Published online Aug 27, 2024. doi: 10.4254/wjh.v16.i8.1091
Revised: June 19, 2024
Accepted: July 3, 2024
Published online: August 27, 2024
Processing time: 136 Days and 5.2 Hours
Hepatitis C virus (HCV) is a significant public health challenge globally, with substantial morbidity and mortality due to chronic liver disease. Despite the availability of highly effective and well-tolerated direct-acting antiviral therapies, widespread disparities remain in hepatitis C screening, access to treatment, linkage to care, and therapeutic outcomes. This review article synthesizes evi
Core Tip: Racial and ethnic disparities in healthcare have been well-documented in numerous conditions, including hepatitis C virus (HCV) infection. Despite guidelines recommending universal screening in specific age groups and populations, racial and ethnic minorities have lesser chances of being screened, accessing treatment, and achieving sustained virologic responses. These disparities are a reflection of broader systemic issues within healthcare systems that must be recognized and reconciled. This review aims to explore these disparities in-depth, assess the factors contributing to them, and examine the existing policies and potential interventions that may alleviate the obstacles faced by underserved populations. By doing so, it intends to shed light on potential paths forward to achieve equity in HCV care and treatment outcomes, ultimately contributing to the global goal of HCV eradication.
- Citation: Alenzi M, Almeqdadi M. Bridging the gap: Addressing disparities in hepatitis C screening, access to care, and treatment outcomes. World J Hepatol 2024; 16(8): 1091-1098
- URL: https://www.wjgnet.com/1948-5182/full/v16/i8/1091.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i8.1091
Hepatitis C virus (HCV) infection remains a critical public health issue with an estimated global prevalence of 71 million individuals chronically infected, leading to liver cirrhosis and hepatocellular carcinoma[1]. According to Centers for Disease Control and Prevention (CDC), there was 129% increase in the number of acute hepatitis C cases reported in the United States compared to 2014[2]. According to most recent World Health Organization report, viral hepatitis, including hepatitis C, remains a significant public health challenge, causing approximately 1.3 million deaths in 2022. This burden is comparable to tuberculosis and second only to coronavirus disease 2019 among communicable diseases. As of 2022, it is estimated that there are approximately 2.4 million people living with hepatitis C in the United States. Unfortunately, only 36.4% of people with hepatitis C were diagnosed by 2022, with a target of 90% by 2030 and only 20% of those diagnosed with hepatitis C were treated by 2022, with a target of 80% by 2030[3].
Despite these significant advancements in therapy, elimination of HCV as a public health threat faces substantial barriers. Amongst these, disparities in hepatitis C screening, access to care, and treatment adherence are the most pre
With the advent of DAA therapies, the landscape of HCV treatment has dramatically shifted, yielding high efficacy with shorter treatment durations and fewer side effects compared to previous interferon-based regimes. These have provided the basis for strategies aiming at the elimination of HCV[3]. However, the optimistic view of DAAs' potential is tempered by the reality of persistent gaps in the hepatitis C care cascade, which often reflect broader societal and heal
Early identification of HCV infection through screening is critical to enable successful linkages to care and treatment. However, discrepancies in screening prevalence among various populations pose a significant challenge to HCV eli
Beyond the issues of screening and diagnosis, access to treatment presents a significant hurdle for many patients with HCV[4]. The high cost of DAA therapies, while reduced compared to their initial market introduction, remains prohi
Socioeconomic status (SES) is intrinsically linked to health disparities, and HCV is no exception. Lower income and educational attainment have been associated with reduced rates of HCV screening and treatment initiation. So
Racial and ethnic disparities in healthcare have been well-documented in numerous conditions, including HCV infe
This review aims to explore these disparities in-depth, assess the factors contributing to them, and examine the existing policies and potential interventions that may alleviate the obstacles faced by underserved populations. By doing so, it intends to shed light on potential paths forward to achieve equity in HCV care and treatment outcomes, ultimately contributing to the global goal of HCV eradication.
While comprehensive screening for HCV is a cornerstone in the battle against the disease, realities on the ground paint a starkly different picture. The CDC has recently changed the guidelines in 2020 and recommend one-time HCV testing for all adults ≥ 18 years of age–a major shift from the prior focus on high-risk populations (“baby boomers” aged ≥ 60 years[12]. Despite that, screening rates are unsatisfactory, with marked variances when dissecting the data demographically. What emerges is a patchwork of screening coverage, spattered with inequities that mirror socioeconomic and racial divides[13,14].
Race and ethnicity profoundly influence HCV screening rates. African Americans, who are disproportionately affected by HCV, often face structural barriers to healthcare access, which translates to reduced screening opportunities. Studies have shown that while African Americans may demonstrate a higher willingness to undergo HCV screening when presented with the opportunity, such opportunities are less frequent compared to their white counterparts[13]. Hispanic Americans, similarly, affected by disparities, present an additional layer of complexity due to language barriers and immigration status, which can further dissuade individuals from seeking screening services. In a recent national trend survey conducted in 2019 to assess public awareness of HCV, 17% of adults never heard of HCV with higher percentages among younger adults < 55 compared to elderly adults. Among young adults < 55, Asian and Hispanic were significantly more likely to never heard of HCV after adjusting for confounders (sex, educational level, household income, English fluency and having a regular provider)[15].
SES factors significantly into the equation, with individuals in lower-income and with less access to education being less likely to receive HCV screening. Factors such as unemployment, housing instability, and lack of health insurance contribute to the underutilization of HCV screening services. Hence, the underprivileged are often diagnosed in later stages of the disease when clinical manifestations prompt medical attention, thus forfeiting the benefits of early intervention. Nili et al[13] had conducted a national health interview survey including a total of 41914 United States Baby Boomers; they found that HCV screening rates were not only lower in Asians compared to Blacks odds ratio = 0.74, P = 0.02 but also people with less education, lower income were significantly less likely to have an HCV screening.
The stigma surrounding HCV poses yet another considerable barrier to screening. People who inject drugs (PWID), despite being a high-risk population for HCV infection, face stigmatization that can discourage them from seeking healthcare services, including HCV screening. This stigma extends to provider biases, which may result in differential offering of screening services, subsequently affecting the screening rates among these populations. In a recent cross-sectional study by Barocas et al[15], beside noted societal stigma, PWID residing in non-urban settings, who have poor access to primary care or who have less education had lower screening rates[16]. These findings highlight the importance of expanded access to primary health care and prevention services, especially in non-urban areas in an attempt to address an unmet need to screen individuals at high risk for HCV.
Despite evidence-based guidelines recommending widespread screening, substantial barriers hinder their successful application across all populations. Moving forward requires not just the provision of resources, but also culturally sensitive approaches that account for, and aim to dismantle, the barriers created by social determinants of health. Reducing disparities in HCV screening and diagnosis is an integral step toward equitable healthcare and the larger goal of HCV elimination.
Unfortunately, recent evidence has shed light on the disparity in healthcare provision among HCV patients based on their insurance status. A recent prospective cohort study revealed markedly higher denial rates among Medicaid beneficiaries (46.3%) compared to those covered by Medicare (5%) or commercial insurance (10.2%), with a significant statistical difference (P < 0.001)[17]. A similar study conducted using Trio Health’s Innervation Platform corroborated these findings. Trio, a distinctive platform, aggregates real-time data from various healthcare sources across the United States. This study found that a staggering 81% of HCV patients prescribed treatment did not initiate it due to insurance or financial constraints. Among these, the Medicaid group had the highest nonstart rate at 35%, while rates for Medicare and commercial insurance were notably lower at 2% and 6%, respectively[9]. Despite evidence of downstream economic benefits associated with treating HCV patients, these disparities persist[18]. This underscores the urgent need to reassess current insurance policies governing HCV treatment coverage, ensuring universal access for all eligible patients and aligning with the World Health Organization's goal of eliminating HCV infection by 2030.
Access to care and treatment for HCV infection is influenced by a complex interplay of factors, including race, ethnicity, and SES. Racial and ethnic minority groups, particularly African American and Hispanic populations, face significant disparities in HCV care access and treatment uptake compared to their Caucasian counterparts. These disparities manifest across various stages of the care continuum, from HCV testing and diagnosis to treatment initiation and adherence[19,20]. Numerous studies have documented the disproportionate burden of HCV among racial and ethnic minorities. For example, African American and Hispanic populations have been found to have higher prevalence rates of HCV infection compared to Caucasians[21,22]. Additionally, disparities in access to healthcare services and treatment options further exacerbate these inequities. Structural barriers within healthcare systems, such as limited access to healthcare facilities, lack of insurance coverage, and cultural and linguistic barriers, contribute to lower rates of HCV testing and treatment initiation among minority populations[23,24].
Socioeconomic factors also play a critical role in shaping disparities in HCV care access and treatment uptake. Individuals with lower SES, including those with lower income levels and educational attainment, are disproportionately affected by HCV-related disparities. These individuals often face financial barriers to accessing healthcare services, including HCV testing and treatment, and may lack resources for adequate disease management and support services[25,26].
Efforts to address disparities in HCV care access and treatment uptake require a multifaceted approach that addresses both structural and individual-level factors. Policy interventions aimed at improving access to healthcare services, increasing insurance coverage, and reducing financial barriers are essential steps towards achieving health equity[27,28]. Additionally, efforts to enhance cultural competence within healthcare systems and provide targeted outreach and education programs to underserved communities can help improve awareness and understanding of HCV and promote uptake of testing and treatment services[29,30].
The role of care models in facilitating HCV linkage to care is critical for improving outcomes and reducing transmission. Care models encompass a variety of approaches aimed at addressing barriers to HCV testing, diagnosis, and treatment initiation, ultimately ensuring that individuals with HCV receive timely and appropriate care.
One significant care model is the integrated care model, which involves embedding HCV screening and treatment services within existing healthcare settings, such as primary care clinics, substance use treatment centers, and human immunodeficiency virus clinics. Integrated care models leverage existing infrastructure and resources to streamline HCV care delivery, improve access to testing and treatment, and facilitate linkage to care for individuals at risk for or living with HCV[25,31].
Another care model gaining traction is the telemedicine or telehealth model, which utilizes technology to provide remote access to HCV care services. Telemedicine platforms enable healthcare providers to conduct virtual consultations, deliver education and counseling, and monitor treatment adherence remotely. This model is particularly valuable for reaching underserved populations in rural or remote areas, as well as individuals facing transportation or mobility barriers[32,33].
Peer-based care models involve trained peers or community health workers who provide support, education, and navigation services to individuals with HCV. Peers share lived experiences and provide culturally relevant assistance, helping to build trust and reduce stigma associated with HCV. Peer-based interventions have been shown to improve engagement in care, treatment adherence, and retention in care, particularly among marginalized populations[34,35].
Pharmacist-led care models involve pharmacists playing a central role in HCV care delivery, including conducting screening, providing education, assisting with treatment initiation, and monitoring treatment outcomes. Pharmacists' expertise in medication management and their accessibility in community settings make them well-positioned to support individuals with HCV throughout their care journey[36,37].
Furthermore, the use of multidisciplinary care teams, which include healthcare providers from diverse disciplines such as hepatology, infectious diseases, addiction medicine, and mental health, allows for comprehensive and holistic care for individuals with HCV. Multidisciplinary teams can address complex medical and psychosocial needs, provide coordinated care, and optimize treatment outcomes[38,39].
Lastly, patient-centered care models prioritize the preferences, needs, and values of individuals with HCV, empowering them to actively participate in decision-making and self-management of their condition. Patient-centered care emphasizes shared decision-making, individualized treatment plans, and ongoing support tailored to each patient's unique circumstances[40,41].
To summarize, Care models play a crucial role in facilitating HCV linkage to care by addressing barriers, improving access, and providing comprehensive support to individuals with HCV. By leveraging diverse care models, healthcare systems can enhance HCV care delivery, promote equitable access to care, and ultimately reduce the burden of HCV-related morbidity and mortality.
Disparities in treatment outcomes and SVR among individuals with HCV infection pose significant challenges in achieving equitable healthcare outcomes. Research has consistently demonstrated variations in SVR rates based on demographic, socioeconomic, and clinical factors, highlighting the need for targeted interventions to address these disparities and improve overall health outcomes.
Socioeconomic factors, such as income level and access to healthcare, play a crucial role in treatment outcomes for HCV. Studies have shown that individuals with lower SES often encounter barriers to accessing HCV treatment, including lack of health insurance coverage and limited healthcare resources[19]. These disparities contribute to differences in SVR rates, with disadvantaged populations experiencing lower treatment success rates compared to their more affluent counterparts.
Racial and ethnic minorities also face disparities in SVR rates, with Black and Hispanic populations exhibiting lower rates of treatment success compared to White individuals[42]. These disparities may be attributed to various factors, including differences in healthcare utilization, provider bias, and SES. Addressing racial and ethnic disparities in treatment outcomes requires culturally sensitive approaches and targeted interventions aimed at improving access to care and reducing barriers to treatment adherence.
Clinical factors, such as liver fibrosis stage and comorbid conditions, further influence SVR rates among individuals with HCV infection. Patients with advanced liver fibrosis or cirrhosis typically exhibit lower SVR rates compared to those with less severe liver disease[43]. Additionally, individuals with comorbid conditions, such as substance use disorders or mental health issues, may face challenges in adhering to HCV treatment regimens, leading to lower SVR rates[44]. Addressing these clinical factors requires a comprehensive approach that includes regular monitoring of liver fibrosis and integration of mental health services.
Disparities in SVR rates are also evident among specific populations, such as PWID and individuals with a history of incarceration. PWID face unique challenges in accessing HCV treatment, including stigma, discrimination, and limited healthcare resources[20]. Studies have shown that PWID often experience lower SVR rates compared to non-PWID, highlighting the need for targeted interventions, harm reduction strategies, and integrated care models to improve treatment outcomes in this population[43].
Addressing disparities in treatment outcomes and SVR requires a multifaceted approach that considers social, economic, and clinical determinants of health. Efforts to improve access to HCV treatment, such as the expansion of healthcare coverage and the implementation of telemedicine and community-based outreach programs, are essential for reducing disparities in SVR rates[44].
Policies to address disparities in HCV treatment and access to care are critical for ensuring equitable healthcare outcomes among individuals affected by this disease. Research has highlighted various factors contributing to disparities in HCV treatment and access, including SES, race and ethnicity, healthcare system barriers, and incarceration history. Im
One significant barrier to HCV treatment access is the high cost of DAA medications. Many individuals, particularly those from lower socioeconomic backgrounds or without adequate health insurance coverage, may face challenges in accessing these life-saving treatments[45]. Policymakers can address this issue by implementing strategies such as price negotiation, bulk purchasing, and generic drug production to reduce medication costs and improve affordability for patients.
Additionally, healthcare system barriers, including limited access to healthcare facilities and providers, can contribute to disparities in HCV treatment access. Individuals residing in rural or underserved areas may face difficulties in accessing specialty care for HCV treatment initiation and management[31]. Policies aimed at expanding access to healthcare services, such as telemedicine programs and mobile clinics, can help overcome these barriers and improve treatment access for underserved populations[46].
As previously mentioned, disparities in HCV treatment access and outcomes exist among racial and ethnic minority populations. Policymakers can address these disparities by implementing culturally sensitive approaches, increasing funding for community-based organizations serving minority populations, and promoting diversity in healthcare workforce.
Incarcerated individuals represent another population facing disparities in HCV treatment access. Correctional facilities often lack adequate healthcare resources and may not offer HCV testing and treatment to inmates[47]. Policies aimed at expanding HCV screening and treatment programs within correctional settings, along with continuity of care upon reentry into the community, are essential for addressing disparities among justice-involved populations[48].
Furthermore, addressing social determinants of health, such as housing instability, food insecurity, and substance use disorders, is crucial for reducing disparities in HCV treatment access and outcomes. Policies that support housing assistance programs, nutritional support services, and substance use treatment can help address these underlying factors and improve overall health outcomes for individuals affected by HCV.
Future directions and research gaps in addressing HCV disparities are critical for advancing efforts towards equitable healthcare access and outcomes for all affected populations. Despite significant progress in HCV treatment and care, disparities persist across various demographic and socioeconomic groups, highlighting the need for targeted inter
One key area for future research is the development and implementation of culturally tailored interventions to address disparities in HCV screening, diagnosis, and treatment initiation among underserved populations. Culturally sensitive approaches, including community-based outreach programs and peer-led interventions, have shown promise in improving HCV awareness and engagement in care among marginalized groups[19].
Furthermore, enhancing access to affordable HCV treatment remains a priority. Research is needed to evaluate the effectiveness of pricing strategies, such as bulk purchasing agreements and generic drug production, in reducing medication costs and improving treatment accessibility for individuals with limited financial resources[45].
Additionally, there is a need for research on innovative healthcare delivery models to expand access to HCV care, particularly in rural and underserved areas. Telemedicine programs, mobile clinics, and decentralized treatment approaches have the potential to overcome geographic barriers and improve access to HCV testing and treatment services[46].
Furthermore, understanding the impact of structural factors, such as healthcare policies and criminal justice system involvement, on HCV disparities is critical for developing targeted interventions and policy reforms. Research is needed to evaluate the effectiveness of policy initiatives, such as Medicaid expansion and prison-based HCV screening and treatment programs, in reducing disparities and improving health outcomes among affected populations[48].
To summarize, future research efforts should focus on developing and implementing culturally tailored interventions, enhancing access to affordable treatment, exploring innovative healthcare delivery models, addressing social determinants of health, and evaluating the impact of structural factors on HCV disparities. By addressing these research gaps, we can advance efforts towards achieving health equity and eliminating HCV-related disparities.
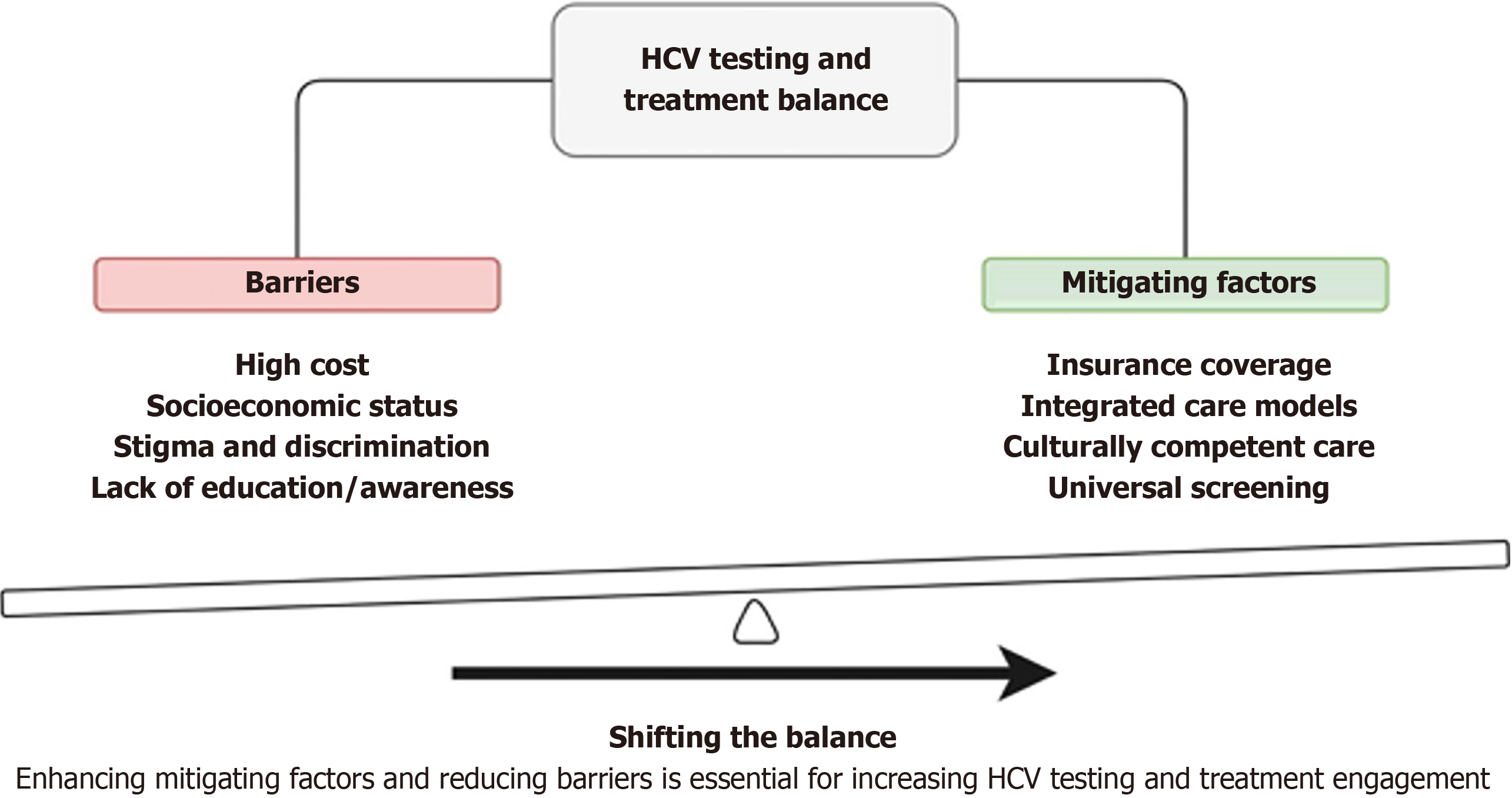
In conclusion, enhancing mitigating factors and reducing barriers are key to increasing HCV screening and treatment engagement, as shown in Figure 1. Addressing disparities in HCV treatment access and outcomes requires multifaceted policy approaches that target various contributing factors, including medication costs, healthcare system barriers, racial and ethnic disparities. By implementing targeted policies and interventions, policymakers can help ensure equitable access to HCV treatment and improve health outcomes for all affected populations.
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1514] [Article Influence: 168.2] [Reference Citation Analysis (0)] |
| 2. | Center for Disease Control and Prevention Viral hepatitis surveillance. Number of reported cases of acute Hepatitis C virus infection and estimated infections-United States, 2014–2021. Available from: https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-c/figure-3.1.htm. |
| 3. | WHO. Global hepatitis report 2024: action for access in low- and middle-income countries. Available from: https://www.who.int/publications/i/item/9789240091672. |
| 4. | Mendizabal M, Alonso C, Silva MO. Overcoming barriers to hepatitis C elimination. Frontline Gastroenterol. 2019;10:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Higashi RT, Jain MK, Quirk L, Rich NE, Waljee AK, Turner BJ, Lee SC, Singal AG. Patient and provider-level barriers to hepatitis C screening and linkage to care: A mixed-methods evaluation. J Viral Hepat. 2020;27:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Coppock D, Chou E, Gracely E, Gross R, Heun-Lee D. Hepatitis C antibody screening and determinants of initial and duplicate screening in the baby boomer patients of six urban primary care clinics. PLoS One. 2020;15:e0235778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Urban K, Payton C, Mamo B, Volkman H, Giorgio K, Kennedy L, Bomber YC, Rodrigues KK, Young J, Tumaylle C, Matheson J, Tasslimi A, Montour J, Jentes E. Hepatitis C Screening and Antibody Prevalence Among Newly Arrived Refugees to the United States, 2010-2017. J Immigr Minor Health. 2023;25:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Stepanova M, Younossi ZM. Interferon-Free Regimens for Chronic Hepatitis C: Barriers Due to Treatment Candidacy and Insurance Coverage. Dig Dis Sci. 2015;60:3248-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Younossi ZM. Disparities in Access to Direct Acting Antiviral Regimens for Hepatitis C Virus (HCV): The Impact of Race and Insurance Status. Am J Gastroenterol. 2018;113:1285-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Saeed YA, Mason K, Mitsakakis N, Feld JJ, Bremner KE, Phoon A, Fried A, Wong JF, Powis J, Krahn MD, Wong WW. Disparities in health utilities among hepatitis C patients receiving care in different settings. Can Liver J. 2023;6:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sims OT, Pollio DE, Hong BA, North CS. Racial Disparities in Hepatitis C Treatment Eligibility. Ann Hepatol. 2017;16:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tran L, Jung J, Feldman R, Riley T 3rd. Disparities in the quality of care for chronic hepatitis C among Medicare beneficiaries. PLoS One. 2022;17:e0263913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Nili M, Luo L, Feng X, Chang J, Tan X. Disparities in hepatitis C virus infection screening among Baby Boomers in the United States. Am J Infect Control. 2018;46:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Islam JY, Spees L, Camacho-Rivera M, Vidot DC, Yarosh R, Wheldon CW. Disparities in Awareness of Hepatitis C Virus Among US Adults: An Analysis of the 2019 Health Information National Trends Survey. Sex Transm Dis. 2021;48:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J. 2014;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (2)] |
| 16. | Lo Re V 3rd, Gowda C, Urick PN, Halladay JT, Binkley A, Carbonari DM, Battista K, Peleckis C, Gilmore J, Roy JA, Doshi JA, Reese PP, Reddy KR, Kostman JR. Disparities in Absolute Denial of Modern Hepatitis C Therapy by Type of Insurance. Clin Gastroenterol Hepatol. 2016;14:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Younossi Z, Gordon SC, Ahmed A, Dieterich D, Saab S, Beckerman R. Treating Medicaid patients with hepatitis C: clinical and economic impact. Am J Manag Care. 2017;23:107-112. [PubMed] |
| 18. | Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, Alter M, Yartel A, Ward JW; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32. [PubMed] |
| 19. | Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, Ryerson AB. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology. 2019;69:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 20. | Trooskin SB, Navarro VJ, Winn RJ, Axelrod DJ, McNeal AS, Velez M, Herrine SK, Rossi S. Hepatitis C risk assessment, testing and referral for treatment in urban primary care: role of race and ethnicity. World J Gastroenterol. 2007;13:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Suraweera D, Sundaram V, Saab S. Treatment of Hepatitis C Virus Infection in Liver Transplant Recipients. Gastroenterol Hepatol (NY). 2016;12:23-30. [PubMed] |
| 22. | Harris AM, Link-Gelles R, Kim K, Chandrasekar E, Wang S, Bannister N, Pong P, Chak E, Chen MS, Bowlus C, Nelson NP. Community-Based Services to Improve Testing and Linkage to Care Among Non–U.S.-Born Persons with Chronic Hepatitis B Virus Infection — Three U.S. Programs, October 2014–September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:541-546. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Falade-Nwulia O, Sulkowski MS, Merkow A, Latkin C, Mehta SH. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat. 2018;25:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Chhatwal J, Chen Q, Aggarwal R. Estimation of Hepatitis C Disease Burden and Budget Impact of Treatment Using Health Economic Modeling. Infect Dis Clin North Am. 2018;32:461-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Spelman T, Morris MD, Zang G, Rice T, Page K, Maher L, Lloyd A, Grebely J, Dore GJ, Kim AY, Shoukry NH, Hellard M, Bruneau J; International Collaborative of Incident HIV and Hepatitis C in Injecting Cohorts (InC3 Study). A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. J Epidemiol Community Health. 2015;69:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, Nakasato C, Boscarino JA, Henkle EM, Nerenz DR, Denniston MM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Fuentes-Afflick E, Hessol NA. Immigration status and use of health services among Latina women in the San Francisco Bay Area. J Womens Health (Larchmt). 2009;18:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Artiga S, Damico A. Health and health coverage in the South: a data update. 2018. Available from: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-and-health-coverage-in-the-south-a-data-update/. |
| 30. | Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122 Suppl 2:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, Akoth E, Thomas A, Ahmed C, Espinosa M, Price A, Rosenthal E, Tang L, Wilson E, Bentzen S, Masur H, Kottilil S; ASCEND Providers. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med. 2017;167:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 32. | Norton BL, Beitin A, Glenn M, DeLuca J, Litwin AH, Cunningham CO. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Tofighi B, Lee JD, Sindhu SS, Chemi C, Leonard NR. Engagement in the Hepatitis C care continuum among people who use drugs. J Subst Use. 2020;25:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Trooskin SB, Poceta J, Towey CM, Yolken A, Rose JS, Luqman NL, Preston TW, Chan PA, Beckwith C, Feller SC, Lee H, Nunn AS. Results from a Geographically Focused, Community-Based HCV Screening, Linkage-to-Care and Patient Navigation Program. J Gen Intern Med. 2015;30:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:754-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Belperio PS, Chartier M, Gonzalez RI. Hepatitis C care cascade among patients diagnosed in primary care and referred to specialty care: a retrospective cohort study. BMJ Open. 2017;7:e015313. |
| 37. | Schinasi DA, Foster CC, Bohling MK, Barrera L, Macy ML. Attitudes and Perceptions of Telemedicine in Response to the COVID-19 Pandemic: A Survey of Naïve Healthcare Providers. Front Pediatr. 2021;9:647937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Al-Tayyib A, Koester S, Langegger S, Raville L. Heroin and Methamphetamine Injection: An Emerging Drug Use Pattern. Subst Use Misuse. 2017;52:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 39. | Butler K, Larney S, Day CA, Burns L. Uptake of direct acting antiviral therapies for the treatment of hepatitis C virus among people who inject drugs in a universal health-care system. Drug Alcohol Rev. 2019;38:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Kramer JR, Puenpatom A, Cao Y, Yu X, El-Serag HB, Kanwal F. Treatment of hepatitis C virus infection in people with opioid use disorder: a real-world study of elbasvir/grazoprevir in a US Department of Veterans Affairs population. Am J Drug Alcohol Abuse. 2022;48:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 41. | Younossi ZM, Kanwal F, Saab S, Brown KA, El-Serag HB, Kim WR, Ahmed A, Kugelmas M, Gordon SC. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2016;44:175-186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 2014;104:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology Commun. 2017;1:513-522. [RCA] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann Intern Med. 2015;163:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 45. | Qiu Y, Liu Y, Ren W, Qiu Y, Ren J. Internet-Based and Mobile-Based General Practice: Cross-Sectional Survey. J Med Internet Res. 2018;20:e266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Larney S, Zaller ND, Dumont DM, Willcock A, Degenhardt L. A systematic review and meta-analysis of racial and ethnic disparities in hepatitis C antibody prevalence in United States correctional populations. Ann Epidemiol. 2016;26:570-578.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, Paar DP. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301:848-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Morris MD, Yen IH, Shiboski S, Evans JL, Page K. Housing Stability and Hepatitis C Infection for Young Adults Who Inject Drugs: Examining the Relationship of Consistent and Intermittent Housing Status on HCV Infection Risk. J Urban Health. 2020;97:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/