Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.465
Peer-review started: October 24, 2023
First decision: January 5, 2024
Revised: January 26, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: March 27, 2024
Processing time: 155 Days and 3.3 Hours
Although hepatitis B virus infection is the leading cause of chronic liver injury globally, nonalcoholic fatty liver disease (NAFLD) is gradually gaining attention as another major chronic liver disease. The number of patients having chronic hepatitis B (CHB) with concomitant hepatic steatosis has increased.
To analyze the effect of NAFLD on the response to antiviral treatment in patients with CHB.
Relevant English studies were systematically searched across PubMed, EMBASE, Web of Science, and Cochrane Library until October 2023. Studies in which the treatment outcomes were compared between patients with CHB only and those with CHB and hepatic steatosis were included.
Of the 2502 retrieved studies, 11 articles were finally included. Biochemical response until 48 wk (OR = 0.87, 95%CI: 0.50–1.53, P = 0.000) and 96 wk (OR = 0.35, 95%CI: 0.24–0.53, P = 0.24) and virological response until 96 wk (OR = 0.80, 95%CI: 0.43–1.49, P = 0.097) were lower in patients with hepatic steatosis than in patients with CHB alone.
Hepatic steatosis lowers the biochemical response to antiviral treatment in patients with CHB.
Core Tip: No consensus is available in the literature about which effect of nonalcoholic fatty liver disease on the response to antiviral treatment in patients with chronic hepatitis B (CHB). This is a systematic review and meta-analysis comparing the response to antiviral treatment between patients with CHB only and those with CHB and hepatic steatosis were included. We investigated these two groups in terms of biochemical responses, serological responses, virological responses the incidence of hepatocellular carcinoma.
- Citation: Liu SY, Wang D, Liu J, Yang LP, Chen GY. Influence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis. World J Hepatol 2024; 16(3): 465-476
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/465.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.465
Chronic hepatitis B (CHB) infection is an important disease globally, particularly in Asia. Epidemiological data indicate that nearly 400 million people have CHB worldwide[1]. If left untreated, approximately one-third of these patients progress to severe end-stage liver diseases, which manifest as liver failure, cirrhosis, and hepatocellular carcinoma (HCC). Therefore, antiviral therapy is crucial for the clinical management of CHB. The currently available antiviral drugs, such as nucleoside/nucleotide analogs (NAs) and interferons (IFNs), can reduce the progression of liver disease, thereby improving the long-term outcomes in CHB patients[2].
Nonalcoholic fatty liver disease (NAFLD) is a common clinicopathologic condition characterized by lipid deposition without or with inflammation in hepatocytes. NAFLD comprises a wide spectrum of liver damage, including simple steatosis, nonalcoholic steatohepatitis, and fibrosis[3]. In recent years, owing to the epidemic of obesity and lifestyle changes, NAFLD has become a common chronic liver disease. The worldwide prevalence of NAFLD in the adult population has been reported to be approximately 25%[4-6]. Hence, there is a surge in patients having CHB with NAFLD. Moreover, the complexity of liver disease has increased, which poses new challenges in clinical diagnosis and treatment. In this scenario, a specific antiviral strategy is warranted for patients having CHB with NAFLD.
Considering the several conflicting observations in the literature on the effect of NAFLD in patients with CHB who are under antiviral treatment, a meta-analysis was conducted to explore the impact of NAFLD on the treatment response in antiviral-treated patients with CHB.
This study was conducted and reported in accordance with the preferred reporting items for systematic reviews and meta-analyses statement[7] and in accordance with the meta-analysis of observational studies in epidemiology guidelines for the meta-analysis of observational studies.
A systematic search was conducted across PubMed, EMBASE, Web of Science, and Cochrane Library databases for articles published until October 2023. The following keywords were used in the search: “chronic hepatitis B” or “hepatitis B antigens” or “hepatitis B virus” or “hepatitis B, chronic”; “fatty liver” or “hepatic steatosis” or “NAFLD”; “antiviral agents” or “nucleoside” or “peginterferon.” Furthermore, the reference lists of key articles were manually and independently reviewed. The potentially eligible studies were reviewed entirely following selection from the initial search.
A total of 11 studies were screened for relevance based on the title, abstract, and entire manuscript. In this study, studies that included patients with CHB and NAFLD with CHB who underwent antiviral treatment (including NAs and IFNs) for at least 96 wk were assessed. Articles were excluded if their subjects were under the age of 18 years if they did not have the reported outcomes, if they did not contain usable primary data, or if they did not setting CHB complicated fatty liver patients. Each article was reviewed by two investigators independently (Liu SY and Wang D). Data were extracted from studies meeting both the inclusion and exclusion criteria following the review of the entire contents of each paper. Any differences were resolved by a third investigator (Chen GY), discussion, or revision.
Data were extracted independently by two authors, and any discrepancies were resolved via consensus. The following information was extracted from each trial: publication details (title, first author, and place of the study), study design (inclusion and exclusion criteria), participant details (number of patients enrolled and their age), intervention details (including type and dose of IFNs, NAs, and mode of administration), duration of treatment, follow-up, and outcomes. Quality assessment of the included studies was performed by two authors using an improved Newcastle–Ottawa Scale (the Newcastle–Ottawa Scale for assessing the quality of nonrandomized studies in meta-analyses). Studies that scored ≥ 9 points were deemed to be of high quality and those with 5–8 points and < 5 points were deemed to be of moderate and low quality, respectively. The risk of bias was rated independently by two authors (Liu SY and Wang D).
The following outcomes were included in the study, biochemical response [time taken for the alanine aminotransferase (ALT) level to return to normal], virological response [time taken for the hepatitis b virus (HBV) DNA to become undetectable], serological response [time taken for the disappearance of hepatitis B e-antigen (HBeAg) and the appearance of anti-HBe], and the incidence of HCC.
Heterogeneity between individual studies was assessed by using the I2 test. The random-effects model was selected a priori due to the anticipated heterogeneity of the included studies. A value of ≥ 75% was considered indicative of substantial heterogeneity, ≥ 50 as moderate heterogeneity, ≥ 25% as mild heterogeneity, and < 25% as the absence of heterogeneity. Publication bias was assessed by constructing a funnel plot of each study’s effect size against the standard error. Funnel plot asymmetry was evaluated using Egger’s test, and P < 0.1 was defined as having a significant publication bias. The Stata 16.0 software was employed for all analyses.
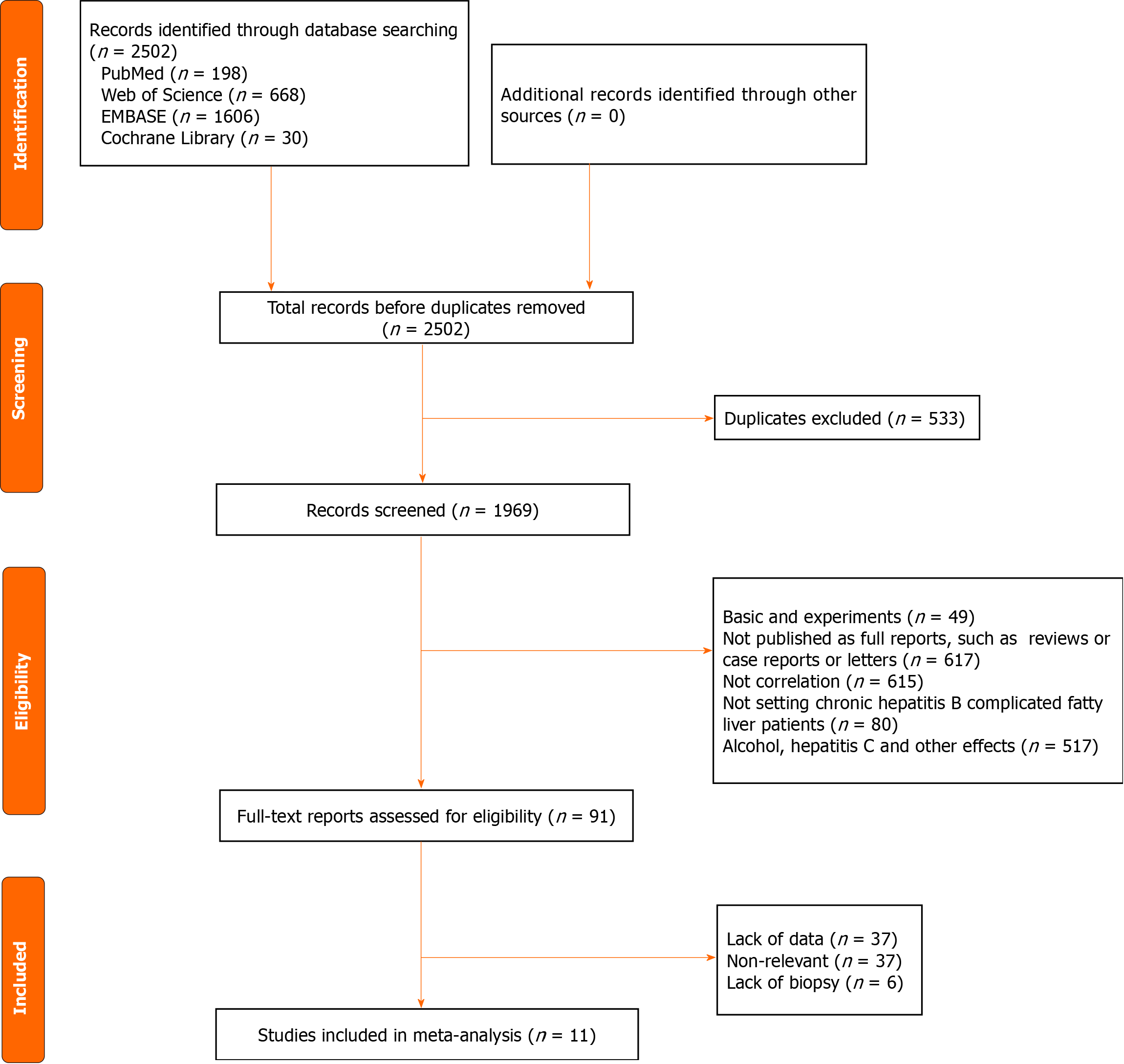
During the initial literature search, a total of 2502 articles were retrieved, of which 533 were eliminated because of duplication. After a careful review of the remaining 1969 titles and abstracts, 617 were excluded because they were not published as full reports (like conference abstracts or letters to the editor); 49 were excluded as they involved animal or cellular experiments; 80 were excluded because they only included patients with CHB not complicated by fatty liver; 615 were excluded because of a lack of correlation; and 517 were excluded due to the presence of other diseases. After a careful review of the 91 full-text articles, 37 were excluded for data insufficiency and 43 because of the absence of correlation. Finally, a total of 11 articles were included in the meta-analysis (Figure 1)[8-18].
General information pertaining to the included studies is presented in Table 1. Of these studies, one was conducted in Turkey[8],six in China[9-12,16,18], three in Korea[13-15], and one in the United States[17]. All studies were published in English. Patients were treated with IFN-α in three studies[8-10] and NAs in eight studies[9,11-17]. Three trials comprised a 48-week IFN-α treatment[8,10,12], with two involving a 48-week follow-up[1,3] and one involving a 96-week follow-up[18]. One trial included NAs and a 24-week follow-up[12], and seven trials included NAs and a > 48-week follow-up[9,11-17]. Five studies were prospective cohort studies[8-11,18], whereas the other six were retrospective cohort studies[12-17].
| Ref. | Ateş et al[8] | Jin et al[9] | Gong et al[10] | Liu et al[11] | Zhu et al[12] | Kim et al[13] | Cho et al[14] | Lee et al[15] | Chen et al[16] | Li et al[17] | Liang et al[18] | |
| Year | 2011 | 2012 | 2015 | 2015 | 2016 | 2019 | 2019 | 2019 | 2020 | 2020 | 2021 | |
| Location | Turkey | China | China | China | China | Korea | Korea | Korea | China | United States | China | |
| Study | Prospective study | Prospective study | Prospective study | Prospective study | Retrospective study | Retrospective study | Retrospective study | Retrospective study | Retrospective study | Retrospective study | Prospective study | |
| Journal | World J Gastroenterol | PLoS One | Transplant Proc | J Interferon Cytokine Res | Drug Des Devel Ther | Clin Mol Hepatol | J Clin Gastroenterol | Clin Mol Hepatol | BMC Gastroenterol | Liver Int | Gastroenterol Res Pract | |
| Therapy regimen | PEG-IFN | Nucleos(t)ide analogues | PEG-IFN | Nucleos(t)ide analogues | Nucleos(t)ide analogues | Nucleos(t)ide analogues | Nucleos(t)ide analogues | Nucleos(t)ide analogues | Nucleos(t)ide analogues | Nucleos(t)ide analogues | PEG-IFN | |
| Male | CHB with NAFLD | 95 | 32 | 20 | 23 | 42 | 91 | 171 | 50 | 43 | 126 | 95 |
| CHB | 82 | 85 | 38 | 12 | 41 | 119 | 333 | 146 | 43 | 211 | 82 | |
| Famale | CHB with NAFLD | 12 | 33 | 11 | 17 | 19 | 69 | 89 | 20 | 13 | 61 | 12 |
| CHB | 3 | 63 | 20 | 8 | 23 | 69 | 233 | 105 | 13 | 157 | 37 | |
| Age | CHB with NAFLD | 50.5 ± 8.7 | 39.56 ± 11.87 | 30.3 ± 7.4 | 37.70 ± 7.90 | 39.26 ± 10.39 | 51.0 (42.0-56.3) | 52.0 ± 9.4 | 45 (36–51) | 39 (31-34) | 47.7 ± 12.5 | 20.87 ± 1.96 |
| CHB | 35.2 ± 8.9 | 39.55 ± 7.83 | 25.1 ± 7.8 | 37.35 ± 8.49 | 39.61 ± 10.87 | 51.0 (43.3-57.0) | 54.0 ± 8.8 | 41 (32–48) | 38.5 (31–44) | 45.5 ± 14.6 | 29.50 ± 5.47 | |
| BMI | CHB with NAFLD | 32.9 ± 3.1 | 26.35 ± 4.19 | 25.3 ± 2.1 | 28.16 ± 1.43 | 26.29 ± 3.99 | 24.7 (22.3-26.7 | 24.5 ± 3.5 | NA | 25.0 ± 3.0 | 25.4 ± 4.3 | NA |
| CHB | 25.7 ± 3.3 | 24.26 ± 3.41 | 22.5 ± 2.9 | 22.06 ± 1.02 | 22.50 ± 2.85 | 22.5 (20.4-24.4) | 23.8 ± 3.2 | NA | 23.0 ± 3.0 | 23.8 ± 4.0 | 20.87 ± 1.96 | |
| ALT | CHB with NAFLD | 128.3 ± 18.9 | 143.3 ± 82.1 | 171.68 ± 46.23 | 227.70 ± 121.14 | 179.87 ± 78.50 | 56 (38-94) | NA | 71 (32–114) | 99 (68-154) | 60 (15 - 1525) | NA |
| CHB | 139.2 ± 52.5 | 157.1 ± 108.3 | 159.18 ± 45.12 | 229.95 ± 137.36 | 187.95 ± 79.88 | 56 (34-90) | NA | 94 (45–171) | 124 (79–216) | 50 (14 - 1079) | 257.39 ± 175.17 | |
| AST | CHB with NAFLD | 90.7 ± 34.8 | 70.2 ± 33.7 | 59.66 ± 13.81 | 183.23 ± 103.70 | NA | NA | NA | 53 (36–86) | 61 (38-84) | 37 (13 - 1465) | NA |
| CHB | 107.0 ± 40.3 | 93.5 ± 72.3 | 56.63 ± 13.13 | 167.60 ± 85.07 | NA | NA | NA | 85 (51–153) | 72 (45–137) | 35 (12 - 863) | 126.72 ± 79.55 | |
| GGT | CHB with NAFLD | 49.8 ± 29.8 | 95.2 ± 73.2 | 42.92 ± 14.83 | 98.75 – 19.90 | NA | NA | NA | NA | NA | NA | NA |
| CHB | 50.0 ± 44.0 | 77.2 ± 89.7 | 46.05 ± 11.36 | 51.41 – 5.46 | NA | NA | NA | NA | NA | NA | NA | |
| TB | CHB with NAFLD | NA | NA | NA | NA | NA | 0.8 (0.7-1.1) | 1.0 ± 0.4 | NA | 0.8 (0.6-1.0) | 1.3 ± 3.0 | NA |
| CHB | NA | NA | NA | NA | NA | 0.9 (0.7-1.2) | 1.1 ± 0.4 | NA | 1.0 (0.7–1.2) | 1.3 ± 2.7 | NA | |
| ALB | CHB with NAFLD | NA | NA | NA | NA | NA | 4.2 (4.0-4.4) | 4.18 ± 0.3 | 4.2 (3.8–4.4) | NA | 4.0 ± 0.6 | NA |
| CHB | NA | NA | NA | NA | NA | 4.2 (3.9-4.4) | 4.25 ± 0.4 | 4.0 (3.7–4.3) | NA | 3.9 ± 0.6 | NA | |
| TC | CHB with NAFLD | 192.2 ± 28.0 | 84.6 ± 14.4 | 80.28 ± 7.92 | 115.02 ± 9 | 68.04 ± 1.26 | 178 (151-204) | NA | 176 (155–203) | NA | 181 ± 40 | NA |
| CHB | 178.0 ± 27.4 | 81.0 ± 14.4 | 78.66 ± 5.76 | 81.18 ± 8.1 | 66.96 ± 17.46 | 170 (151-190) | NA | 159 (137–179) | NA | 177 ± 41 | 0.56 ± 0.45 | |
| TG | CHB with NAFLD | 188.3 ± 52.0 | 34.2 ± 14.4 | 27.54 ± 6.84 | 48.06 ± 10.98 | 32.4 ± 16.2 | NA | NA | 103 (81–137) | NA | 189 ± 153 | NA |
| CHB | 114.2 ± 44.2 | 21.6 ± 9 | 19.98 ± 7.02 | 25.92 ± 2.88 | 23.94 ± 11.52 | NA | NA | 80 (63–109) | NA | 86 ± 42 | 4.68 ± 0.80 | |
| HDL | CHB with NAFLD | NA | NA | NA | NA | 15.48 ± 6.12 | NA | NA | 44.4 (35.4–54.4) | NA | 40 ± 9 | NA |
| CHB | NA | NA | NA | NA | 19.26 ± 6.3 | NA | NA | 48.3 (39.4–59.4) | NA | 58 ± 19 | NA | |
| LDL | CHB with NAFLD | NA | NA | NA | NA | 41.58 ± 12.96 | 113 (93-129) | NA | 105 (87–129) | NA | 111 ± 37 | NA |
| CHB | NA | NA | NA | NA | 38.88 ± 12.24 | 101 (80-126) | NA | 90 (75–108) | NA | 106 ± 32 | NA | |
| Glucose | CHB with NAFLD | 102.7 ± 27.7 | 4.8 ± 0.6 | 5.46 ± 1.37 | NA | 5.11 ± 0.85 | 96.0 (89.3-107.0) | NA | 103 (92–117) | NA | 115 ± 37 | NA |
| CHB | 96.7 ± 19.0 | 4.5 ± 0.5 | 5.07 ± 0.92 | NA | 4.89 ± 0.80 | 9 4.0 (87.3-102) | NA | 98 (90–108) | NA | 105 ± 26 | NA | |
| Quality scores | 6 | 7 | 7 | 6 | 6 | 7 | 8 | 6 | 7 | 6 | 6 | |
Baseline data, including ALT, aspartate aminotransferase, gamma-glutamyl transferase, total cholesterol, triglyceride, total bilirubin, albumin, high-density lipoprotein, low-density lipoprotein, and glucose, are shown in Table 1.
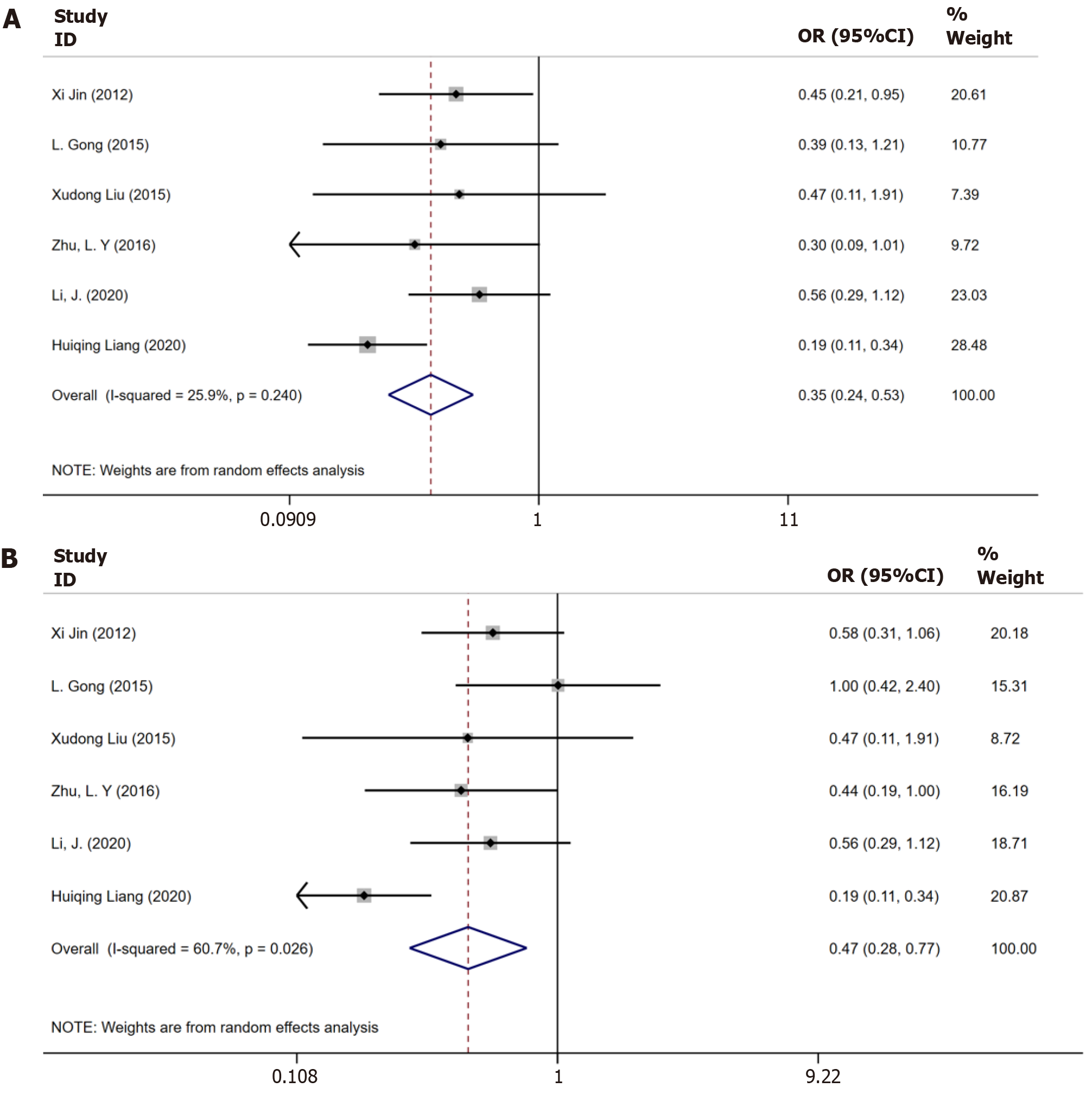
Six trials[9-12,17,18] had a combined study population of 459 patients having CHB plus steatosis and 695 patients had only CHB reported data on biochemical responses until 48 wk. The result is shown in Figure 2A. Moderate substantial heterogeneity was observed among these studies (I2 = 60.7%, P = 0.026), and a random-effects model was applied for the analysis. Patients with CHB plus steatosis demonstrated a lower rate of biochemical response until 48 wk when compared to those with only CHB [odds ratio (OR) = 0.43, 95%CI: 0.28–0.77, P = 0.03, Figure 2A].
Six studies[9-12,17,18] reported data on biochemical response until 96 wk, which displayed heterogeneity (I2 = 25.9%, P = 0.024). The P value indicated a significantly lower sustained biochemical response in patients with CHB and steatosis than in those with only CHB (OR = 0.35, 95%CI: 0.24–0.53, P = 0.47, Figure 2B).
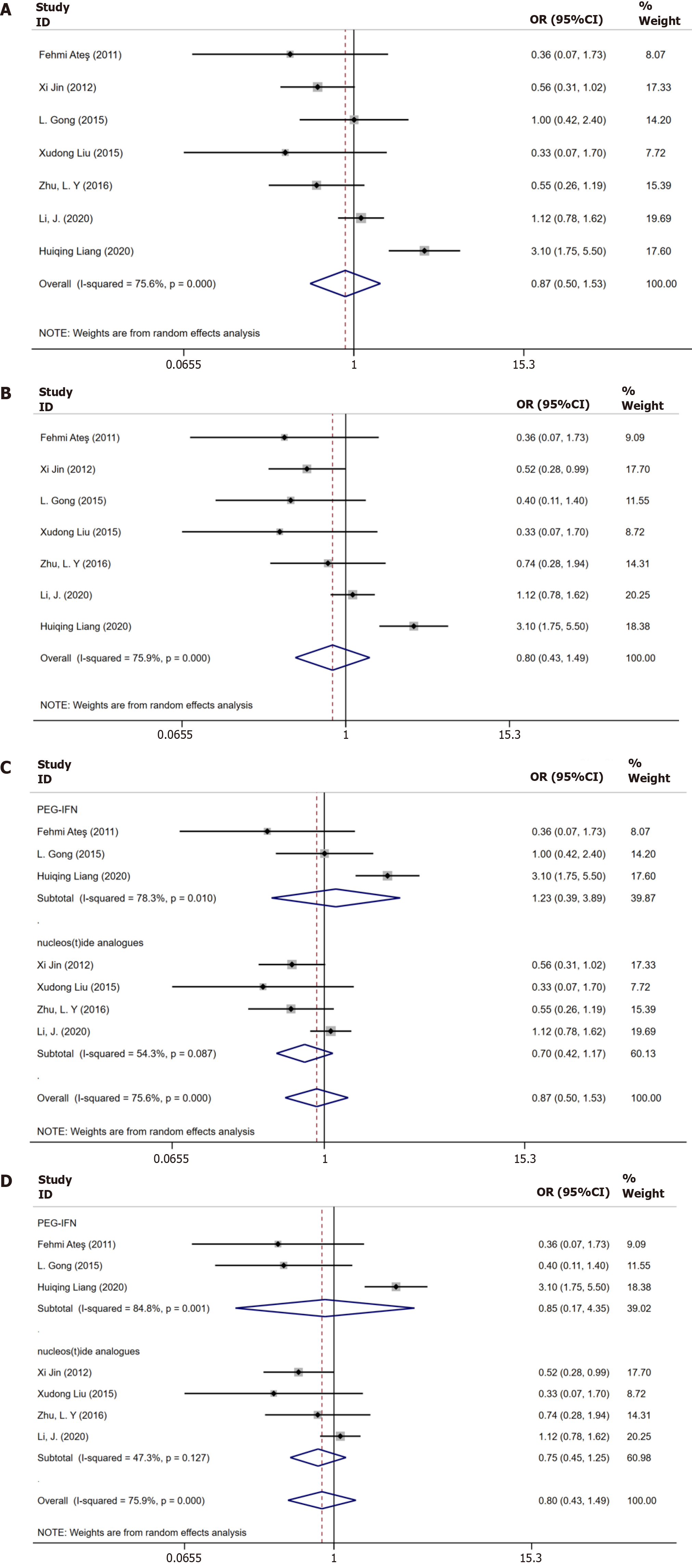
Seven studies[8-12,17,18] reported data on virological response until 48 wk. Substantial heterogeneity was noted (I2 = 75.6%, P = 0.000), and the random effects model was applied. No significant between-groups difference was observed with respect to the sustained virological response until 48 wk (OR = 0.87, 95%CI: 0.50–1.53, P = 0.112, Figure 3A).
Data on virological response until 96 wk was available for seven trials[8-12,17,18]. The P value indicated no significant between-group difference (OR = 0.80, 95%CI: 0.43–1.49, P = 0.097). Heterogeneity was observed among these studies (I2 = 75.90%, P = 0.000), and the random-effects model was applied (Figure 3B).
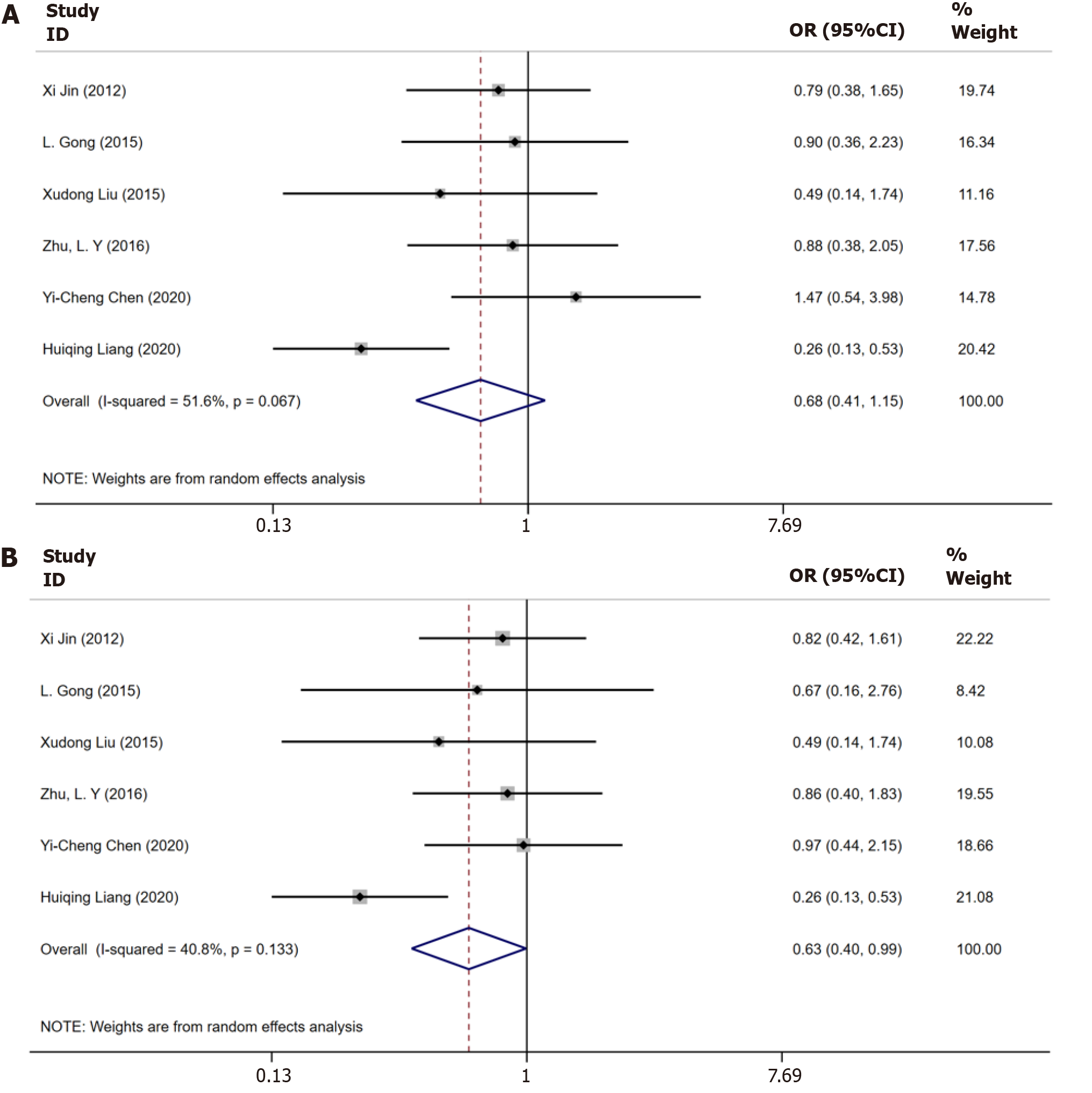
Six trials[9-12,16,18] examined the serological response to antiviral treatment until 48 wk. No statistically significant heterogeneity was observed among these studies (I2 = 51.6%, P = 0.067), and the P value showed no significant between-group difference (OR = 0.68, 95%CI: 0.41–1.15, P = 0.51, Figure 4A).
Six studies[9-12,16,18] documented the results of serological response until 96 wk. As no substantial heterogeneity was detected, the random-effects model was applied for the analysis (I2 = 40.8%, P = 0.1333). The P value showed a significantly lower sustained biochemical response in patients with CHB and steatosis than in those with only CHB (OR = 0.63, 95%CI: 0.40–0.99, P = 0.047, Figure 4B).
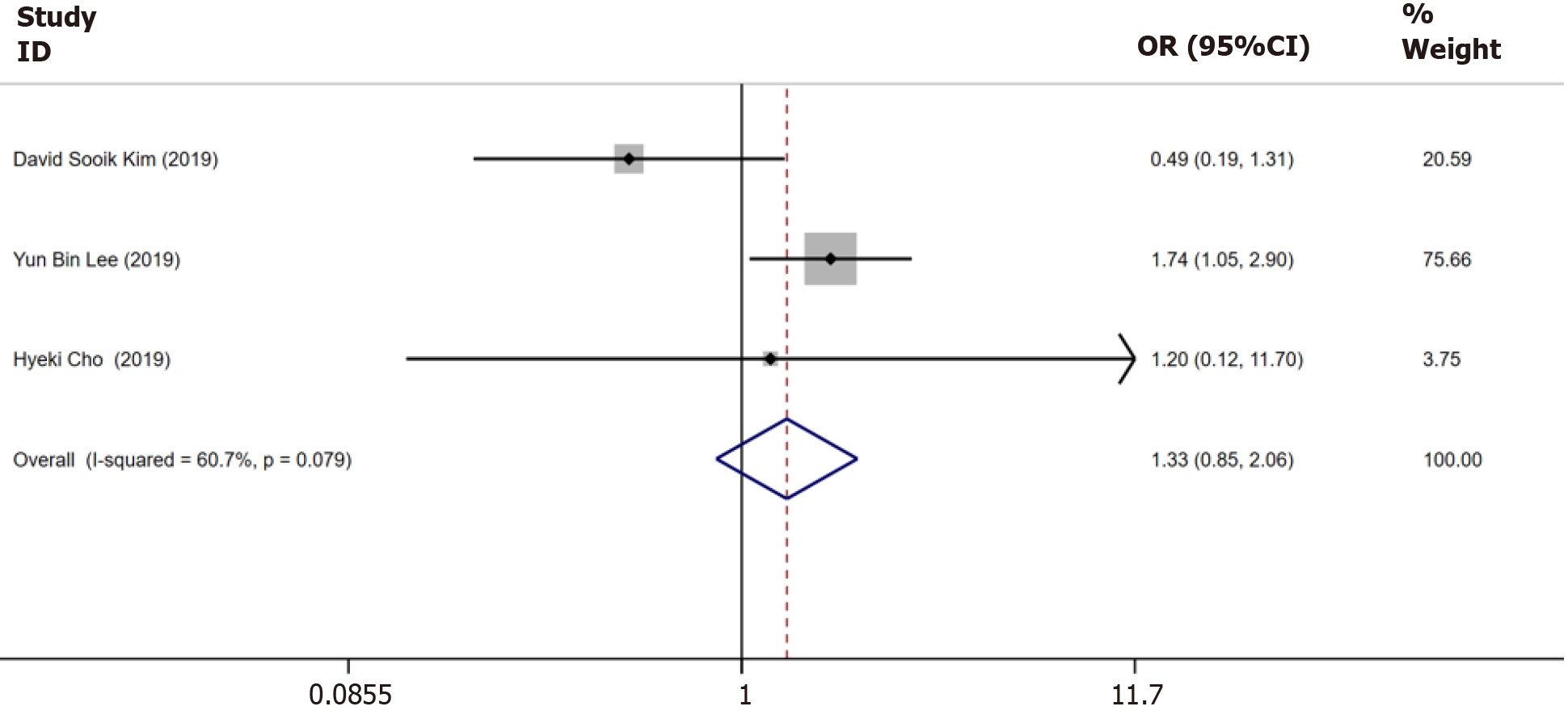
Three trials[13-15] reported data on the incidence of HCC until 5 years, which showed no heterogeneity (I2 = 60.7%, P = 0.079). The estimated pooled OR value showed no significant between-group difference (OR = 1.33, 95%CI: 0.85–2.06, P = 0.15, Figure 5).
Three studies[8,10,18] treating patients using IFNs and four studies[7,11,12,17] using NAs achieved a virological response until 48 wk and 96 wk, respectively. Thus, subgroup analysis was performed according to the treatment regimens: NAs or IFNs. Subgroup analysis implied that if patients were treated with NAs, there was no significant difference in the virological response until 48 wk (OR = 0.70, 95%CI: 0.42–1.17, P = 0.80, Figure 3C) and 96 wk (OR = 0.75, 95%CI: 0.45–1.25, P = 0.75, Figure 3D). No significant differences were observed in the virological response until 48 wk (OR = 1.23, 95%CI: 0.39–3.89, P = 0.96, Figure 3C) and 96 wk (OR = 0.85, 95%CI: 0.17–1.25, P = 1.01, Figure 3D) between the two groups if the patients were treated with IFNs.
Funnel plots of publication bias based on biochemical, virological, and serological responses did not demonstrate any obvious asymmetry. The interpretation of these plots was limited by the limited number of studies. Egger’s tests for biochemical (P = 0.434), virological (P = 0.328), and serological responses (P = 0.429) until 48 wk were not significant. Egger’s tests for biochemical (P = 0.517), virological (P = 0.231), and serological responses (P = 0.985) until 96 wk were also not significant.
The incidence of NAFLD is on the rise owing to the increase in the consumption of a fat-rich diet coupled with a sedentary lifestyle. Thus, hepatic steatosis is encountered frequently in patients with CHB. Definitive evidence is not available for the effect of hepatic steatosis on the efficacy of antiviral therapy in patients with CHB. In this meta-analysis, 11 cohort studies published between 2011 and 2020 with a combined population of 1903 patients having CHB plus hepatic steatosis and 1042 patients with only CHB were included. Almost all patients received IFNs or NAs for > 96 wk. In the meta-analysis, hepatic steatosis lowered the biochemical response until 48 wk and 96 wk and serological response until 96 wk to antiviral treatment in patients with CHB. On the contrary, virological responses until 48 wk and 96 wk, serological response until 48 wk, and the incidence of HCC until 5 years were not significantly different in patients with hepatic steatosis than in those without the condition. Our finding signifies that hepatic steatosis lowers the response to antiviral therapy in patients with CHB.
With regard to the biochemical response, hepatic steatosis and inflammation could also cause an elevation in ALT, which may mask the real ALT change caused by HBV activation, thereby resulting in the misclassification of patients with CHB into antiviral therapy[9]. Therefore, suitable criteria for antiviral therapy are required for patients having CHB with hepatic steatosis. Whether NAFLD should be first treated until selecting patients having CHB with NAFLD for anti-HBV therapy is an interesting question that warrants further investigation.
In addition, subgroup analysis was performed based on the treatment regimens. The results indicated that when patients were treated with IFNs or NAs, those with CHB and hepatic steatosis did not exhibit any significant difference from those with only CHB. The outcome of treatment with oral antivirals or interferons appears to be unaffected by HS[19]. Some studies have reported that fat accumulation in hepatocytes may minimize the contact area between drugs and hepatocytes, which can result in a low antiviral response to Nas[20]. Moreover, the declined activity of hepatic cyto
HBeAg seroconversion is one of the therapeutic goals in patients with HBeAg-positive CHB. Considering the HBeAg seroconversion rate, past studies have shown that NAFLD may affect the rate of long-term serological response in CHB but does not affect the serological response rate during early treatment. In these studies, the degree of hepatic steatosis was unclear in most cases and the duration of therapy was short in patients with an HBeAg-positive status. Hence, drawing a definitive conclusion was difficult.
The findings from this study suggested that the coexistence of fatty liver associated with an increased risk of HCC development in patients with CHB was unclear. Nevertheless, only three trials reported data on the incidence of HCC; therefore, the relationship between hepatic steatosis and HCC warrants further investigations with more numbers of subjects. Notably, some studies suggested that NAFLD promotes HCC development via direct and indirect mechanisms. NAFLD not only directly affects hepatocytes but also immensely alters the local microenvironment in the liver and enhances HCC development. Dysregulation of lipid metabolism and accumulation of lipids in the liver causes the selective loss of intrahepatic CD4+ T lymphocytes and results in accelerated hepatocarcinogenesis[15]. Inflammatory cytokines, endoplasmic reticulum stress, and circadian dysregulation mediate hepatocyte injury and progression of NAFLD. Furthermore, reshaped local immune systems with altered microbial metabolites foster a tumor-promoting environment and contribute to NAFLD-mediated hepatocarcinogenesis. The association between HCC in hepatic steatosis and HBV is unclear, but the influence of NAFLD on HCC development may have additive effects in patients with CHB. Although CHB affects the incidence of NAFLD, there is no conclusive evidence linking HS to liver fibrosis, cirrhosis, and HCC in patients with CHB infection. NASH, a severe form of NAFLD, shows a rapid progression in fibrosis, and it is the major cause of liver fibrosis, cirrhosis, and HCC in advanced NAFLD. However, the degree of hepatic steatosis was unclear in most studies, with no reference to NASH. The coexistence of NAFLD may independently increase the risk of HCC development, which is likely to be the same mechanism through which NAFLD alone induces HCC[19].
There are several limitations to this meta-analysis. First, most studies included in the meta-analysis were retrospective, single-center studies. Second, the sample size in certain studies was small. Both factors could have introduced an element of bias and affected the results of the meta-analysis. Hence, more prospective, multicenter observational studies are needed to validate the current findings.
Hepatic steatosis lowers the biochemical response to antiviral treatment in patients with CHB. This condition might become a protective factor of disease progression when present in patients affected by HBV. The significant effect of hepatic steatosis on the therapeutic response in patients with CHB should be demonstrated through larger prospective studies.
There is a surge in patients having chronic hepatitis B (CHB) with nonalcoholic fatty liver disease (NAFLD). However, there are several conflicting observations in the literature on the effect of NAFLD in patients with CHB who are under antiviral treatment.
A meta-analysis was conducted to explore the impact of NAFLD on the treatment response in antiviral-treated patients with CHB.
The complexity of liver disease has increased, which poses new challenges in clinical diagnosis and treatment. In this scenario, a specific antiviral strategy is warranted for patients having CHB with NAFLD.
This is a systematic review and meta-analysis that compared the response to antiviral treatment between patients with CHB alone and those with CHB and hepatic steatosis. We investigated these two groups in terms of biochemical responses, serological responses, and virological responses to the incidence of hepatocellular carcinoma (HCC).
In the meta-analysis, hepatic steatosis lowered the biochemical response until 48 wk and 96 wk and serological response until 96 wk to antiviral treatment in patients with CHB. On the contrary, virological responses until 48 wk and 96 wk, serological response until 48 wk, and the incidence of HCC until 5 years were not significantly different in patients with hepatic steatosis than in those without the condition. Our finding signifies that hepatic steatosis lowers the response to antiviral therapy in patients with CHB.
Hepatic steatosis lowers the biochemical response to antiviral treatment in patients with CHB. This condition might become a hazard factor of disease progression when present in patients affected by HBV.
The significant effect of hepatic steatosis on the therapeutic response in patients with CHB should be demonstrated through larger prospective studies.
| 1. | World Health Organization. Global hepatitis report. 2017. Available from: https://www.who.int. |
| 2. | Tao Y, Wu D, Zhou L, Chen E, Liu C, Tang X, Jiang W, Han N, Li H, Tang H. Present and Future Therapies for Chronic Hepatitis B. Adv Exp Med Biol. 2020;1179:137-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 4. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 845] [Article Influence: 93.9] [Reference Citation Analysis (2)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7944] [Article Influence: 794.4] [Reference Citation Analysis (8)] |
| 6. | Aller R, Fernández-Rodríguez C, Lo Iacono O, Bañares R, Abad J, Carrión JA, García-Monzón C, Caballería J, Berenguer M, Rodríguez-Perálvarez M, Miranda JL, Vilar-Gómez E, Crespo J, García-Cortés M, Reig M, Navarro JM, Gallego R, Genescà J, Arias-Loste MT, Pareja MJ, Albillos A, Muntané J, Jorquera F, Solà E, Hernández-Guerra M, Rojo MÁ, Salmerón J, Caballería L, Diago M, Molina E, Bataller R, Romero-Gómez M. Consensus document. Management of non-alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol Hepatol. 2018;41:328-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11320] [Article Influence: 665.9] [Reference Citation Analysis (0)] |
| 8. | Ateş F, Yalnız M, Alan S. Impact of liver steatosis on response to pegylated interferon therapy in patients with chronic hepatitis B. World J Gastroenterol. 2011;17:4517-4522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS One. 2012;7:e34198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Gong L, Liu J, Wang J, Lou GQ, Shi JP. Hepatic Steatosis as a Predictive Factor of Antiviral Effect of Pegylated Interferon Therapy in Patients With Hepatitis B. Transplant Proc. 2015;47:2886-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Liu X, Shen Z, Zhang H, Liang J, Lin H. Interleukin-21 Is Associated with Early Antiviral Response in Patients with Hepatitis B e Antigen-Positive Chronic Hepatitis B and Nonalcoholic Fatty Liver Disease. J Interferon Cytokine Res. 2016;36:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Zhu LY, Wang YG, Wei LQ, Zhou J, Dai WJ, Zhang XY. The effects of the insulin resistance index on the virologic response to entecavir in patients with HBeAg-positive chronic hepatitis B and nonalcoholic fatty liver disease. Drug Des Devel Ther. 2016;10:2739-2744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kim DS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin Mol Hepatol. 2019;25:283-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Cho H, Chang Y, Lee JH, Cho YY, Nam JY, Lee YB, Lee DH, Cho EJ, Yu SJ, Kim YJ, Lee JM, Yoon JH. Radiologic Nonalcoholic Fatty Liver Disease Increases the Risk of Hepatocellular Carcinoma in Patients With Suppressed Chronic Hepatitis B. J Clin Gastroenterol. 2020;54:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Chen YC, Jeng WJ, Hsu CW, Lin CY. Impact of hepatic steatosis on treatment response in nuclesos(t)ide analogue-treated HBeAg-positive chronic hepatitis B: a retrospective study. BMC Gastroenterol. 2020;20:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Li J, Le AK, Chaung KT, Henry L, Hoang JK, Cheung R, Nguyen MH. Fatty liver is not independently associated with the rates of complete response to oral antiviral therapy in chronic hepatitis B patients. Liver Int. 2020;40:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Liang H, Liu Y, Jiang X, Zheng X, Tang J, Yang J, Zhuang H, Lai P, Peng L, Guo Z, Cai S, Luo D, Xu L, Mao Q, Chen S. Impact of Hepatic Steatosis on the Antiviral Effects of PEG-IFNα-2a in Patients with Chronic Hepatitis B and the Associated Mechanism. Gastroenterol Res Pract. 2020;2020:1794769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Tong X, Song Y, Yin S, Wang J, Huang R, Wu C, Shi J, Li J. Clinical impact and mechanisms of hepatitis B virus infection concurrent with non-alcoholic fatty liver disease. Chin Med J (Engl). 2022;135:1653-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Taliani G, Duca F, Lecce R, Livoli D, Pasquazzi C, De Bac C. Hepatic lidocaine metabolism in chronic hepatitis C virus hepatitis with or without steatosis. Hepatology. 1995;21:1760-1761. [PubMed] [DOI] [Full Text] |
| 21. | Leclercq I, Horsmans Y, Desager JP, Delzenne N, Geubel AP. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. J Hepatol. 1998;28:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Atalay R, Sayar S, Ayrancı FG, Çakmak Ş, Tanboğa İH, Doğanay L, Özdil K. Does Hepatic Steatosis Influence the Virological Response with Chronic Hepatitis B Patients Treated with Entecavir or Tenofovir Disoproxil Fumarate? Turk J Gastroenterol. 2022;33:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Losurdo G, Italy; Yesil A, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Guo X