Published online Jun 27, 2024. doi: 10.4254/wjh.v16.i6.920
Revised: April 28, 2024
Accepted: May 17, 2024
Published online: June 27, 2024
Processing time: 132 Days and 12.9 Hours
Studies with large size samples on the liver histological changes of indeterminate phase chronic hepatitis B (CHB) patients were not previously conducted.
To assess the liver histological changes in the indeterminate phase CHB patients using liver biopsy.
The clinical and laboratory data of 1532 untreated CHB patients were collected, and all patients had least once liver biopsy from January 2015 to December 2021. The significant differences among different phases of CHB infection were compared with t-test, and the risk factors of significant liver histological changes were analyzed by the multivariate logistic regression analysis.
Among 1532 untreated CHB patients, 814 (53.13%) patients were in the indeter
Our results suggested that significant liver histological changes were not rare among the untreated CHB patients in indeterminate phase, and additional strategies are urgently required for the management of these patients.
Core Tip: In this study, we investigated liver histological changes in 814 chronic hepatitis B patients in the indeterminate phase, in which significant liver histological changes (defined as biopsy score ≥ G2 and/or ≥ S2) were found in 488/814 (59.95%) patients, which suggested that additional strategies are urgently required for management of these patients.
- Citation: Huang DL, Cai QX, Zhou GD, Yu H, Zhu ZB, Peng JH, Chen J. Liver histological changes in untreated chronic hepatitis B patients in indeterminate phase. World J Hepatol 2024; 16(6): 920-931
- URL: https://www.wjgnet.com/1948-5182/full/v16/i6/920.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i6.920
Hepatitis B virus (HBV) infection is an important public health concern worldwide, and also one of the leading causes of liver inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[1-3]. At present, chronic hepatitis B (CHB) infection is mainly divided into four phases, including immune tolerant phase, HBeAg-positive immune active phase, inactive phase, and HBeAg-negative immune active phase[1-3]. The concept of CHB phase is clinically important to determine the history and risk of developing CHB- related complications, as well as assisting clinicians in the therapy of CHB. Antiviral therapy is currently recommended for CHB patients in HBeAg- positive and -negative immune active phases to reduce the risk of cirrhosis and HCC, while regular monitoring of CHB patients in inactive and immune tolerant phases is essential[1-4].
Nevertheless, there are still some “gray-zone” patients beyond the definition of CHB phase[5]; for instance, corresponding to CHB patients in HBeAg-positive immune tolerant phase whose alanine aminotransferase (ALT) level is normal and serum HBV-DNA level > 106 IU/m, patients with normal ALT level and serum HBV-DNA level ≤ 106 IU/L that would be beyond the clinical criteria of tolerant phase, these “gray-zone” patients cannot be assigned into none of the four phases and allocated into indeterminate phase, and the main management strategy for them is monitoring[1,6-8]. Recently, studies showed that the disease progression of these patients was inconsistent. For instance, a study performed in the United States showed that Caucasian patients with indeterminate phase CHB without antiviral treatment had a good outcome in the long-term follow-up, and antiviral therapy could be safely avoided or delayed[8]. Nevertheless, studies found that untreated indeterminate CHB patients were at a higher risk of HCC than the immune tolerant and inactive CHB patients, although majority of those patients remained indeterminate during follow-up, had a higher 10-year cumulative HCC incidence and a higher risk of HCC than those remained in the inactive phase[9,10]. Therefore, it remains controversial whether these patients deserve long-term monitoring or require further positive intervention. Therefore, the present study was conducted to explore liver histological changes in the untreated indeterminate phase CHB patients by liver biopsy.
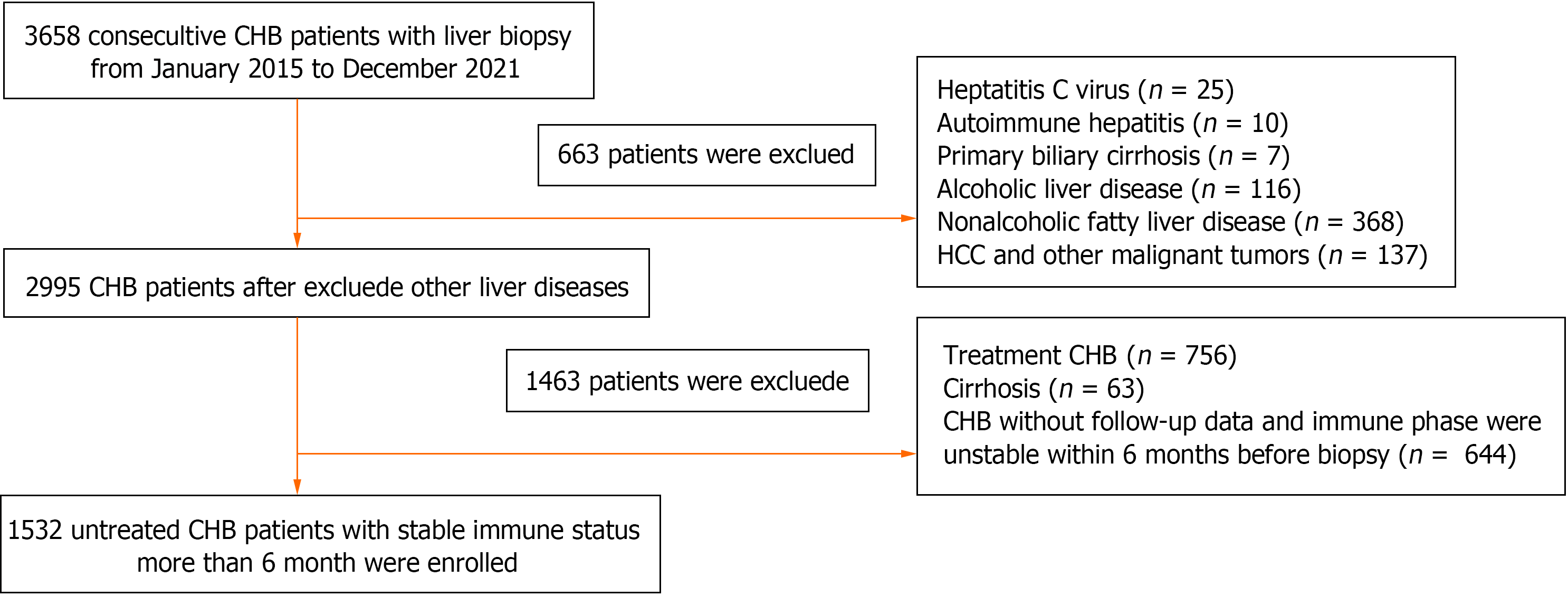
A total of 3658 CHB patients who underwent liver biopsy in the Third People’s Hospital of Shenzhen (Shenzhen, China) from January 2015 to December 2021 were enrolled. These adult patients (older than 18), with HBsAg positive more than 6 months and never received antiviral therapy. Patients had a stable state of hepatitis B related antigen, and antibodies, HBV-DNA level, and ALT level within 6 months before liver biopsy. Patients with the following conditions were excluded: Co-infection with other viruses, including hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, Epstein-Barr virus, cytomegalovirus, herpes virus infection, human immunodeficiency virus infection; co-existence of decompensated liver cirrhosis, HCC, and other malignant liver tumors; concurrent with other chronic liver diseases, such as autoimmune hepatitis, alcoholic liver disease, primary biliary cirrhosis, nonalcoholic fatty liver disease; immune phase were unstable within 6 months before liver biopsy; and a history of undergoing liver transplantation before the enrolment. Finally, 1532 CHB patients were met the inclusion criteria and included in the study (Figure 1). All data were collected from electronic medical record system of our hospital, including age, gender, disease history, albumin (ALB), ALT, aspartate aminotransferase (AST), glutamate aminotransferase (GGT), total bilirubin (TBIL), white blood cell (WBC), platelet (PLT), HBsAg, HBeAg, HBeAb, HBcAb, HBV-DNA level, imaging findings and liver biopsy report. The present study was approved by the Ethics Committee of the Third People’s Hospital of Shenzhen (Approval No. 2022-003), and patients’ informed consent was waived due to the retrospective nature of the study.
According to the clinical staging criteria presented by the American Association for the Study of Liver Diseases (AASLD) guideline (ver. 2018)[1], patients with CHB were divided into the four phases, including immune tolerant phase, HBeAg-positive immune active phase, inactive phase, and HBeAg-negative immune active phase. The upper limits of normal (ULN) of ALT was 35 U/L for males and 25 U/L for females based on the AASLD guideline (ver. 2018)[1,11].
Patients who did not meet the clinical criteria of four phases were classified as indeterminate phase. According to the different statuses of HBeAg, and the levels of ALT and HBV-DNA, corresponding to the four-phase, patients in the indeterminate phase were further divided into 4 subgroups: The indeterminate A phase (patients with HBeAg-positive, normal ALT level, and serum HBV-DNA level ≤ 106 IU/mL); the indeterminate B phase (patients with HBeAg-positive, ALT ≥ 2 ULN and serum HBV- DNA level < 20000 IU/mL, or patients with HBeAg-positive, ALT equal to 1-2 ULN, regardless of HBV-DNA level); the indeterminate C phase (patients with HBeAg- negative, normal ALT level, and serum HBV-DNA level ≥ 2000 IU/mL); and indeterminate D phase (patients with HBeAg-negative, ALT ≥ 2 ULN and serum HBV-DNA level < 2000 IU/mL, or patients with HBeAg-negative, ALT equal to 1-2 ULN, regardless of HBV- DNA level (Supplementary Table 1)[5].
In the present study, aspartate aminotransferase-to-PLT ratio index (APRI), fibrosis-4 (FIB-4) index, and gamma-glutamyl transpeptidase to PLT ratio (GPR) were used to assess liver histological changes. Studies have shown that APRI < 1.0, FIB-4 < 1.45, and GPR ≤ 0.32 were the critical values for excluding patients with advanced fibrosis and liver cirrhosis[1-4,12,13].
Liver tissues were obtained by percutaneous liver biopsy using a 16-gauge disposable needle. The inflammation and fibrosis of the liver were assessed by the Scheuer scoring system. The histological grading of hepatic inflammation ranged from G0 to G4, and fibrosis from S0 to S4. Liver inflammation ≥ G2 was defined as significant inflammation, liver fibrosis ≥ S2 was defined as significant fibrosis, and liver inflammation ≥ G2 and/or liver fibrosis ≥ S2 was defined as significant liver histological changes. Liver samples were evaluated and reviewed by two experienced pathologists who were blinded to patients’ biochemical data.
Data were presented as frequency or percentage for categorical variables, and as mean ± SD or median interquartile range for continuous variables. Univariate and multivariate logistic regression analyses were performed to determine factors associated with liver histological changes. Odds ratios (ORs) and 95% confidence intervals (CIs) with P-values were presented. A two-tailed P < 0.05 was considered statistically significant. Data were analyzed using SPPS 24.0 software (IBM, Armonk, NY, United States), the R 3.1.2 statistical package (R Foundation for Statistical Computing, Vienna, Austria), and EmpowerStats software (X&Y Solutions, Inc., Boston, MA, United States).
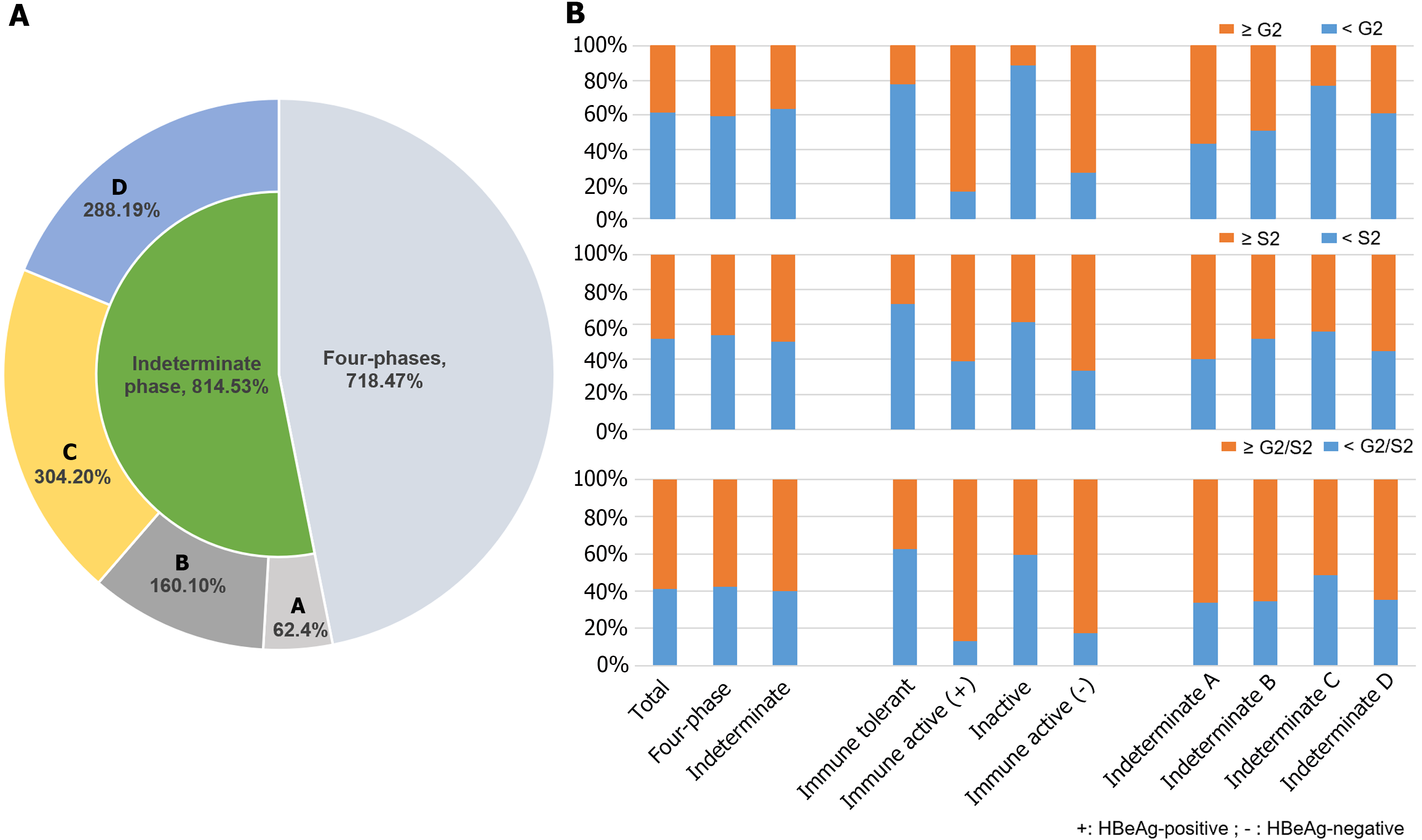
The mean age of patients was 38.09 ± 9.07 years, 69.97% of patients were male, and 118 (7.70%), 197 (12.86%), 317 (20.69%), and 86 (5.61%) patients were in the immune tolerant phase, HBeAg-positive immune active phase, immune inactive phase, and HBeAg-negative immune active phase, respectively. Moreover, 814 (53.13%) patients were assigned into the indeterminate phase (Figure 2A). The characteristics of all patients were shown as Table 1. There were 62 (7.62%), 160 (19.66%), 304 (37.35%), and 288 (35.38%) patients in the indeterminate A, B, C, and D phases, respectively (Figure 2A). The characteristics of the indeterminate phase patients as shown in Table 2. Patients in the indeterminate C and D phases were older than those in the indeterminate A and B phases. The levels of ALT, AST, TBIL, HBsAg, and HBV-DNA in the indeterminate B phase were higher than those in other indeterminate phases, while the levels of ALB, GGT, WBC and PLT had no significant.
| Phase | Immune-Tolerant CHB | HBeAg-positive immune active CHB | Inactive CHB | HBeAg-negative Immune Active CHB | Indeterminate | P value |
| N | 118 (7.70) | 197 (12.86) | 317 (20.70) | 86 (5.61) | 814 (53.13) | |
| Age, yr | 33.11 ± 7.52 | 32.41 ± 7.15 | 40.13 ± 9.37 | 40.43 ± 9.48 | 39.15 ± 8.73 | < 0.001 |
| Male | 53 (44.92) | 131 (66.50) | 222 (70.03) | 68 (79.07) | 598 (73.46) | < 0.001 |
| HBsAg, log10, IU/mL | 4.62 ± 0.59 | 4.18 ± 0.73 | 2.38 ± 1.26 | 3.28 ± 0.65 | 3.18 ± 1.02 | < 0.001 |
| HBeAg | < 0.001 | |||||
| Negative | 0 (0.00) | 0 (0.00) | 317 (100.00) | 86 (100.00) | 592 (72.73) | |
| Positive | 118 (100.00) | 197 (100.00) | 0 (0.00) | 0 (0.00) | 222 (27.27) | |
| HBV-DNA, log10, IU/mL | 8.03 ± 0.54 | 7.60 ± 1.01 | 2.47 ± 0.56 | 5.68 ± 1.40 | 4.71 ± 1.73 | < 0.001 |
| ALT, (IQR), U/L | 19.00 (15.00-23.00) | 138.00 (88.00-233.00) | 18.00 (14.00-25.00) | 106.50 (79.00-245.00) | 33.00 (23.00-45.00) | < 0.001 |
| AST, (IQR), U/L | 20.00 (17.00-22.75) | 74.00 (50.00-129.00) | 20.00 (17.00-23.00) | 57.00 (42.25-108.00) | 26.00 (21.00-33.00) | < 0.001 |
| GGT, (IQR), U/L | 15.00 (12.00-20.00) | 52.00 (29.00-89.00) | 18.00 (14.00-27.00) | 50.50 (34.25-80.25) | 25.00 (17.00-40.00) | < 0.001 |
| TBIL, (IQR), umol/L | 11.25 (8.20-14.47) | 15.20 (10.90-21.60) | 12.40 (9.30-16.70) | 15.60 (11.83-21.20) | 12.75 (9.90-17.30) | < 0.001 |
| ALB, g/L | 44.53 ± 3.42 | 42.52 ± 4.26 | 44.85 ± 3.23 | 43.57 ± 5.68 | 44.50 ± 3.91 | < 0.001 |
| WBC, 109/L | 5.62 ± 1.24 | 5.55 ± 1.59 | 5.69 ± 1.44 | 5.85 ± 1.97 | 5.85 ± 1.60 | 0.062 |
| PLT, 109/L | 228.04 ± 58.84 | 198.12 ± 54.38 | 204.09 ± 57.04 | 200.98 ± 65.66 | 205.85 ± 59.42 | < 0.001 |
| Stage of Inflammation | < 0.001 | |||||
| 0 | 2 (1.69) | 0 (0.00) | 11 (3.47) | 0 (0.00) | 14 (1.72) | |
| 1 | 90 (76.27) | 31 (15.74) | 271 (85.49) | 23 (26.74) | 506 (62.16) | |
| 2 | 26 (22.03) | 113 (57.36) | 33 (10.41) | 48 (55.81) | 263 (32.31) | |
| 3 | 0 (0.00) | 51 (25.89) | 2 (0.63) | 15 (17.44) | 26 (3.19) | |
| 4 | 0 (0.00) | 2 (1.02) | 0 (0.00) | 0 (0.00) | 5 (0.61) | |
| Degree of fibrosis | < 0.001 | |||||
| 0 | 3 (2.54) | 5 (2.54) | 14 (4.42) | 1 (1.16) | 17 (2.09) | |
| 1 | 82 (69.49) | 72 (36.55) | 181 (57.10) | 28 (32.56) | 390 (47.91) | |
| 2 | 29 (24.58) | 65 (32.99) | 80 (25.24) | 32 (37.21) | 219 (26.90) | |
| 3 | 4 (3.39) | 31 (15.74) | 25 (7.89) | 8 (9.30) | 96 (11.79) | |
| 4 | 0 (0.00) | 24 (12.18) | 17 (5.36) | 17 (19.77) | 92 (11.30) | |
| ≥ G2 | 26 (22.03) | 166 (84.26) | 35 (11.04) | 63 (73.26) | 294 (36.12) | < 0.001 |
| ≥ S2 | 33 (27.97) | 120 (60.91) | 122 (38.49) | 57 (66.28) | 407 (50.00) | < 0.001 |
| G2/S2 | 44 (37.29) | 171 (86.80) | 129 (40.69) | 71 (82.56) | 488 (59.95) | < 0.001 |
| Phase | Indeterminate-A | Indeterminate-B | Indeterminate-C | Indeterminate-D | P value |
| N | 62 (7.62) | 160 (19.66) | 304 (37.35) | 288 (35.38) | |
| Age, yr | 36.42 ± 7.56 | 34.30 ± 7.70 | 40.93 ± 8.08 | 40.56 ± 9.05 | < 0.001 |
| Male | 46 (74.19) | 111 (69.38) | 211 (69.41) | 230 (79.86) | 0.019 |
| HBsAg, log10, IU/mL | 3.58 ± 0.81 | 4.12 ± 0.79 | 2.99 ± 0.73 | 2.77 ± 1.08 | < 0.001 |
| HBeAg | < 0.001 | ||||
| Negative | 0 (0.00) | 0 (0.00) | 304 (100.00) | 288 (100.00) | |
| Positive | 62 (100.00) | 160 (100.00) | 0 (0.00) | 0 (0.00) | |
| HBV-DNA, log10, IU/mL | 4.33 ± 1.22 | 7.03 ± 1.72 | 4.28 ± 0.79 | 3.88 ± 1.40 | < 0.001 |
| ALT, (IQR), U/L | 24.00 (18.25-28.00) | 45.00 (38.00-55.00) | 21.00 (16.00-26.00) | 43.00 (37.00-57.25) | < 0.001 |
| AST, (IQR), U/L | 23.00 (20.00-26.38) | 32.00 (27.80-40.00) | 21.00 (18.00-24.00) | 29.50 (25.00-38.00) | < 0.001 |
| GGT, (IQR), U/L | 20.00 (16.00-32.80) | 32.15 (18.00-53.25) | 19.00 (14.00-25.23) | 32.00 (21.75-49.25) | 0.167 |
| TBIL, (IQR), umol/L | 13.10 (8.98-16.71) | 13.55 (10.50-17.42) | 12.55 (9.70-16.35) | 12.90 (10.07-17.72) | < 0.001 |
| ALB, g/L | 43.83 ± 4.28 | 43.96 ± 4.05 | 44.71 ± 3.65 | 44.73 ± 3.98 | 0.144 |
| WBC, 109/L | 6.14 ± 1.64 | 5.70 ± 1.49 | 5.76 ± 1.61 | 5.96 ± 1.62 | 0.085 |
| PLT, 109/L | 207.32 ± 69.37 | 203.07 ± 59.53 | 210.12 ± 55.75 | 202.57 ± 60.84 | 0.477 |
| APRI stage | < 0.001 | ||||
| < 1 | 59 (95.16) | 145 (90.62) | 301 (99.01) | 268 (93.06) | |
| 2 | 2 (3.23) | 9 (5.62) | 3 (0.99) | 6 (2.08) | |
| > 2 | 1 (1.61) | 6 (3.75) | 0 (0.00) | 14 (4.86) | |
| FIB-4 stage | 0.136 | ||||
| < 1.45 | 49 (79.03) | 131 (81.88) | 257 (84.54) | 225 (78.12) | |
| 1.45- 3.25 | 10 (16.13) | 21 (13.12) | 43 (14.14) | 47 (16.32) | |
| > 3.25 | 3 (4.84) | 8 (5.00) | 4 (1.32) | 16 (5.56) | |
| GPR stage | < 0.001 | ||||
| ≤ 0.32 | 42 (67.74) | 75 (46.88) | 247 (81.25) | 133 (46.18) | |
| > 0.32 | 20 (32.26) | 85 (53.12) | 57 (18.75) | 155 (53.82) | |
| Stage of inflammation | < 0.001 | ||||
| 0 | 4 (6.45) | 2 (1.25) | 4 (1.32) | 4 (1.39) | |
| 1 | 23 (37.10) | 80 (50.00) | 231 (75.99) | 172 (59.72) | |
| 2 | 28 (45.16) | 67 (41.88) | 68 (22.37) | 100 (34.72) | |
| 3 | 7 (11.29) | 10 (6.25) | 0 (0.00) | 9 (3.12) | |
| 4 | 0 (0.00) | 1 (0.62) | 1 (0.33) | 3 (1.04) | |
| Degree of fibrosis | < 0.001 | ||||
| 0 | 2 (3.23) | 2 (1.25) | 6 (1.97) | 7 (2.43) | |
| 1 | 23 (37.10) | 81 (50.62) | 164 (53.95) | 122 (42.36) | |
| 2 | 12 (19.35) | 34 (21.25) | 89 (29.28) | 84 (29.17) | |
| 3 | 8 (12.90) | 16 (10.00) | 33 (10.86) | 39 (13.54) | |
| 4 | 17 (27.42) | 27 (16.88) | 12 (3.95) | 36 (12.50) | |
| ≥ G2 | 35 (56.45) | 78 (48.75) | 69 (22.70) | 112 (38.89) | < 0.001 |
| ≥ S2 | 37 (59.68) | 77 (48.12) | 134 (44.08) | 159 (55.21) | 0.019 |
| G2/S2 | 41 (66.13) | 105 (65.62) | 156 (51.32) | 186 (64.58) | 0.002 |
APRI, FIB-4, and GPR scores are presented in Supplementary Tables 2 and 3. The proportions of APRI < 1, FIB-4 < 1.45, and GPR ≤ 0.32 in the indeterminate patients were 94.96%, 81.33% and 61.06%, respectively. Similarly, APRI < 1 and FIB-4 < 1.45 had the highest proportions in the different indeterminate sub-phases.
Of 1532 CHB patients, significant inflammation, fibrosis, and liver histological changes were found among 584 (38.12%), 739 (48.24%), and 903 (58.94%) patients, respectively. The proportions of significant inflammation in the immune tolerant, HBeAg-positive immune active, inactive, HBeAg-negative immune active, and indeterminate phases were 22.03%, 84.26%, 11.04%, 73.26%, and 36.12%, respectively; the proportions of significant fibrosis were 27.97%, 60.91%, 38.49%, 66.28%, and 50.00%, respectively; and the proportions of significant liver histological changes were 37.29%, 86.80%, 40.69%, 82.56%, and 59.95%, respectively (Table 1 and Figure 2B). The proportions of significant inflammation in indeterminate A, B, C, and D phases were 56.45%, 48.75%, 22.70%, and 38.89%, respectively; the proportions of significant fibrosis in indeterminate A, B, C, and D phases were 59.68%, 48.12%, 44.08%, and 55.21%, respectively; and the proportions of significant liver histological changes in indeterminate A, B, C, and D phases were 66.13%, 65.62%, 51.32%, and 64.58%, respectively (Table 2 and Figure 2B).
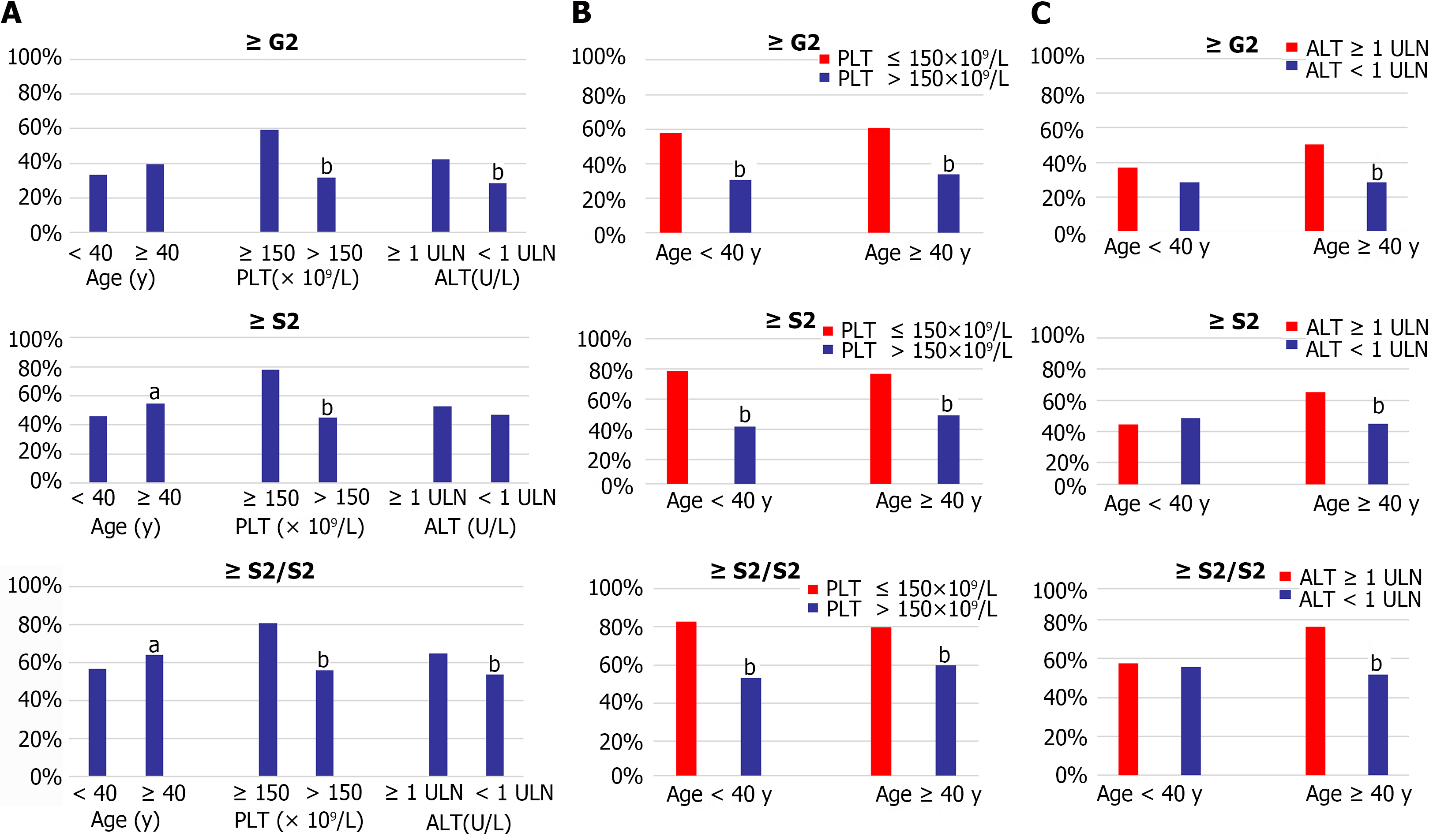
Liver histological changes of the indeterminate patient were also analyzed by different levels of age, PLT, and ALT. The proportion of significant liver histological changes in patients with age ≥ 40 years old, PLT ≤ 150 × 109/L, and ALT ≥ 1ULN were significantly higher than that in age < 40 years old, PLT > 150 × 109/L, and ALT < 1ULN groups, respectively, as shown in Figure 2A. There was no significant difference in inflammation between the patients who were younger and older than 40 years, and no discernible difference in the significant fibrosis between patients with ALT ≥ 1ULN and < 1ULN (Figure 3A).
Patients were further categorized by levels of PLT and ALT in the different age subgroups (Figure 3B and C). Regardless of age was ≥ 40 or < 40 years, individuals with PLT ≤ 150 × 109/L had considerably greater proportions of significant liver fibrosis, inflammation, and histological changes than those with PLT > 150 × 109/L (Figure 3B). When compared with the ALT ≥ 1ULN and < 1ULN groups, the proportion of significant liver inflammation, significan fibrosis, and significant liver histological changes in the patients with ALT ≥ 1ULN was higher than those with ALT < 1ULN group when age was ≥ 40 years (Figure 3C).
The analysis of risk factors of liver histological changes in all patients showed that age ≥ 45 years old, HBeAg-positive, high HBV-DNA level (≥ 3 × log10 IU/mL), PLT ≤ 150 × 109/L, and abnormal ALT level were independent risk factors of significant inflammation and significant liver histological changes (Supplementary Table 4).
According to the results of the multivariate logistic regression analysis of indeterminate patients (Table 3), age ≥ 40 years old adjust odd risk (aOR), 1.44; 95%CI: 1.06-1.97; P = 0.02), PLT ≤ 150 × 109/L (aOR, 2.99; 95%CI: 1.85-4.83; P < 0.0001), and ALT ≥ 1 ULN (aOR, 1.48; 95%CI: 1.08, 2.05, P = 0.0163) were independent risk factors for significant liver histological changes. Age ≥ 40 years old and PLT ≤ 150 × 109/L were independent risk factors for significant inflammation and fibrosis; HBeAg-positive and ALT ≥ 1ULN were also positively correlated with significant inflammation.
| Univariable | Multivariable1 | |||
| Significant necroinflammation | OR, (95%CI) | P value | Adjusted OR, (95%CI) | P value |
| Age, yr | ||||
| < 40 | Referent | Referent | ||
| ≥ 40 | 1.29 (0.97, 1.72) | 0.0846 | 1.75 (1.25, 2.44) | 0.001 |
| Sex | ||||
| Male | Referent | Referent | ||
| Female | 0.76 (0.54, 1.06) | 0.1007 | 0.75 (0.52, 1.07) | 0.1098 |
| HBeAg | ||||
| - | Referent | Referent | ||
| + | 2.36 (1.72, 3.24) | < 0.0001 | 2.55 (1.77, 3.68) | < 0.0001 |
| HBV-DNA log10, IU/mL | ||||
| < 3 | Referent | Referent | ||
| ≥ 3 | 0.98 (0.63, 1.53) | 0.9258 | 1.10 (0.67, 1.80) | 0.7017 |
| PLT, 109/L | ||||
| > 150 | Referent | Referent | ||
| ≤ 150 | 3.16 (2.14, 4.66) | < 0.0001 | 2.77 (1.83, 4.19) | < 0.0001 |
| ALT, U/L | ||||
| < ULN | Referent | Referent | ||
| ≥ ULN | 1.85 (1.38, 2.49) | < 0.0001 | 1.60 (1.15, 2.25) | 0.006 |
| Significant fibrosis | OR, (95%CI) | P value | Adjusted OR, (95%CI) | P value |
| Age, yr | ||||
| < 40 | Referent | Referent | ||
| ≥ 40 | 1.42 (1.07, 1.87) | 0.0136 | 1.36 (1.00, 1.85) | 0.049 |
| Sex | ||||
| Male | Referent | Referent | ||
| Female | 0.88 (0.65, 1.20) | 0.4272 | 0.97 (0.70, 1.35) | 0.8752 |
| HBeAg | ||||
| - | Referent | Referent | ||
| + | 1.08 (0.79, 1.47) | 0.6367 | 1.14 (0.80, 1.62) | 0.4734 |
| HBV-DNA log10, IU/mL | ||||
| < 3 | Referent | Referent | ||
| ≥ 3 | 0.85 (0.56, 1.31) | 0.4636 | 0.93 (0.58, 1.49) | 0.7537 |
| PLT,109/L | ||||
| > 150 | Referent | Referent | ||
| ≤ 150 | 4.30 (2.75, 6.71) | < 0.0001 | 3.92 (2.49, 6.17) | < 0.0001 |
| ALT, U/L | ||||
| < ULN | Referent | Referent | ||
| ≥ ULN | 1.27 (0.96, 1.68) | 0.0907 | 1.15 (0.84, 1.59) | 0.3758 |
| Significant liver histological changes | OR, (95%CI) | P value | Adjusted OR, (95%CI) | P value |
| Age, yr | ||||
| < 40 | Referent | Referent | ||
| ≥ 40 | 1.37 (1.03, 1.82) | 0.0308 | 1.44 (1.06, 1.97) | 0.0214 |
| Sex | ||||
| Male | Referent | Referent | ||
| Female | 0.97 (0.71, 1.33) | 0.8423 | 1.05 (0.76, 1.47) | 0.7551 |
| HBeAg | ||||
| - | Referent | Referent | ||
| + | 1.41 (1.03, 1.95) | 0.0347 | 1.40 (0.97, 2.01) | 0.0689 |
| HBV-DNA log10, IU/mL | ||||
| < 3 | Referent | Referent | ||
| ≥ 3 | 0.97 (0.63, 1.49) | 0.8813 | 1.13 (0.70, 1.81) | 0.626 |
| PLT,109/L | ||||
| > 150 | Referent | Referent | ||
| ≤ 150 | 3.34 (2.09, 5.35) | < 0.0001 | 2.99 (1.85, 4.83) | < 0.0001 |
| ALT, U/L | ||||
| < ULN | Referent | Referent | ||
| ≥ ULN | 1.57 (1.19, 2.09) | 0.0016 | 1.48 (1.08, 2.05) | 0.0163 |
Risk factors of liver histological changes in the subgroups of indeterminate patient by different status of HBeAg were also analyzed (Supplementary Tables 5 and 6). Age ≥ 40 years old and PLT ≤ 150 × 109/L were both of independent risk factors for significant inflammation and significant liver histological changes in the HBeAg positive indeterminate patients (Supplementary Table 5). In the HBeAg positive indeterminate patients, both PLT ≤ 150 × 109/L and ALT ≥ 1 ULN were independent risk factors for significant inflammation, fibrosis and liver histological changes, and age ≥ 40 years was the risk factor for significant necroinflammation (Supplementary Table 6). These results were similar to the results of the overall indeterminate patients. In addition, the results suggested that different gender had different risks in different HBeAg status populations, which is worthy of further exploration in later studies.
The available CHB management guidelines have provided detailed diagnostic and treatment criteria, in which timely antiviral therapy was recommended for patients in the immune active phase, and monitoring of ALT level and liver histological changes were found essential for patients in the immune tolerant and inactive phases[1,2,4]. However, patients in the indeterminate phase are beyond the definition of four phases, and no histological evidence to confirm the necessity of antiviral therapy. The monitoring of ALT level and liver histological changes was recommended by the relevant guidelines. Our results showed that patients had a high rate of histological progression, and over half of patients (59.9%) had G2 inflammation and/or S2 fibrosis; even in CHB patients in indeterminate A and C phases whose ALT level was normal, the proportions of liver histological changes in patients reached 66.13% and 51.32%, respectively, suggesting the majority of patients in the indeterminate phase were in disease progression.
Recently, a large sample size cohort performed in the United States showed that among patients with cirrhosis, 9% of patients were in the indeterminate phase[10]. Another study from the United States assessed the phenotype of HBV in 1390 adult participants enrolled in the Hepatitis B Research Network Cohort Study, of whom 524 patients were in the indeterminate phase, 88 (19%) and 5 (1%) patients obtained APRI scores > 0.50-2.0 and > 2, and 78 (17%) and 13 (3%) patients achieved FIB-4 scores equal to 1.45–3.25 and > 3.25, respectively[14]. Hsu et al[15] evaluated 198 untreated Asian-American CHB patients with a mean follow-up time of 21 months, and using the modified ALT criteria, it was revealed that 43 (28.1%) patients had phase-based changes, of whom 31/43 (72.1%) patients were shifted from phase 1 and indeterminate phase to phases 2 and 4, as being more active CHB phases. However, our histological findings showed higher percentages of inflammation and fibrosis than the above studies. Our study showed 294 (36.12%) and 407 (50.00%) patients were ≥ G2 and ≥ S2, respectively; 20 (2.46%) and 21 (2.58%) patients obtained APRI scores of 1-2 and > 2, respectively; 4 (3.39%) and 31 (3.81%) patients achieved APRI scores of 121 (14.86%) and > 3.25, respectively. Recently, Chinese scholars investigated liver histological changes of 106 HBeAg-negative CHB patients with normal ALT level and HBV-DNA level ≥ 2000 IU/mL, equal to patients in the indeterminate C phase, and the proportions of significant inflammation, significant fibrosis, and significant liver histological changes were found in 58.5%, 67.9% and 74.5% of patients, respectively[16]. The differences between liver biopsy and APRI/FIB-4 scores could be mainly attributed to the low diagnostic accuracy of APRI/FIB-4 scores. Several studies have found that the diagnostic accuracy of APRI and FIB-4 for significant liver fibrosis in CHB patients was low[13,17-20] and not ideal substitutes for liver biopsy[21].
To date, few studies have concentrated on the HBeAg-positive indeterminate CHB patients. In the present study, we found that 50.90%, 51.35% and 57.60% of HBeAg-positive indeterminate CHB patients had significant inflammation, fibrosis, and liver histological changes, respectively. In the indeterminate A phase, with HBeAg-positive, normal ALT level, and low HBV-DNA level, more than 50% of patients had significant inflammation and significant fibrosis. Additionally, our study revealed that more than 60% of patients had significant liver histological changes in the indeterminate B and D phases with a mild abnormal ALT level (more than 1 ULN, while lower than 2 ULN), and HBV-DNA level was relatively low in HBeAg-positive or -negative patients.
Some studies have assessed risk factors for liver inflammation and fibrosis in indeterminate CHB patients. In our study, the multivariate logistic regression analysis revealed that age ≥ 40 years old, PLT ≤ 150 × 109/L, and ALT level ≥ ULN were independent risk factors for significant fibrosis and liver histological changes in the indeterminate CHB patients. Previous studies have suggested that age was a risk factor for long-term prognosis in patients with chronic HBV infection, and the incidence of adverse events increased with age[22,23]. A study on HBeAg-negative CHB patients with persistently normal ALT level showed that older age was an independent predictor of significant liver fibrosis and liver histological damage[24]. Additionally, another study revealed that age ≥ 40 years old was a risk factor for HCC in indeterminate patients[9]. Numerous studies showed that abnormal ALT level was related to liver fibrosis and cirrhosis, and the traditional normal range of ALT level has remained controversial[11,25]. Studies have demonstrated that ULN of lower ALT could better reflect the liver histological changes[11,25]. A Korean study suggested that compared with ALT level < 20 U/L, the risk of inflammation in patients with ALT levels of 20-29 and 30-39 U/L significantly increased[26]. A study on CHB patients with persistently normal ALT level also revealed that ALT level > 0.5 ULN (20 U/L) was associated with liver fibrosis and inflammation[16]. In our study, according to ALT level of 35 IU/L in male and 25 IU/L in female patients, which were lower than the reference levels, an abnormal ALT level was found as a risk factor for significant inflammation and fibrosis in indeterminate CHB patients. Therefore, a lower ALT level used as a reference level could be more beneficial to the management of CHB patients. In addition, previous studies demonstrated that PLT was an independent predictor of liver fibrosis and cirrhosis in the CHB patients, regardless of undergoing treatment[17,19,27,28]. Our study further supported this finding in the indeterminate phase patients. Portal hypertension and hypersplenism are factors leading to thrombocytopenia, accompanied by the development of liver fibrosis and cirrhosis. Other studies on patients undergoing liver transplantation have suggested that thrombocytopenia was associated with the progression of liver fibrosis, leading to the decreased production of thrombopoietin by hepatocytes[27,28].
Previous studies suggested that CHB patients in the indeterminate phase had a better outcome and with a lower risk of development to adverse prognoses, such as cirrhosis and HCC[8,29]. But, these studies mainly concentrated on Caucasians with relatively small sample size and the follow-up time was no longer enough. Recently, some studies have shown that indeterminate phase patients had a higher risk of development to HCC during follow-up[9,10,30]. A retrospective study that included 3336 untreated CHB patients from the United States and Taiwan showed that, after 10 years of follow-up, the cumulative incidence of HCC in 1303 indeterminate phase patients was 4.5-fold higher than those in inactive patients (2.7% vs 0.6%), and the cumulative incidence of HCC in patients who remained indeterminate after follow-up was 9 times higher than those remained inactive (4.6% vs 0.5%)[9].
Our study have some limitations. This was a cross-sectional and single-center study, and long-time multicenter follow-up data were absent, thus, we could not determine the results of disease progression with inclusion of cirrhosis and HCC. Multicenter and well-designed prospective studies are therefore required to confirm the findings of the present study. In addition, the initial liver biopsy for the majority of patients in the indeterminate phase was conducted between 2020 and early 2021. Due to the impact of the coronavirus disease 2019 pandemic, many of these patients were unable to undergo a second paired liver biopsy, and as a result, the dynamic changes in liver pathology are not addressed in this article. This is another limitation of our research. Our research team has initiated relevant paired clinical studies to offer more robust evidence for future clinical practice.
In summary, this is the largest cohort study that enrolled CHB patients who were in all the phases of indeterminate using liver biopsy. Our study indicated that about 60% of indeterminate CHB patients had histopathological changes. Age ≥ 40 years old, PLT ≤ 150 × 109/L, and ALT ≥ ULN were independent risk factors for significant liver fibrosis or significant histological changes in the indeterminate CHB patients. Liver biopsy should be recommended for indeterminate patients to evaluate liver histopathological changes, especially in those patients with ≥ 40 years old, or PLT ≤ 150 × 109/L. More optimal ALT cutoff value in CHB patients may still need to be adjusted to identify more populations in need of treatment. Antiviral therapy of indeterminate patients is worthy of consideration in the management of CHB patients.
We would like to thank all those who participated in the study for their time and involvement.
| 1. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3095] [Article Influence: 386.9] [Reference Citation Analysis (1)] |
| 2. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4011] [Article Influence: 445.7] [Reference Citation Analysis (1)] |
| 3. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1631] [Article Influence: 163.1] [Reference Citation Analysis (2)] |
| 4. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2055] [Article Influence: 205.5] [Reference Citation Analysis (12)] |
| 5. | Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, Wu W, Liu Y, Chen Y, Zhang Z, Li J, Huang R, Wu C. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Hadziyannis SJ. Unrevealing the natural course of the so-called "inactive HBsAg or HBV carrier state". Hepatol Int. 2007;1:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, Bose S. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Bonacci M, Lens S, Mariño Z, Londoño MC, Rodríguez-Tajes S, Mas A, García-López M, Pérez-Del-Pulgar S, Sánchez-Tapias JM, Forns X. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment Pharmacol Ther. 2018;47:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, Zhang J, Li J, Wong C, Cheung RC, Yang HI, Nguyen MH. Natural History and Hepatocellular Carcinoma Risk in Untreated Chronic Hepatitis B Patients With Indeterminate Phase. Clin Gastroenterol Hepatol. 2022;20:1803-1812.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 10. | Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, Lu M, Boscarino JA, Schmidt MA, Trinacty CM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006-2013. Aliment Pharmacol Ther. 2016;44:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 11. | Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1381] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 13. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (1)] |
| 14. | Di Bisceglie AM, Lombardero M, Teckman J, Roberts L, Janssen HL, Belle SH, Hoofnagle JH; Hepatitis B Research Network (HBRN). Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat. 2017;24:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Hsu YN, Pan CQ, Abbasi A, Xia V, Bansal R, Hu KQ. Clinical presentation and disease phases of chronic hepatitis B using conventional versus modified ALT criteria in Asian Americans. Dig Dis Sci. 2014;59:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Duan M, Chi X, Xiao H, Liu X, Zhuang H. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B. Hepatol Int. 2021;15:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (3)] |
| 17. | Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, Mak B, Oo AM, Lim SG. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD, Schall RE, Dinh P, Yee LJ, Martins EB, Lim SG, Loomba R, Petersen J, Buti M, Marcellin P. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Dong XQ, Wu Z, Zhao H, Wang GQ; China HepB-Related Fibrosis Assessment Research Group. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J Viral Hepat. 2019;26:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Sonneveld MJ, Brouwer WP, Chan HL, Piratvisuth T, Jia JD, Zeuzem S, Liaw YF, Hansen BE, Choi H, Wat C, Pavlovic V, Gaggar A, Xie Q, Buti M, de Knegt RJ, Janssen HLA. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: results from the SONIC-B study. Lancet Gastroenterol Hepatol. 2019;4:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 22. | Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, Realdi G, Ruol A. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Tan Y, Ye Y, Zhou X, Chen L, Wen D. Age as a predictor of significant fibrosis features in HBeAg-negative chronic hepatitis B virus infection with persistently normal alanine aminotransferase. PLoS One. 2015;10:e0123452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1063] [Article Influence: 44.3] [Reference Citation Analysis (4)] |
| 26. | Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 27. | Adinolfi LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, Ragone E, Durante Mangoni E, Ruggiero G. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol. 2001;113:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 28. | Kawasaki T, Takeshita A, Souda K, Kobayashi Y, Kikuyama M, Suzuki F, Kageyama F, Sasada Y, Shimizu E, Murohisa G, Koide S, Yoshimi T, Nakamura H, Ohno R. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94:1918-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Oliveri F, Surace L, Cavallone D, Colombatto P, Ricco G, Salvati N, Coco B, Romagnoli V, Gattai R, Salvati A, Moriconi F, Yuan Q, Bonino F, Brunetto MR. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver Int. 2017;37:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Choi GH, Kim GA, Choi J, Han S, Lim YS. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment Pharmacol Ther. 2019;50:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/