Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.101798
Revised: November 13, 2024
Accepted: November 22, 2024
Published online: January 27, 2025
Processing time: 101 Days and 17.9 Hours
Helicobacter pylori (H. pylori) infection is a known inducer of various gastroin
Core Tip: The association between Helicobacter pylori (H. pylori) infection and metabolic dysfunction-associated steatohepatitis (MASH) has been confirmed in large-scale multicenter studies, suggesting that H. pylori is an independent risk factor for MASH development. This study revealed that H. pylori may influence MASH through chronic inflammation, insulin resistance, and the gut-liver axis, providing new insights into the pathogenesis of metabolic liver diseases. On the basis of these findings, H. pylori eradication therapy may offer a novel strategy for the prevention and treatment of MASH. Future research should focus on the molecular mechanisms linking H. pylori infection and MASH, particularly genetic susceptibility, gene polymorphisms, and metabolic pathways, in the treatment of MASH.
- Citation: Gou GE, Li T, Liu CR, Meng T, Li YP. Potential mechanisms and therapeutic prospects of the association between Helicobacter pylori infection and metabolic dysfunction-associated steatohepatitis. World J Hepatol 2025; 17(1): 101798
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/101798.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.101798
We are delighted to read the high-quality article ‘Prevalence and risk factors associated with metabolic dysfunction-associated steatohepatitis in patients with Helicobacter pylori infection: A population-based study’ by Abdel-Razeq et al[1], which will be published in the World Journal of Hepatology. Helicobacter pylori (H. pylori) is a common gastrointestinal pathogen classified as a Group 1 carcinogen by the International Agency for Research on Cancer and is widely recognized as a major factor leading to gastritis, gastric ulcers, and gastric cancer. Additionally, studies have indicated its role in promoting the development of colorectal cancer[2-4]. However, as research has progressed, the impact of H. pylori infection has not been limited to gastrointestinal effects, and its association with metabolic diseases has gradually become a research hotspot[2,5,6]. In recent years, metabolic dysfunction-associated steatohepatitis (MASH), a liver disease closely related to metabolic syndrome, has drawn increasing attention. MASH syndrome is characterized by liver cell steatosis, inflammation, and fibrosis, with metabolic syndrome as the central driver[7]. Unlike nonalcoholic steatohepatitis (NASH), which primarily focuses on nonalcoholic factors as the cause of liver damage, MASH explicitly highlights metabolic dysfunction as the primary pathogenesis[8]. Moreover, H. pylori has been implicated in both MASH and NASH through mechanisms involving chronic inflammation, oxidative stress, gut-liver axis disruption, and metabolic dysregulation, further linking metabolic and liver diseases[9]. The pathogenesis of MASH is still not fully understood[10]. This study systematically evaluated the potential association between H. pylori infection and MASH via a large-scale multicenter database and proposed that H. pylori might be involved in the development of MASH through complex inflammatory and metabolic mechanisms, providing a new perspective for further exploration of the role of H. pylori in metabolic liver diseases.
This study analyzed data from a multicenter database of more than 69.23 million patients, setting a precedent in the relevant field with its unprecedented scale. Compared with previous small-scale, regional studies, this study overcomes issues related to small sample sizes and limited conclusions, significantly enhancing the reliability of the statistical results. The study revealed that H. pylori infection is an independent risk factor for MASH development, with high statistical significance (OR: 2.51, 95%CI: 2.31-2.73). Even after controlling for known metabolic syndrome risk factors, such as obesity, diabetes, hypertension, and hyperlipidemia, H. pylori infection remained an important independent factor influencing MASH, revealing its potential pathogenic effect on the liver. This discovery provides a rationale for considering H. pylori as a potential therapeutic target for MASH.
The mechanisms by which H. pylori leads to or exacerbates MASH are not yet clear, but this study provides new perspectives for exploring potential pathophysiological mechanisms. While the exact mechanism by which H. pylori affects liver metabolism remains to be further studied, combining the literature and new evidence provided by this paper suggests that H. pylori infection may promote MASH development and progression by impairing liver function, disturbing glucose and lipid metabolism, triggering inflammatory responses, and exacerbating metabolic syndrome[11]. The OR values for H. pylori infection in MASH patients range from 1.13 to 1.38[10,12].
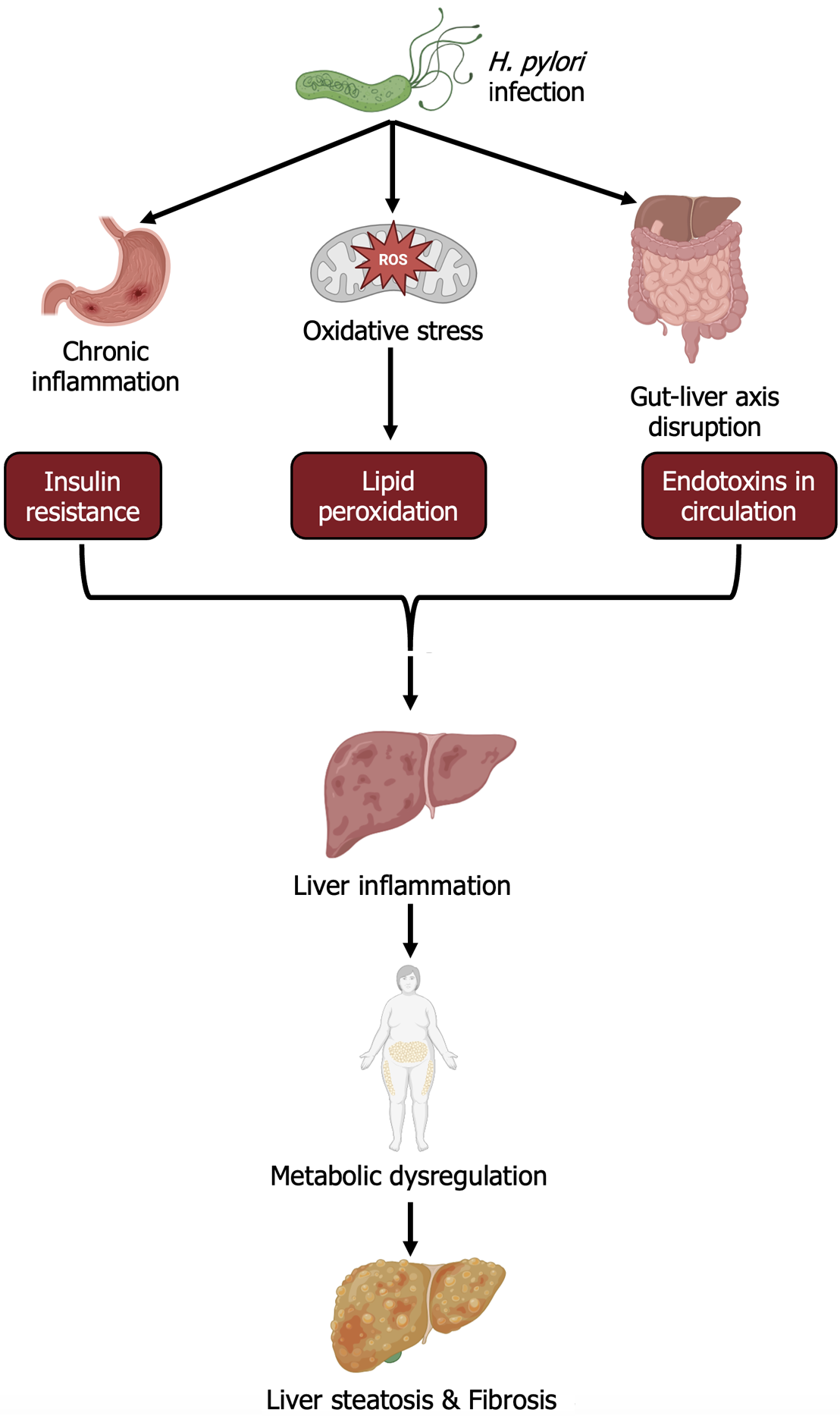
H. pylori infection exacerbates high-fat diet-induced MASH by disrupting gastric epithelial regeneration, leading to chronic inflammation and immune activation. These events trigger elevated production of reactive oxygen species (ROS), which increases epithelial and endothelial permeability, facilitating the systemic entry of bacterial components[13]. The resulting oxidative stress and lipid peroxidation cause endothelial lipid deposition, impaired apoptosis, and cell lysis. These changes increase low-density lipoprotein and triglyceride levels while reducing high-density lipoprotein levels, promoting liver steatosis, fibrosis, inflammation, and systemic complications such as obesity and atherosclerosis[13,14]. Additionally, H. pylori, through virulence factors such as CagA and VacA, may trigger inflammatory responses not only in the stomach but also via the gut-liver axis, affecting liver function. These virulence factors can damage the intestinal mucosal barrier, allowing bacterial metabolites such as endotoxins to enter the liver and induce local inflammation and fat degeneration, thereby exacerbating MASH[2,9]. Second, higher H. pylori infection rates in diabetic patients reveal a link between H. pylori infection and insulin resistance. The underlying mechanism involves the activation of proinflammatory mediators such as C-reactive protein (CRP) and tumor necrosis factor-alpha by H. pylori infection, alongside the production of ROS, ghrelin, and leptin, as well as the release of lipopolysaccharides[11]. Conversely, virulent strains of H. pylori (cag+) induce the production of inflammatory factors, including interleukin-6 and CRP, and chronic inflammation. This inflammatory cascade disrupts the regulation of gastroduodenal hormones mediated by insulin, impairing insulin signaling pathways. Collectively, these changes contribute to the development of insulin resistance, which predisposes individuals to obesity and type 2 diabetes[15,16]. Hyperglycemia and insulin resistance are key pathological mechanisms of MASH[17]. H. pylori may exacerbate insulin resistance and disrupt lipid metabolism by inducing chronic systemic low-grade inflammation, thus promoting liver steatosis and inflammatory responses[18]. This study provides new directions for further exploration of the role of H. pylori in liver metabolic dysfunction. Future studies should further clarify H. pylori’s specific pathogenic pathways in liver inflammation, steatosis, and fibrosis, particularly in chronic liver disease patients. The mechanisms are shown in Figure 1.
Although this paper does not perform a genetic analysis, it proposes new genetic hypotheses for future research, particularly with respect to genetic susceptibility. For example, the relationship between H. pylori infection and MASH may be influenced by specific single-nucleotide polymorphisms (SNPs) or gene mutations, particularly in genes involved in inflammation and lipid metabolism pathways. These genetic variations may increase the influence of H. pylori on metabolic liver disease in specific populations. Recent studies suggest a potential genetic interaction between H. pylori infection and MASH. Specifically, the PNPLA3 gene SNP rs738409 is considered to increase the risk of metabolic liver disease[19,20]. This variation impacts lipid metabolism and may exacerbate liver damage in the context of chronic inflammation (e.g., H. pylori-induced inflammation).
The results of this study not only reveal the association between H. pylori and MASH but also offer new clinical treatment perspectives. Traditional MASH treatments focus primarily on lifestyle interventions and managing metabolic syndrome-related diseases[21]. This study suggests that H. pylori could become a new therapeutic target for MASH. Clinical studies have shown that H. pylori eradication can improve several metabolic abnormalities, including insulin sensitivity and lipid levels[17,22,23]. Future clinical trials should further explore the actual therapeutic effects of H. pylori eradication in MASH patients, especially in high-risk populations with concomitant metabolic syndrome.
By integrating H. pylori eradication into treatment, MASH management may evolve into a more comprehensive approach, combining metabolic syndrome management, anti-inflammatory therapy, and H. pylori infection control. This multidimensional treatment strategy holds promise for improving the long-term prognosis of MASH patients.
Although the study suggested that H. pylori eradication may improve metabolic dysfunction, clinical trial data to support its efficacy in MASH and NASH patients are lacking. Future randomized controlled trials are needed to evaluate the therapeutic benefits of H. pylori eradication in patients with metabolic liver diseases. Future studies should focus on the following: Exploring the specific molecular mechanisms by which H. pylori infection leads to liver metabolic dysfunction, especially in terms of inflammation, insulin resistance, and the gut-liver axis; designing prospective clinical trials to verify the therapeutic effects of H. pylori eradication in MASH patients; and conducting genomic studies to explore the potential genetic susceptibility between H. pylori infection and MASH development. By further investigating the role of H. pylori in metabolic liver diseases, we can not only better understand the pathogenesis of MASH but also develop new, multidimensional therapeutic approaches to promote personalized medicine.
In this study, a large-scale multicenter database was used to establish H. pylori as an independent risk factor for MASH. These findings provide new insights into the etiology of MASH and highlight the potential of H. pylori eradication therapy as a novel approach for managing metabolic liver diseases, paving the way for future research and clinical applications.
| 1. | Abdel-Razeq R, Bitar L, Bitar ER, Onwuzo C, Abu-Hammour MN, Eren B, Mohamed I, Johnson A, Boustany A, Onwuzo S, Asaad I. Prevalence and risk factors associated with metabolic dysfunction-associated steatohepatitis in patients with Helicobacter pylori infection: A population-based study. World J Hepatol. 2024;16:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53:33-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 3. | Inoue M. Epidemiology of Gastric Cancer-Changing Trends and Global Disparities. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Ralser A, Dietl A, Jarosch S, Engelsberger V, Wanisch A, Janssen KP, Middelhoff M, Vieth M, Quante M, Haller D, Busch DH, Deng L, Mejías-Luque R, Gerhard M. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut. 2023;72:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 5. | Doulberis M, Srivastava S, Polyzos SA, Kountouras J, Papaefthymiou A, Klukowska-Rötzler J, Blank A, Exadaktylos AK, Srivastava DS. Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Tzitiridou-Chatzopoulou M, Kazakos E, Orovou E, Andronikidi PE, Kyrailidi F, Mouratidou MC, Iatrakis G, Kountouras J. The Role of Helicobacter pylori and Metabolic Syndrome-Related Mast Cell Activation Pathologies and Their Potential Impact on Pregnancy and Neonatal Outcomes. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Wang S, Friedman SL. Found in translation-Fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci Transl Med. 2023;15:eadi0759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 8. | Sergi CM. NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Martin-Nuñez GM, Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Gut Microbiota: The Missing Link Between Helicobacter pylori Infection and Metabolic Disorders? Front Endocrinol (Lausanne). 2021;12:639856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Zhang D, Wang Q, Bai F. Bidirectional relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease: insights from a comprehensive meta-analysis. Front Nutr. 2024;11:1410543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Chen C, Zhang C, Wang X, Zhang F, Zhang Z, Ma P, Feng S. Helicobacter pylori infection may increase the severity of nonalcoholic fatty liver disease via promoting liver function damage, glycometabolism, lipid metabolism, inflammatory reaction and metabolic syndrome. Eur J Gastroenterol Hepatol. 2020;32:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Heydari K, Yousefi M, Alizadeh-Navaei R, Lotfi P, Sheydaee F, Raei M, Vahdatinia A, Hessami A, Rafati S, Moosazadeh M, Ghasemian R, Salehi F, Massoudi H, Ghaffari-Saravi F, Rismantab S. Helicobacter pylori Infection and Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Turk J Gastroenterol. 2022;33:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | He C, Cheng D, Wang H, Wu K, Zhu Y, Lu N. Helicobacter pylori infection aggravates diet-induced nonalcoholic fatty liver in mice. Clin Res Hepatol Gastroenterol. 2018;42:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Tomaszewska A, Gonciarz W, Rechcinski T, Chmiela M, Kurdowska AK, Krupa A. Helicobacter pylori components increase the severity of metabolic syndrome and its hepatic manifestations induced by a high fat diet. Sci Rep. 2024;14:5764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Mansori K, Dehghanbanadaki H, Naderpour S, Rashti R, Moghaddam AB, Moradi Y. A systematic review and meta-analysis of the prevalence of Helicobacter pylori in patients with diabetes. Diabetes Metab Syndr. 2020;14:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Azami M, Baradaran HR, Dehghanbanadaki H, Kohnepoushi P, Saed L, Moradkhani A, Moradpour F, Moradi Y. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Bołdys A, Bułdak Ł, Maligłówka M, Surma S, Okopień B. Potential Therapeutic Strategies in the Treatment of Metabolic-Associated Fatty Liver Disease. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Chen X, Peng R, Peng D, Liu D, Li R. Helicobacter pylori infection exacerbates metabolic dysfunction-associated steatotic liver disease through lipid metabolic pathways: a transcriptomic study. J Transl Med. 2024;22:701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Maiorana F, Neschuk M, Caronia MV, Elizondo K, Robledo ML, Schneider A, Veron G, Zapata PD, Barreyro FJ. The interplay between Helicobacter pylori infection and rs738409 PNPLA3 in metabolic dysfunction-associated steatotic liver disease. PLoS One. 2024;19:e0310361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (3)] |
| 20. | Mayerle J, den Hoed CM, Schurmann C, Stolk L, Homuth G, Peters MJ, Capelle LG, Zimmermann K, Rivadeneira F, Gruska S, Völzke H, de Vries AC, Völker U, Teumer A, van Meurs JB, Steinmetz I, Nauck M, Ernst F, Weiss FU, Hofman A, Zenker M, Kroemer HK, Prokisch H, Uitterlinden AG, Lerch MM, Kuipers EJ. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013;309:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Beygi M, Ahi S, Zolghadri S, Stanek A. Management of Metabolic-Associated Fatty Liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: From Medication Therapy to Nutritional Interventions. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 22. | Huang Y, Wang X, Zhang L, Zheng K, Xiong J, Li J, Cong C, Gong Z, Mao J. Effect of Probiotics Therapy on Nonalcoholic Fatty Liver Disease. Comput Math Methods Med. 2022;2022:7888076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Jamali A, Karbalai S, Tefagh G, Jamali R, Ahmadi A. The Effects of Helicobacter Pylori Eradication on Liver Function and Metabolic Profile in Non-diabetic Non-alcoholic Steatohepatitis: A 5-year Randomized Clinical Trial. Middle East J Dig Dis. 2022;14:85-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/