Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.684
Revised: April 8, 2024
Accepted: April 15, 2024
Published online: May 27, 2024
Processing time: 95 Days and 10 Hours
In this editorial we comment on the review by Zhou et al reviewing the landscape of nanomedicine in the treatment of hepatocellular carcinoma (HCC). We focus on the immense potential of nanotechnology, particularly ligand-receptor mediated nanotherapy, in revolutionizing the treatment landscape of HCC. Despite advan
Core Tip: Despite recent advances in locoregional and systemic therapy for the treatment of hepatocellular carcinoma, current conventional treatment of advanced disease only offers limited survival benefit. Systemic therapy is particularly constrained by the inability for precise targeting of tumor cells, thus leading to systemic adverse effects and decreased efficacy in treatment. Nanotherapy holds the potential to overcome these traditional constraints but remains primarily a proof of concept at this juncture with in vivo studies in animal models. Further research and collaboration will ultimately lead to clinical applicability.
- Citation: Lee HD, Yuan LY. Nano-revolution in hepatocellular carcinoma: A multidisciplinary odyssey - Are we there yet? World J Hepatol 2024; 16(5): 684-687
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/684.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.684
Hepatocellular carcinoma (HCC) continues to pose a significant threat to global health. Despite rapid growth in multidisciplinary treatment and the evolution in systemic therapy in recent years, the overall survival in patients with advanced HCC remains poor owing to the tumor’s extraordinary heterogeneity, challenges in the treatment landscape and drug resistance[1,2]. In pursuing effective therapeutic strategies, the convergence of nanotechnology and precision medicine to enhance drug delivery, improve efficacy, and minimize adverse effects has led researchers to explore the vast potential of nanoparticles. As highlighted in the review titled “Precision targeting in hepatocellular carcinoma: Exploring ligand-receptor mediated nanotherapy” by Zhou et al[3], the advent of ligand-receptor mediated nanotherapy represents a groundbreaking approach, offering unparalleled precision and efficacy in HCC treatment. Zhou et al[3] illuminates the multifaceted landscape of ligand-receptor interactions and their transformative potential, while addressing the challenges we face in this new era of medicine.
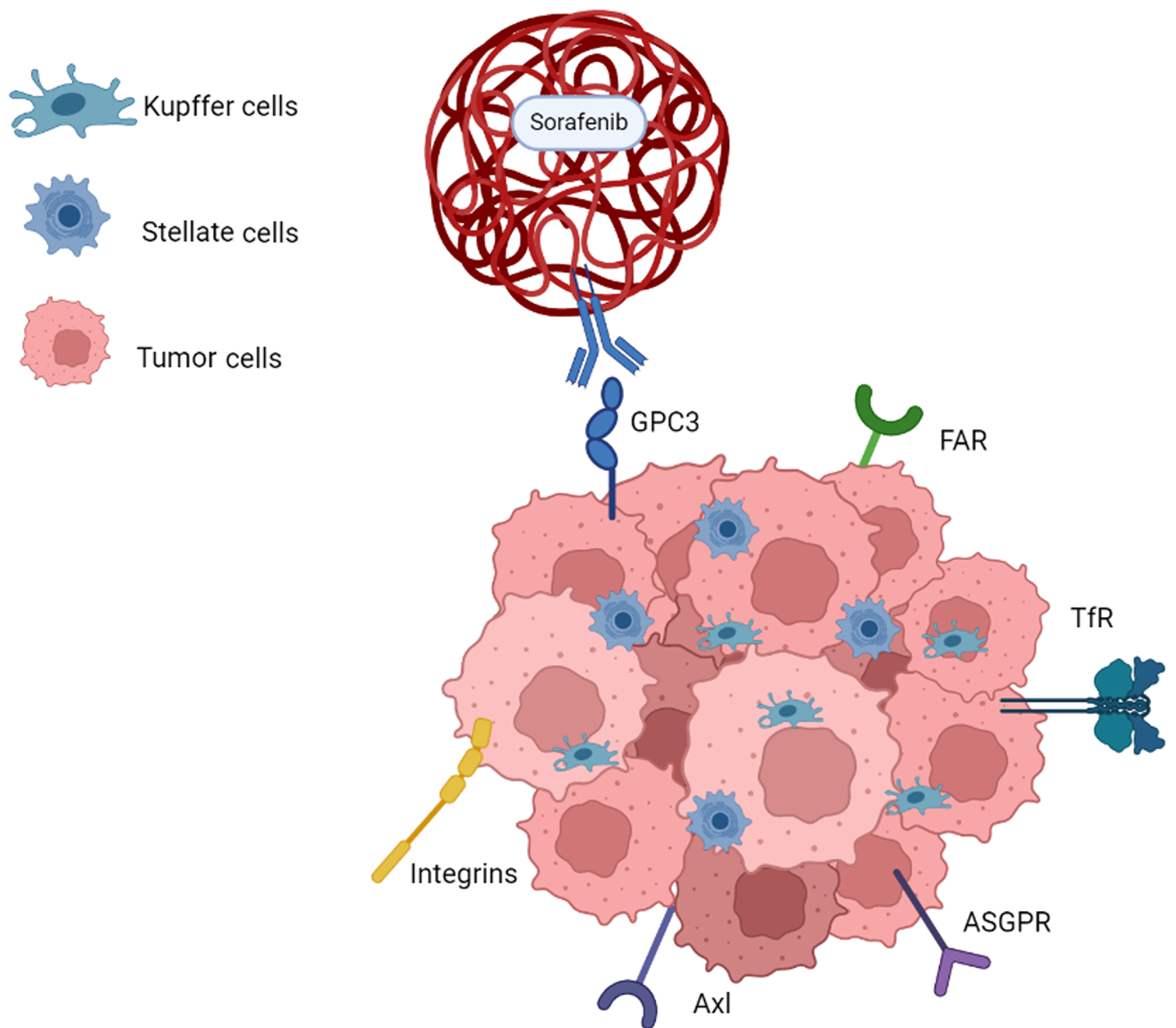
At the heart of this nanotherapeutic revolution lies the ability of nanoscale carriers to precisely deliver therapeutic drugs to the tumor site[4]. Alterations in expression of oncogenes and tumor suppressor genes have been identified as potential targets for therapeutic intervention[5]. The diverse tumor microenvironment created by the interaction among cells serves as a critical element affecting the management and prognosis of patients with advanced HCC[6] (Figure 1). Ligand--mediated approaches, such as coupling technology, enhance targeting specificity by covalently binding different components like lipids, peptides, antibodies, and sugars with respective receptors[7]. These carriers enhance drug solubility and stability, thereby improving its efficacy and reducing the adverse side effects that often accompany conventional treatments. For instance, anti-glypican-3, with its significant overexpression in HCC cells, serves as a prime target for sorafenib-loaded polymer nanoparticles[8,9]. GalNAc coupling to asialoglycoprotein, a receptor abundantly expressed on hepatocytes, also emerges as a promising strategy, overcoming hurdles associated with drug resistance and improving the efficiency of liver-specific drug delivery[10,11]. The proliferation of receptors such as transferrin receptor and folic acid receptor that correspond to the tumor’s increased demand of essential nutrients are further targets that show promises for ligand-receptor mediated drug delivery[12,13]. Lastly, strategies to facilitate endosome escape, such as glutathione-responsive environmentally sensitive nanocarriers, are additional focuses in ligand-mediated nanotherapy[7,14].
In addition to improving the specificity of drug delivery, ligand-modified nanoparticles also hold potential both in combatting the complexities of drug resistance and aggressive tumor biology. Nanomedicine holds the unique ability to bypass efflux pumps and navigate the liver's intricate enzyme system and offers hope in overcoming resistance mechanisms that often impede traditional chemotherapy regimens[15]. Overexpressed glycoprotein and certain integrins were previously considered markers of poor prognosis[16]. The advent of ligand-receptor mediated therapy takes advantage of these biomarkers for specifically targeting and delivering the medications to the tumors[7,11]. The exploration of dual-ligand nanoparticle modification is an additional sophisticated strategy that simultaneously targets multiple receptors or pathways on cancer cells. This approach not only enhances targeting and specificity but also opens avenues for synergistic therapeutic agents, providing a comprehensive and dynamic approach to HCC treatment[17,18].
The integration of nanotherapy in the treatment landscape of HCC holds immense promise, yet it is not devoid of challenges. Achieving precise targeted drug delivery to the intricate microenvironment of liver tumors remains a challenge that demands innovative solutions. Additionally, immune responses pose a significant obstacle, as the body often identifies nanoparticles as foreign entities. Overcoming these immune reactions is crucial for maintaining the therapeutic effectiveness of nanocarriers. The synthesis of nanoparticles with consistent properties is another hurdle, requiring advancements to ensure reproducibility and reliability in the manufacturing process. Scaling up production for mass market availability while addressing cost-effectiveness and adhering to stringent regulatory and ethical considerations further complicates the landscape[7,19]. Addressing each of these limitations demands a multidisciplinary approach and is vital for optimizing clinical care and realizing the full potential of nanomedicine in HCC treatment.
Despite these challenges, the future of ligand-receptor mediated nanotherapy in HCC is bright. Innovations in ligand-receptor interactions and dual-ligand modifications are expected to refine targeting strategies. Researchers envision controlled drug release mechanisms that respond to stimuli, enabling on-demand drug delivery at tumor sites. The future of nanotherapy in HCC is likely to be personalized, tailoring nanoparticle formulations based on individual patient profiles for enhanced treatment outcomes. The development of multifunctional nanoparticles, such as the combination of chemotherapy and immunotherapy, capable of delivering synergistic therapeutic agents, is anticipated to revolutionize HCC treatment. Advances in nanomaterials science will contribute to the synthesis of nanoparticles with uniform and reproducible properties, addressing current challenges in manufacturing. Streamlining large-scale production processes and ensuring cost-effectiveness are critical for making nanotherapies widely accessible.
Continued collaboration across scientific and medical disciplines is needed to navigate the challenges and unlock the full potential of nanomedicine. Overcoming current limitations requires a multidisciplinary approach, with ongoing research aimed at refining targeting strategies, improving drug release mechanisms, and addressing production challenges. As innovations unfold, personalized and multifunctional nanotherapies may revolutionize HCC treatment, offering hope for more effective, precise, and accessible clinical care. The future, it seems, holds the promise of a nano-revolution in HCC therapy-a multidisciplinary odyssey towards precision targeting and transformative outcomes.
| 1. | Huang Z, Xia H, Cui Y, Yam JWP, Xu Y. Ferroptosis: From Basic Research to Clinical Therapeutics in Hepatocellular Carcinoma. J Clin Transl Hepatol. 2023;11:207-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (35)] |
| 2. | Katoch S, Sharma V, Patial V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: Experimental and clinical scenarios. World J Gastroenterol. 2022;28:3535-3554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 3. | Zhou XQ, Li YP, Dang SS. Precision targeting in hepatocellular carcinoma: Exploring ligand-receptor mediated nanotherapy. World J Hepatol. 2024;16:164-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (15)] |
| 4. | Abd-Rabou AA, Ahmed HH, Mohamed SH, Kotob SE, Kishta MS. Nanotherapy: New Approach for Impeding Hepatic Cancer Microenvironment via Targeting Multiple Molecular Pathways. Asian Pac J Cancer Prev. 2022;23:4261-4274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (8)] |
| 5. | Holstein E, Binder M, Mikulits W. Dynamics of Axl Receptor Shedding in Hepatocellular Carcinoma and Its Implication for Theranostics. Int J Mol Sci. 2018;19:4111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 6. | Dai Z, Wang Y, Sun N, Zhang C. Characterizing ligand-receptor interactions and unveiling the pro-tumorigenic role of CCL16-CCR1 axis in the microenvironment of hepatocellular carcinoma. Front Immunol. 2023;14:1299953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (9)] |
| 7. | Ren X, Su D, Shi D, Xiang X. The improving strategies and applications of nanotechnology-based drugs in hepatocellular carcinoma treatment. Front Bioeng Biotechnol. 2023;11:1272850. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (24)] |
| 8. | Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 9. | Shen J, Cai W, Ma Y, Xu R, Huo Z, Song L, Qiu X, Zhang Y, Li A, Cao W, Zhou S, Tang X. hGC33-Modified and Sorafenib-Loaded Nanoparticles have a Synergistic Anti-Hepatoma Effect by Inhibiting Wnt Signaling Pathway. Nanoscale Res Lett. 2020;15:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 10. | Li YL, Zhu XM, Liang H, Orvig C, Chen ZF. Recent Advances in Asialoglycoprotein Receptor and Glycyrrhetinic Acid Receptor-Mediated and/or pH-Responsive Hepatocellular Carcinoma- Targeted Drug Delivery. Curr Med Chem. 2021;28:1508-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (17)] |
| 11. | Pranatharthiharan S, Patel MD, Malshe VC, Pujari V, Gorakshakar A, Madkaikar M, Ghosh K, Devarajan PV. Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv. 2017;24:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 12. | Adachi M, Kai K, Yamaji K, Ide T, Noshiro H, Kawaguchi A, Aishima S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 13. | Wang H, Wu D, Wang P, Gao C, Teng H, Liu D, Zhao Y, Du R. Albumin nanoparticles and their folate modified counterparts for delivery of a lupine derivative to hepatocellular carcinoma. Biomed Pharmacother. 2023;167:115485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 14. | Lee YW, Luther DC, Kretzmann JA, Burden A, Jeon T, Zhai S, Rotello VM. Protein Delivery into the Cell Cytosol using Non-Viral Nanocarriers. Theranostics. 2019;9:3280-3292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 15. | Heinrich S, Castven D, Galle PR, Marquardt JU. Translational Considerations to Improve Response and Overcome Therapy Resistance in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel). 2020;12:2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 16. | Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, Gu Y, Zhao N, Xiang Q, Cui Y. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 789] [Article Influence: 263.0] [Reference Citation Analysis (13)] |
| 17. | Xiang Y, Huang W, Huang C, Long J, Zhou Y, Liu Y, Tang S, He DX, Tan XW, Wei H, Yu CY. Facile Fabrication of Nanoparticles with Dual-Targeting Ligands for Precise Hepatocellular Carcinoma Therapy In Vitro and In Vivo. Mol Pharm. 2020;17:3223-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 18. | Cheng Y, Zhao P, Wu S, Yang T, Chen Y, Zhang X, He C, Zheng C, Li K, Ma X, Xiang G. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int J Pharm. 2018;545:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 19. | Alhalmi A, Beg S, Kohli K, Waris M, Singh T. Nanotechnology Based Approach for Hepatocellular Carcinoma Targeting. Curr Drug Targets. 2021;22:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Shah SIA, Pakistan S-Editor: Che XX L-Editor: A P-Editor: Cai YX