Published online Apr 27, 2024. doi: 10.4254/wjh.v16.i4.601
Peer-review started: November 13, 2023
First decision: January 23, 2024
Revised: February 4, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: April 27, 2024
Processing time: 162 Days and 17.2 Hours
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver condition that typically arises in the middle and late stages of pregnancy. Short-chain fatty acids (SCFAs), prominent metabolites of the gut microbiota, have significant connections with various pregnancy complications, and some SCFAs hold poten
To investigate the metabolic profiles and differences in SCFAs present in the maternal and cord blood of patients with ICP and determine the clinical signifi
Maternal serum and cord blood samples were collected from both patients with ICP (ICP group) and normal pregnant women (NP group). Targeted metabolo
Significant differences in maternal SCFAs were observed between the ICP and NP groups. Most SCFAs exhibited a consistent declining trend in cord blood samples from the ICP group, mirroring the pattern seen in maternal serum. Correlation analysis revealed a positive correlation between maternal serum SCFAs and cord blood SCFAs [r (Pearson) = 0.88, P = 7.93e-95]. In both maternal serum and cord blood, acetic and caproic acids were identified as key metabolites contributing to the differences in SCFAs between the two groups (variable importance for the projection > 1). Receiver operating characteristic analysis demonstrated that multiple SCFAs in maternal blood have excellent diagnostic capabilities for ICP, with caproic acid exhibiting the highest diagnostic efficacy (area under the curve = 0.97).
Compared with the NP group, significant alterations were observed in the SCFAs of maternal serum and cord blood in the ICP group, although they displayed distinct patterns of change. Furthermore, the SCFA levels in maternal serum and cord blood were significantly positively correlated. Notably, certain maternal serum SCFAs, specifically caproic and acetic acids, demonstrated excellent diagnostic efficiency for ICP.
Core Tip: Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver condition that typically arises in the middle and late stages of pregnancy. Short-chain fatty acids (SCFAs), prominent metabolites of the gut microbiota, have significant connections with various pregnancy complications. This work assesses the SCFA levels in maternal serum and cord blood samples which are collected from both patients with ICP and normal pregnant women by using targeted metabolomics, then the correlation between maternal and cord blood SCFAs are explored. At the same time, the clinical diagnostic potential of key differential SCFAs are assessed.
- Citation: Ren SJ, Feng JT, Xiang T, Liao CL, Zhou YP, Xuan RR. Expression and clinical significance of short-chain fatty acids in patients with intrahepatic cholestasis of pregnancy. World J Hepatol 2024; 16(4): 601-611
- URL: https://www.wjgnet.com/1948-5182/full/v16/i4/601.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i4.601
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver condition that typically arises in the middle and late stages of pregnancy. ICP is characterized by increased bile acid levels in maternal blood and persistent skin itching[1]. Although the clinical symptoms in patients with ICP often subside quickly after delivery, the perinatal mortality rate for fetuses and newborns can be as high as 5%. Additionally, ICP tends to recur in up to 70% of subsequent pregnancies[2]. While the exact pathogenesis of ICP remains incompletely understood, it is believed to be influenced by genetic, hormonal, and environmental factors[2].
Recent research has shed light on the role of gut microbiota and their metabolites in the progression of ICP[3]. Short-chain fatty acids (SCFAs), prominent metabolites of the gut microbiota, play a pivotal role in preserving host metabolism, maintaining intestinal barrier function, fostering immune tolerance, and regulating autoimmune activity[4]. SCFAs have significant connections with various pregnancy complications[5], and some SCFAs hold potential for treating such complications[6]. However, the metabolic profile of SCFAs in patients with ICP remains unclear. Hence, we herein used targeted metabolomics technology to analyze SCFAs in the maternal and cord blood of both patients with ICP and normal pregnant (NP) women. The primary objectives were to investigate the changes in SCFAs in patients with ICP and their offspring, examine the correlation between maternal and umbilical blood SCFAs, and assess their clinical significance and diagnostic value. Ultimately, the study aims to identify novel biomarkers for diagnosing and treating ICP by focusing on gut microbiota metabolites.
In this study, we selected 34 patients with ICP (ICP group) who delivered at our hospital between October 2020 and March 2022 to comprise the study group. Additionally, we included 30 NP women from the same period as controls (NP group), based on predefined inclusion and exclusion criteria. The inclusion criteria encompassed meeting the diagnostic standards for ICP as established by the Chinese Medical Association[7] and having reached a gestational age of ≥ 28 wk with no other pregnancy-related complications. Furthermore, participants needed to volunteer for the study and provide informed consent.
The exclusion criteria specified that participants should not have a history of major diseases affecting organs or systems, including cardiovascular, cerebrovascular, pulmonary, hepatic, renal, or endocrine conditions. They should also be free from severe internal or external complications apart from ICP and should not have taken antibiotics, probiotics, or prebiotics within one month before the sample collection. Additionally, they should not have experienced diarrhea or other gastrointestinal symptoms. The study received approval from the Ethics Committee, and all participants signed written informed consent forms. All methods used in this study adhered to the principles of the Helsinki Declaration.
The demographic and clinical information for both groups of participants, encompassing data such as age, pre-pregnancy body mass index, and pregnancy-related weight gain, was meticulously collected. Fasting venous blood samples were obtained from the subjects before delivery, and umbilical cord blood samples were collected during the delivery process. Following collection, the samples were promptly stored at 4 °C and allowed to stand for a duration of 4 h. Subsequently, the samples underwent centrifugation, and the resulting serum was extracted and stored at −80 °C.
Furthermore, clinical indicators, including but not limited to hemoglobin, total bile acid, and D-dimer levels; fetal biparietal diameter; abdominal circumference; and fetal birth weight, were extracted from the medical record system. These clinical indicators encompassed blood parameters before delivery, fetal ultrasound data, and the outcome of the pregnancies. The ultrasound metrics for the fetuses were derived from the last obstetric ultrasound conducted by the subjects within one week before the delivery.
The maternal and cord blood samples were meticulously collected and subjected to a process involving a mixture of 50% H2SO4 and an extraction solution. This extraction solution comprised an internal standard, 2-methyl pentanoic acid (25 mg/L), and methyl tert-butyl ether. The procedure involved a sequence of steps, including vortexing, oscillation, low-temperature ultrasound, and centrifugation, culminating in allowing the mixture to stand before extracting the supernatant. Subsequently, the supernatant underwent detection of SCFAs, specifically acetic acid, propionic acid, butyric acid, isobutyric acid, isovaleric acid, valeric acid, and caproic acid, utilizing a gas chromatography-mass spectrometer (SHIMADZU GC2030-QP2020 NX, J&W Scientific, Folsom, CA, United States).
To assess intergroup differences in SCFAs between maternal and cord blood samples, principal component analysis and orthogonal projections to latent structures discriminant analysis were used. Important differential metabolites were identified based on variable importance for the projection (VIP) values. Cluster and correlation analyses of SCFAs were conducted using R software (v.4.2.2), utilizing the pheatmap package, cor() function, and cor.test() function. Furthermore, potential biomarkers were evaluated and screened using the receiver operating characteristic (ROC) curve.
For pathway analysis, the seven types of SCFAs were integrated into the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, leading to the identification of 24 pathways (Supplementary Table 1). Finally, the KEGG pathway enrichment results were visualized using the website https://www.bioinformatics.com.cn.
Statistical analysis was conducted using SPSS (v.26.0) and GraphPad Prism (v.8.0.2) software. The Kolmogorov-Smirnov test was used to inspect the normality and homogeneity of variance of all the data. For quantitative data that adhered to a normal distribution, group-wise statistical differences were assessed using Student's t-tests. The data was presented as mean ± SD. In cases where the quantitative data did not follow a normal distribution, the Wilcoxon rank sum test was used. Values were presented as median and interquartile range (IQR) for data that were not normally distributed for continuous variables. Categorical data was expressed as percentages (%), and the statistical distinctions between groups were examined using the chi-square test or Fisher's exact test. To explore the correlation between SCFAs and clinical indicators, the Spearman correlation coefficient was used. Additionally, the relationship between maternal serum and cord blood SCFAs was analyzed using linear regression and the Pearson correlation coefficient. The resultant plots were generated utilizing R software (v.4.2.2), particularly with the ggplot2 (v.3.4.2) package. Statistical significance was defined as P < 0.05.
In this study, a total of 64 subjects were included, 34 with ICP and 30 NP women. The baseline data and clinical indicators for all participants were presented in Supplementary Tables 2-5, with Table 1 highlighting the indicators that exhibited statistical differences. The analysis revealed no statistically significant differences (P > 0.05) between the two groups in terms of age, years of education, and the number of pregnancies and births. However, the ICP group exhibited lower uterine height, and abdominal circumference during pregnancy than the NP group (P < 0.05). Also, Compared with the NP group, the ICP group showed a lower trend in weight gain during pregnancy (P > 0.05). Additionally, the hemoglobin level of the ICP group was significantly lower than that of the NP group (P < 0.05), while the serum alanine aminotransferase, total bile acid, and glycyrrhetinic acid levels were significantly higher in the ICP group (P < 0.001).
| Index | NP (n = 30) | ICP (n = 34) | P value | |
| Baseline information | Maternal fundal height (cm) | 34.60 ± 2.43 | 32.79 ± 3.19 | 0.014 |
| Maternal abdominal circumference (cm) | 102.17 ± 5.89 | 97.47 ± 8.73 | 0.016 | |
| Blood routine | Hemoglobin (g/L) | 4.20 ± 0.60 | 3.79 ± 0.44 | 0.003 |
| Hematocrit | 127.33 ± 12.14 | 117.41 ± 12.32 | 0.002 | |
| Leukocyte (× 109/L) | 10.70 ± 3.23 | 8.10 ± 2.34 | < 0.001 | |
| Percentage of neutrophils (%) | 77.96 ± 6.69 | 72.50 ± 8.86 | 0.008 | |
| Liver function | Direct bilirubin (μmol/L) | 1.45 ± 0.73 | 2.37 ± 2.09 | 0.018 |
| Glutamic-pyruvic transaminase (U/L) | 9.40 ± 3.19 | 38.33 ± 48.76 | < 0.001 | |
| Total bile acid (μmol/L) | 2.65 ± 1.35 | 22.64 ± 18.11 | < 0.001 | |
| Cholyglycine (mg/L) | 1.38 ± 0.18 | 6.58 ± 8.92 | < 0.001 | |
| Fetal ultrasound | Biparietal diameter (mm) | 93.40 ± 3.56 | 91.49 ± 4.09 | 0.032 |
| Fetal head circumference (mm) | 334.40 ± 11.03 | 327.86 ± 12.40 | 0.029 | |
| Fetal abdominal circumference (mm) | 337.20 ± 19.36 | 326.57 ± 18.67 | 0.028 | |
| Fetal femur length (mm) | 71.47 ± 3.47 | 68.89 ± 3.87 | 0.007 | |
| Pregnancy outcome | Gestational week of delivery (wk) | 39.39 ± 0.84 | 37.82 ± 1.64 | < 0.001 |
| Vaginal delivery [n (%)] | 23(76.67) | 11(32.35) | < 0.001 | |
| Neonatal birth weight (g) | 3476.00 ± 436.12 | 3063.14 ± 386.62 | < 0.001 | |
Pre-delivery ultrasound examinations indicated that the ICP group had smaller biparietal diameter, fetal head circumference, abdominal circumference, and femoral length than the NP group (P < 0.05). Furthermore, perinatal outcomes revealed that the ICP group had an earlier gestational delivery, a higher rate of cesarean section, and lower birth weights for newborns than the NP group (P < 0.001).
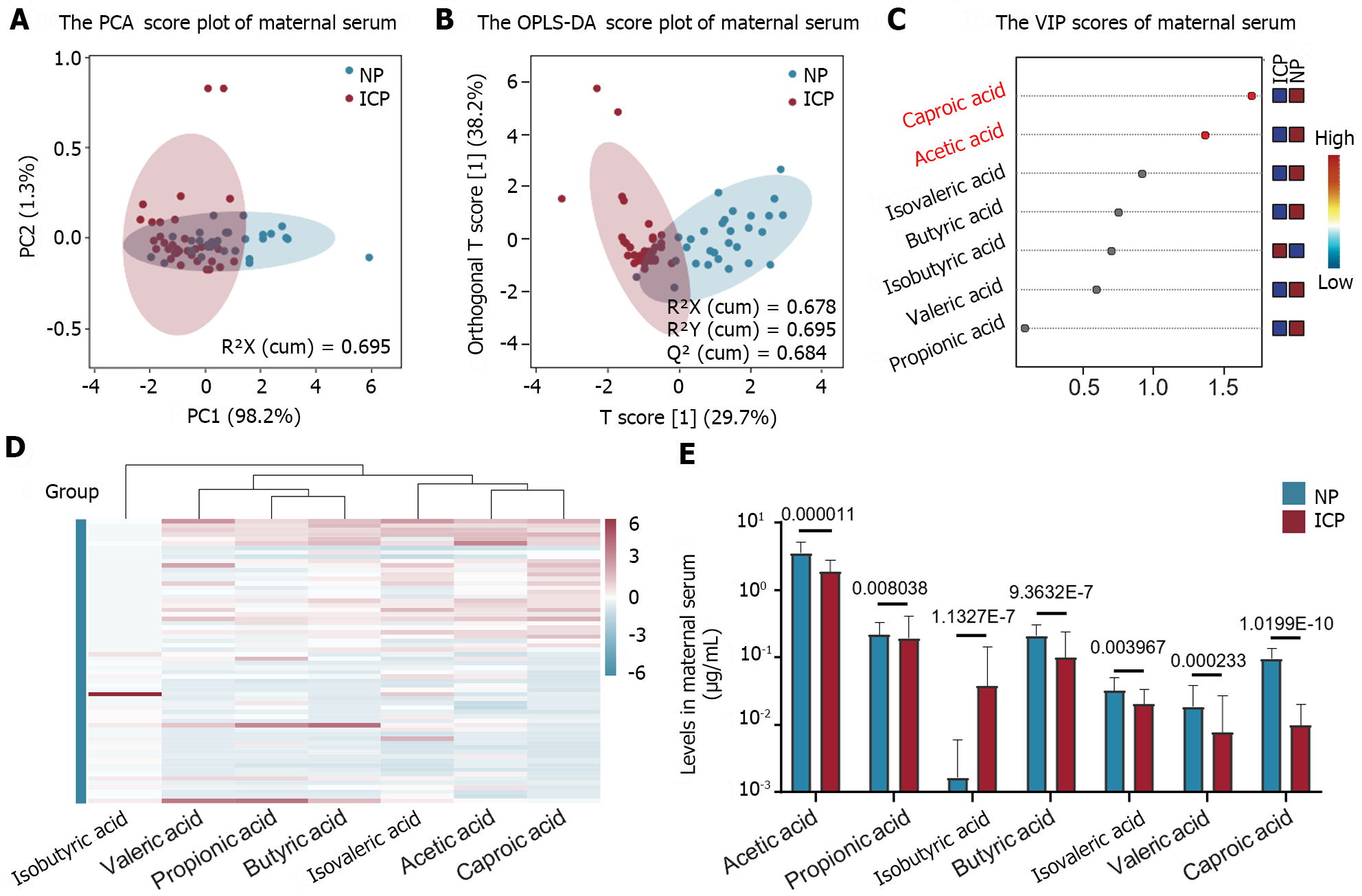
The metabolic profile of maternal serum SCFAs in the ICP and NP groups exhibited significant differences, as indicated by the results of targeted metabolomics quantitative analysis (Figure 1A and B). In the serum of the ICP group, all SCFAs, except for isobutyric acid, demonstrated a decreasing trend (Figure 1C-E). Notably, acetic acid and caproic acid were identified as key metabolites responsible for the differences in serum SCFAs between the two groups, each with a VIP value greater than 1 (Figure 1C). Quantitative analysis further confirmed that acetic acid represented the most abundant SCFA in both the ICP and NP groups. Furthermore, isobutyric acid displayed a notable difference in serum content between the two groups, with significantly higher levels in the ICP group than the NP group, while being present at very low levels in the NP group (Figure 1E).
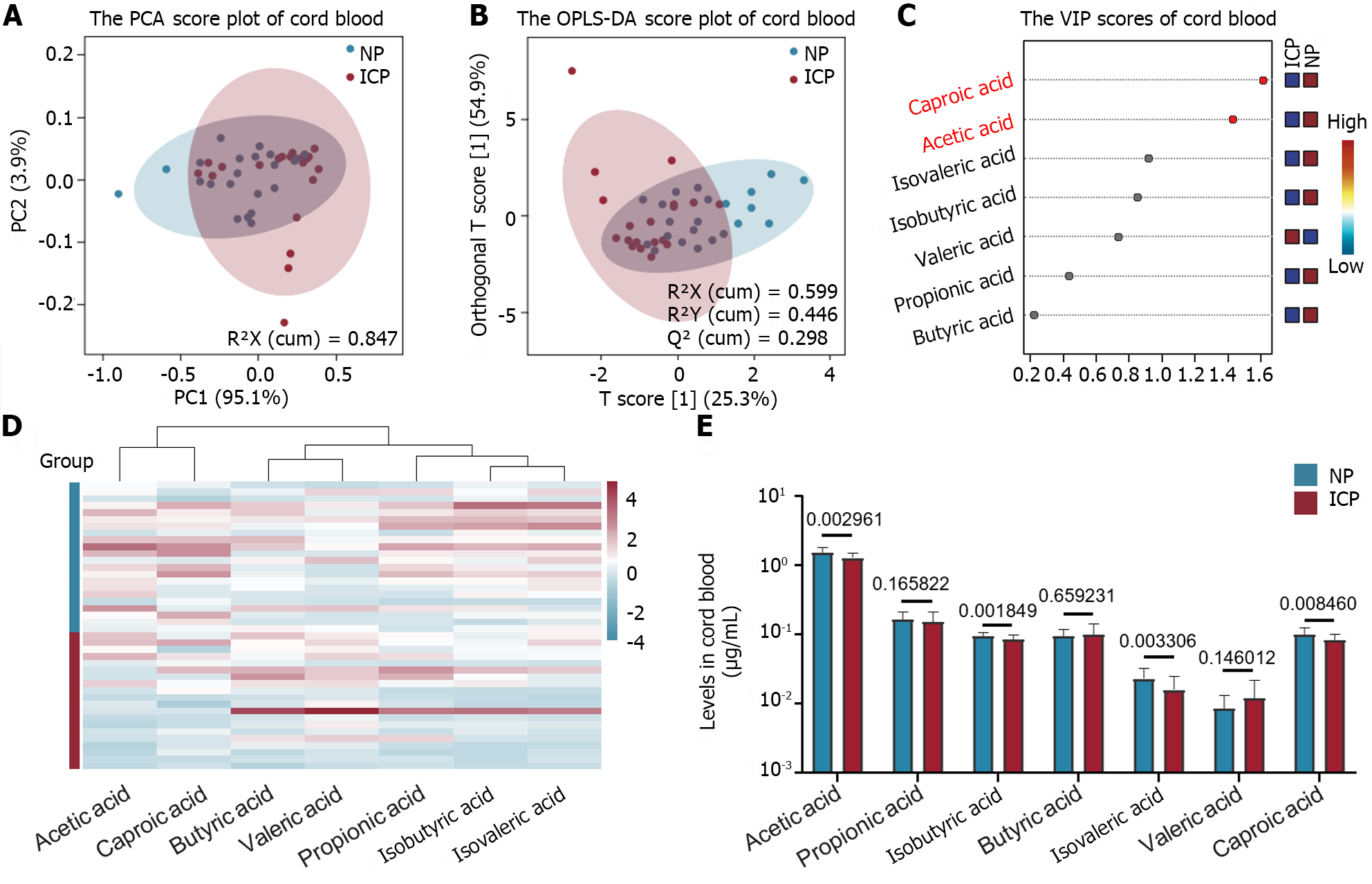
The metabolic profile of SCFAs in cord blood was investigated in both ICP and NP groups. Targeted metabolomics analysis revealed that there were no significant differences in the metabolic profiles of SCFAs between the two groups (Figure 2A and B). However, the VIP diagram indicated that acetic acid and caproic acid were key metabolites contributing to the differences in SCFAs between the two groups, each with a VIP value greater than 1 (Figure 2C). Most SCFAs exhibited a decreasing trend in the ICP group compared with the NP group (Figure 2D and E). Among the seven SCFAs detected, the acetic acid level was the highest in cord blood, and its level was significantly lower in the ICP group than in the NP group (P < 0.01, Figure 2E). Additionally, the isobutyric acid levels in cord blood showed an opposite trend to that in maternal serum, with a significant decrease observed in the ICP group compared with that in the NP group (P < 0.01, Figure 2E).
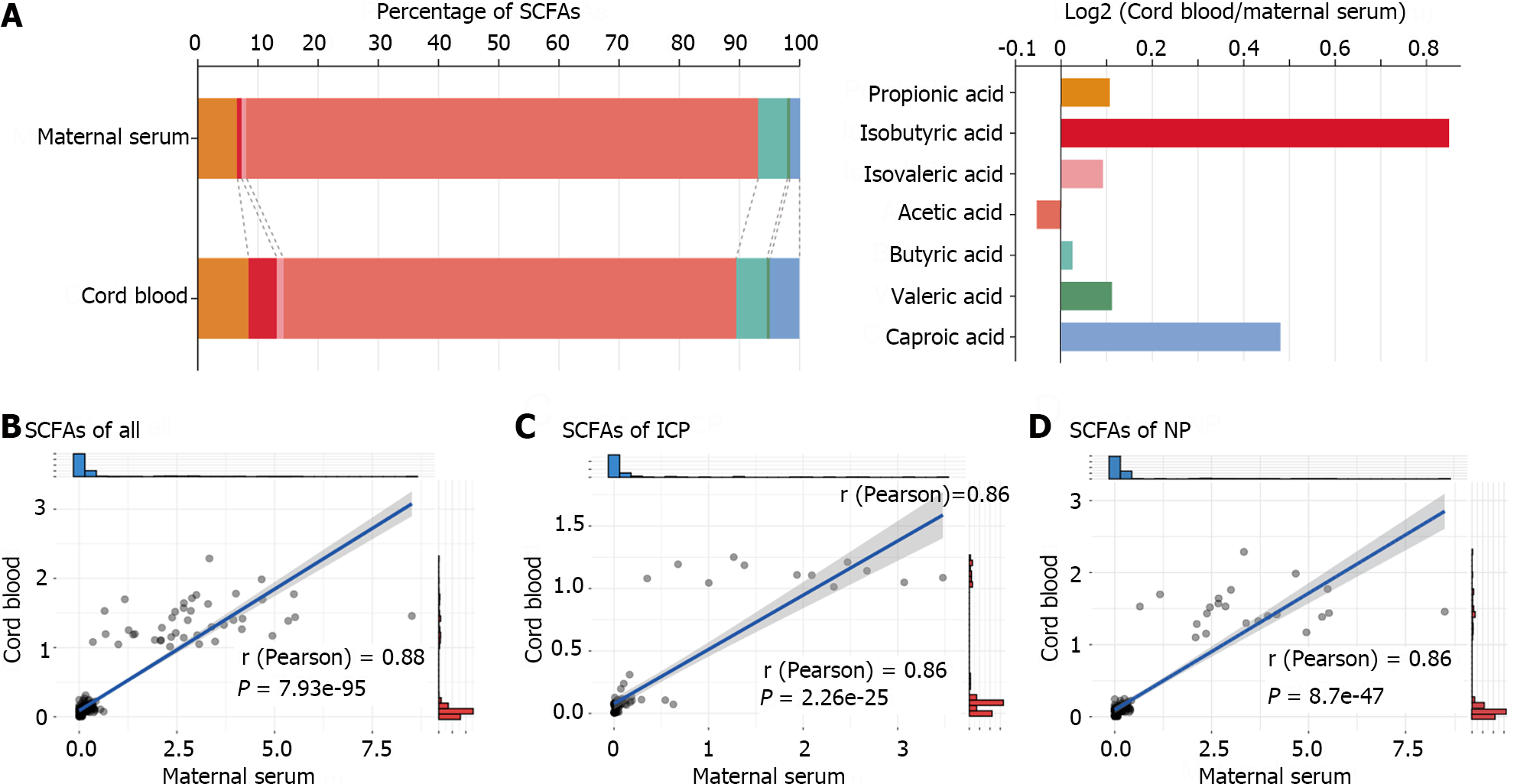
Further analysis was conducted to explore the correlation between SCFAs in maternal blood and SCFAs in cord blood. The stacked bar chart depicting the percentage of SCFAs in the blood samples of the subjects (Figure 3A) highlighted that the isobutyric acid and caproic acid levels in the total SCFAs of maternal serum were exceptionally low but significantly increased in cord blood SCFAs. Notably, the acetic acid levels were the highest in both maternal serum and cord blood. However, the acetic acid levels were notably lower in cord blood than in maternal serum (75.18% vs 84.99%).
The scatter plot of the linear regression (Figure 3B-D) indicated a robust positive correlation between the total SCFAs in cord blood of the ICP and NP groups and the SCFAs in maternal serum (P < 0.001). These findings suggest a close correlation between SCFAs in cord blood and maternal serum. However, among all subjects, only acetic acid and caproic acid exhibited a significant positive correlation for an individual SCFA (P < 0.05, Table 2).
| ALL | NP | ICP | ||||
| r (Pearson) | P value | r (Pearson) | P value | r (Pearson) | P value | |
| Acetic acid | 0.33 | 0.03 | 0.03 | 0.90 | -0.24 | 0.45 |
| Propionic acid | 9.42E-04 | 1.00 | 0.02 | 0.93 | -0.12 | 0.70 |
| Isobutyric acid | -0.21 | 0.19 | 0.21 | 0.35 | -0.17 | 0.61 |
| Butyric acid | 0.11 | 0.48 | 0.05 | 0.82 | 0.35 | 0.26 |
| Isovaleric acid | 0.05 | 0.76 | -0.12 | 0.59 | 0.04 | 0.89 |
| Valeric acid | -0.17 | 0.29 | -0.23 | 0.30 | -0.03 | 0.92 |
| Caproic acid | 0.38 | 0.01 | 0.22 | 0.33 | 0.18 | 0.58 |
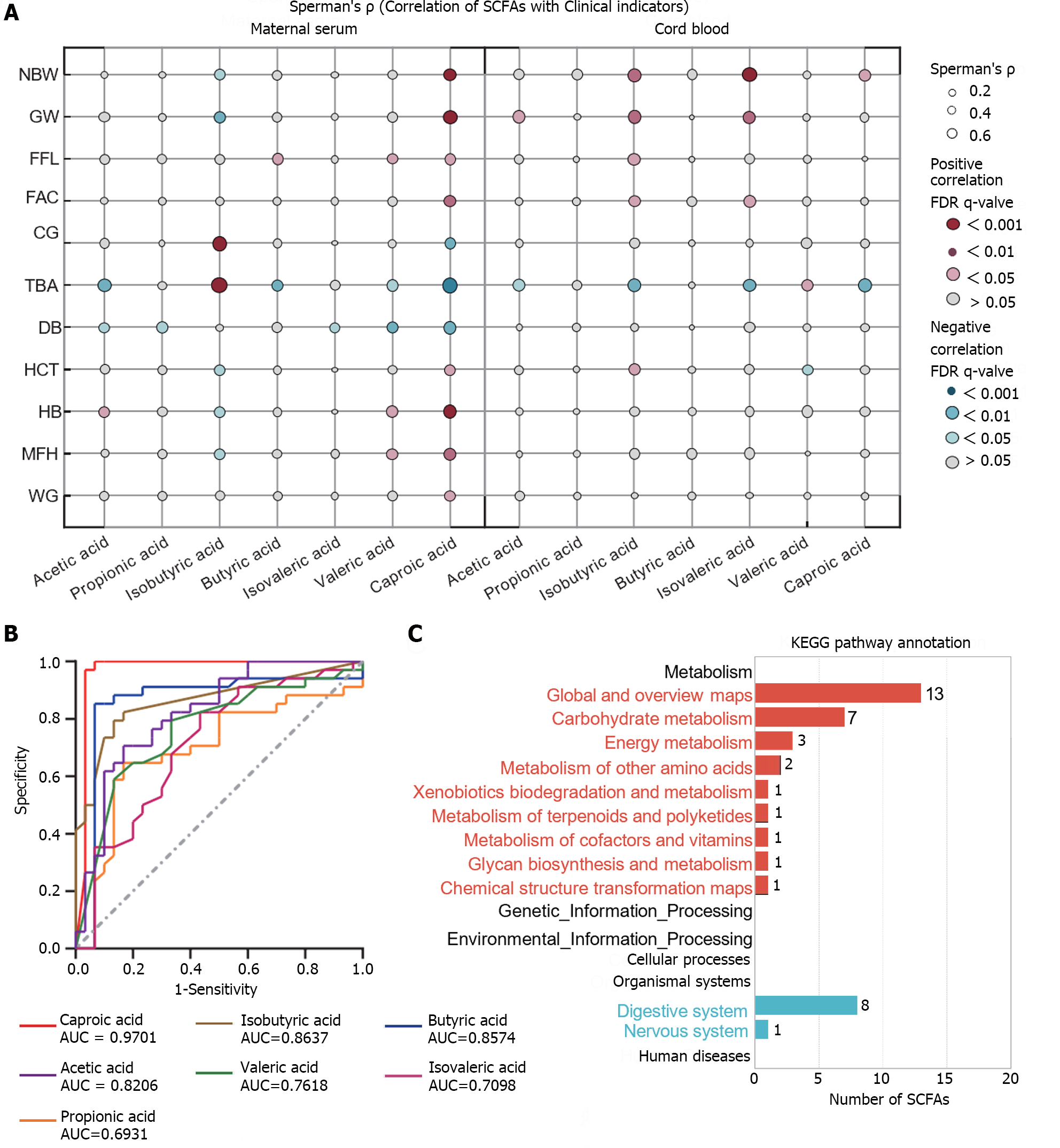
To further explore the correlation between SCFAs and clinical indicators, SCFAs in maternal serum and cord blood from the two subject groups with statistically different clinical indicators were analyzed (Spearman analysis, Figure 4A). The results revealed that the correlation between acetic acid and caproic acid in maternal serum and cord blood exhibited a consistent trend with clinical indicators. For instance, both of these SCFAs displayed a negative correlation with total bile acid levels (P < 0.05), and caproic acid demonstrated a significant positive correlation with neonatal birth weight (P < 0.001). However, isobutyric acid showed a significant positive correlation with total bile acids in maternal serum but exhibited an opposite trend in cord blood (P < 0.05). ROC curve analysis showed that multiple SCFAs in maternal serum possessed excellent diagnostic capabilities for ICP (area under the curve > 0.8), with caproic acid demonstrating the highest diagnostic efficacy (area under the curve = 0.97, Figure 4B). To gain insights into the function of SCFAs, a KEGG pathway enrichment analysis was conducted. It was observed that SCFAs participate in 24 pathways, primarily involving metabolic and biological pathways, such as carbohydrate metabolism, energy metabolism, the digestive system, and the nervous system (Supplementary Table 5, Figure 4C). Among these pathways, the digestion and absorption of proteins in the digestive system were predominantly influenced by SCFAs. Acetic acid was involved in most pathway metabolisms, isobutyric acid participated in four of them, while caproic acid was not linked to the mentioned pathways. For detailed information, please refer to Supplementary Table 5, which may provide valuable insights into the metabolism and digestive status of the ICP group.
SCFAs are the byproducts of dietary fiber fermentation in the gut microbiota under anaerobic conditions. Different gut microbiota can produce varying SCFAs, and the top three SCFAs in the human body are acetic acid, propionic acid, and butyric acid[8]. Prior studies have indicated that, during pregnancy, the total SCFAs in maternal peripheral blood circulation decrease significantly compared with those in non-pregnancy, with reductions in acetic acid and propionic acid levels, while butyric acid levels increase[9]. As pregnancy progresses, the level of butyric acid in peripheral blood circulation increases, while the acetic acid, propionic acid, and isobutyric acid levels remain relatively stable[10]. SCFAs are associated with various pregnancy complications and can impact fetal growth and development in the womb[11-13]. A recent report in Science Advances found that SCFAs produced by the maternal microbiome play a role in supporting normal placental development, and their absence can limit placental growth and damage placental vascularization[14]. However, the alterations in SCFAs in the maternal blood of patients with ICP and their influence on fetal health remain unclear.
This study uncovered that both ICP and NP groups exhibited significantly higher acetic acid, propionic acid, and butyric acid levels in maternal serum SCFAs than other SCFAs. In umbilical cord blood SCFAs, acetic and propionic acids were the most abundant, with similar butyric acid, isobutyric acid, and caproic acid levels, while the isovaleric acid and valeric acid levels were the lowest. These changes may be linked to placental transport functions. Correlation analysis revealed that both acetic acid and caproic acid in maternal and cord blood were negatively correlated with total bile acids in peripheral blood. ROC analysis indicated that multiple SCFAs in maternal blood exhibited good diagnostic capabilities for ICP, with caproic acid demonstrating the highest diagnostic efficacy. KEGG pathway analysis suggested that acetic acid is involved in most pathway metabolisms, which may be related to the metabolism in patients with ICP.
Acetic acid is the most abundant SCFA in the human body and is produced by species in the Bacteroidetes phylum, one of the most abundant microbial groups in the intestine[13]. Acetic acid can promote the recruitment of immune cells in the intestine, thereby regulating intestinal inflammation[15]. Moreover, acetic acid has an inhibitory effect on liver adipogenesis, reducing lipid aggregation in adipose tissue[16]. Research has shown that when acetic acid is added only to the drinking water of pregnant mice during pregnancy, their offspring are immune to induced allergic airway disease[13], suggesting that acetic acid can traverse the placenta and have an impact on the fetus. This study discovered that compared with the NP group, acetic acid in the maternal serum and cord blood of the ICP group exhibited a significant decline and was significantly negatively correlated with total bile acids in maternal circulation. These results suggest that acetic acid plays a protective role in the progression of ICP, although the precise mechanisms in intrahepatic cholestasis and ICP require further investigation.
Caproic acid, as a putrefactive SCFA, results from the fermentation of amino acids or proteins that are not digested or absorbed in the small intestine, leading to the production of protein breakdown products[17]. Animal experiments have demonstrated that supplementation of exogenous caproic acid can increase phospholipid metabolism in the mother and enhance progesterone synthesis in the ovaries, potentially improving the embryonic survival rate in early pregnancy[18]. Interestingly, obese pregnant women have been observed to have lower caproic acid levels in their feces than normal-weight pregnant women[19]. In the context of this study, both maternal serum and cord blood caproic acid levels displayed a decreasing trend in patients with ICP, and maternal serum caproic acid levels exhibited a positive correlation with weight gain during pregnancy, which might be related to metabolic adaptation. Previous research has indicated that caproic acid can inhibit NF-κB transactivation and possess anti-inflammatory effects[20]. However, further exploration is necessary to determine whether it exerts anti-inflammatory and protective effects on patients with ICP and their fetuses.
In contrast to other SCFAs, this study identified a significant increase in isobutyric acid in the maternal serum of patients with ICP. Your previous research has also noted that the level of isobutyric acid in the peripheral blood circulation of pregnant women with pregnancy complications, such as pre-eclampsia and gestational diabetes, significantly increased compared with the NP group, aligning with the findings of this study[5,21]. This suggests that isobutyric acid plays a pivotal role in promoting the progression of pregnancy complications. Serino[22] has commented that SCFAs produced by gut microbiota are linked to host health. While it is generally believed that SCFAs have a more significant positive impact on host metabolism than harm, in specific situations, excessive production of SCFAs can have detrimental effects on the host, making it challenging to definitively classify SCFAs as beneficial or harmful to the host. The results of this study imply that elevated isobutyric acid levels in maternal blood circulation not only lead to pathological changes in the mother but also impact the growth and development of the fetus in the uterus.
This study conducted an analysis of SCFAs in both maternal serum and umbilical cord blood from the ICP and NP groups, elucidating the trends of SCFA changes in the two sample types. It was observed that a significant positive correlation exists between maternal serum SCFAs and umbilical cord blood SCFAs, distinguishing it from most existing studies that concentrate on fecal or human serum samples. The comprehensive analysis of changes in SCFAs in patients with ICP provides valuable insights. However, this study has certain limitations. Firstly, the sample size was relatively small, and samples were collected from a single hospital within the same period, which may affect the robustness of the results. Expanding the clinical sample size and collecting samples from other regions in synchrony would enhance the reliability of the data. Secondly, SCFAs, being closely related to factors such as place of residence, lifestyle, diet, and medication, were not fully accounted for in the subjects' information, potentially introducing bias into the results. Thirdly, the effects and mechanisms of SCFAs, especially acetic acid, caproic acid, and isobutyric acid, on patients with ICP and their fetuses have not been comprehensively explored. Further in vivo and in vitro experiments are required to elucidate their mechanisms of action, providing a solid theoretical foundation for SCFAs as diagnostic and therapeutic targets for ICP.
Intrahepatic cholestasis of pregnancy (ICP) is a liver condition specific to pregnancy. Short-chain fatty acids (SCFAs), important metabolites produced by the gut microbiota, are significantly linked to several pregnancy complications.
However, the metabolic profile of SCFAs in patients with ICP is still uncertain.
The study aimed to examine the correlation between maternal and umbilical blood SCFAs and investigate the changes in SCFAs in patients with ICP and their offspring. Additionally, the research sought to assess the clinical significance and diagnostic value of these SCFAs. Ultimately, the study aimed to identify novel biomarkers for diagnosing and treating ICP by focusing on gut microbiota metabolites.
Therefore, in this study, we utilized targeted metabolomics technology to analyze SCFAs in the maternal and cord blood of patients with ICP and normal pregnant (NP) women.
The study revealed that maternal serum SCFAs in both the ICP and NP groups showed significantly higher levels of acetic acid, propionic acid, and butyric acid compared to other SCFAs. In umbilical cord blood, acetic and propionic acids were found to be the most abundant, with similar levels of butyric acid, isobutyric acid, and caproic acid, while isovaleric acid and valeric acid levels were the lowest. Furthermore, the correlation analysis indicated a negative correlation between both acetic acid and caproic acid in maternal and cord blood, and total bile acids in peripheral blood.
Significant alterations were observed in the SCFAs of maternal serum and cord blood in the ICP group, compared with the NP group. It is notable that the SCFA levels in maternal serum and cord blood were significantly positively correlated in the ICP group. Additionally, certain maternal serum SCFAs, specifically caproic and acetic acids, exhibited excellent diagnostic efficiency for ICP.
Additional in vivo and in vitro experiments are needed to clarify the mechanisms of action of SCFAs, establishing a strong theoretical basis for their use as diagnostic and therapeutic targets for ICP.
| 1. | Hobson S, Gandhi S, Sobel M. Intrahepatic cholestasis of pregnancy. CMAJ. 2022;194:E1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 2. | Terrault NA, Williamson C. Pregnancy-Associated Liver Diseases. Gastroenterology. 2022;163:97-117.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Tang B, Tang L, Li S, Liu S, He J, Li P, Wang S, Yang M, Zhang L, Lei Y, Tu D, Tang X, Hu H, Ouyang Q, Chen X, Yang S. Gut microbiota alters host bile acid metabolism to contribute to intrahepatic cholestasis of pregnancy. Nat Commun. 2023;14:1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 4. | Kim CH. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol. 2023;20:341-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 233] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 5. | Chen S, Li J, Ren S, Gao Y, Zhou Y, Xuan R. Expression and clinical significance of short-chain fatty acids in pregnancy complications. Front Cell Infect Microbiol. 2022;12:1071029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 6. | Yong W, Zhao Y, Jiang X, Li P. Sodium butyrate alleviates pre-eclampsia in pregnant rats by improving the gut microbiota and short-chain fatty acid metabolites production. J Appl Microbiol. 2022;132:1370-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Obstetrics Subgroup; Chinese Society of Obstetrics. Guidelines for the management of intrahepatic cholestasis of pregnancy (2015). Zhonghua Fuchanke Zazhi. 2015;50:481-485. [DOI] [Full Text] |
| 8. | Ziętek M, Celewicz Z, Szczuko M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 9. | Luo H, Li W, Wu L, Zhong S, Du C, Liu Y, Xu Y, Huang X, Bahru AH, Tang X, Zhou J, Wang D, Lou X, Bin X, Xiao X. Differences in cognition, short-chain fatty acids and related metabolites in pregnant vs non-pregnant women: a cross-sectional study. BMC Pregnancy Childbirth. 2022;22:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Hernández-Martínez C, Canals J, Voltas N, Martín-Luján F, Arija V. Circulating Levels of Short-Chain Fatty Acids during Pregnancy and Infant Neurodevelopment. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Yu L, Zhong X, He Y, Shi Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol Res. 2020;160:105082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Liu X, Li X, Xia B, Jin X, Zou Q, Zeng Z, Zhao W, Yan S, Li L, Yuan S, Zhao S, Dai X, Yin F, Cadenas E, Liu RH, Zhao B, Hou M, Liu Z, Liu X. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021;33:923-938.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 13. | van der Hee B, Wells JM. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021;29:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 660] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 14. | Pronovost GN, Yu KB, Coley-O'Rourke EJL, Telang SS, Chen AS, Vuong HE, Williams DW, Chandra A, Rendon TK, Paramo J, Kim RH, Hsiao EY. The maternal microbiome promotes placental development in mice. Sci Adv. 2023;9:eadk1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Ikeda T, Nishida A, Yamano M, Kimura I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol Ther. 2022;239:108273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 152] [Reference Citation Analysis (0)] |
| 16. | Mazhar M, Zhu Y, Qin L. The Interplay of Dietary Fibers and Intestinal Microbiota Affects Type 2 Diabetes by Generating Short-Chain Fatty Acids. Foods. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 17. | Xiong J, Liao XS, Yin T, Liu XC, Bao L, Li LQ. Alterations of the gut microbiota and short chain fatty acids in necrotizing enterocolitis and food protein-induced allergic protocolitis infants: A prospective cohort study. Front Cell Infect Microbiol. 2022;12:1030588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Ye Q, Cai S, Wang S, Zeng X, Ye C, Chen M, Qiao S. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J Nutr Biochem. 2019;69:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Szczuko M, Kikut J, Maciejewska D, Kulpa D, Celewicz Z, Ziętek M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Tayyeb JZ, Popeijus HE, Mensink RP, Konings MCJM, Mokhtar FBA, Plat J. Short-Chain Fatty Acids (Except Hexanoic Acid) Lower NF-kB Transactivation, Which Rescues Inflammation-Induced Decreased Apolipoprotein A-I Transcription in HepG2 Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Li J, Wang L, Chen H, Yang Z, Chen S, Wang J, Zhou Y, Xuan R. The Diagnostic Potential of Gut Microbiota-Derived Short-Chain Fatty Acids in Preeclampsia. Front Pediatr. 2022;10:878924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Serino M. SCFAs - the thin microbial metabolic line between good and bad. Nat Rev Endocrinol. 2019;15:318-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Popovic DD, Serbia S-Editor: Gong ZM L-Editor: A P-Editor: Cai YX