounting evidence, prompting a comprehensive bibliometric analysis in this study, which aimed to examine the emerging research trends and focuses in the relationship between the two fields. Over the past ten years, there has been a substantial rise in studies exploring the connection between gut microbiota and NAFLD. These investigations mainly concentrate on the function of gut microbiota in the development and potential therapy of NAFLD. By shedding light on the pathogenesis of NAFLD, these studies not only augment our comprehension of the disease's origin but also furnish novel therapeutic strategies and insights.
- Citation: Huang CY, Luo ZZ, Huang WP, Lin LP, Yao YT, Zhuang HX, Xu QY, Lai YD. Research hotspots and trends in gut microbiota and nonalcoholic fatty liver disease: A bibliometric study. World J Hepatol 2025; 17(1): 102034
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/102034.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.102034
Nonalcoholic fatty liver disease (NAFLD) is a prevalent hepatic disorder characterized by the excessive accumulation of abnormal lipids in the liver, exceeding 5% of the organ's total weight, in the absence of significant alcohol consumption[1]. NAFLD is intricately associated with obesity, type 2 diabetes, and metabolic syndrome, impacting approximately 25% of the global population and swiftly emerging as the most prevalent chronic liver disease worldwide[2,3]. Concurrently, the term "gut microbiota" denotes the intricate ecosystem of microorganisms inhabiting the human gut, such as bacteria, fungi, viruses, and other microscopic life forms. This ecosystem supports various physiological functions, such as fortifying intestinal integrity, facilitating nutrient extraction, defending against pathogens and regulating host immune responses[4]. The interplay between gut microbiota and NAFLD has been a subject of extensive research, revealing a significant association between the two. Given the intricate anatomical and physiological link between the liver and the intestine, substances and immune elements from the gut can reach the liver through the portal vein, participating in intricate liver functions. This communication pathway is often referred to as the gut-liver axis (GLA)[5]. The gut mi
However, current research on the relationship between the gut microbiota and NAFLD remains nascent, with many unresolved questions and controversies. Bibliometric analysis can objectively identify relevant literature, provide a clear overview of the field's development, determine the current status, and offer insights into future advancements[7]. This type of analysis is extensively utilized in the domains of oncology, ophthalmology, complementary medicine, and alternative medicine, among others[8,9]. Although there has been some literature available as of 2021, the field of bibliometric studies exploring the connection between gut microbiota and NAFLD remains underexplored[10]. Due to the impact of coronavirus disease 2019, further updates are needed for bibliometric studies on this topic.
This study conducts an extensive bibliometric analysis of research articles published from 2013 to 2023, shedding light on the prevailing landscape, evolving patterns, and priority themes within the domain of gut microbiota and its re
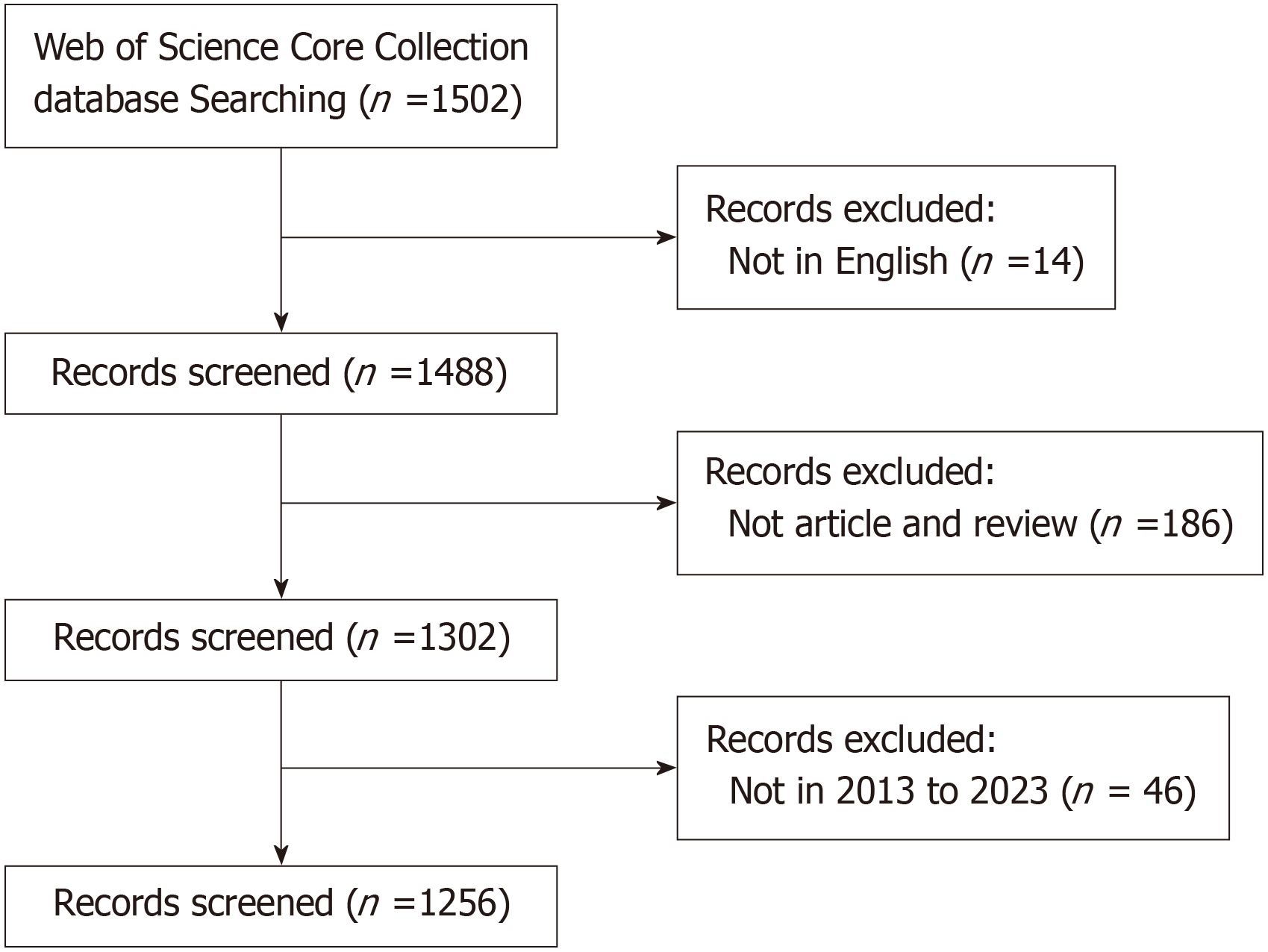
The screening of publications adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methodology[11]. To ensure focus, the inclusion criteria were limited to English-language articles specifically examining the connection between gut microbiota and NAFLD. Publications that did not pertain to this specific topic, such as non-English texts, retracted articles, letters, conference abstracts, and editorial content, were excluded from the selection.
The source of data for this study was the Web of Science Core Collection (WoSCC). The search parameters were defined as follows: Searching for articles or reviews published between January 1, 2013 and December 31, 2023, written in English, and containing any of the following keywords in the title or subject fields: (TI = "NAFLD" or "nonalcoholic fatty liver disease" or "non-alcoholic fatty liver disease" or "MAFLD" or "metabolic dysfunction-associated fatty liver disease" or "metabolic associated fatty liver disease") and (TS = "gut flora" or "gut microbiota" or "gut microbiome" or "gut microflora" or "gut bacteria" or "intestinal flora" or "intestinal microbiota" or "intestinal microbiome" or "intestinal microflora" or "intestinal bacteria").
Two independent reviewers evaluated articles using pre-established inclusion and exclusion criteria. When disa
Using VOSviewer 1.6.20, a bibliometric mapping and cluster analysis were performed to create a visual representation of a bibliometric network. In this visualization, nodes on the map symbolize distinct parameters, such as countries, in
The bibliometric tool, CiteSpace can identify publication trends and hotspots, thereby facilitating the exploration of expertise within the field as well as emerging research topics[13]. Keyword citation bursts, references, double graphs, and timeline views were analyzed using CiteSpace 6.1R6 Basic software. Data statistics and tabulation were performed using Origin 2023.
The search performed on 21 February 2024 yielded a total of 1502 potentially relevant records. The PRISMA 2020 guidelines were adhered to in the study selection process, which is depicted in Figure 1. Following a thorough screening, a comprehensive bibliometric analysis was conducted on an initial pool of 1256 studies that met the established inclusion criteria.
The data presented in Figure 2 demonstrate a consistent upward trajectory in publications pertaining to the correlation between gut microbiota and NAFLD, with a notable surge observed within the past two years. This observation implies an escalating interest among researchers regarding the intricate association between the gut microbiota and NAFLD.
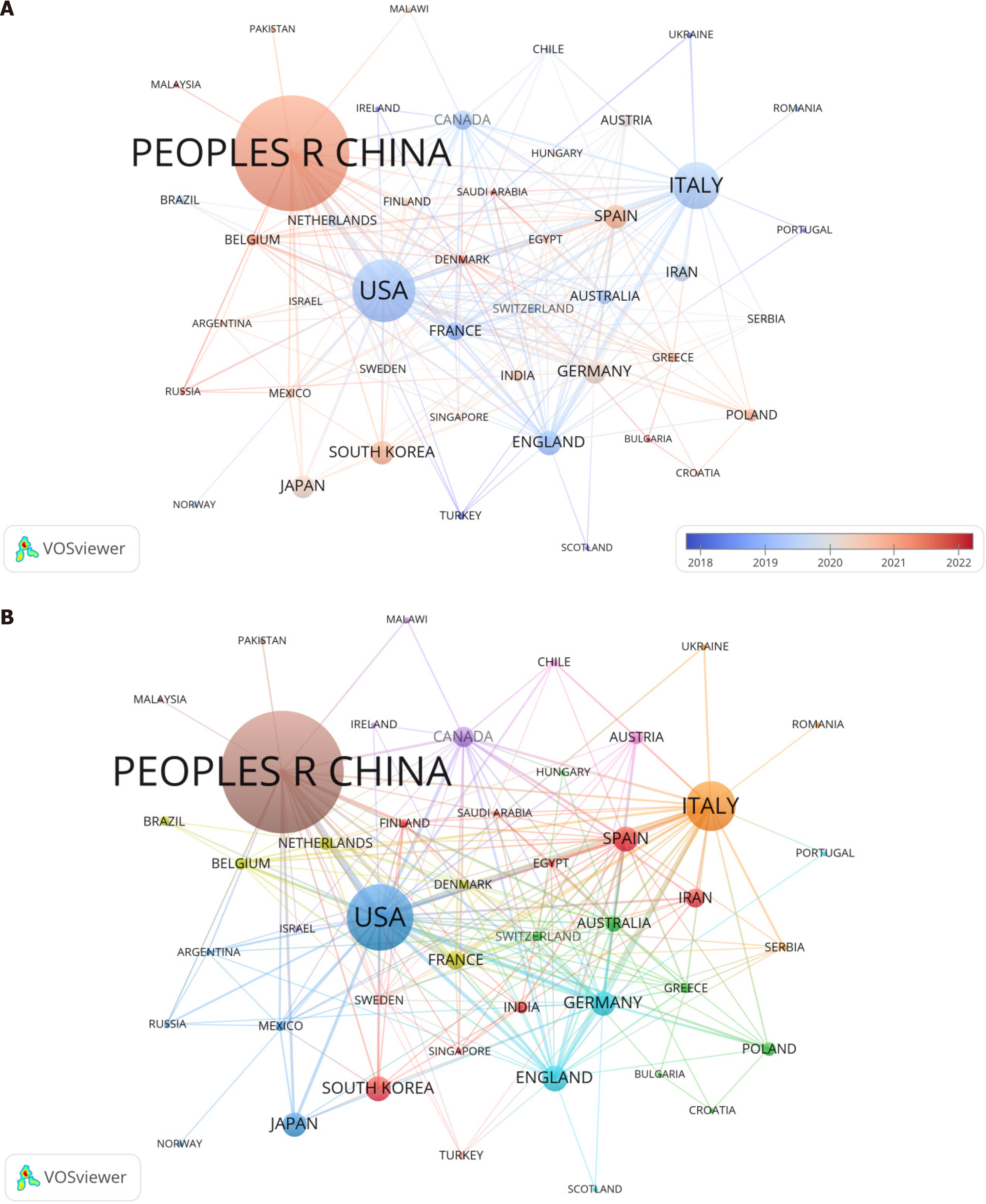
Table 1 outlines the top 15 countries that have explored the connection between gut microbiota and NAFLD in 64 countries. China dominates this research area with 549 publications (33.85%), reflecting the highest number of annual publications in the field. The United States stands as the second leading contributor with 224 papers (13.81%), followed by Italy, which has published 147 studies (9.06%). In terms of total citation count, the United States leads with the highest number of citations (15,261), followed by China (12409), Italy (9552), the United Kingdom (6470), and France (4763). The substantial number of citations signifies the exceptional quality, influence, and significance of these research outputs. Figure 3A illustrates that there are a total of thirty-six countries/regions contributing more than five publications each, while Figure 3B shows that seventeen countries/regions exceed the threshold of twenty published articles.
| Ranking | Country | Documents | Citations |
| 1 | China | 549 | 12409 |
| 2 | United States | 224 | 15261 |
| 3 | Italy | 147 | 9552 |
| 4 | United Kingdom | 57 | 6470 |
| 5 | Spain | 56 | 1852 |
| 6 | South Korea | 56 | 1728 |
| 7 | Germany | 54 | 2624 |
| 8 | Japan | 54 | 2191 |
| 9 | Canada | 41 | 2775 |
| 10 | France | 37 | 4763 |
| 11 | Iran | 37 | 1486 |
| 12 | Australia | 29 | 3366 |
| 13 | Austria | 24 | 2777 |
| 14 | Netherlands | 24 | 2003 |
| 15 | Poland | 24 | 341 |
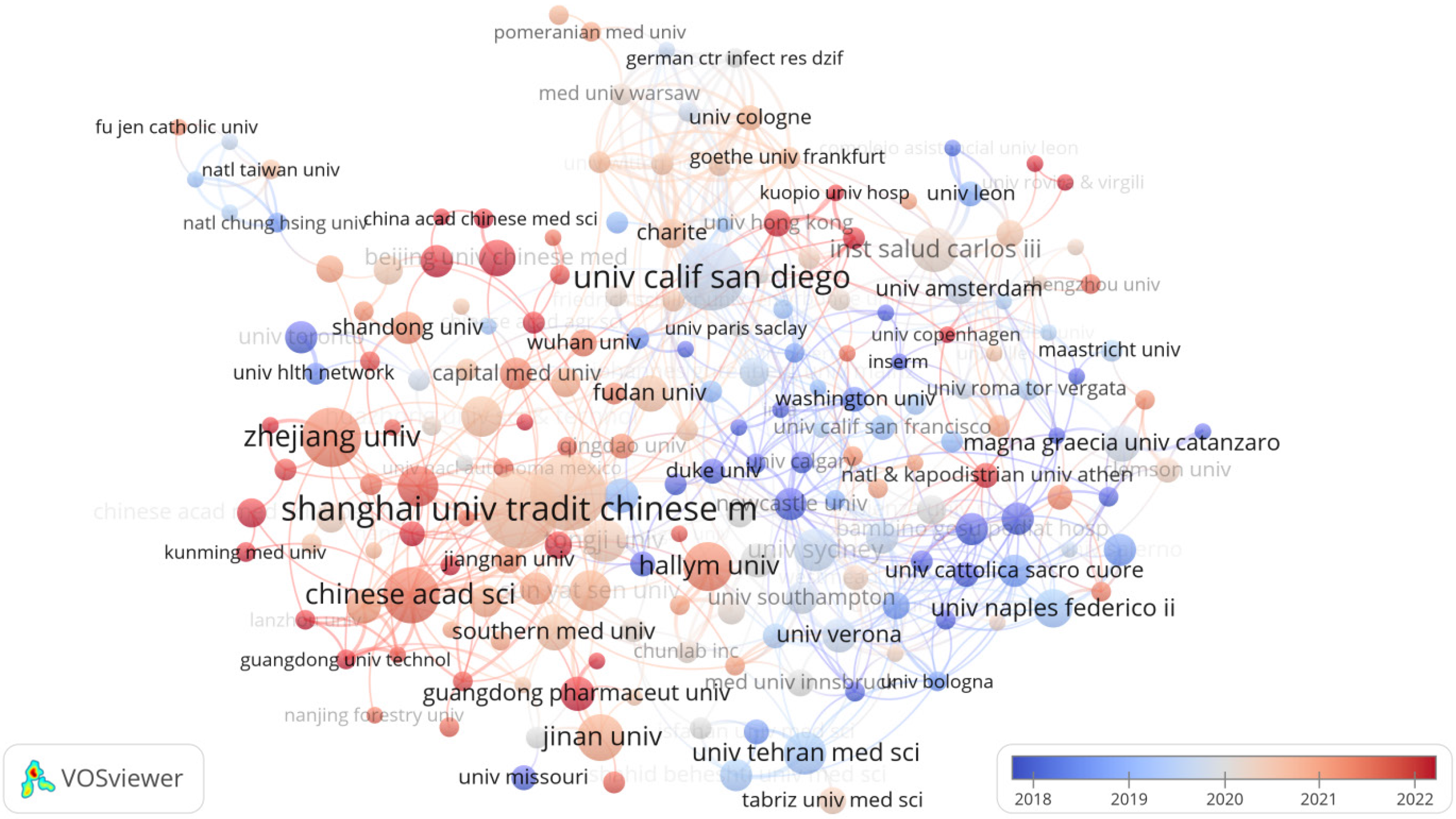
Ranked first among the world's 15 most productive research institutions, as detailed in Table 2, is Shanghai University of Traditional Chinese Medicine, with 35 papers published. Trailing closely behind are Shanghai Jiao Tong University with 34 papers, and the University of California, San Diego (UCSD) ranks third with 29 papers. Notably, UCSD leads in citations with an impressive 2372, followed by the University of Sydney at 1471 citations. The third most cited institution is Shanghai Jiao Tong University (1013), indicating the strong influence of these research institutions in the field. Of these institutions, nine are from China, indicating that more institutions in China are focusing on the study of gut microbiota and NAFLD. Figure 4 shows a map illustrating the worldwide distribution of institutions engaged in research pertaining to the gut microbiota and NAFLD. A total of 1755 institutions participated in the study, and 150 institutions published more than 5 papers. The size of the nodes in Figure 4 symbolizes the quantity of research papers published by each institution.
| Ranking | Organization | Documents | Citations |
| 1 | Shanghai Univ Tradit Chinese Medicine | 35 | 762 |
| 2 | Shanghai Jiao Tong Univ | 34 | 1013 |
| 3 | Univ Calif, San Diego | 29 | 2372 |
| 4 | Zhejiang Univ | 24 | 704 |
| 5 | Chinese Acad Sci | 22 | 857 |
| 6 | Hallam Univ | 18 | 360 |
| 7 | Jinan Univ | 17 | 591 |
| 8 | Inst Salud Carlos Iii | 16 | 861 |
| 9 | Tongji Univ | 15 | 555 |
| 10 | Univ Sydney | 15 | 1471 |
| 11 | Univ Tehran Med Sci | 15 | 919 |
| 12 | Hua Zhong University Sci & Technol | 14 | 532 |
| 13 | Sun Yat Sen Univ | 14 | 945 |
| 14 | Zhejiang Chinese Med Univ | 14 | 143 |
| 15 | Univ Naples Federico Ii | 13 | 572 |
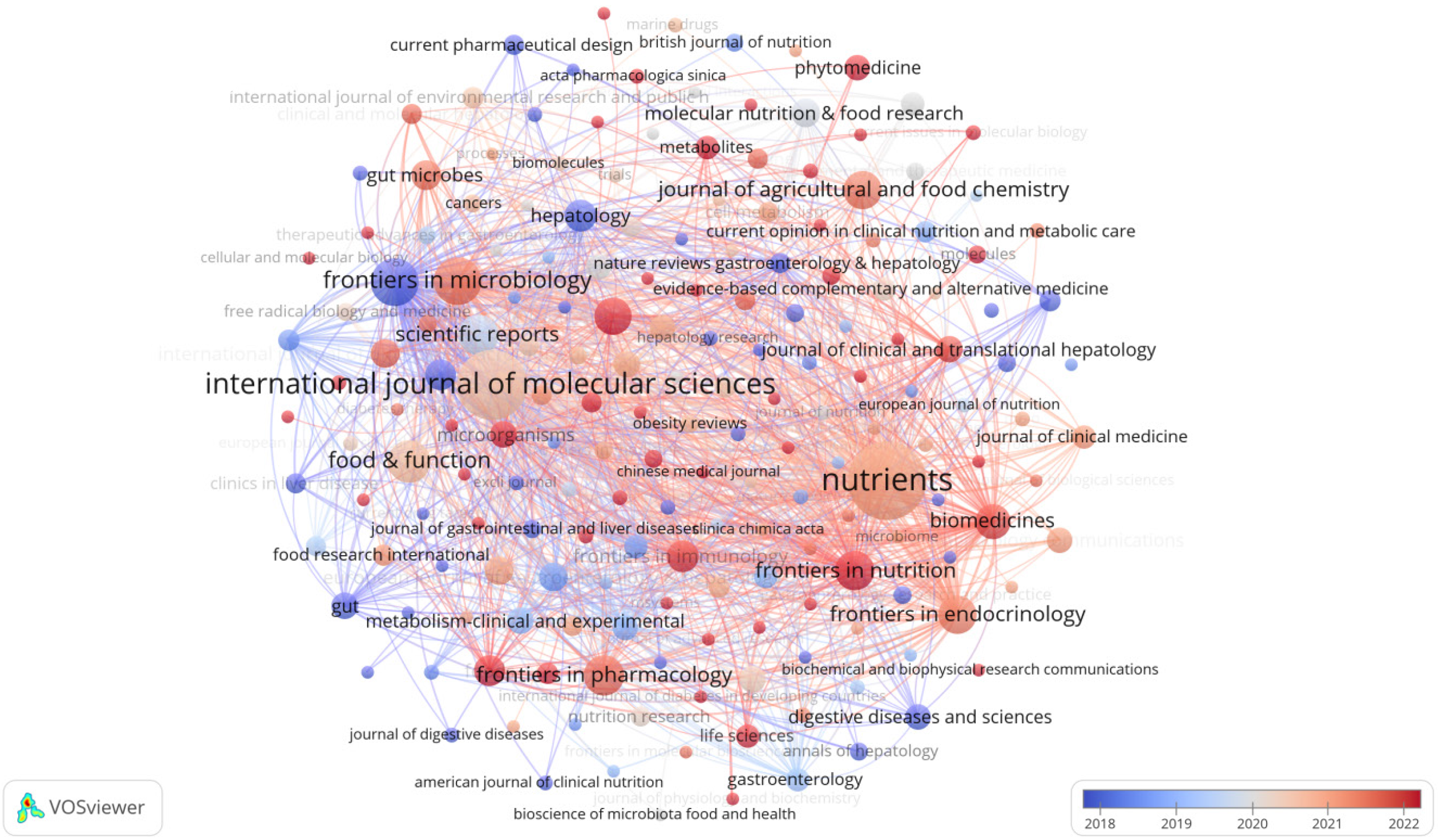
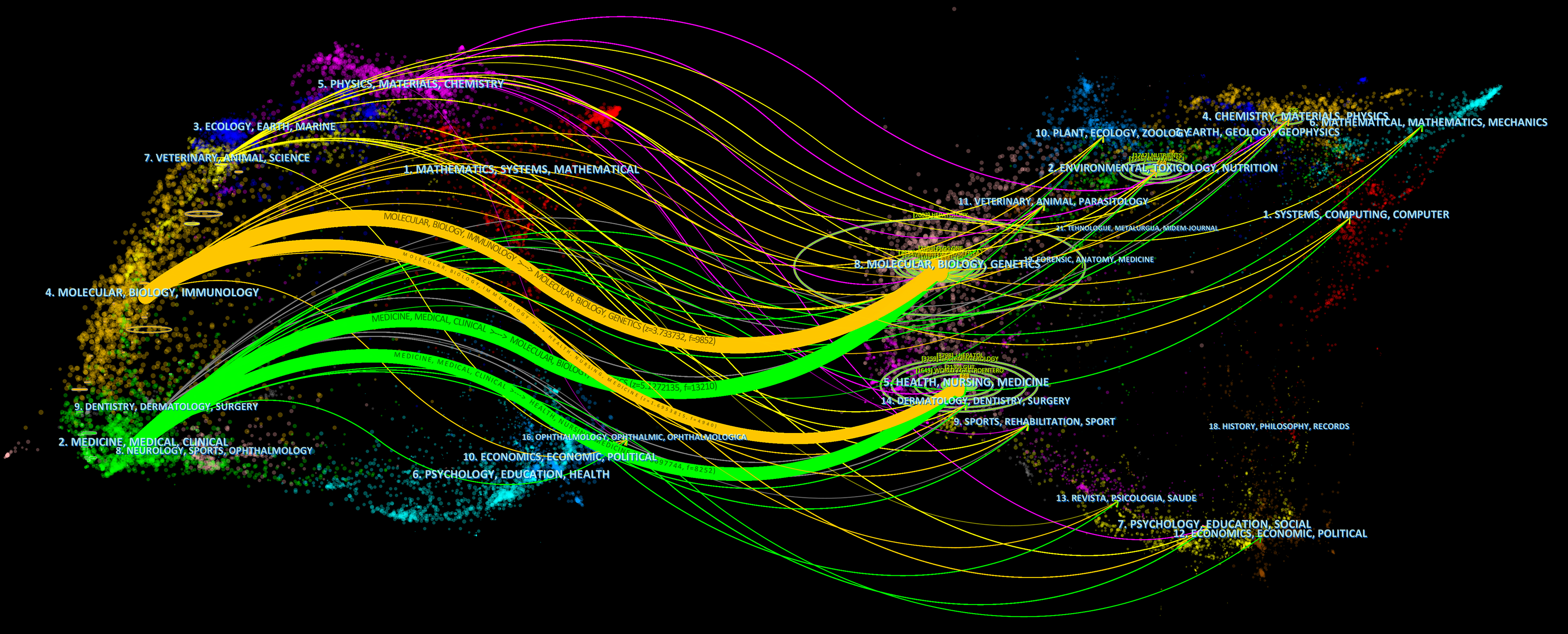
Table 3 highlights the top 15 key publications focusing on gut microbiota and NAFLD. Notably, "Nutrients" stands out as the most productive journal, accounting for 76 articles, trailed closely by "International Journal of Molecular Sciences" with 60 contributions. "Frontiers in Microbiology" and "World Journal of Gastroenterology" tied for third place, each having published 28 articles. In terms of citation impact, "Hepatology" led with an impressive 3817 citations, albeit having only 13 papers. "World Journal of Gastroenterology" ranked second in citation rates (2119 times), followed by "Nutrients" with 1890 citations. This indicates the high scholarly standing and influence of these journals, as citation rates reflect the quality and significance of their research. As visualized in Figure 5, the journal network map, 409 journals have contributed to the field of gut microbiota and NAFLD studies, with 63 of them having over 5 publications. To explore the relationships between cited and co-cited journals, a double-graph overlay analysis was conducted (Figure 6), where lines connecting nodes on both sides demonstrate citation pathways and the interconnections among diverse research areas.
| Ranking | Source | Documents | Citations |
| 1 | Nutrients | 76 | 1890 |
| 2 | International Journal of Molecular Sciences | 60 | 1787 |
| 3 | Frontiers in Microbiology | 28 | 530 |
| 4 | World Journal of Gastroenterology | 28 | 2119 |
| 5 | Food & Function | 23 | 478 |
| 6 | Frontiers in Pharmacology | 20 | 372 |
| 7 | Scientific Reports | 20 | 1269 |
| 8 | Frontiers in Nutrition | 19 | 143 |
| 9 | Frontiers In Endocrinology | 18 | 215 |
| 10 | Journal of Agricultural and Food Chemistry | 18 | 449 |
| 11 | Frontiers in Cellular and Infection Microbiology | 17 | 166 |
| 12 | Biomedicines | 15 | 252 |
| 13 | Frontiers In Immunology | 13 | 154 |
| 14 | Hepatology | 13 | 3817 |
| 15 | Foods | 12 | 66 |
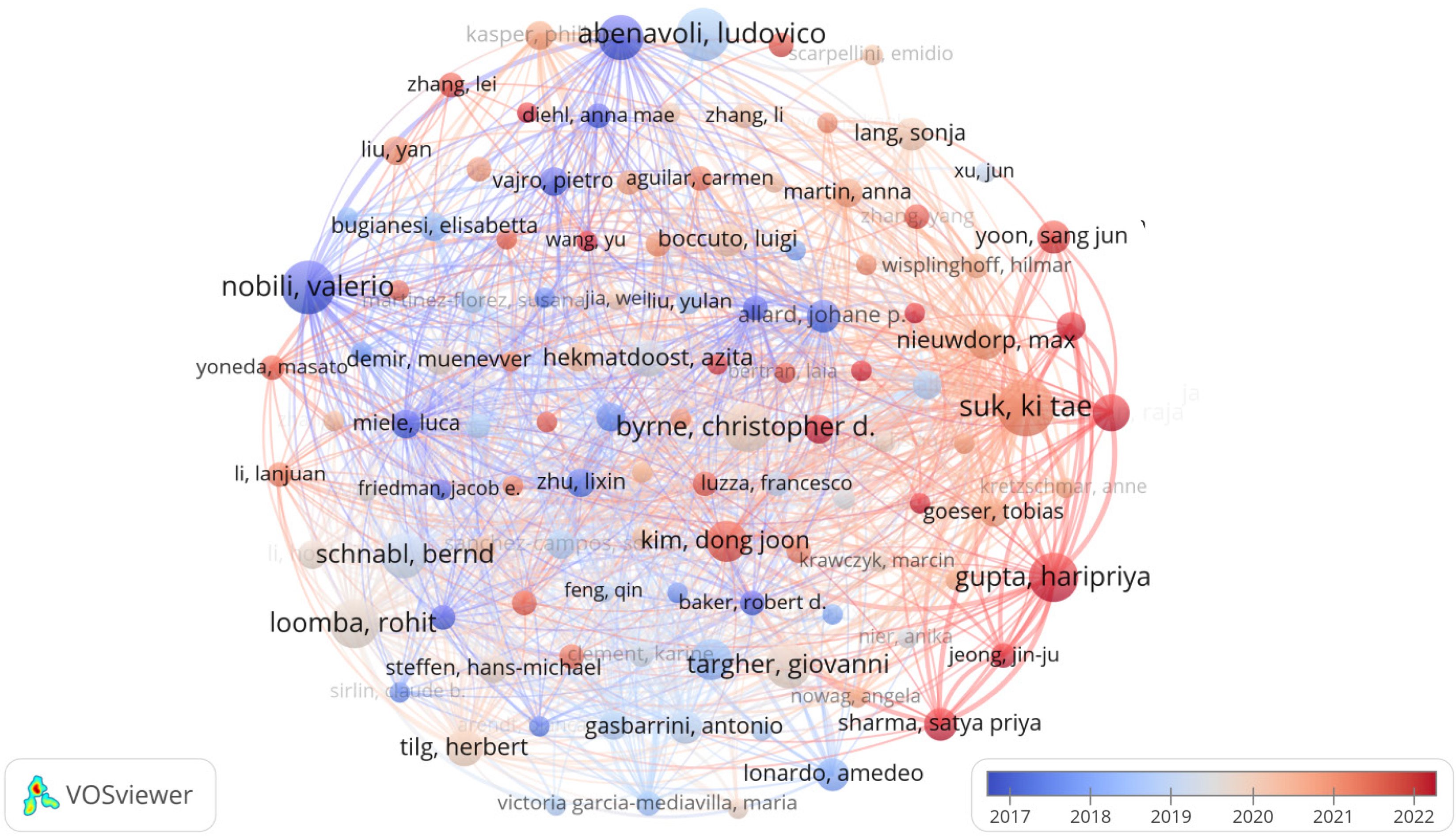
It can be seen in Table 4 that, Suk Ki Tae from South Korea was the most prolific author in the field of gut microbiota and NAFLD with 14 publications (340 citations), followed by Abenavoli Ludovico from Italy (13 publications, 603 citations) and Nobili Valerio (13 publications, 1258 citations). The most cited authors were Targher Giovanni from Italy (2023 citations), followed by Tilg Herbert from Austria (1403 citations) and Schnabl Bernd from Germany (1308 citations), indicating that the studies of these authors are of interest to researchers. Figure 7 shows a network map of the authors. As of now, 7111 authors have contributed to research on the subject of gut microbiota in relation to NAFLD, with a notable group of 112 authors having published over five articles each on this topic.
| Ranking | Authors | Documents | Citations |
| 1 | Suk Ki Tae | 14 | 340 |
| 2 | Abenavoli Ludovico | 13 | 603 |
| 3 | Nobili Valerio | 13 | 1258 |
| 4 | Byrne Christopher D. | 12 | 1245 |
| 5 | Gupta Hari Priya | 12 | 217 |
| 6 | Roomba Rohit | 12 | 1302 |
| 7 | Alisi Anna | 11 | 980 |
| 8 | Schnabl Bernd | 11 | 1308 |
| 9 | Targher Giovanni | 11 | 2023 |
| 10 | Bergheim Ina | 10 | 456 |
| 11 | Kim Dong Joon | 10 | 181 |
| 12 | Ganesan Raja | 9 | 54 |
| 13 | Hekmatdoost Azita | 9 | 552 |
| 14 | Nieuwdorp Max | 9 | 703 |
| 15 | Tilg Herbert | 9 | 1403 |
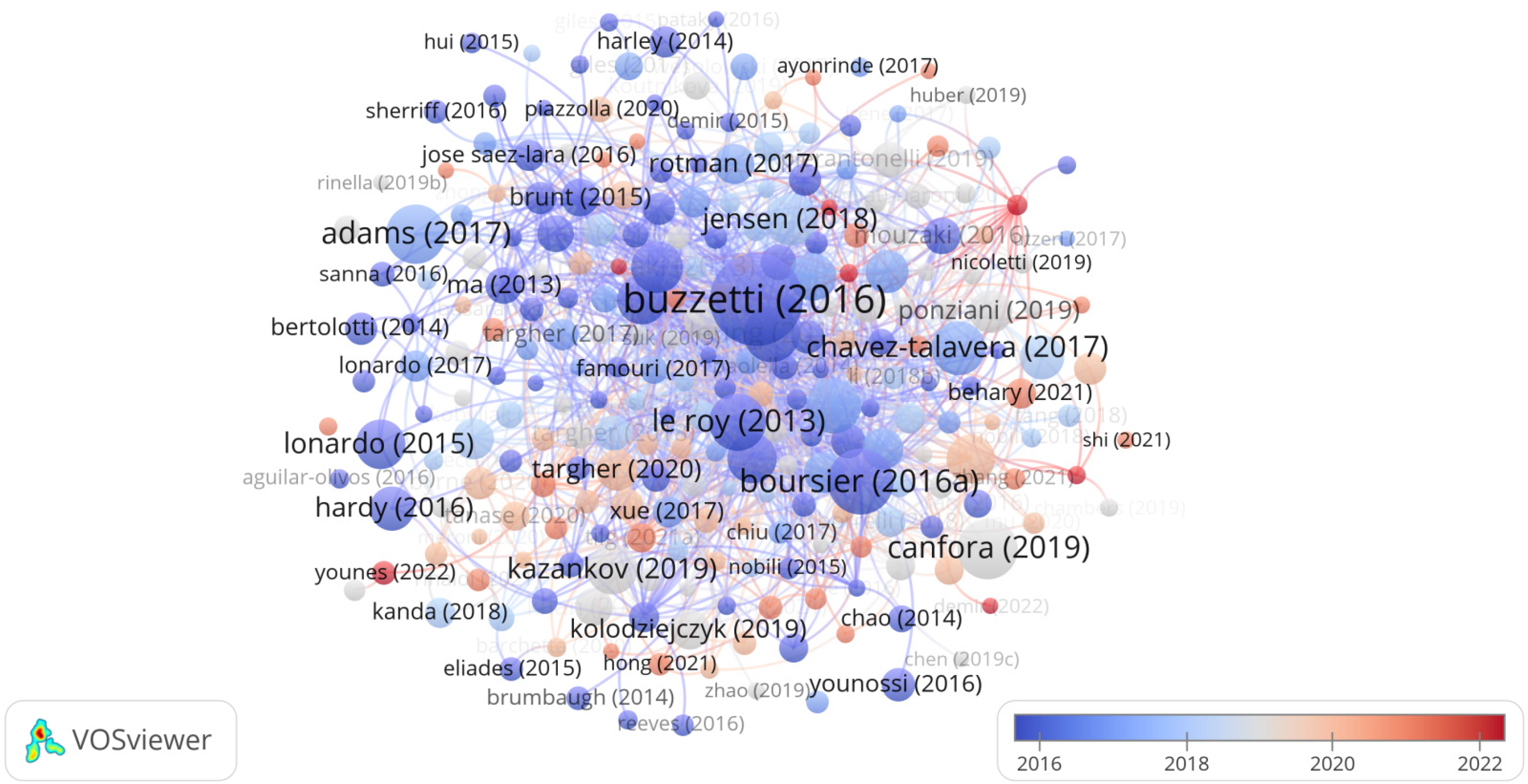
From 2013 to 2023, Table 5 documents the annual global citation counts (GCS) for the top 10 most cited articles, showcasing the cumulative number of times these articles have been referenced as recorded in the Web of Science database. The GCS represents the total citations garnered by a particular article over the years in question. It is used as a measure of the influence and scientific importance of the literature under consideration. Table 5 reveals that Buzzetti E's article published in Metabolism-Clinical and Experimental in 2016 obtained the highest GCS score. Ranking second on the GCS list was Boursier J's article published in Hepatology in 2016, followed by Canfora EE's article published in Nature Reviews Endocrinology in 2019. The network map depicting highly cited articles is illustrated in Figure 8.
| Ranking | Title | First author | Journal | Year | Total citations |
| 1 | The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) | Buzzetti E | Metabolism-Clinical and Experimental | 2016 | 1764 |
| 2 | The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut; Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota | Boursier J | Hepatology | 2016 | 869 |
| 3 | Gut microbial metabolites in obesity, NAFLD and T2DM | Canfora EE | Nature Reviews Endocrinology | 2019 | 724 |
| 4 | Non-alcoholic fatty liver disease and its relationship with cardiovascular; disease and other extrahepatic diseases | Adams LA | Gut | 2017 | 712 |
| 5 | Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice | Le Roy T | Gut | 2013 | 660 |
| 6 | The role of the gut microbiota in NAFLD | Leung C | Nature Reviews Gastroenterology & Hepatology | 2016 | 624 |
| 7 | Gut Microbiome-Based Metagenomic Signature for Non-invasive; Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease | Loomba R | Cell Metabolism | 2017 | 613 |
| 8 | Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2; Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease | Chávez-Talavera O | Gastroenterology | 2017 | 580 |
| 9 | Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease | Mouzaki M | Hepatology | 2013 | 526 |
| 10 | Fructose and sugar: A major mediator of non-alcoholic fatty liver disease | Jensen T | Journal of Hepatology | 2018 | 521 |
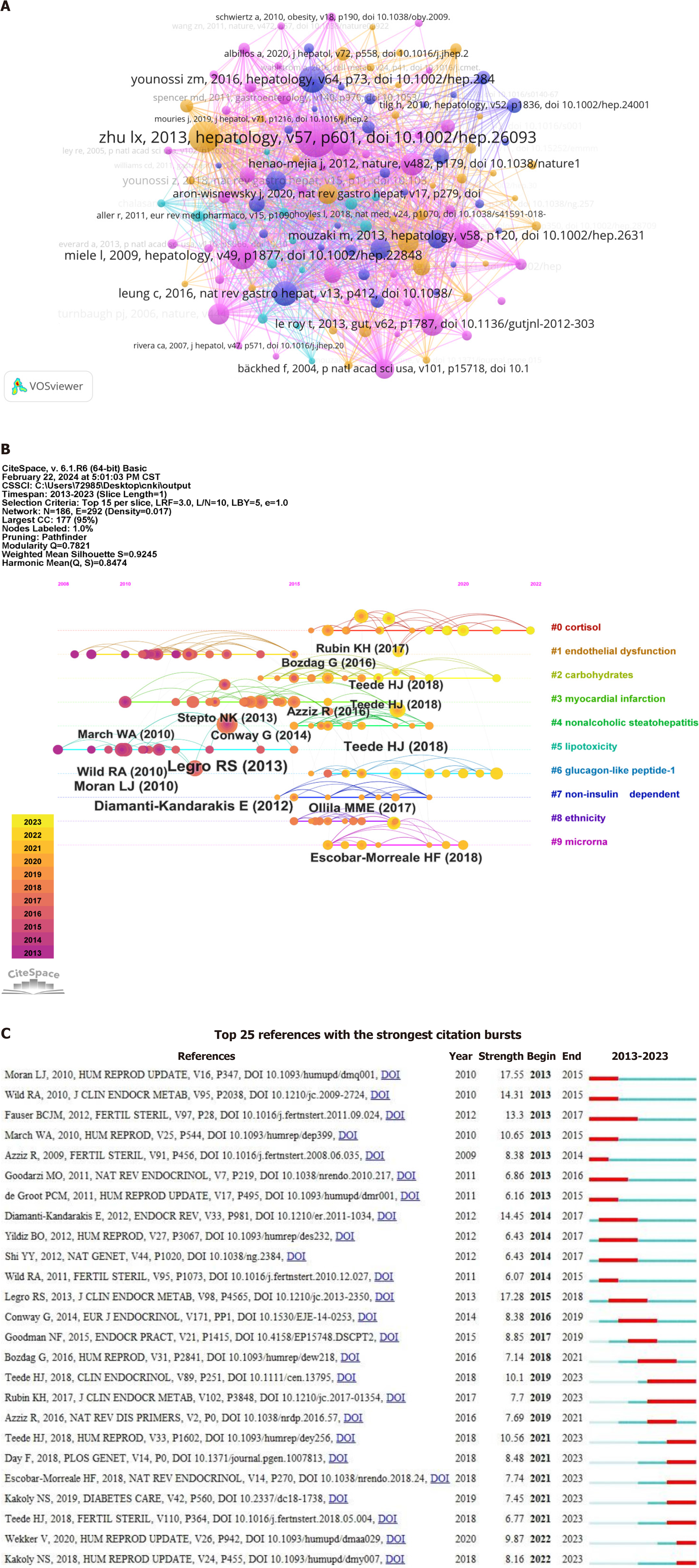
Figure 9A depicts a co-citation analysis of the cited references. Co-citation refers to the relationship between two or more references when they are simultaneously cited by another reference (published later). This phenomenon of co-citation serves as an indicator of the correlation and mutual influence between documents. A total of 49,633 articles were analyzed using VOSviewer, with nodes connected by lines denoting their citation in the same publication; shorter lines indicate a closer relationship.
Figure 9B shows the 10 major clusters of co-cited references, namely cortisol, endothelial dysfunction, carbohydrate metabolism, myocardial infarction, non-alcoholic steatohepatitis, lipotoxicity, glucagon-like peptide-1, non-islet depen
Figure 9C displays the top 25 most frequently cited references. Among the burst strength results, Moran LJ exhibited the highest burst strength of 17.55. In addition, Legro RS had burst strength of 17.28, Diamanti-Kandarakis E had burst strength of 14.45, Wild RA had burst strength of 14.31, Fauser BCJM had burst strength of 13.3 and March WA had burst strength of 10.65. These references have received more attention.
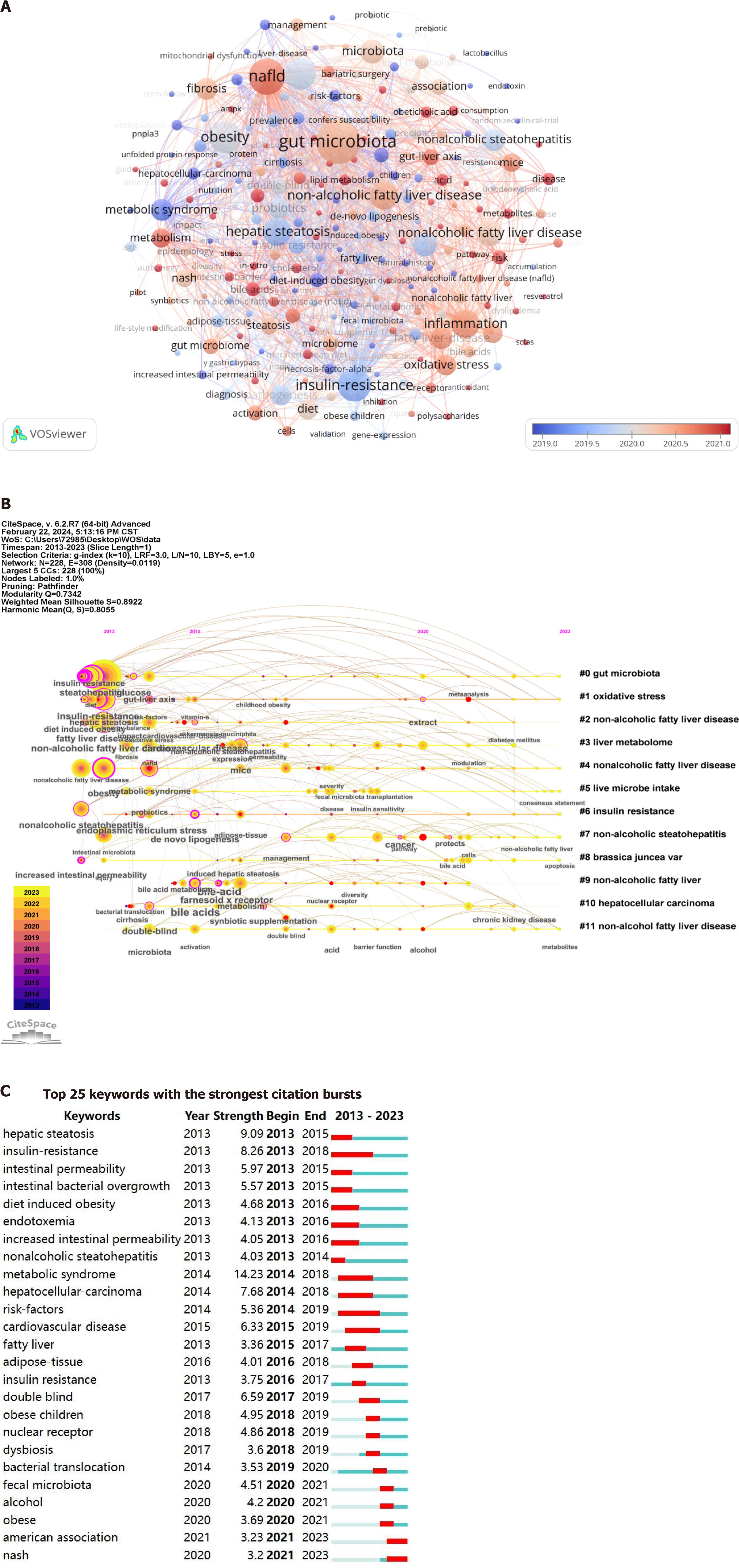
Keywords serve as crucial indicators that reflect research hotspots and trends, while also refining the core content of literature. The analysis of keywords can shed light on the prominent research areas and evolving trends within an academic discipline. As depicted in Table 6, these are the 20 most recurrent keywords pertaining to gut microbiota and NAFLD from 2013 to 2023. The most common keyword was gut microbiota with 610 occurrences (5215 for connection strength), followed by NAFLD with 406 occurrences (3589 for connection strength). The third most frequent keyword was insulin resistance, which had a frequency of 336 times (with a connection strength of 3181 times). The keywords obesity, steatohepatitis, inflammatory, metabolic syndrome, fibrosis, oxidative stress, probiotics, and dysbiosis exhibited a higher frequency and stronger connections (keywords with similar meanings have been excluded). These keywords encapsulate the prevailing research hotspots and trends within the field of gut microbiota and NAFLD. The network visualization corresponding to these keywords is illustrated in Figure 10A. The dimensions of each node correspond to the frequency with which the pertinent keywords are represented in the literature. Node color is typically used to distinguish different categories or attributes of keywords, while lines represent associations between keywords, with line color often indicating the nature or intensity of these associations. Furthermore, as shown in Figure 10B, gut microbiota, oxidative stress, non-alcoholic fatty liver disease, liver metabolome, liver microbial intake, insulin resistance, brassica juncea var, and hepatocellular carcinoma have long been a focus of research on gut microbiota and NAFLD. The most popular topics of the last 10 years are illustrated in Figure 10C, encompassing metabolic syndrome (citation burst strength 14.23), hepatic steatosis (citation burst strength 9.09), insulin resistance (citation burst strength 8.26), hepatocellular carcinoma (citation burst strength 7.68) and cardiovascular disease (citation burst strength 6.33).
| Ranking | Keywords | Frequencies | Total link strength |
| 1 | Gut microbiota | 610 | 5215 |
| 2 | NAFLD | 406 | 3589 |
| 3 | Insulin-resistance | 336 | 3181 |
| 4 | Obesity | 328 | 2869 |
| 5 | Steatohepatitis | 311 | 2899 |
| 6 | Inflammation | 256 | 2326 |
| 7 | Non-alcoholic fatty liver disease | 250 | 2204 |
| 8 | Hepatic Steatosis | 243 | 2286 |
| 9 | Nonalcoholic fatty liver disease | 216 | 1865 |
| 10 | Microbiota | 177 | 1571 |
| 11 | Intestinal microbiota | 169 | 1643 |
| 12 | Nonalcoholic steatohepatitis | 154 | 1514 |
| 13 | Metabolic syndrome | 149 | 1379 |
| 14 | Fatty liver disease | 148 | 1263 |
| 15 | Fibrosis | 146 | 1343 |
| 16 | Oxidative stress | 136 | 1197 |
| 17 | Probiotics | 134 | 1388 |
| 18 | Pathogenesis | 129 | 1184 |
| 19 | Diet | 127 | 1169 |
| 20 | Dysbiosis | 119 | 1053 |
Utilizing VOSviewer and CiteSpace, we analyzed the literature's temporal and spatial distribution, keyword frequency, citation data, and co-citation indicators. These tools enabled us to map out the current state of research, highlight key areas of focus, and identify evolving trends within the field of gut microbiota and NAFLD studies. By examining these insights, our study intends to offer valuable guidance for researchers exploring liver disease, thereby contributing to the advancement of knowledge in this domain.
A total of 1256 articles were included in our analysis. Between 2013 and 2023, we observed a consistent upward trend in the number of publications focusing on gut microbiota and NAFLD, which has attracted significant attention from researchers worldwide.
Among the 64 nations contributing to the field, China stands out as the leading producer of research papers, with the United States and Italy trailing closely behind. The surge in China's research output can be attributed to the rising incidence of NAFLD within the country. A growing focus of Chinese scientists has been the complex interactions between gut microbiota and NAFLD, as they diligently examine the disease's causality and potential therapies. These studies not only contribute to enhancing China's academic standing in this field but also serve as important references for related research worldwide. In terms of research into the association between gut microbiota and NAFLD, the United States exhibited the highest citation rate, signifying its predominant influence in this field. This notable advantage can be attributed to prominent scholars who possess advanced research facilities and cutting-edge technology.
The Shanghai University of Traditional Chinese Medicine leads in the number of published papers related to gut microbiota and NAFLD research, reflecting its substantial focus and support in these fields. Following closely are Shanghai Jiao Tong University and the UCSD in terms of publication volume. In terms of citation impact, the UCSD stands at the forefront, succeeded by the University of Sydney and Shanghai Jiao Tong University, demonstrating the significant influence of their research in this domain. It is noteworthy that nine out of the top 15 institutions engaged in this research are based in China, highlighting the growing interest and dedication of Chinese institutions to the study of gut microbiota and NAFLD.
High-impact factor journals are esteemed platforms for publishing top-tier research, thus articles appearing in these journals are considered to be of superior quality and significant contributions to the respective academic discipline. However, within gut microbiota and NAFLD research, there is a notable scarcity of publications in such prestigious journals (impact factor < 10 for most journals). Nutrients, a journal with an impact factor of 6.6, has significantly con
Suk Ki Tae, a researcher from the Institute of Liver Digestion at Hanlim University School of Medicine in South Korea, holds the distinction of being the most productive author in the field of gut microbiota and NAFLD research. He has authored 14 papers that have cumulatively been cited 340 times. Suk Ki Tae's primary area of interest lies in exploring the role of gut microbiota and its metabolites in the development and progression of NAFLD. Notably, his research prominently features studies on probiotics, investigating their potential as a promising therapeutic approach for treating NAFLD[14-16]. The article suggests that the consumption of probiotics like Lactobacillus acidophilus, Lactobacillus fermentans, and Lactobacillus plantarum may potentially alleviate non-alcoholic steatosis by helping to reduce cholesterol levels and thus enhance the condition's positive progression[17]. Another study has demonstrated that short-chain fatty acids and indole compounds produced by Bifidobacteria can alleviate NAFLD through the modulation of the GLA pathway[18]. Abenavoli Ludovico and Nobili Valerio (both with 13 publications each) have secured the second position in the ranking and amassed 603 and 1258 citations respectively. Abenavoli Ludovico's research interests closely parallel Suk Ki Tae's work[19,20]. Nobili, Valerio's research centers on the investigation of the gut microbiota's influence in the development of pediatric NAFLD. The study reveals that children with NAFLD exhibit heightened intestinal permeability, a factor that positively correlates with the severity of the disease[21]. He dedicated significant attention to exploring the therapeutic potential of probiotics in managing NAFLD. A review indeed confirmed the effectiveness of probiotics for treating NAFLD in pediatric patients[22]. A recent study suggests that Bifidobacterium could play a protective role in preventing the de
Papers with high citation counts can serve as indicators of the prevailing issues of interest within a specific research domain. The papers written by Buzzetti E published in Metabolism-Clinical and Experimental in 2016 were ranked first in the GCS. This article primarily focuses on elucidating the pathogenesis of NAFLD. The "two-strike" hypothesis has been criticized for inadequately explaining some molecular and metabolic changes seen in NAFLD patients. A more comprehensive theory proposes that multiple factors, acting in synergy, contribute to the development of NAFLD in genetically prone individuals. These factors encompass insulin resistance, hormones from adipose tissue, dietary influences, gut microbiota composition, and genetic and epigenetic effects[24]. Boursier J's 2016 study in Hepatology explored the role of gut microbiota dysregulation in determining the severity of NAFLD, ranking second in significance[25]. Canfora EE's article, published in Nature Reviews Endocrinology in 2019, secured the third spot on the GCS list by conducting a systematic review that illuminated the connection between microbial fermentation products from carbohydrates and proteins with obesity, obesity-related insulin resistance, type 2 diabetes mellitus, and NAFLD, further elucidating the mechanisms at play[26]. The involvement of gut microbiota in the pathogenesis of NAFLD is a persistent area of interest, as these studies demonstrate.
The visualization of keywords and their co-cited references through time can potentially uncover significant trends and focuses in the domain of gut microbiota and NAFLD research. According to the timeline of co-cited literature, cortisol, and endothelial dysfunction were the common focus, revealing their important roles in the pathophysiological me
As research into gut microbiota and NAFLD advances, the research hotspot changes. The publications which exhibited the highest citation frequency, average annual keyword views, and emergence mapping effectively illustrate the prevailing research hotspots and trajectories. A literature review from the past five years underscored the primary research directions concerning gut microbiota and NAFLD, which are listed as follows: Aron-Wisnewsky et al[33] describe the challenges of distinguishing gut microbiota signatures specific to NAFLD from those associated with obesity and type 2 diabetes. They call for advanced metagenomics to better understand these signatures. Kolodziejczyk et al[34] investigated the impact of gut microbiota and their byproducts on hepatic metabolism and inflammation, thereby con
The analysis of these keywords indicated that research on the gut microbiota and NAFLD predominantly centers on pathogenesis and therapeutic interventions. It is unequivocal that future investigations should explore the underlying mechanisms of NAFLD as well as the influence of gut microbiota on NAFLD treatment.
To objectively assess the connection between gut microbiota and NAFLD, an extensive citation analysis was conducted using CiteSpace and VOSviewer software. This investigation illuminated the current state, evolving patterns, and pivotal themes pertaining to gut microbiota and its association with NAFLD. Despite providing valuable insights, the study has limitations, chiefly the restriction to the WoSCC database and the exclusive inclusion of English-language publications, potentially leading to selection bias.
Bibliometric research showed that publications on gut microbiota and NAFLD are increasing worldwide, and this disease is receiving significant attention. The results showed that China was the most prolific country in terms of the number of publications, the United States was the most influential country, Shanghai University of Traditional Chinese Medicine had a greater number of publications, and the UCSD was the most cited institution. The journal Nutrients was the most published journal, and Hepatology was the most cited journal. Suk Ki Tae from South Korea was the most prolific author.
The co-cited keyword cluster labels revealed the presence of ten major clusters, namely cortisol, endothelial dysfunction, carbohydrate metabolism, myocardial infarction, NASH, lipotoxicity, glucagon-like peptide-1, non-islet dependent, ethnicity and microRNA. Intense research efforts in the field have uncovered key areas of focus, including metabolic syndrome, hepatic steatosis, insulin resistance, hepatocellular carcinoma, cardiovascular disease, intestinal permeability, and intestinal bacterial overgrowth. These topics have emerged as critical subjects of investigation in the outbreak analysis.
The relationship between gut microbiota and its influence on the development and management of NAFLD has emerged as a key area of research. Studies in this domain aim to elucidate the function of gut microbiota in the initiation and progression of NAFLD, as well as its potential role in the therapeutic approach to NAFLD. These investigations not only advance our comprehension of NAFLD's etiology but also propose innovative strategies and techniques for its treatment.
We are grateful to Web of Science for granting access to their data, and CiteSpace and VOSviewer for their invaluable support in the analysis of the results.
| 1. | Rinaldi L, Pafundi PC, Galiero R, Caturano A, Morone MV, Silvestri C, Giordano M, Salvatore T, Sasso FC. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 2. | Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020;158:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 905] [Article Influence: 150.8] [Reference Citation Analysis (2)] |
| 3. | Pais R, Maurel T. Natural History of NAFLD. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 898] [Article Influence: 149.7] [Reference Citation Analysis (1)] |
| 5. | Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic Fatty Liver Disease and the Gut-Liver Axis: Exploring an Undernutrition Perspective. Gastroenterology. 2022;162:1858-1875.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Nonalcoholic Fatty Liver Disease: Modulating Gut Microbiota to Improve Severity? Gastroenterology. 2020;158:1881-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 7. | Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A Bibliometric Analysis of Top-Cited Journal Articles in Obstetrics and Gynecology. JAMA Netw Open. 2019;2:e1918007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 8. | Qi X, Li Y, Liu W, Wang Y, Chen Z, Lin L. Research Trend of Publications Concerning Antibody-Drug Conjugate in Solid Cancer: A Bibliometric Study. Front Pharmacol. 2022;13:921385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Song L, Jia J, Tian W, Lai R, Zhang Z, Li J, Ju J, Xu H. Knowledge Mapping of Necroptosis From 2012 to 2021: A Bibliometric Analysis. Front Immunol. 2022;13:917155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Li Y, Zhou Y, Wang L, Lin X, Mao M, Yin S, Zhu L, Jiao Y, Yu W, Gao P, Yang L. Emerging trends and hotspots in the links between the gut microbiota and MAFLD from 2002 to 2021: A bibliometric analysis. Front Endocrinol (Lausanne). 2022;13:990953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51767] [Article Influence: 10353.4] [Reference Citation Analysis (2)] |
| 12. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4505] [Cited by in RCA: 5693] [Article Influence: 334.9] [Reference Citation Analysis (2)] |
| 13. | Chen C, Chen Y. Searching for clinical evidence in CiteSpace. AMIA Annu Symp Proc. 2005;2005:121-125. [PubMed] |
| 14. | Park JW, Kim SE, Lee NY, Kim JH, Jung JH, Jang MK, Park SH, Lee MS, Kim DJ, Kim HS, Suk KT. Role of Microbiota-Derived Metabolites in Alcoholic and Non-Alcoholic Fatty Liver Diseases. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Gupta H, Min BH, Ganesan R, Gebru YA, Sharma SP, Park E, Won SM, Jeong JJ, Lee SB, Cha MG, Kwon GH, Jeong MK, Hyun JY, Eom JA, Park HJ, Yoon SJ, Choi MR, Kim DJ, Suk KT. Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Lee NY, Shin MJ, Youn GS, Yoon SJ, Choi YR, Kim HS, Gupta H, Han SH, Kim BK, Lee DY, Park TS, Sung H, Kim BY, Suk KT. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin Mol Hepatol. 2021;27:110-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Yoon SJ, Yu JS, Min BH, Gupta H, Won SM, Park HJ, Han SH, Kim BY, Kim KH, Kim BK, Joung HC, Park TS, Ham YL, Lee DY, Suk KT. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front Microbiol. 2023;14:1129904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 19. | Abenavoli L, Scarpellini E, Rouabhia S, Balsano C, Luzza F. Probiotics in non-alcoholic fatty liver disease: which and when. Ann Hepatol. 2013;12:357-363. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis. 2018;27:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 21. | Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Putignani L, Alisi A, Nobili V. Pediatric NAFLD: the future role of patient-tailored probiotics therapy. J Pediatr Gastroenterol Nutr. 2016;63:S6-8. [DOI] [Full Text] |
| 23. | Nobili V, Putignani L, Mosca A, Del Chierico F, Vernocchi P, Alisi A, Stronati L, Cucchiara S, Toscano M, Drago L. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: which strains act as health players? Arch Med Sci. 2018;14:81-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2307] [Article Influence: 230.7] [Reference Citation Analysis (1)] |
| 25. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1114] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 26. | Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 1025] [Article Influence: 146.4] [Reference Citation Analysis (1)] |
| 27. | Demori I, Grasselli E. The Role of the Stress Response in Metabolic Dysfunction-Associated Fatty Liver Disease: A Psychoneuroendocrineimmunology-Based Perspective. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Yang K, Song M. New Insights into the Pathogenesis of Metabolic-Associated Fatty Liver Disease (MAFLD): Gut-Liver-Heart Crosstalk. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 29. | Wang G, Han Q, Yan X, Feng L, Zhang Y, Zhang R, Zhang Y. Polyphenols-rich extracts from walnut green husk prevent non-alcoholic fatty liver disease, vascular endothelial dysfunction and colon tissue damage in rats induced by high-fat diet. J Funct Foods. 2021;87:104853. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Caturano A, Acierno C, Nevola R, Pafundi PC, Galiero R, Rinaldi L, Salvatore T, Adinolfi LE, Sasso FC. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes. 2021;9:135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 31. | Musso G, Cassader M, Cohney S, Pinach S, Saba F, Gambino R. Emerging Liver-Kidney Interactions in Nonalcoholic Fatty Liver Disease. Trends Mol Med. 2015;21:645-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Shen B, Gu T, Shen Z, Zhou C, Guo Y, Wang J, Li B, Xu X, Li F, Zhang Q, Cai X, Dong H, Lu L. Escherichia coli Promotes Endothelial to Mesenchymal Transformation of Liver Sinusoidal Endothelial Cells and Exacerbates Nonalcoholic Fatty Liver Disease Via Its Flagellin. Cell Mol Gastroenterol Hepatol. 2023;16:857-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 785] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 34. | Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 420] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 35. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 379] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 36. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 564] [Article Influence: 70.5] [Reference Citation Analysis (1)] |
| 37. | Pierantonelli I, Svegliati-Baroni G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation. 2019;103:e1-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/