Published online Aug 27, 2024. doi: 10.4254/wjh.v16.i8.1145
Revised: July 3, 2024
Accepted: July 23, 2024
Published online: August 27, 2024
Processing time: 94 Days and 10 Hours
Previous research has highlighted correlations between blood cell counts and chronic liver disease. Nonetheless, the causal relationships remain unknown.
To evaluate the causal effect of blood cell traits on liver enzymes and nonalcoholic fatty liver disease (NAFLD) risk.
Independent genetic variants strongly associated with blood cell traits were extracted from a genome-wide association study (GWAS) conducted by the Blood Cell Consortium. Summary-level data for liver enzymes were obtained from the United Kingdom Biobank. NAFLD data were obtained from a GWAS meta-analysis (8434 cases and 770180 controls, discovery dataset) and the Fingen GWAS (2275 cases and 372727 controls, replication dataset). This analysis was conducted using the inverse-variance weighted method, followed by various sensitivity analyses.
One SD increase in the genetically predicted haemoglobin concentration (HGB) was associated with a β of 0.0078 (95%CI: 0.0059-0.0096), 0.0108 (95%CI: 0.0080-0.0136), 0.0361 (95%CI: 0.0156-0.0567), and 0.0083 (95%CI: 00046-0.0121) for alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase, and gamma-glutamyl transferase, respectively. Genetically predicted haematocrit was associated with ALP (β = 0.0078, 95%CI: 0.0052-0.0104) and ALT (β = 0.0057, 95%CI: 0.0039-0.0075). Genetically determined HGB and the reticulocyte fraction of red blood cells increased the risk of NAFLD [odds ratio (OR) = 1.199, 95%CI: 1.087-1.322] and (OR = 1.157, 95%CI: 1.071-1.250). The results of the sensitivity analyses remained significant.
Novel causal blood cell traits related to liver enzymes and NAFLD development were revealed through Mendelian randomization analysis, which may facilitate the diagnosis and prevention of NAFLD.
Core Tip: Mendelian randomization analysis revealed a novel evidence for a causal role of genetically predicted blood cell traits in liver injury and nonalcoholic fatty liver disease (NAFLD). The study found that genetically determined increases in hemoglobin concentration (HGB) and hematocrit levels were associated with elevated levels of liver enzymes. In addition, genetic determinants of HGB and reticulocyte ratio are associated with an increased risk of NAFLD. These findings may help in the diagnosis and prevention of NAFLD.
- Citation: Hu B, Wan AH, Xiang XQ, Wei YH, Chen Y, Tang Z, Xu CD, Zheng ZW, Yang SL, Zhao K. Blood cell counts and nonalcoholic fatty liver disease: Evidence from Mendelian randomization analysis. World J Hepatol 2024; 16(8): 1145-1155
- URL: https://www.wjgnet.com/1948-5182/full/v16/i8/1145.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i8.1145
Chronic liver disease (CLD), including nonalcoholic fatty liver disease (NAFLD), is a significant cause of mortality and liver cancer, accounting for 3.5% of all deaths worldwide[1]. The incidence of NAFLD, a chronic metabolic stress-related liver disease, is increasing, contributing to the rapid increase in the global burden of liver disease[2]. The insidious onset and complex pathogenesis of NAFLD are not fully understood, but the disease is closely associated with insulin resistance (IR), genetic predisposition, and an increased risk of developing cirrhosis, end-stage liver disease, and hepatocellular carcinoma over time[3-5]. Serum liver enzymes, including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), are considered important markers in the clinical assessment of liver injury, and they are associated with the risk of developing NAFLD[6,7]. Given the invasive nature of the gold standard for NAFLD biopsy and the limitations of diagnostic imaging criteria, there is a need to search for noninvasive circulating biomarkers. In addition, determining the causal relationships among diseases is the first step towards rational and individualized treatment.
Blood cell counts are quantitative clinical laboratory measures that characterize the production of haematopoietic progenitor cells, the synthesis of haemoglobin, and the clearance of mature or senescent blood cells from the circulation[8]. Considering the vital functions of blood cells in delivering tissue oxygen, managing inflammatory responses, addressing atherosclerosis, and preventing thrombosis, factors contributing to interpopulation variations in blood cell traits may significantly impact the development of CLD and contribute to health disparities among populations[9,10]. However, the limitations of these studies include potential confounding factors, reverse causality, and the inability to establish causality because of their cross-sectional or retrospective nature. Determining if blood cell counts are causally involved in the progression of NAFLD is of significant clinical importance
Mendelian randomization (MR), an emerging method, offers a novel approach to address these limitations. By utilizing single-nucleotide polymorphisms (SNPs) identified from genome-wide association studies (GWASs) as instrumental variables (IVs), MR analysis leveraging genetic variants randomly allocated at conception helps mitigate biases from confounders and reverse causation, resembling a "natural" randomized controlled trial[11]. Many powerful GWASs have identified thousands of SNPs associated with blood cell-related traits or circulating liver enzyme levels[9,12,13]. This creates an opportunity to test genetic, potentially causal relationships between blood cell traits and relevant CLD-associated traits using the MR approach.
Therefore, in this context, we conducted a two-sample bidirectional MR design and systematically assessed the potential causal relationships between blood cell counts and CLD-associated traits.
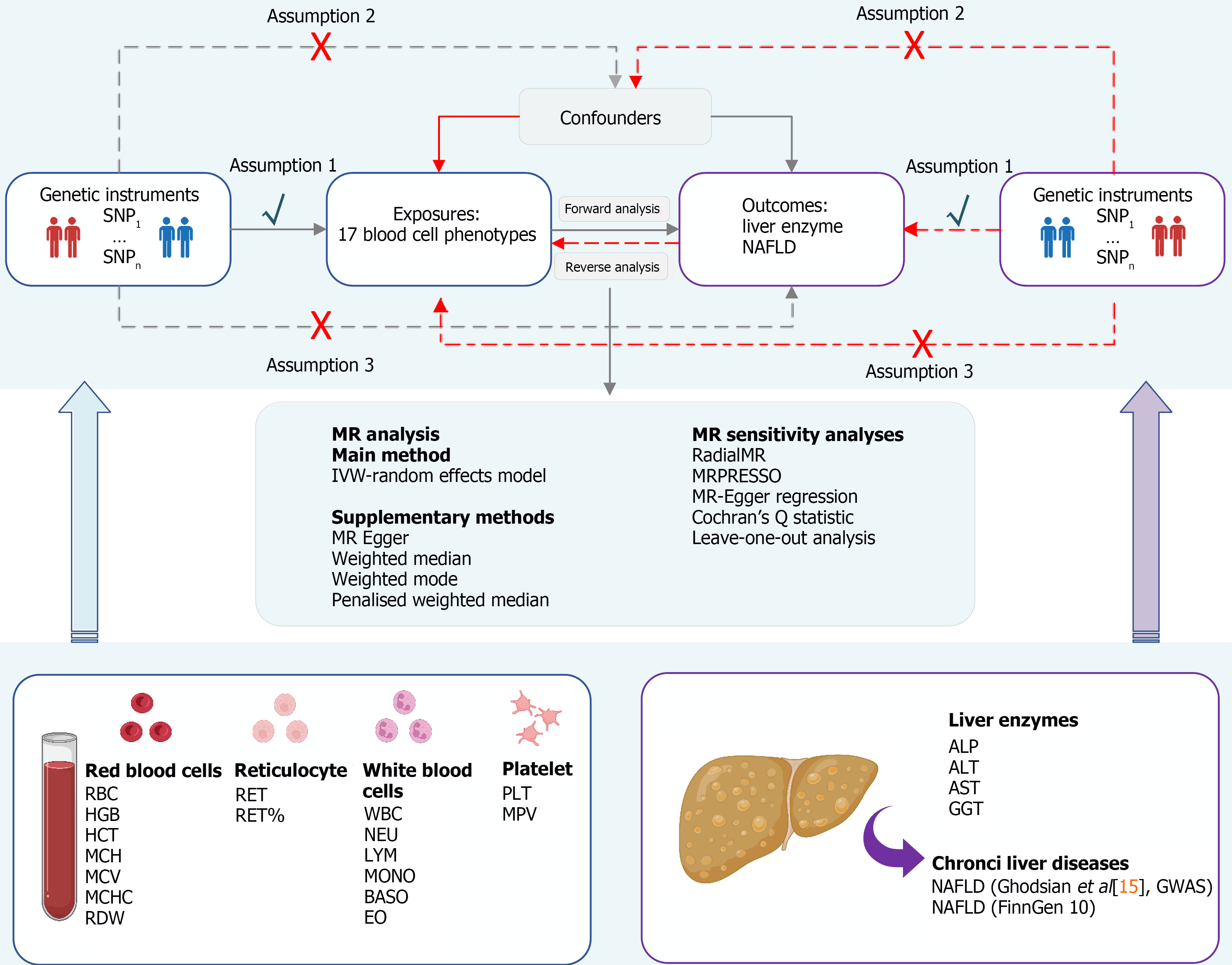
A summary of the research methodology is depicted in Figure 1. The study is reported according to the STROBE-MR checklist guidelines (https://www.strobe-mr.org/)[14]. The summary data from the GWAS employed in this research are accessible to the public for download, and every original investigation secured written consent from the subjects involved, with endorsement from ethical review boards. An overview of the GWAS data sources used for MR analysis is shown in Table 1.
| Phenotypes | Trait name | Unit | Sample size | No. of cases | Ethnicity | Adjustment | Ref. |
| Red blood cells | RBC | per pL | 563946 | 545203 | European | Age, sex, the first 10 principal components, and cohort-specific covariates were corrected in the original GWAS | [9] |
| HGB | g/dL | 563946 | 563946 | European | |||
| HCT | % | 563946 | 562259 | European | |||
| MCH | pg | 563946 | 491553 | European | |||
| MCV | fL | 563946 | 544127 | European | |||
| MCHC | g/dL | 563946 | 491553 | European | |||
| RDW | fL | 563946 | 531774 | European | |||
| Immature red cells | RET | pL | 408112 | 408112 | European | [10] | |
| RET% | % | 408112 | 408112 | European | |||
| White blood cells | WBC | per nL | 563946 | 562243 | European | [9] | |
| NEU | per nL | 563946 | 519288 | European | |||
| LYM | per nL | 563946 | 524923 | European | |||
| MONO | per nL | 563946 | 521594 | European | |||
| BASO | per nL | 563946 | 474001 | European | |||
| EO | per nL | 563946 | 474237 | European | |||
| Platelets | PLT | per nL | 563946 | 542827 | European | ||
| MPV | fL | 563946 | 460935 | European | |||
| Covariates | WHR | SD | 694649 | 694649 | European | BMI | [27] |
| T2D | Log (OR) | 933970 | 80154 | European | Age, sex, study-specific covariates, and principal components | [28] | |
| Liver enzymes | ALP | SD | 437438 | 437438 | European | Age and sex and principal components of genetically inferred ancestry | [12] |
| ALT | SD | 437267 | 437267 | European | |||
| GGT | SD | 437194 | 437194 | European | |||
| AST | SD | 411048 | 411048 | European | Age sex, BMI, and the first 12 principal components of genetic ancestry | [13] | |
| Chronic liver diseases | NAFLD (Ghodsian et al[15], 2020) | Log (OR) | 778614 | 8434 | European | BMI | [15] |
| NAFLD (Fingen 10) | Log (OR) | 375002 | 2275 | European | Age, sex, the first 10 genetic principal components, and genotyping batch | - |
Summary statistics for the 17 blood cell traits were derived from comprehensive GWASs conducted by the Blood Cell Consortium and the United Kingdom Biobank (UKB), which represents the largest GWAS on blood cell traits to date, encompassing 563085 participants of European ancestry[9,10]. In the original GWAS, meticulous efforts were made to correct for potential confounders, including age, sex, the first 10 principal components of genetic ancestry, and cohort-specific covariates, to ensure the robustness of the genetic associations identified. The specific blood cell traits analysed included 8 red blood cell traits [red blood cell count (RBC); haemoglobin concentration (HGB); haematocrit (HCT); mean corpuscular haemoglobin (MCH); mean corpuscular volume; mean corpuscular haemoglobin concentration; red cell distribution width (RDW)], 2 immature red cell traits [reticulocyte count (RET); reticulocyte fraction of red cells (RET%)], 6 white blood cell traits [white blood cell count (WBC); neutrophil count (NEU); lymphocyte count (LYM); monocyte count (MONO); basophil count (BASO); eosinophil count (EO)], and 2 platelet traits [platelet count (PLT); mean platelet volume (MPV)]. Complete summary statistics from GWASs of blood cell traits can be downloaded from the GWAS catalogue (https://www.ebi.ac.uk/gwas/).
Genetic associations with ALP, ALT, and GGT were extracted from GWASs conducted by Pazoki et al[12]. We utilized data from the UKB and included 437267 individuals aged between 40 and 69 years. Study participants were identified through the United National Health Service Registers across 22 centres in the UKB between the years 2006 and 2010. The study included individuals of European ancestry, following quality measures and exclusions such as sex, high missingness, and/or heterozygosity. The summary datasets of AST were derived from a GWAS meta-analysis of 411048 individuals in the UKB (adjusted for various factors, including the age at recruitment, sex, body mass index, and the first 12 principal components of genetic ancestry)[13].
The summary level GWAS statistics for NAFLD in the primary analysis were obtained from the Ghodsian et al’s study[15], which included 8434 NAFLD cases and 770180 controls of European ancestry (discovery dataset). The study integrated data from four additional GWASs: The Electronic Medical Records and Genomics, the FinnGen consortium, the UKB, and the Estonian Biobank. NAFLD diagnosis was determined on the basis of electronic health records or hospital records for all participants. Specifically, NAFLD was defined by the use of EHR codes and the International Classification of Diseases (ICD) (ICD9: 571.5, ICD9: 571.8, ICD9: 571.9, ICD10: K75.81, ICD10: K76.0 and ICD10: K76.9). To validate our results via replication analysis and meta-analysis, we used NAFLD (2275 NAFLD cases and 375002 controls) and data from the FinnGen consortium (replication dataset), which is publicly available at the following website (https://R10.finngen.fi/).
We extracted all SNPs that strongly and independently predicted 17 blood cell traits at the threshold of genome-wide significance (P < 5 × 10-8). After the significant SNPs corresponding to each blood cell trait were extracted, a linkage disequilibrium (LD) evaluation was conducted utilizing the European genetic profiles from a cohort of 1000 subjects as a benchmark and excluding potential LD (with R2 < 0.001 within a 10000 kb region), palindromic structural SNPs (minor allele frequency > 0.42), and incompatible SNPs[16]. We calculated the F statistic (F = β2/SE2) for each SNP (F statistic range 29.75 to 1600.00), F > 10, suggesting the absence of weak instrumental bias[17]. In addition, we aimed to estimate the causal effects of genetically predicted liver enzymes and NAFLD on blood cell traits. We further performed reverse MR analyses. Data harmonization and MR methods were also performed as described in the forwards MR analysis. Comprehensive details regarding these exposures, outcomes, datasets, and IVs are presented in Supplementary Table 1.
Before conducting the MR analysis, SNPs directly related to the liver enzymes and NAFLD data were removed to avoid potential horizontal pleiotropy (P < 5 × 10-8). The primary analysis used inverse-variance weighted (IVW) with a random effects model[18]. In addition, a range of sensitivity analyses, such as MR-Egger[19], weighted median[20], weighted mode[21], simple mode, and penalized weighted median methods, were performed to evaluate the potential bias in the MR setting. In addition, RadialMR and MR pleiotropy residual sum and outlier (MRPRESSO, NbDistribution = 3000) were utilized to identify outliers with multiple effects across all levels for variables exhibiting significant causal links, followed by a re-evaluation of the causality estimates subsequent to the exclusion of these outliers[22,23]. Furthermore, we also performed sensitivity analyses using MR-Egger regression (assessing the presence of directional pleiotropy) to determine the robustness of the IVW results, in which a P value for the intercept < 0.05 was considered statistically significant[24]. Cochran's Q statistic checks the heterogeneity of individual causal effects, and leave-one-out analysis examines the effects of outlying and pleiotropic SNPs on causal estimates[25,26]. To control the influence of pleiotropy within this research, we proceeded to perform multivariable MR (MVMR) analyses, which accounted for the waist-to-hip ratio (WHR)[27] and type 2 diabetes (T2D)[28]. MVMR facilitates the concurrent assessment of causal relationships among various predictors and outcomes[29]. MVMR was executed employing the IVW approach as the principal technique and the Egger regression method for supplementary evaluations.
All the statistical analyses were performed using the TwoSampleMR (version 0.5.4), RadialMR (version 1.0), and MRPRESSO (version 1.0) packages in R software (version 4.2.2). The MR estimates are reported as effect sizes (β) or odds ratios (ORs) along with 95%CIs. The statistical significance threshold was defined as a P value < 5.88 × 10-4 (0.05/17 exposure × 5 outcome) after Bonferroni correction to address multiple testing issues[30]. If a P value was between 5.58 × 10-4 and 0.05, it was considered to indicate a potential causal relationship with nominal significance. For the MVMR analysis, a P value < 0.05 was considered statistically significant.
Specifically, IVW MR analysis revealed a statistically significant association between genetically predicted higher RBC levels and ALP (β = 0.0043, 95%CI: 0.002-0.0065, PIVW = 1.84E-04 per 1-SD increase). Higher HGB levels were significantly associated with ALP (β = 0.0079, 95%CI: 0.0051-0.0107, PIVW = 3.07E-08), ALT (β = 0.0128, 95%CI: 0.0085-0.0170, PIVW = 4.69E-09), AST (β = 0.0713, 95%CI: 0.0395-0.1031, PIVW = 1.12E-05) and GGT (β = 0.0108, 95%CI: 00049-0.0168, PIVW = 3.73E-04; Supplementary Table 2). In addition, genetically determined higher HCT levels (per 1-SD increase) were significantly associated with the levels of ALP (β = 0.0060, 95%CI: 0.0033-0.0087, PIVW = 1.14E-05) and ALT (β = 0.0075, 95%CI: 0.0034-0.0116, PIVW = 3.05E-04). The RadialMR and MRPRESSO tests were used to identify any level of multieffect outliers and reassessed the causal effect estimates after removing outliers, and the results remained significant (Supplementary Table 3).
Another aspect, genetically determined higher circulating levels of WBC, NEU, and BASO, was associated with increased levels of ALP (β = 0.0031, 95%CI: 0.0005-0.0056, PIVW = 1.89E-02 per 1-SD increase in WBC; β = 0.0035, 95%CI: 0.0007-0.0062, PIVW = 1.25E-02 per 1-SD increase in NEU; β = 0.0041, 95%CI: 0.0004-0.0077, PIVW = 2.87E-02 per 1-SD increase in BASO; Supplementary Table 2). Genetically predicted RBC, MCH, RET, RET%, and LYW were associated with the levels of ALT (β = 0.0040, 95%CI: 0.0005-0.0075, PIVW = 2.42E-02 per 1-SD increase in RBC; β = 0.0042, 95%CI: 0.0012-0.0071, PIVW = 5.26E-03 per 1-SD increase in MCH; β = 0.0052, 95%CI: 0.0017-0.0087, PIVW = 4.00E-03 per 1-SD increase in RET; β = 0.0050, 95%CI: 0.0019-0.0080, PIVW = 1.58E-03 per 1-SD increase in RET%; β = 0.005, 95%CI: 0.0013-0.0086, PIVW = 7.88E-03 per 1-SD increase in LYM).
We detected a positive correlation between HCT levels and AST (β = 0.0452, 95%CI: 0.0162-0.0742, PIVW = 2.23E-03 per 1-SD increase in HCT). Similarly, we observed that the levels of RBC, HCT, and PLT were suggestively associated with the levels of GGT, with β values of 0.0078 (95%CI: 0.003-0.0126 PIVW = 1.35E-03), 0.0064 (95%CI: 0.001-0.0118 PIVW = 2.09E-02), and β of 0.0068 (95%CI: 0.0027-0.0109 PIVW = 1.06E-03), respectively. Interestingly, genetically predicted higher RDWs were inversely associated with the levels of ALT (β = -0.0034, 95%CI: -0.0067 to -0.0002, PIVW = 3.95E-02) and AST (β = -0.0248, 95%CI: -0.046 to -0.0035, PIVW = 2.21E-02). No significant causal associations were found between other genetically driven blood cell traits and liver enzymes.
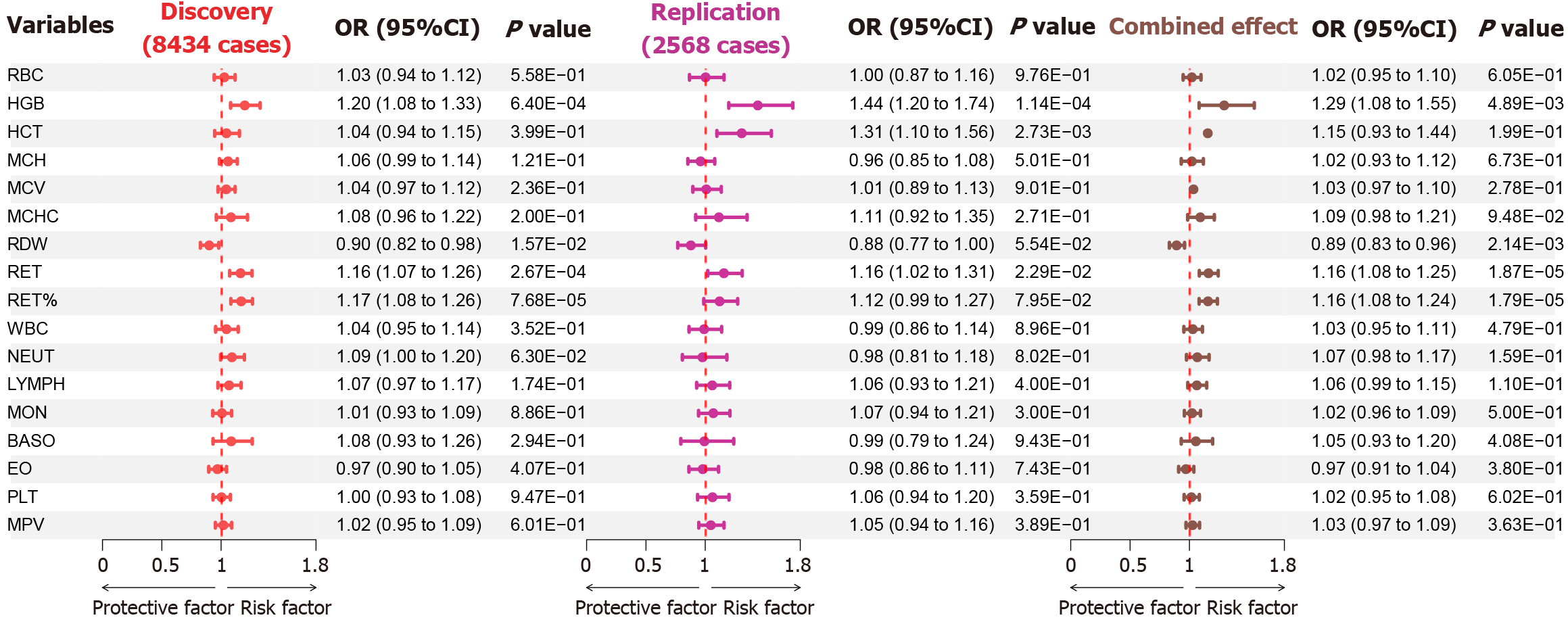
After removing outliers, the MR results revealed that genetically predicted HGB and RET% increased the risk of NAFLD (OR = 1.199, 95%CI: 1.087-1.322, PIVW = 2.70E-04) and (OR = 1.157, 95%CI: 1.071-1.250, PIVW = 2.24E-04; Supplementary Table 4). Nevertheless, we observed a potential positive correlation between genetically elevated levels of RET and the risk of NAFLD (OR = 1.137, 95%CI: 1.051-1.229, PIVW = 1.29E-03). In addition, MR analysis suggested that a genetic predisposition to higher HGB, RET, and RET% was causally associated with an increased risk of NAFLD in both discovery, replication, and meta-analyses (Figure 2).
No evidence of heterogeneity (Cochranes’s Q, P value > 0.05) or horizontal pleiotropy (MR-Egger intercept, P value > 0.05) was observed in the MR estimates (Supplementary Table 5). The global tests from RadialMR and MRPRESSO were utilized to identify outliers with multiple effects across all levels for associations with significant causality and to reevaluate the causality estimates after these outliers were removed. The results remained stable (Supplementary Tables 3 and 4). Additionally, a leave-one-out sensitivity analysis was conducted, successively excluding each SNP associated with risk factors and recalculating the MR outcomes for the remaining SNPs. Notably, this analysis confirmed the stability of the outcomes (Supplementary Figures 1-10). The visual representations, including scatter plots, funnel plots, forest plots, and leave-one-out plots, are shown in Supplementary Figures 1-10.
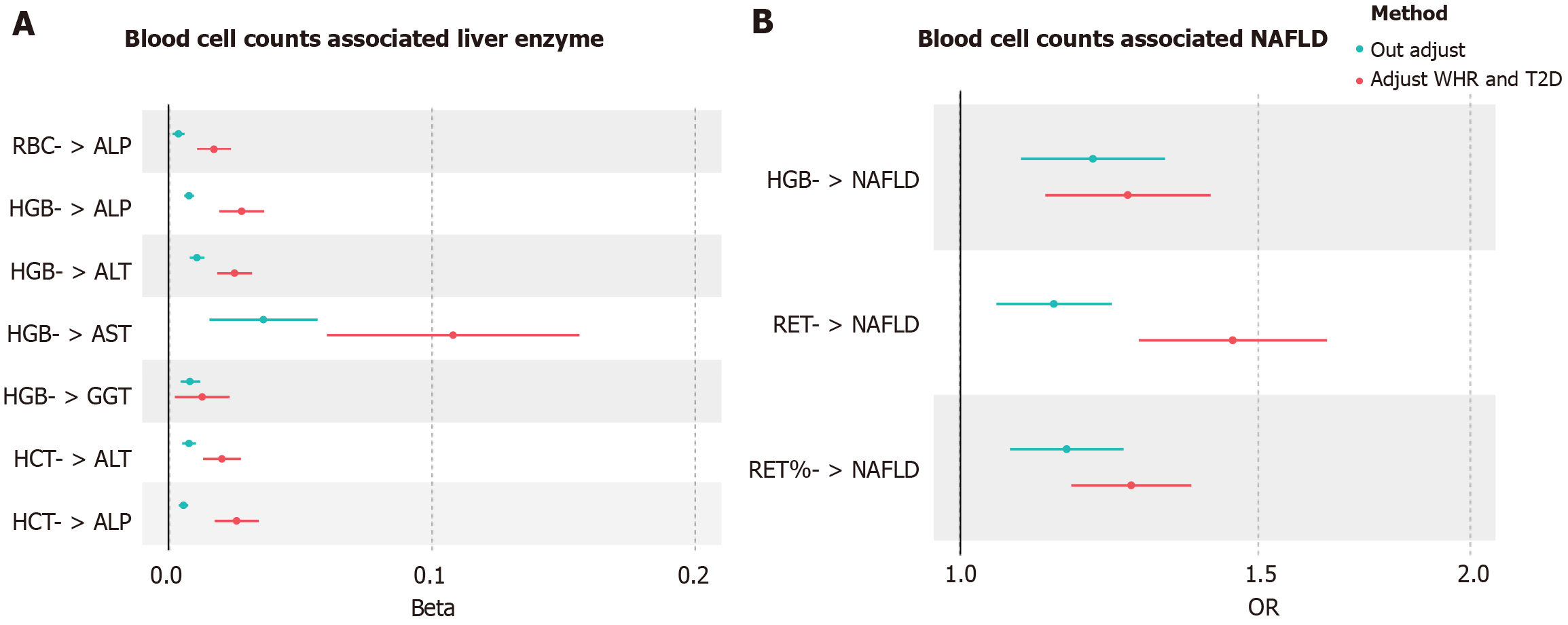
Within the MVMR framework, we adjusted for two key confounding factors (WHR and T2D). Our findings revealed that the causal relationships remained robust and consistent, notwithstanding the consideration of a range of influencing factors (Figure 3A and Supplementary Table 6). However, the associations between genetically determined HGB and GGT levels were nonsignificant (β = 0.0128, 95%CI: 0.0024-0.0233, PIVW = 1.62E-02 per 1-SD increase in HGB) (MVMR-IVW method) and (β = 0.0134, 95%CI: 0.0028-0.0238, PIVW = 1.26E-02 per 1-SD increase in HGB) (MVMR-Egger method). Importantly, most of the P values of the MVMR-Egger intercept were > 0.05, suggesting a low likelihood of pleiotropy.
The positive effects of RET (OR = 1.266, 95%CI: 1.070-1.498, PIVW = 1.29E-03, per 1-SD increase) and RET% (OR = 1.264, 95%CI: 1.165-1.371, PIVW = 1.59E-08) remained following adjustments for WHR and T2D in the multivariable MR analysis (Figure 3B and Supplementary Table 6). Furthermore, there was an inconsistency between the MVMR-Egger results and the MVMR-IVW analysis results. For example, genetically predicted HGB and the risk of NAFLD became nonsignificant (OR = 1.165, 95%CI: 0.987-1.373, PIVW = 7.03E-04, per 1-SD increase in HGB). In addition, most of the P values of the MVMR-Egger intercept were > 0.05, indicating a low likelihood of pleiotropy.
We performed reverse MR analysis on the significant results of the positive MR (after Bonferroni correction). Characteristics of the genetic variants were associated with liver enzyme levels and NAFLD (Supplementary Table 7). The IVW MR analysis revealed that ALP had significant causal effects on increases in RBC (β = 0.5365, 95%CI: 0.2636-0.8094, PIVW = 1.17E-04) and HCT (β = 0.4562, 95%CI: 0.2046-0.7077, PIVW = 3.78E-04; Supplementary Table 8). In addition, higher ALT levels were significantly associated with HGB levels (β = 0.3576, 95%CI: -0.1262 to 0.8412, PIVW = 5.83E-05). All additional methods yielded similar results, except for MR-Egger and weighted methods (Supplementary Table 8). In addition, genetic susceptibility to NAFLD risk was not associated with HGB (β = 0.2892, 95%CI: -0.0139 to 0.5923, PIVW = 3.52E-03), RET (β = 0.0014, 95%CI: -0.0731 to 0.0759, PIVW = 9.70E-01), or RET% (β = -0.0097, 95%CI: -0.0876 to 0.0683, PIVW = 8.08E-01).
In this MR study, we provided new novel evidence for the causal roles of 17 genetically predicted blood cell traits in liver injury and the development of NAFLD. We found that genetically predicted HGB and HCT were causally associated with a wide range of levels of liver injury. Elevated HGB, RET, and RET% were strong indicators of an increased risk of NAFLD. Similarly, the multivariate MR and sensitivity analyses remained significant. These findings highlight the roles of blood cell traits in the pathogenesis of NAFLD and provide new evidence for understanding the impact of abnormal liver function on blood biology, which may facilitate its diagnosis and prevention.
Blood is described as a viscous non-Newtonian fluid, with more than 95% of blood being red blood cells, thus determining its critical role in whole blood viscosity (WBV). WBV is a crucial physiological and pathological marker that often increases with the onset of chronic conditions such as inflammation, aging, and NAFLD[31-33]. Understanding the pivotal role of WBV as a physiological and pathological marker provides valuable insights into the interconnected mechanisms at play. Prior research has underscored the robust correlation between elevated RBC levels and various health conditions, such as IR, metabolic syndrome (Mets), and NAFLD, and emphasized the significance of hematological parameters in assessing metabolic health[34-36]. Moreover, in the context of NAFLD, elevated RBC counts have been identified as an independent predictor of disease progression, suggesting their potential utility as a diagnostic biomarker[37]. The widely accepted "multiple parallel hits" hypothesis offers a comprehensive understanding of NAFLD pathogenesis, highlighting the interplay of IR, oxidative stress, and inflammatory responses within this intricate framework[38,39]. As ongoing research continues to unveil the nuanced connections between blood traits and systemic health, these findings contribute to a broader understanding of the intricate web of factors influencing metabolic and liver-related disorders.
HGB is the most common component of erythrocytes and is the carrier of carbon dioxide and oxygen through the vascular network to body cells. The intricate relationship between elevated HGB levels and increased risk of NAFLD is underscored by various physiological mechanisms. Elevated HGB not only is positively correlated with NAFLD risk but also serves as a diagnostic indicator for disease progression[39-41]. The repercussions of increased HGB include increased blood viscosity, compromised hepatic blood flow perfusion, and impaired microcirculation, culminating in hepatocyte ischemia and hypoxia. Furthermore, the cascade of events includes hypoxia-induced oxidative stress, disruptions in hepatic glycogen deposition, and diminished insulin sensitivity. This complex interplay also involves the upregulation of hepatic lipid synthesis genes and the downregulation of lipid metabolism genes[33,42]. These molecular and physiological alterations collectively contribute to increased susceptibility to NAFLD. Additionally, the robustness of these findings is supported by MR analysis, which establishes a causal relationship between genetically predisposed HGB levels and the levels of four liver enzymes, as well as the incidence of NAFLD. Importantly, even after adjusting for confounding factors such as the WHR and T2D status in multivariable MR analyses, the persistent causal association underscores the critical role of HGB in the development and progression of NAFLD and its impact on liver health.
RET serves as an indicator reflecting the haematopoietic function of bone marrow, drawing considerable attention from scholars worldwide for its applications in diagnosing and treating anaemic conditions, as well as in radiotherapy for patients with tumours. Notably, previous findings revealed marked increases in RET% and RET levels within the chronic hepatitis cohort, suggesting a potential link between RET and liver health[43]. Additionally, other studies have confirmed that RET can indicate liver fibrosis severity to a certain extent, thus serving as a diagnostic marker for liver fibrosis and its routine monitoring[44]. Moreover, anaemia resulting from malabsorption of iron, folic acid, and vitamin B12 in patients with CLDs may present as elevated RET levels[45]. Expanding on these observations, our MR study adds a genetic perspective, indicating that genetically predicted higher RET% levels increase the risk of NAFLD. This aligns with findings from independent datasets, collectively reinforcing the validity of the conclusion and providing robust genetic support for previously observed associations. The convergence of evidence from various sources underscores the multifaceted role of RET in liver health and positions it as a valuable marker for both haematopoietic function and liver-related conditions.
A key advantage of this research is its use of the MR approach, which effectively mitigates the impact of confounding factors and the issue of reverse causality typically encountered in observational research. This method provides a thorough examination of the causal links between blood cell trait circulation and the levels of liver enzymes, as well as the risk of NAFLD. In addition, we used data from an independent GWAS in NAFLD to validate our findings in the discovery data and combined causal effect estimates from both datasets through meta-analysis to increase statistical power and improve estimation accuracy. The associations remained consistent in the sensitivity analyses, and no indication of unbalanced pleiotropy was detected. Furthermore, we performed MVMR analyses for traits with strong phenotypes, and the adjusted associations remained substantially stable.
Interpreting the results of our MR study requires careful consideration of several limitations. First, MR provides only a preliminary assessment of the causality between exposure and disease, without delving into the specific biological pathways that underpin these relationships. Our study acknowledges the potential for residual confounding factors that may not have been identified or documented in the current literature. Additionally, MR studies do not offer individual-level data, which limits the ability to examine potential nonlinear relationships. Consequently, robust individual-level data are essential to better understand the impact of elevated blood cell counts on the development of NAFLD.
Finally, the findings from this MR study are grounded in GWAS summary statistics from the European population. However, the generalizability of these results to other ethnic groups remains an open question that necessitates further investigation. To address this, cross-ethnic group studies should be undertaken. These studies compare genetic rela
Taking into account both our MR and MVMR results, the associations between high HGB, HCT, and RET% with liver injury and the risk of NAFLD may act independently. These findings have clinical implications, as they suggest the future potential for evaluating blood cell traits as targets for liver injury and NAFLD prevention or treatment.
The authors acknowledge the researchers of the original studies for sharing the GWAS data used in this project.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2467] [Article Influence: 352.4] [Reference Citation Analysis (1)] |
| 2. | Samanta A, Sen Sarma M. Metabolic dysfunction-associated steatotic liver disease: A silent pandemic. World J Hepatol. 2024;16:511-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 3. | Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018;27:22-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 557] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 4. | En Li Cho E, Ang CZ, Quek J, Fu CE, Lim LKE, Heng ZEQ, Tan DJH, Lim WH, Yong JN, Zeng R, Chee D, Nah B, Lesmana CRA, Bwa AH, Win KM, Faulkner C, Aboona MB, Lim MC, Syn N, Kulkarni AV, Suzuki H, Takahashi H, Tamaki N, Wijarnpreecha K, Huang DQ, Muthiah M, Ng CH, Loomba R. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72:2138-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 200] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 5. | Vargas M, Cardoso Toniasso SC, Riedel PG, Baldin CP, Dos Reis FL, Pereira RM, Brum MCB, Joveleviths D, Alvares-da-Silva MR. Metabolic disease and the liver: A review. World J Hepatol. 2024;16:33-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (3)] |
| 6. | Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, Xu C. The associations between modifiable risk factors and nonalcoholic fatty liver disease: A comprehensive Mendelian randomization study. Hepatology. 2023;77:949-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 7. | Newton KP, Lavine JE, Wilson L, Behling C, Vos MB, Molleston JP, Rosenthal P, Miloh T, Fishbein MH, Jain AK, Murray KF, Schwimmer JB; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis. Hepatology. 2021;73:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Chen MH, Raffield LM, Mousas A, Sakaue S, Huffman JE, Moscati A, Trivedi B, Jiang T, Akbari P, Vuckovic D, Bao EL, Zhong X, Manansala R, Laplante V, Chen M, Lo KS, Qian H, Lareau CA, Beaudoin M, Hunt KA, Akiyama M, Bartz TM, Ben-Shlomo Y, Beswick A, Bork-Jensen J, Bottinger EP, Brody JA, van Rooij FJA, Chitrala K, Cho K, Choquet H, Correa A, Danesh J, Di Angelantonio E, Dimou N, Ding J, Elliott P, Esko T, Evans MK, Floyd JS, Broer L, Grarup N, Guo MH, Greinacher A, Haessler J, Hansen T, Howson JMM, Huang QQ, Huang W, Jorgenson E, Kacprowski T, Kähönen M, Kamatani Y, Kanai M, Karthikeyan S, Koskeridis F, Lange LA, Lehtimäki T, Lerch MM, Linneberg A, Liu Y, Lyytikäinen LP, Manichaikul A, Martin HC, Matsuda K, Mohlke KL, Mononen N, Murakami Y, Nadkarni GN, Nauck M, Nikus K, Ouwehand WH, Pankratz N, Pedersen O, Preuss M, Psaty BM, Raitakari OT, Roberts DJ, Rich SS, Rodriguez BAT, Rosen JD, Rotter JI, Schubert P, Spracklen CN, Surendran P, Tang H, Tardif JC, Trembath RC, Ghanbari M, Völker U, Völzke H, Watkins NA, Zonderman AB; VA Million Veteran Program, Wilson PWF, Li Y, Butterworth AS, Gauchat JF, Chiang CWK, Li B, Loos RJF, Astle WJ, Evangelou E, van Heel DA, Sankaran VG, Okada Y, Soranzo N, Johnson AD, Reiner AP, Auer PL, Lettre G. Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations. Cell. 2020;182:1198-1213.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 490] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 10. | Vuckovic D, Bao EL, Akbari P, Lareau CA, Mousas A, Jiang T, Chen MH, Raffield LM, Tardaguila M, Huffman JE, Ritchie SC, Megy K, Ponstingl H, Penkett CJ, Albers PK, Wigdor EM, Sakaue S, Moscati A, Manansala R, Lo KS, Qian H, Akiyama M, Bartz TM, Ben-Shlomo Y, Beswick A, Bork-Jensen J, Bottinger EP, Brody JA, van Rooij FJA, Chitrala KN, Wilson PWF, Choquet H, Danesh J, Di Angelantonio E, Dimou N, Ding J, Elliott P, Esko T, Evans MK, Felix SB, Floyd JS, Broer L, Grarup N, Guo MH, Guo Q, Greinacher A, Haessler J, Hansen T, Howson JMM, Huang W, Jorgenson E, Kacprowski T, Kähönen M, Kamatani Y, Kanai M, Karthikeyan S, Koskeridis F, Lange LA, Lehtimäki T, Linneberg A, Liu Y, Lyytikäinen LP, Manichaikul A, Matsuda K, Mohlke KL, Mononen N, Murakami Y, Nadkarni GN, Nikus K, Pankratz N, Pedersen O, Preuss M, Psaty BM, Raitakari OT, Rich SS, Rodriguez BAT, Rosen JD, Rotter JI, Schubert P, Spracklen CN, Surendran P, Tang H, Tardif JC, Ghanbari M, Völker U, Völzke H, Watkins NA, Weiss S; VA Million Veteran Program, Cai N, Kundu K, Watt SB, Walter K, Zonderman AB, Cho K, Li Y, Loos RJF, Knight JC, Georges M, Stegle O, Evangelou E, Okada Y, Roberts DJ, Inouye M, Johnson AD, Auer PL, Astle WJ, Reiner AP, Butterworth AS, Ouwehand WH, Lettre G, Sankaran VG, Soranzo N. The Polygenic and Monogenic Basis of Blood Traits and Diseases. Cell. 2020;182:1214-1231.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 509] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 11. | Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 2651] [Article Influence: 530.2] [Reference Citation Analysis (0)] |
| 12. | Pazoki R, Vujkovic M, Elliott J, Evangelou E, Gill D, Ghanbari M, van der Most PJ, Pinto RC, Wielscher M, Farlik M, Zuber V, de Knegt RJ, Snieder H, Uitterlinden AG; Lifelines Cohort Study, Lynch JA, Jiang X, Said S, Kaplan DE, Lee KM, Serper M, Carr RM, Tsao PS, Atkinson SR, Dehghan A, Tzoulaki I, Ikram MA, Herzig KH, Järvelin MR, Alizadeh BZ, O'Donnell CJ, Saleheen D, Voight BF, Chang KM, Thursz MR, Elliott P; VA Million Veteran Program. Genetic analysis in European ancestry individuals identifies 517 loci associated with liver enzymes. Nat Commun. 2021;12:2579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Ward LD, Tu HC, Quenneville CB, Tsour S, Flynn-Carroll AO, Parker MM, Deaton AM, Haslett PAJ, Lotta LA, Verweij N, Ferreira MAR; Regeneron Genetics Center; Geisinger-Regeneron DiscovEHR Collaboration, Baras A, Hinkle G, Nioi P. GWAS of serum ALT and AST reveals an association of SLC30A10 Thr95Ile with hypermanganesemia symptoms. Nat Commun. 2021;12:4571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2570] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 15. | Ghodsian N, Abner E, Emdin CA, Gobeil É, Taba N, Haas ME, Perrot N, Manikpurage HD, Gagnon É, Bourgault J, St-Amand A, Couture C, Mitchell PL, Bossé Y, Mathieu P, Vohl MC, Tchernof A, Thériault S, Khera AV, Esko T, Arsenault BJ. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease. Cell Rep Med. 2021;2:100437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 16. | Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4747] [Cited by in RCA: 5623] [Article Influence: 702.9] [Reference Citation Analysis (0)] |
| 17. | Bowden J, Del Greco M F, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, Thompson J, Davey Smith G. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48:728-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (1)] |
| 18. | Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG; EPIC- InterAct Consortium. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 1341] [Article Influence: 121.9] [Reference Citation Analysis (0)] |
| 19. | Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2275] [Cited by in RCA: 7157] [Article Influence: 650.6] [Reference Citation Analysis (1)] |
| 20. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 6515] [Article Influence: 651.5] [Reference Citation Analysis (0)] |
| 21. | Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 643] [Cited by in RCA: 2508] [Article Influence: 313.5] [Reference Citation Analysis (0)] |
| 22. | Bowden J, Spiller W, Del Greco M F, Sheehan N, Thompson J, Minelli C, Davey Smith G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47:1264-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 580] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 23. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 6203] [Article Influence: 775.4] [Reference Citation Analysis (0)] |
| 24. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 3375] [Article Influence: 375.0] [Reference Citation Analysis (0)] |
| 25. | Greco M FD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34:2926-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 1158] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 26. | Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 1468] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 27. | Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, Yengo L, Ferreira T, Marouli E, Ji Y, Yang J, Jones S, Beaumont R, Croteau-Chonka DC, Winkler TW; GIANT Consortium, Hattersley AT, Loos RJF, Hirschhorn JN, Visscher PM, Frayling TM, Yaghootkar H, Lindgren CM. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 887] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 28. | Mahajan A, Spracklen CN, Zhang W, Ng MCY, Petty LE, Kitajima H, Yu GZ, Rüeger S, Speidel L, Kim YJ, Horikoshi M, Mercader JM, Taliun D, Moon S, Kwak SH, Robertson NR, Rayner NW, Loh M, Kim BJ, Chiou J, Miguel-Escalada I, Della Briotta Parolo P, Lin K, Bragg F, Preuss MH, Takeuchi F, Nano J, Guo X, Lamri A, Nakatochi M, Scott RA, Lee JJ, Huerta-Chagoya A, Graff M, Chai JF, Parra EJ, Yao J, Bielak LF, Tabara Y, Hai Y, Steinthorsdottir V, Cook JP, Kals M, Grarup N, Schmidt EM, Pan I, Sofer T, Wuttke M, Sarnowski C, Gieger C, Nousome D, Trompet S, Long J, Sun M, Tong L, Chen WM, Ahmad M, Noordam R, Lim VJY, Tam CHT, Joo YY, Chen CH, Raffield LM, Lecoeur C, Prins BP, Nicolas A, Yanek LR, Chen G, Jensen RA, Tajuddin S, Kabagambe EK, An P, Xiang AH, Choi HS, Cade BE, Tan J, Flanagan J, Abaitua F, Adair LS, Adeyemo A, Aguilar-Salinas CA, Akiyama M, Anand SS, Bertoni A, Bian Z, Bork-Jensen J, Brandslund I, Brody JA, Brummett CM, Buchanan TA, Canouil M, Chan JCN, Chang LC, Chee ML, Chen J, Chen SH, Chen YT, Chen Z, Chuang LM, Cushman M, Das SK, de Silva HJ, Dedoussis G, Dimitrov L, Doumatey AP, Du S, Duan Q, Eckardt KU, Emery LS, Evans DS, Evans MK, Fischer K, Floyd JS, Ford I, Fornage M, Franco OH, Frayling TM, Freedman BI, Fuchsberger C, Genter P, Gerstein HC, Giedraitis V, González-Villalpando C, González-Villalpando ME, Goodarzi MO, Gordon-Larsen P, Gorkin D, Gross M, Guo Y, Hackinger S, Han S, Hattersley AT, Herder C, Howard AG, Hsueh W, Huang M, Huang W, Hung YJ, Hwang MY, Hwu CM, Ichihara S, Ikram MA, Ingelsson M, Islam MT, Isono M, Jang HM, Jasmine F, Jiang G, Jonas JB, Jørgensen ME, Jørgensen T, Kamatani Y, Kandeel FR, Kasturiratne A, Katsuya T, Kaur V, Kawaguchi T, Keaton JM, Kho AN, Khor CC, Kibriya MG, Kim DH, Kohara K, Kriebel J, Kronenberg F, Kuusisto J, Läll K, Lange LA, Lee MS, Lee NR, Leong A, Li L, Li Y, Li-Gao R, Ligthart S, Lindgren CM, Linneberg A, Liu CT, Liu J, Locke AE, Louie T, Luan J, Luk AO, Luo X, Lv J, Lyssenko V, Mamakou V, Mani KR, Meitinger T, Metspalu A, Morris AD, Nadkarni GN, Nadler JL, Nalls MA, Nayak U, Nongmaithem SS, Ntalla I, Okada Y, Orozco L, Patel SR, Pereira MA, Peters A, Pirie FJ, Porneala B, Prasad G, Preissl S, Rasmussen-Torvik LJ, Reiner AP, Roden M, Rohde R, Roll K, Sabanayagam C, Sander M, Sandow K, Sattar N, Schönherr S, Schurmann C, Shahriar M, Shi J, Shin DM, Shriner D, Smith JA, So WY, Stančáková A, Stilp AM, Strauch K, Suzuki K, Takahashi A, Taylor KD, Thorand B, Thorleifsson G, Thorsteinsdottir U, Tomlinson B, Torres JM, Tsai FJ, Tuomilehto J, Tusie-Luna T, Udler MS, Valladares-Salgado A, van Dam RM, van Klinken JB, Varma R, Vujkovic M, Wacher-Rodarte N, Wheeler E, Whitsel EA, Wickremasinghe AR, van Dijk KW, Witte DR, Yajnik CS, Yamamoto K, Yamauchi T, Yengo L, Yoon K, Yu C, Yuan JM, Yusuf S, Zhang L, Zheng W; FinnGen; eMERGE Consortium, Raffel LJ, Igase M, Ipp E, Redline S, Cho YS, Lind L, Province MA, Hanis CL, Peyser PA, Ingelsson E, Zonderman AB, Psaty BM, Wang YX, Rotimi CN, Becker DM, Matsuda F, Liu Y, Zeggini E, Yokota M, Rich SS, Kooperberg C, Pankow JS, Engert JC, Chen YI, Froguel P, Wilson JG, Sheu WHH, Kardia SLR, Wu JY, Hayes MG, Ma RCW, Wong TY, Groop L, Mook-Kanamori DO, Chandak GR, Collins FS, Bharadwaj D, Paré G, Sale MM, Ahsan H, Motala AA, Shu XO, Park KS, Jukema JW, Cruz M, McKean-Cowdin R, Grallert H, Cheng CY, Bottinger EP, Dehghan A, Tai ES, Dupuis J, Kato N, Laakso M, Köttgen A, Koh WP, Palmer CNA, Liu S, Abecasis G, Kooner JS, Loos RJF, North KE, Haiman CA, Florez JC, Saleheen D, Hansen T, Pedersen O, Mägi R, Langenberg C, Wareham NJ, Maeda S, Kadowaki T, Lee J, Millwood IY, Walters RG, Stefansson K, Myers SR, Ferrer J, Gaulton KJ, Meigs JB, Mohlke KL, Gloyn AL, Bowden DW, Below JE, Chambers JC, Sim X, Boehnke M, Rotter JI, McCarthy MI, Morris AP. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet. 2022;54:560-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 29. | Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 1198] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 30. | Curtin F, Schulz P. Multiple correlations and Bonferroni's correction. Biol Psychiatry. 1998;44:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 527] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Gyawali P, Richards RS. Association of altered hemorheology with oxidative stress and inflammation in metabolic syndrome. Redox Rep. 2015;20:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 216] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Yu KJ, Zhang MJ, Li Y, Wang RT. Increased whole blood viscosity associated with arterial stiffness in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Choi KM, Lee J, Kim YH, Kim KB, Kim DL, Kim SG, Shin DH, Kim NH, Park IB, Choi DS, Baik SH; Koreans-Southwest Seoul (SWS) Study. Relation between insulin resistance and hematological parameters in elderly Koreans-Southwest Seoul (SWS) Study. Diabetes Res Clin Pract. 2003;60:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Ben-Assayag H, Brzezinski RY, Berliner S, Zeltser D, Shapira I, Rogowski O, Toker S, Eldor R, Shenhar-Tsarfaty S. Transitioning from having no metabolic abnormality nor obesity to metabolic impairment in a cohort of apparently healthy adults. Cardiovasc Diabetol. 2023;22:226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Lin JD, Chiou WK, Chang HY, Liu FH, Weng HF, Liu TH. Association of hematological factors with components of the metabolic syndrome in older and younger adults. Aging Clin Exp Res. 2006;18:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Zhong F, Guan L, Lin H, Zhao M, Qin Y, Li Q, Yuan Z, Yang G, Gao L, Zhao J. Red Blood Cell Count: An Unrecognized Risk Factor for Nonalcoholic Fatty Liver Disease. Front Endocrinol (Lausanne). 2021;12:760981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 39. | Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 40. | Chung GE, Yim JY, Kim D, Kwak MS, Yang JI, Chung SJ, Yang SY, Kim JS. Associations between hemoglobin concentrations and the development of incidental metabolic syndrome or nonalcoholic fatty liver disease. Dig Liver Dis. 2017;49:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Bennett RW. The status of the state-federal tuberculosis eradication program. Proc Annu Meet U S Anim Health Assoc. 1979;488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Fiorentino TV, Marini MA, Succurro E, Andreozzi F, Sciacqua A, Hribal ML, Perticone F, Sesti G. Association between hemoglobin glycation index and hepatic steatosis in non-diabetic individuals. Diabetes Res Clin Pract. 2017;134:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Huang CF, Huang CI, Yeh ML, Hou C, Hou NJ, Hsieh MY, Huang JF, Chen SC, Lin ZY, Dai CY, Chuang WL, Yu ML. Disease severity and erythropoiesis in chronic hepatitis C. J Gastroenterol Hepatol. 2017;32:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Parker R, Armstrong MJ, Bruns T, Hodson J, Rowe IA, Corbett CD, Reuken PA, Gunson BK, Houlihan DD, Stephenson B, Malessa C, Lester W, Ferguson JW. Reticulocyte count and hemoglobin concentration predict survival in candidates for liver transplantation. Transplantation. 2014;97:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Brugnara C. Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci. 2000;37:93-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/