Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.477
Peer-review started: November 12, 2023
First decision: December 7, 2023
Revised: January 3, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 27, 2024
Processing time: 135 Days and 22.5 Hours
The neutrophil-to-lymphocyte ratio (NLR) is commonly utilized as a prognostic indicator in end-stage liver disease (ESLD), encompassing conditions like liver failure and decompensated cirrhosis. Nevertheless, some studies have contested the prognostic value of NLR in ESLD.
To investigate the ability of NLR to predict ESLD.
Databases, such as Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Weipu, and Wanfang, were comprehensively searched to identify studies published before October 2022 assessing the pro
A total of thirty studies involving patients with end-stage liver disease (ESLD) were included in the evaluation. Among the pooled results of eight studies, it was observed that the Neutrophil-to-Lymphocyte Ratio (NLR) was significantly higher in non-survivors compared to survivors (random-effects model: standar
Increased NLR in patients with ESLD is associated with a higher risk of mortality, particularly in Asian patients. NLR is a useful prognostic biomarker in patients with ESLD.
Core Tip: This meta-analysis examines the association between neutrophil-to-lymphocyte ratio (NLR) and mortality in patients with end-stage liver disease (ESLD). It finds that elevated NLR is correlated with higher risk of death. Specifically, NLR levels were higher in non-survivors than survivors, and high NLR predicted increased mortality risk as indicated by univariate and multivariate hazards ratios and odds ratios. Moreover, NLR had stronger prognostic value in Asian populations, suggesting it may be a useful biomarker for identifying high-risk ESLD patients, particularly in Asia.
- Citation: Cai XH, Tang YM, Chen SR, Pang JH, Chong YT, Cao H, Li XH. Prognostic value of neutrophil-to-lymphocyte ratio in end-stage liver disease: A meta-analysis. World J Hepatol 2024; 16(3): 477-489
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/477.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.477
End-stage liver disease (ESLD) is defined as the final stage of liver disease caused by various factors. Globally, cirrhosis and liver cancer are ranked as the eleventh and sixteenth leading causes of death, respectively, accounting for 3.5% of all deaths each year worldwide[1]. The burden of ESLD is expected to increase in the future[2]. Because liver transplantation remains the only curative treatment for ESLD, it is crucial to identify predictors of ESLD prognosis to differentiate between patients who require immediate transplantation and those who can be managed with intensive medical care for a longer period.
The neutrophil-to-lymphocyte ratio (NLR) is a readily measurable parameter that has been shown to reflect disease severity[3]. NLR has been widely used as a biomarker for prognostic evaluation of patients with various diseases and has diagnostic value in distinguishing among certain conditions[4]. For example, NLR has shown promise in predicting poor prognosis in cancer patients[4]. Because Kupffer cells and inflammatory cells, such as macrophages, T lymphocytes, neutrophils, and dendritic cells, have been found to contribute to liver inflammation and fibrosis in patients with liver disease[5], NLR is often utilized as a prognostic factor in these patients. NLR has also been associated with prognosis in patients with hepatocellular carcinoma, suggesting its potential as a prognostic indicator after liver transplantation[6,7]. Moreover, NLR has been used to predict the prognosis of patients with other liver diseases, such as acute-on-chronic liver failure (ACLF) and decompensated liver cirrhosis (DC)[8-10], although the prognostic value of NLR in patients with ACLF and DC remains unclear. Most studies indicate that NLR is linked to poor prognosis in patients with ACLF or DC, although other studies have reported no association[11]. Most of these studies, however, focused solely on patients with ACLF or DC, with few examining whether NLR is a prognostic factor for ESLD, the broader condition.
The objective of this systematic review and meta-analysis was to thoroughly assess the correlation between NLR and prognosis in patients with ESLD. The aim was to identify a reliable and easily measurable parameter that could help identify patients in need of immediate liver transplantation.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2009 statement guidelines[12] were followed to report the results of this systematic review. The protocol was registered in the Prospective Register of Systematic Review [CRD42022367423].
The databases OVID Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Weipu, and Wanfang were systematically searched for studies on the associations of NLR with ESLD published from 1 January 1980 to 30 October 2022 in English or Chinese. Search terms included “end-stage liver disease”tOR “liver cirrhosis”rOR “hepatic cirrhosis”rOR “liver fibrosis”bOR “liver failure”iOR “hepatic failure”iOR “liver trans
Studies were selected if they were (1) observational studies, including cross-sectional, cohort, and case-control studies; (2) included adults aged ≥18 years; (3) involved patients who were diagnosed with ESLD; and (4) measured NLR in both survivors and non-survivors or reported a hazard ratio (HR) or odds ratio (OR) reflecting the association between NLR and mortality. Conference abstracts, case reports, systematic reviews, dissertations, expert opinions, and editorials or commentaries were excluded, as were studies that included fewer than 100 participants and studied published in Chinese journals limited to the Chinese Scientific and Technical Papers and Citation Database, the Chinese Science Citation Database, and the Chinese core journal criterion of Peking University. If multiple studies involved the same dataset, the study with the larger number of participants was included. After removing duplicates, two authors (CXH and TYM) independently reviewed the titles and abstracts to remove irrelevant studies. The full texts of the remaining studies were examined with a record of reasons for exclusion. A third author (LXH) resolved disagreements when necessary.
ESLD was defined as chronic or acute-on-chronic liver failure according to the standard criteria of the Asian Pacific Association for the Study of the Liver (APASL)[13] or the European Association for the Study of the Liver[14]. Included were patients with liver cirrhosis who were diagnosed pathologically or by clear ultrasound with at least an index clinical complication of decompensation and candidates for liver transplantation due to liver failure or cirrhosis. Patients aged < 18 years and patients with acute liver failure or other terminal diseases were excluded.
Data were extracted from included articles using a standardized form in Microsoft Excel. Data extracted from these studies included the name of the first author; the year of publication; the location of the study; the number of patients analyzed, as well as their sex and mean or median age; the etiology of ESLD; the mean or median NLR and NLR cutoff value; the primary outcome of the study; and univariate and/or multivariate HRs or ORs, along with their associated 95% confidence intervals (CIs). Two authors (CXH and TYM) independently extracted these daga, with disagreements resolved by consensus.
Two authors (CXH and TYM) independently assessed the quality of each study using the Newcastle-Ottawa Scale. This tool consists of three items, selection, comparability and outcome/exposure, which included four, two, and three sub-items, respectively, to which star-based scores were assigned. Studies with scores ≥ 6 were considered high-quality studies, those with scores of 4-5 were regarded as having a moderate risk of bias, and those with scores < 4 were regarded as having a high risk of bias.
Statistical analyses were performed using Stata 15.1 (Stata Corp, College Station, TX, United States) and Comprehensive Meta-analysis software (2.0). The main pooled outcomes were the HRs or ORs with their 95%CIs of the associations between NLR and ESLD. HRs and ORs were analyzed separately, as were univariate and multivariate HRs and ORs.
The heterogeneity of the studies was assessed using I2 statistics, with I2 values of 25%, 50%, 75%, and ≥ 75% indicating low, moderate, high, and very high heterogeneity, respectively[15]. If heterogeneity was high or very high, a random-effects model was used. Study heterogeneity and some potential moderators were explored using subgroup analyses and meta-regression. These variables included the mean age of the patients (categorized as < 50 or > 50 years), location (categorized as Asia or non-Asian regions), etiology, and duration of follow-up. Publication bias was assessed by visual inspection of funnel plots, and by Begg’egand Eggersed ons). When necessary, trim-and-fill analyses and sensitivity analyses were performed.
All statistical tests were two-sided, with the level of significance set at P < 0.05.
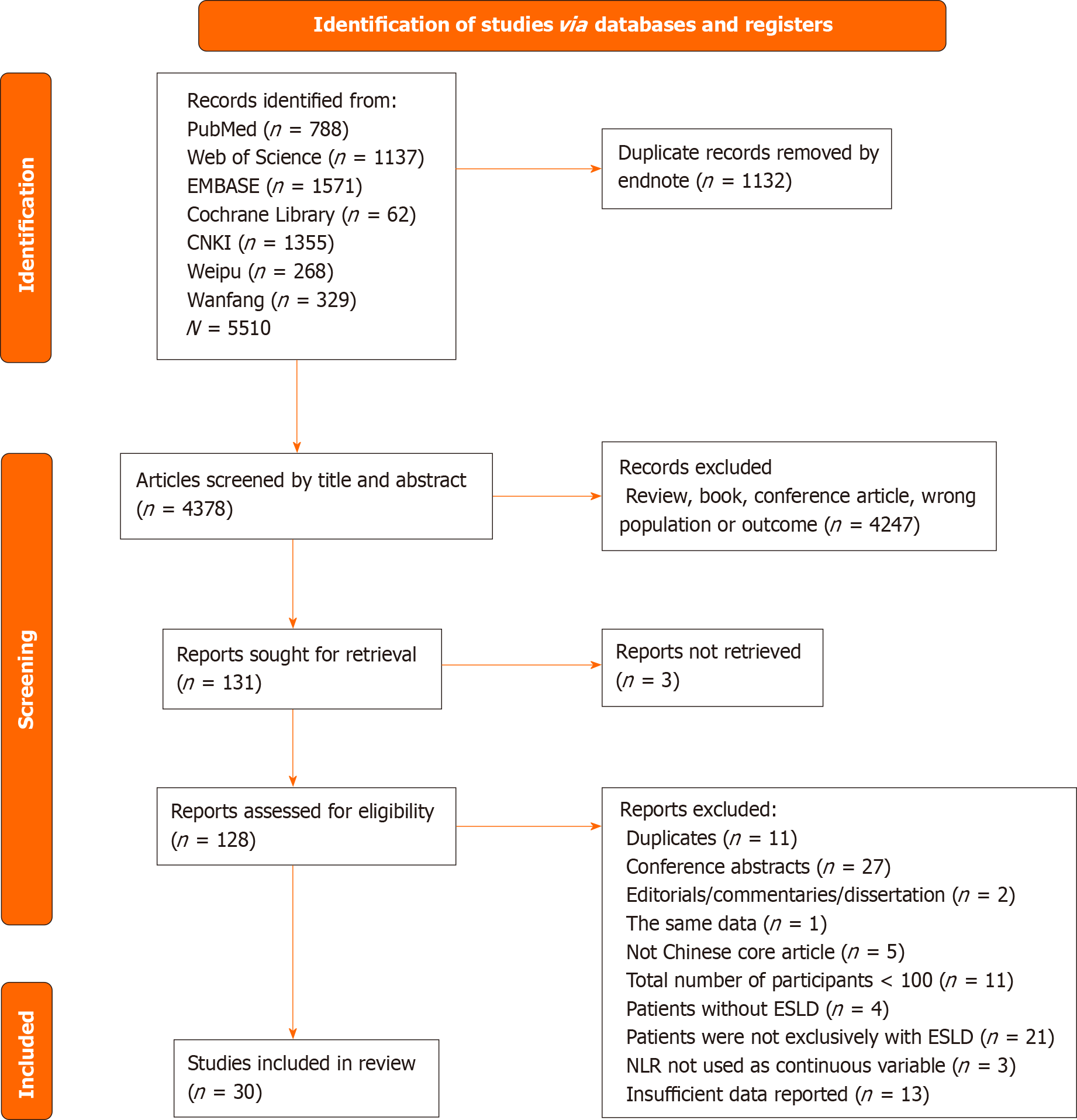
A search of the databases yielded 5510 studies. Analysis using EndNote Version 9.0 software found that 1132 of these studies were duplicates. The remaining 4378 studies were screened by reading their titles and abstracts, resulting in the removal of 4247 studies. A full-text review of the remaining 131 studies resulted in the inclusion of 30 of these studies. The literature search strategy is described in a PRISMA flow diagram (Figure 1).
The 30 studies consisted of 21 published in English and nine published in Chinese. Table 1 shows the main characteristics of the included studies.
| Ref. | Year | Location | Population | Patient number (male) | Mean age | Outcome | Etiology | NLR cutoff value | Analysis | NOS scores |
| Agiasotelli et al[8] | 2016 | Greece | ACLF patients | 108 (80) | 60.5 | 30-d & 180-d mortality | Mixed | NR | HR (Univariate & Multivariate) | 8 |
| Bernsmeier et al[27] | 2020 | Britain | DCC & ACLF patients | 617 (386) | NR | 90-d mortality | Mixed | 30 | OR (Univariate & Multivariate) | 8 |
| Cai et al[28] | 2018 | China | ACLF patients | 203 (151) | 51.14 | 90-d mortality | HBV | 5.09 | HR (Univariate & Multivariate) | 8 |
| Cai et al[18] | 2017 | China | ACLF patients | 637 (486) | 54 | 6-month, 1-yr & 3-yr mortality | Mixed | 5.7 | HR (Multivariate) | 8 |
| Chiriac et al[29] | 2020 | Romania | ACLF patients | 70 (49) | 62 | In-hospital mortality | Mixed | 5 | NR | 7 |
| Fan et al[30] | 2017 | China | ACLF patients | 560 (487) | 44.9 | 30-d mortality | HBV | NR | OR (Multivariate) | 8 |
| Gao et al[31] | 2017 | China | ACLF patients | 573 (478) | 43.5 | 90-d mortality | HBV | NR | HR (Univariate & Multivariate) | 8 |
| Guan et al[32] | 2019 | China | ACLF patients | 174 (135) | 49.60 | Mortality | HBV | 6.5 | OR (Univariate) | 6 |
| Li et al[33] | 2022 | China | LC patients with UGIB | 376 (235) | 60.25 | 1-yr mortality | Mixed | 3.76 | OR (Univariate) | 7 |
| Li et al[10] | 2020 | China | DCC patients | 174 (139) | 53.6 | 28-d mortality | HBV | 3.78 | HR (Univariate & Multivariate) | 8 |
| Liang et al[34] | 2020 | China | ACLF patients | 227 (202) | 46.4 | 90-d mortality | HBV | 5.38 | HR (Univariate) | 6 |
| Lin et al[35] | 2018 | China | DCC patients | 235 (133) | 60 | 30-d mortality | Mixed | NR | HR (Multivariate) | 9 |
| Liu et al[36] | 2014 | China | ACLF patients | 216 (183) | 45.58 | 8-wk mortality | HBV | 6.12 | NR | 8 |
| Liu et al[37] | 2021 | China | ACLF patients | 160 (145) | 46.1 | 28-d mortality | HBV | 4.5 | OR (Univariate) | 7 |
| Maccali et al[38] | 2021 | Brazil | DCC patients | 320 (235) | 55.67 | 90-d mortality | Mixed | NR | HR (Univariate & Multivariate) | 8 |
| Moreau et al[39] | 2018 | Belgium | ACLF patients | 105 (72) | 58 | 90-d mortality | Mixed | 6.2 | HR (Univariate & Multivariate) | 7 |
| Oikonomou et al[11] | 2020 | Greece | DCC patients | 132 (NR) | NR | 10-month mortality | Mixed | NR | HR (Univariate) | 7 |
| Qi et al[26] | 2021 | China | DCC patients | 144 (115) | 54.0 | 30-d mortality | HBV | 3.78 | OR (Univariate & Multivariate) | 8 |
| Qiang et al[40] | 2021 | China | ACLF patients | 577 (494) | 48.20 | 90-d mortality | HBV | 4.09 | HR (Univariate & Multivariate) | 7 |
| Shi et al[41] | 2022 | China | LC patients with HE | 402 (323) | 52 | 30-d mortality | HBV | 4 | HR (Univariate & Multivariate) | 7 |
| Sun et al[9] | 2021 | China | ACLF patients | 412 (351) | NR | 28-d & 90-d mortality | HBV | 4.79 | OR (Univariate & Multivariate) | 9 |
| Sun et al[42] | 2021 | China | ACLF patients | 290 (252) | 44 | 90-d mortality | HBV | 4.78 | HR (Univariate & Multivariate) | 9 |
| Wang et al[43] | 2019 | China | ACLF patients | 270 (228) | 46.56 | 90-d mortality | HBV | NR | OR (Univariate) | 6 |
| Wang et al[44] | 2020 | China | ACLF patients | 102 (75) | 42.9 | 90-d mortality | HBV | 4.22 | OR (Univariate) | 6 |
| Wu et al[45] | 2018 | China | ACLF patients | 100 (89) | 47.3 | 28-d mortality | HBV | NR | NR | 6 |
| Xue et al[46] | 2021 | China | LC patients with HE | 116 (74) | 60 | 30-d mortality | Mixed | 4.4 | OR (Univariate) | 6 |
| Zhang et al[47] | 2016 | China | DCC patients | 148 (118) | 53.2 | 30-d mortality | HBV | 5 | HR (Univariate & Multivariate) | 7 |
| Zhang et al[48] | 2018 | China | ACLF patients | 133 (108) | 44.9 | 90-d mortality | HBV | 2.06 | OR (Univariate) | 5 |
| Zhang et al[49] | 2022 | United State | DCC patients | 264 (122) | 58.31 | 30-d & 90-d mortality | Mixed | 10.6 | HR (Univariate & Multivariate) | 9 |
| Zhou et al[50] | 2022 | China | LC patients with acute UGIB | 676 (398) | 62.29 | 6-wk mortality | Mixed | 5.04 | OR (Univariate) | 9 |
All studies were published after 2014, with the largest number, seven, published in 2021. The studies included were from three continents, with the largest number, 22, from Asia. Sixteen studies included patients with ACLF, 13 included patients with acute decompensation (AD), and one included patients with both ACLF and AD. Eighteen studies analyzed patients with hepatitis B virus (HBV)-related ESLD. The mean quality assessment score of the 30 studies was 7.4 (range: 5–9).
Eight studies provided NLR data for both survivors and non-survivors. Twelve studies used logistic regression analysis to determine the association between NLR and mortality in patients with ESLD, whereas 15 studies used Cox regression analysis to determine this association.
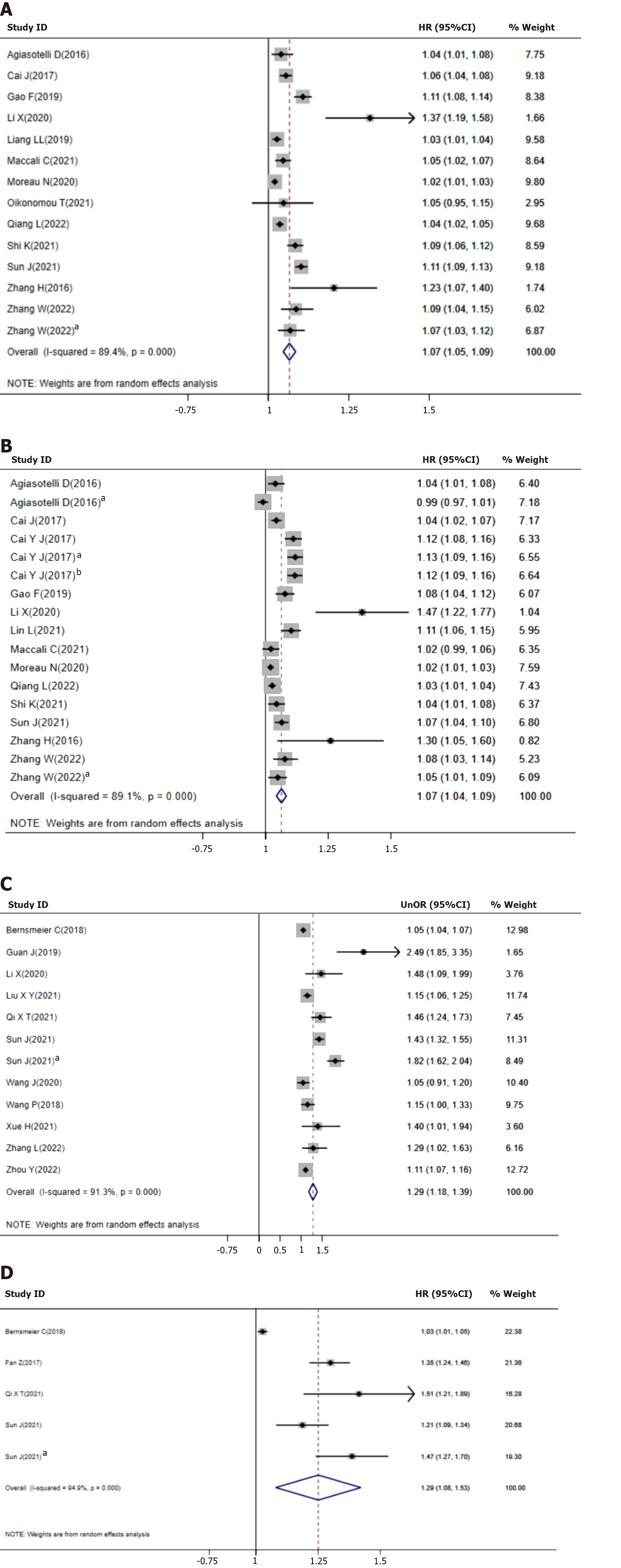
Univariate HR: Thirteen studies reported the association between NLR and mortality as univariate HR, with a meta-analysis finding that increased NLR was predictive of increased mortality (Figure 2, Panel A, HR = 1.07, 95%CI = 1.05-1.09). There was significant heterogeneity among these studies (I2 = 89.4%, P < 0.001). Subgroup (Table 2) and meta-regression (Supplementary Table 1) analyses showed that patient age, sex ratio, region, population, primary outcome, and etiology of ESLD did not affect the prognostic value of NLR. On publication bias tests, Begg’s test was non-significant, whereas Egger linear regression indicated possible bias (Supplementary Figure 1, P < 0.05). Using trim-and-fill analyses, two studies were imputed into the meta-analysis, but this did not significantly change the results (Supplementary Figure 2, HR = 1.06, 95%CI = 1.04-1.08). Sensitivity analysis showed similar results when each study was excluded.
| Number of subgroup data from studies | Effect size | 95%CI | I2 | Q between subgoup | |
| Univariate HR | |||||
| Mean age | |||||
| > 50 | 9 | 1.072 | 1.043-1.101 | 85.63c | 0.492 |
| ≤ 50 | 3 | 1.056 | 1.016-1.097 | 92.65c | |
| Study location | |||||
| Not Asian | 6 | 1.049 | 1.018-1.082 | 63.92a | 2.168 |
| Asian | 8 | 1.082 | 1.054-1.110 | 91.32c | |
| Population | |||||
| AD | 8 | 1.076 | 1.044-1.110 | 85.60c | 0.376 |
| ACLF | 6 | 1.062 | 1.032-1.093 | 92.03c | |
| Primary outcome | |||||
| ≤ 30 mortality | 5 | 1.097 | 1.057-1.139 | 80.06c | 2.852 |
| Long term mortality | 9 | 1.057 | 1.034-1.080 | 90.95c | |
| Etiology | |||||
| HBV | 8 | 1.082 | 1.054-1.110 | 91.95c | 2.168 |
| Mixed | 6 | 1.049 | 1.018-1.082 | 63.92a | |
| Multivariate HR | |||||
| Mean age | |||||
| > 50 | 12 | 1.082 | 1.051-1.113 | 90.10c | 0.621 |
| ≤ 50 | 2 | 1.052 | 0.986-1.121 | 83.28b | |
| Study location | |||||
| Not Asian | 6 | 1.030 | 1.000-1.061 | 70.153b | 7.728b |
| Asian | 11 | 1.087 | 1.061-1.113 | 87.451c | |
| Population | |||||
| AD | 11 | 1.046 | 1.038-1.054 | 91.00c | 3.472 |
| ACLF | 6 | 1.031 | 1.022-1.040 | 82.00c | |
| Mortality | |||||
| ≤ 30 mortality | 6 | 1.087 | 1.044-1.131 | 77.08c | 1.297 |
| Long term mortality | 11 | 1.058 | 1.033-1.083 | 91.38c | |
| Etiology | |||||
| HBV | 7 | 1.069 | 1.030-1.110 | 78.04c | 0.864 |
| Mixed | 10 | 1.065 | 1.036-1.095 | 92.38c | |
| Univariate OR | |||||
| Mean age | |||||
| > 50 | 4 | 1.323 | 1.077-1.625 | 78.80b | 0.036 |
| ≤ 50 | 5 | 1.289 | 1.080-1.538 | 85.73c | |
| Population | |||||
| AD | 4 | 1.329 | 1.058-1.669 | 78.80b | 0.095 |
| ACLF | 7 | 1.388 | 1.179-1.634 | 92.12c | |
| Mortality | |||||
| ≤ 30 mortality | 3 | 1.329 | 1.127-1.567 | 87.61b | 0.301 |
| Long term mortality | 7 | 1.256 | 1.117-1.412 | 93.79c | |
| Etiology | |||||
| HBV | 8 | 1.375 | 1.247-1.515 | 90.93c | 3.558 |
| Mixed | 4 | 1.166 | 1.013-1.081 | 76.32b |
Multivariate HR: Thirteen studies also reported the association between NLR and mortality as multivariate HR, with a meta-analysis finding that increased NLR was predictive of increased mortality (Figure 2, Panel B, HR = 1.07, 95%CI = 1.04-1.09). There was significant heterogeneity among these studies (I2 = 89.1%, P < 0.001). Similar to the results of univariate HR analysis, subgroup (Table 2), and meta-regression (Supplementary Table 1) analyses showed that age, sex ratio, population, primary outcome, and etiology of ESLD did not affect the prognostic value of NLR. In contrast, subgroup analysis revealed that studies in Asia (HR = 1.87, 95%CI = 1.06-1.11) and studies not in Asia (HR = 1.03, 95%CI = 1.00-1.06) yielded significant effects (P = 0.005). Both Begg and Egger test showed possible publication biases (Supplementary Figure 3, P < 0.05). By trim-and-fill analyses, two studies were imputed into the meta-analysis, but this did not significantly change the results (Supplementary Figure 4, HR = 1.06, 95%CI = 1.04-1.08). Sensitivity analysis showed similar result when each study was excluded.
Univariate OR: Eleven studies reported the association between NLR and mortality as univariate OR, with a meta-analysis showing that increased NLR was predictive of increased mortality (Figure 2, Panel C, OR = 1.29, 95%CI = 1.18-1.39). There was significant heterogeneity among these studies (I2 = 91.3%, P < 0.001). Subgroup (Table 2) and meta-regression (Supplementary Table 1) analyses showed that age, sex ratio, region, population, primary outcome, and etiology of ESLD did not affect the prognostic value of NLR. In the publication bias test, Begg’s test was non-significant (p=0.81). However, Egger’s linear regression showed the possible presence of bias (Supplementary Figure 5, P < 0.05). No study was imputed into the meta-analysis by trim-and-fill analyses (Supplementary Figure 6). Because the number of studies was small, the possibility of publication bias could not be completely excluded. Sensitivity analysis showed similar results when each study was excluded.
Multivariate OR: Four studies reported the association between NLR and mortality as multivariate OR, with a meta-analysis indicating that increased NLR was predictive of increased mortality (Figure 2, Panel D, OR = 1.29, 95%CI = 1.09-1.49). There was significant heterogeneity among these studies (I2 = 93.4%, P < 0.001). Because the number of studies was not adequate, subgroup and meta-regression analyses were not performed. In publication bias tests, Begg test was not significant (P = 0.81), whereas Egger linear regression showed possible bias (Supplementary Figure 7, P < 0.05). No study was imputed into the meta-analysis by trim-and-fill analyses. Because the number of studies was small, the possibility of publication bias could not be excluded completely. Sensitivity analysis showed similar result when each study was excluded.
Eight studies compared NLR in surviving and non-surviving patients with ESLD. A meta-analysis showed that NLR was significantly higher in non-survivors than in survivors (Supplementary Figure 8, random-effects model: SMD = 1.02 95%CI; 0.67–1.37).
To our knowledge, this systematic review is the first to report a relationship between NLR and mortality in patients with ESLD. The pooled results of this study indicated that NLR was associated with mortality (random-effects model; univariate HR = 1.07, 95%CI = 1.05-1.09; multivariate HR = 1.07, 95%CI = 1.07-1.09; univariate OR = 1.29, 95%CI = 1.18-1.39; multivariate OR = 1.29, 95%CI = 1.09-1.49). Furthermore, the pooled results of eight studies showed that NLR levels were significantly higher in non-survivors than in survivors with ESLD (random-effects model: SMD = 1.02, 95%CI = 0.67-1.37).
Mortality rates are high in patients with ESLD, such as liver failure and decompensated cirrhosis. Systemic inflammatory reactions are closely related to the severity and prognosis of liver disease in patients with severe cirrhosis, with the occurrence of systemic inflammatory response syndrome increasing mortality rates in patients with cirrhosis[16]. It is therefore crucial to identify and treat infections and systemic inflammation in patients with ESLD. Although routine tests, including measurements of C-reactive protein and procalcitonin (PCT) concentrations and white blood cell (WBC) counts, are commonly used to assess bacterial infection and systemic inflammation, these tests may not fully meet the demands of patients with ESLD. High serum total bilirubin concentrations in these patients can influence the diagnostic sensitivity of PCT[17]. Additionally, patients with ESLD often have lower baseline WBC counts, which can impair the predictive value of WBC in detecting infections. A study included in this review confirmed that NLR is superior to WBC or PCT for assessing infection in patients with ACLF[9]. NLR may also be a useful indicator of systemic inflammatory response syndrome or infection in patients with decompensated cirrhosis[18]. Taken together, these findings suggest that NLR strongly correlates with infection and systemic inflammatory response syndrome in patients with ESLD and that NLR may be predictive of mortality. These findings are consistent with the majority of the included studies and the final pooled results.
NLR has also been shown to be an indicator of inflammation in other conditions, such as colorectal cancer and myocardial infarction[19]. Peripheral neutrophil counts have been reported to serve as markers for both acute and chronic inflammation[20]. Activation of these neutrophils can inhibit T lymphocyte activation through the production of reactive oxygen and arginase[21]. Peripheral T-lymphocyte subsets were found to be significantly lower in ACLF patients than in healthy controls[22], and lower lymphocyte cell counts have been associated with poorer immune responses in patients with chronic liver disease[23]. These findings suggest that NLR may be a practical indicator that reflects the balance between inflammation and immune reactions. Furthermore, the inflammatory process has been shown to play a significant role in the development of liver fibrosis and cirrhosis. A meta-analysis suggested that NLR may be a marker of the degree of fibrosis and predictor of prognosis in patients with chronic liver disease[24]. NLR may also be predict of for prognosis in patients with ESLD.
Subgroup and meta-regression analyses revealed that the predictive value of NLR was not influenced by patient age, sex ratio, or the etiology of ESLD, suggesting that NLR is a reliable predictor of ESLD prognosis across different patient populations. NLR is considered a cost-effective and practical tool for predicting mortality in critically ill patients with liver failure and for screening patients with severe liver disease. Unlike other prognostic biomarkers, neutrophils and lymphocytes can be easily obtained and measured in clinical practice. Subgroup analysis of multivariate HR from 13 studies showed that NLR was strongly associated with mortality in Asian patients with ESLD, possibly due to the high prevalence of hepatitis B infection in Asian populations. HBV-ACLF patients exhibit lower levels of circulating lymphocytes and significantly higher levels of liver infiltrating lymphocytes[25]. Subgroup analysis, however, did not find significant differences in NLR between patients with HBV and those with mixed etiology. This may have been due to confounding factors and high heterogeneity in the mixed etiology group.
It is worth mentioned, the severity of the neutropenia and the overall status of the patient should be taken into account. Profound neutropenia may signify a more severe inflammatory or immunocompromised state, potentially affecting the NLR's ability to reflect the underlying inflammatory process accurately. In these patients, it can potentially impact the accuracy of NLR as a marker of systemic inflammation. Future research should focus on large-scale longitudinal studies to assess the predictive value of the NLR in ESLD patients with neutropenia, subgroup analyses to account for specific clinical characteristics, mechanistic studies to understand the underlying pathophysiology.
While NLR was identified as the strongest independent predictor in this study, other ratios such as platelet-to-lymphocyte ratio (PLR) and platelet-to-neutrophil ratio (PNR) have also been investigated for prognosis in liver diseases. However, study have shown that NLR had good predictive ability for mortality, higher than PNR[26]. In the setting of ESLD, PLR and PNR may be less reliable due to various thrombocytopenia mechanisms associated with advanced liver dysfunction. This is aruably a more direct assessment of the disease stage and prognosis in decompensated cirrhosis patients. For this reason, this article focused on NLR rather than PLR or PNR, though future studies could explore whether a combination of ratios provides even stronger predictive ability than individual markers alone.
The present review and meta-analysis had several limitations. First, there was high heterogeneity among the studies included in this analysis, similar to other prognostic reviews, despite the use of a random-effects model. Second, most of the included studies reported positive results, which may have introduced latent publication bias, although Begg's test and Egger's test did not show significant biases. Moreover, the number of studies that utilized multivariate OR analysis to assess the association between NLR and mortality was too small for determination of publication bias. Third, the critical cut-off value of NLR for determining prognosis remains unclear. Due to limitations in the original studies, the present analysis could not determine an exact ideal cut-off value.
This meta-analysis highlights the significance of NLR as a valuable prognostic biomarker in patients with ESLD, with higher NLRs indicating an increased risk of mortality. These findings especially emphasize the strong association between higher NLRs and prognosis in the Asian patients with ESLD. The continuing absence of a critical cut-off value of NLR for determining prognosis suggests the need for additional research to clarify this matter.
End-stage liver disease (ESLD) carries a high mortality risk. Identifying reliable prognostic factors is important to guide management, but studies on the prognostic value of neutrophil-to-lymphocyte ratio (NLR) in ESLD have reported conflicting results.
To comprehensively evaluate the association between NLR and ESLD prognosis through a systematic review and meta-analysis of existing literature.
To establish whether NLR is a useful prognostic biomarker for predicting mortality in patients with ESLD.
A systematic literature search was conducted through multiple databases. Studies evaluating the relationship between NLR and mortality in ESLD patients were selected and their data extracted. Pooled effect sizes were calculated using meta-analysis.
Higher NLR levels were associated with increased mortality risk in ESLD based on meta-analysis of 27 studies reporting hazard/odds ratios. NLR also distinguished survivors from non-survivors. The prognostic value of NLR was not influenced by patient characteristics but differed regionally.
NLR is clinically useful for prognostic assessment in ESLD patients, especially Asian populations, but optimal cut-off values require further investigation.
NLR represents a promising, readily available prognostic tool for risk stratifying ESLD patients. Future research should establish standardized NLR cut-offs and evaluate its utility accounting for potential confounders like severity of neutropenia.
We would like to express our gratitude to Professor Xing-Fa Zhang, the statistician who provided statistical support for this article.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2472] [Article Influence: 353.1] [Reference Citation Analysis (1)] |
| 2. | Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, Kyu HH, Barber RM, Wagner J, Cercy K, Kravitz H, Coggeshall M, Chew A, O'Rourke KF, Steiner C, Tuffaha M, Charara R, Al-Ghamdi EA, Adi Y, Afifi RA, Alahmadi H, AlBuhairan F, Allen N, AlMazroa M, Al-Nehmi AA, AlRayess Z, Arora M, Azzopardi P, Barroso C, Basulaiman M, Bhutta ZA, Bonell C, Breinbauer C, Degenhardt L, Denno D, Fang J, Fatusi A, Feigl AB, Kakuma R, Karam N, Kennedy E, Khoja TA, Maalouf F, Obermeyer CM, Mattoo A, McGovern T, Memish ZA, Mensah GA, Patel V, Petroni S, Reavley N, Zertuche DR, Saeedi M, Santelli J, Sawyer SM, Ssewamala F, Taiwo K, Tantawy M, Viner RM, Waldfogel J, Zuñiga MP, Naghavi M, Wang H, Vos T, Lopez AD, Al Rabeeah AA, Patton GC, Murray CJ. Global burden of diseases, injuries, and risk factors for young people's health during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:2383-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 681] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 3. | Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5-14. [PubMed] |
| 4. | Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 5. | Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 992] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Peng C, Cheng Z, Wang X, Wu L, Li J, Huang C, Guo Q, Cai H. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: A systematic review and meta-analysis. Int J Surg. 2018;55:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Al-Ameri AAM, Wei X, Wen X, Wei Q, Guo H, Zheng S, Xu X. Systematic review: risk prediction models for recurrence of hepatocellular carcinoma after liver transplantation. Transpl Int. 2020;33:697-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Agiasotelli D, Alexopoulou A, Vasilieva L, Kalpakou G, Papadaki S, Dourakis SP. Evaluation of neutrophil/Leukocyte ratio and organ failure score as predictors of reversibility and survival following an acute-on-chronic liver failure event. Hepatology Research. 2016;46:514-520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 9. | Sun J, Guo H, Yu X, Chen J, Zhu H, Qi X, Zhang X, Han J, Liu X, Yang J, Wang J, Qian Z, Huang Y, Mao R, Zhang J. Evaluation of prognostic value of neutrophil-to-lymphocyte ratio in patients with acute-on-chronic liver failure or severe liver injury from chronic HBV infection. Eur J Gastroenterol Hepatol. 2021;33:e670-e680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Li X, Wu J, Mao W. Evaluation of the neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and red cell distribution width for the prediction of prognosis of patients with hepatitis B virus-related decompensated cirrhosis. J Clin Lab Anal. 2020;34:e23478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Oikonomou T, Goulis I, Kiapidou S, Tagkou N, Akriviadis E, Papatheodoridis G, Cholongitas E. The significance of C-reactive protein to albumin ratio in patients with decompensated cirrhosis. Ann Gastroenterol. 2020;33:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 9015] [Article Influence: 530.3] [Reference Citation Analysis (0)] |
| 13. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 649] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 14. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 464] [Article Influence: 77.3] [Reference Citation Analysis (2)] |
| 15. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48655] [Article Influence: 2115.4] [Reference Citation Analysis (4)] |
| 16. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Qu J, Feng P, Luo Y, Lü X. Impact of hepatic function on serum procalcitonin for the diagnosis of bacterial infections in patients with chronic liver disease: A retrospective analysis of 324 cases. Medicine (Baltimore). 2016;95:e4270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Cai YJ, Dong JJ, Dong JZ, Chen Y, Lin Z, Song M, Wang YQ, Chen YP, Shi KQ, Zhou MT. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45:1413-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Doğan M, Akyel A, Bilgin M, Erat M, Çimen T, Sunman H, Efe TH, Açıkel S, Yeter E. Can Admission Neutrophil to Lymphocyte Ratio Predict Infarct-Related Artery Patency in ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2015;21:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2954] [Cited by in RCA: 4045] [Article Influence: 311.2] [Reference Citation Analysis (0)] |
| 21. | Müller I, Munder M, Kropf P, Hänsch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Lou YF, Dong W, Ye B, Lin S, Mao WL. Changes in peripheral T-lymphocyte subsets in acute-on-chronic liver failure patients with artificial liver support system. Hepatogastroenterology. 2012;59:814-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | O'Keefe SJ, El-Zayadi AR, Carraher TE, Davis M, Williams R. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 1980;2:615-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Peng Y, Li Y, He Y, Wei Q, Xie Q, Zhang L, Xia Y, Zhou X, Feng X, Chen K, Chen S, Chen W, Long Q, Chai J. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: a systematic review. Expert Rev Gastroenterol Hepatol. 2018;12:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 25. | Zou Z, Xu D, Li B, Xin S, Zhang Z, Huang L, Fu J, Yang Y, Jin L, Zhao JM, Shi M, Zhou G, Sun Y, Wang FS. Compartmentalization and its implication for peripheral immunologically-competent cells to the liver in patients with HBV-related acute-on-chronic liver failure. Hepatol Res. 2009;39:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Qi X, Wang C, Shan X. Peripheral Blood Cell Ratios as Prognostic Predictors of Mortality in Patients with Hepatitis B Virus-Related Decompensated Cirrhosis. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Bernsmeier C, Cavazza A, Fatourou EM, Theocharidou E, Akintimehin A, Baumgartner B, Dhar A, Auzinger G, Thursz M, Bernal W, Wendon JA, Karvellas CJ, Antoniades CG, McPhail MJW. Leucocyte ratios are biomarkers of mortality in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. Aliment Pharmacol Ther. 2020;52:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Cai J, Wang K, Han T, Jiang H. Evaluation of prognostic values of inflammation-based makers in patients with HBV-related acute-on-chronic liver failure. Medicine (Baltimore). 2018;97:e13324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Chiriac S, Stanciu C, Singeap AM, Sfarti CV, Cuciureanu T, Trifan A. Prognostic value of neutrophil-to-lymphocyte ratio in cirrhotic patients with acute-on-chronic liver failure. Turk J Gastroenterol. 2020;31:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Fan Z, EnQiang C, Yao DL, LiBo Y, Hong L, Lang B, Ping F, Hong T. Neutrophil-lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS One. 2017;12:e0175332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Gao F, Sun L, Ye X, Liu Y, Liu H, Geng M, Li X, Yang X, Li Y, Wang R, Chen J, Wan G, Jiang Y, Wang X. Development and validation of a prognostic model for acute-on-chronic hepatitis B liver failure. Eur J Gastroenterol Hepatol. 2017;29:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Guan J, Xu Y, Xian Y, Huang C, Lai R, Wang X, Xie J, Cai W, Xie Q. [Prognostic value of the neutrophil-lymphocyte ratio in peripheral blood in acute and chronic liver failure]. Chinese Hepatology. 2019;24:130-132. [DOI] [Full Text] |
| 33. | Li X, Ji Y, Sun Y, Gao Y. [The role of ALBI score combined with NLR in the prognosis of esophageal variceal hemorrhage in cirrhosis]. Modern Digestion & Intervention. 2022;27. |
| 34. | Liang L, Jin S, Du J, Hu T, Hu Y, Yan H. [Prognostic value of neutrophil-lymphocyte ratio combined with CLIF-OF scores in the short-term outcomes of patients with hepatitis B virus-associated acute-on-chronic liver failure]. Modern Practical Medicine. 2020;32:450-452+563. |
| 35. | Lin L, Yang F, Wang Y, Su S, Su Z, Jiang X, Zheng Y, Deng Y, Lv H, Zhao J, Lin R, Wang B, Sun C. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int Immunopharmacol. 2018;56:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Liu H, Zhang H, Wan G, Sang Y, Chang Y, Wang X, Zeng H. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat. 2014;21:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Liu XY, He X, Cai M, Peng SQ. Prognostic Value of Complete Blood Cell Count-Derived Inflammatory Markers in Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Maccali C, Augustinho FC, Zocche TL, Silva TE, Narciso-Schiavon JL, Schiavon LL. Neutrophil-lymphocyte ratio predicts short-term mortality in patients hospitalized for acute decompensation of cirrhosis. Arq Gastroenterol. 2021;58:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Moreau N, Wittebole X, Fleury Y, Forget P, Laterre PF, Castanares-Zapatero D. Neutrophil-to-Lymphocyte Ratio Predicts Death in Acute-on-Chronic Liver Failure Patients Admitted to the Intensive Care Unit: A Retrospective Cohort Study. Shock. 2018;49:385-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Qiang L, Qin J, Sun C, Sheng Y, Chen W, Qiu B, Chen X, Chen Y, Liu F, Wu G. [Prognostic evaluation of a prediction model based on neutrophil/Lymphocyte ratio and red blood cell distribution width of inflammatory factors on hepatitis B virus-related acute-on-chronic liver failure]. Jounral of Chongqing Medical University. 2021;46. |
| 41. | Shi K, Huang Y, Zhang Q, Ran C, Hou J, Zhang Y, Bi Y, Wang X. A dynamic nomogram to predict transplant-free mortality in patients with hepatitis B-related cirrhosis and overt hepatic encephalopathy. Int Immunopharmacol. 2022;108:108879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Sun J, Guo H, Yu X, Zhu H, Zhang X, Yang J, Wang J, Qian Z, Shen Z, Mao R, Zhang J. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol. 2021;21:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Wang J, Cheng N, Xiang T, Li S, Xiao J, Wu X. [Establishment and evaluation of short-term prognostic model for hepatitis B virus-related acute-on-chronic liver failure]. Journal of Clinical Hepatology. 2019;35. |
| 44. | Wang P, Zhang Q, Li Y, Yan X, Ding Q. [Usefulness of red blood cell distribution width and neutrophil to lymphocyte ratio model for a short-term prognosis of patients with hepatitis B virus-induced acute-on-chronic liver failure]. Journal of Practical Hepatology. 2020;23:682-686. |
| 45. | Wu D, Jin L, Gao Y, Ye J, Xia G, Li f, Zhou G. [Value of neutrophil-lymphocyte ratio in evaluating the short-time prognosis of patients with acute-on-chronic liver failure]. Journal of Clinical Hepatology. 2018;34:1945-1949. |
| 46. | Xue H, Shen J, Ju L, Zhang X, Shao J, Bian Z. [Correlation between neutrophil/Lymphocyte ratio, platelet/Lymphocyte ratio and prognosis in patients with cirrhotic encephalopathy]. International Journal of Laboratory Medicine. 2021;42. |
| 47. | Zhang J, Hu Y, Gao G, Hu A, Dong F. [Evaluation of the neutrophil/Lymphocyte ratio and end-stage liver disease model for short-term prognosis in patients with chronic severe hepatitis]. Modern Practical Medicine. 2016;28. |
| 48. | Zhang L, Chen W, Sheng Y, Deng C. [Value of Model for End-Stage Liver Disease score combined with neutrophil-lymphocyte ratio in predicting the short-term prognosis of patients with HBV-related acute-on-chronic liver failure]. Journal of Clinical Hepatology. 2018;34. |
| 49. | Zhang W, Aryan M, Chen Z, Khan W, Thompson B, Kwenda E, Geller B, Morelli G. Prognostic value of neutrophil-to-lymphocyte ratio in cirrhosis patients undergoing transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2022;34:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Zhou YF, Xu Y, Ding YF, Yu XJ, Wu YL, Chen P, Zou DW. Novel nomogram model for predicting 6-week mortality in liver cirrhosis patients with acute upper gastrointestinal bleeding. J Dig Dis. 2022;23:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shelat VG, Singapore S-Editor: Liu JH L-Editor: A P-Editor: Guo X