Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.428
Peer-review started: December 10, 2023
First decision: December 28, 2023
Revised: January 25, 2024
Accepted: February 29, 2024
Article in press: February 29, 2024
Published online: March 27, 2024
Processing time: 107 Days and 18.3 Hours
Long-term abdominal drains (LTAD) are a cost-effective palliative measure to ma
To compare the effectiveness and safety of palliative LTAD and LVP in refractory ascites secondary to end-stage chronic liver disease.
A retrospective, observational cohort study comparing the effectiveness and safety outcomes of palliative LTAD and regular palliative LVP as a treatment for refractory ascites in consecutive patients with end-stage chronic liver disease followed-up at our United Kingdom tertiary centre between 2018 and 2022 was conducted. Fisher’s exact tests and the Mann-Whitney U test were used to compare qualitative and quantitative variables, respectively. Kaplan-Meier sur
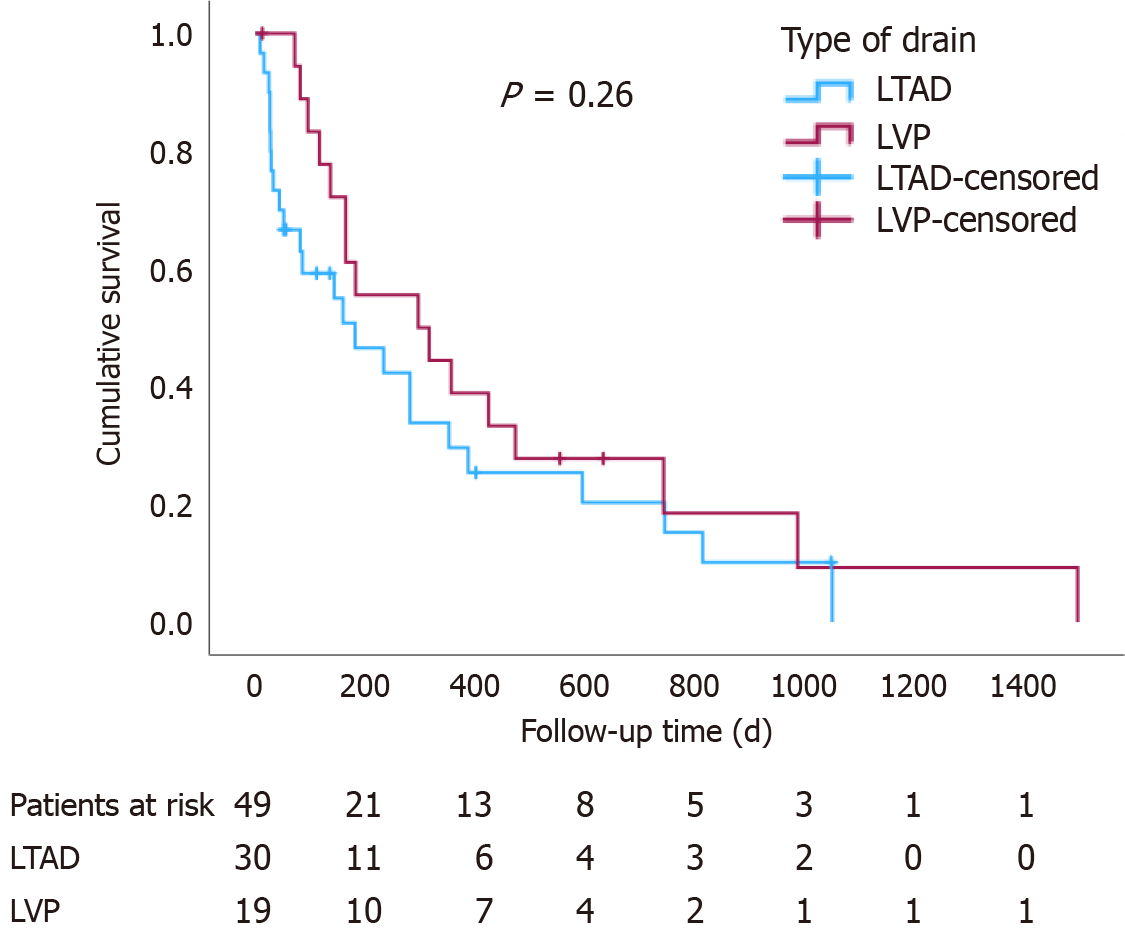
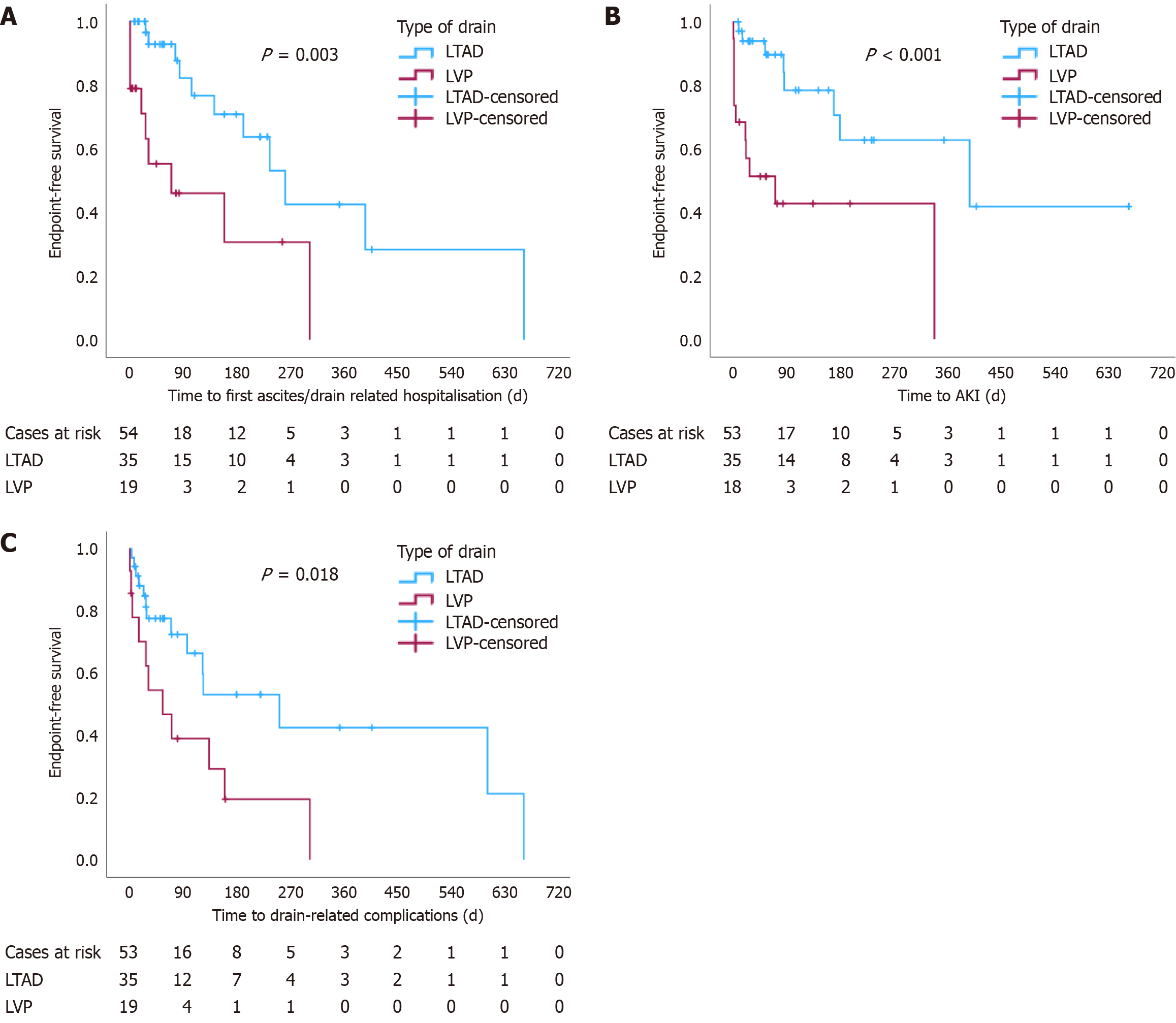
Thirty patients had a total of 35 indwelling abdominal drains and nineteen patients underwent regular LVP. The baseline characteristics were similar between the groups. Prophylactic antibiotics were more frequently prescribed in patients with LTAD (P = 0.012), while the incidence of peritonitis did not differ between the two groups (P = 0.46). The incidence of acute kidney injury (P = 0.014) and ascites/drain-related hospital admissions (P = 0.004) were significantly higher in the LVP group. The overall survival was similar in the two groups (log-rank P = 0.26), but the endpoint-free survival was significantly shorter in the LVP group (P = 0.003, P < 0.001, P = 0.018 for first ascites/drain-related admission, acute kidney injury and drain-related complications, respectively).
The use of LTAD in the management of refractory ascites in palliated end-stage liver disease is effective, safe, and may reduce hospital admissions and utilisation of healthcare resources compared to LVP.
Core Tip: The standard treatment of refractory ascites in palliated patients with end-stage liver disease is repeated large volume paracentesis (LVP) with albumin infusion. This study focuses on real-world data comparing the effectiveness and safety of long-term abdominal drains (LTAD) in comparison with LVP. The incidence of acute kidney injury, ascites and drain-related hospital admissions was lower in the LTAD group. There was no difference in the overall survival between the two groups, but time to acute kidney injury, first ascites/drain-related hospital admission and drain-related complications were shorter in the LTAD group.
- Citation: Kaur S, Motta RV, Chapman B, Wharton V, Collier JD, Saffioti F. Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease. World J Hepatol 2024; 16(3): 428-438
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/428.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.428
In Europe, liver-related mortality has risen from 2.3% of all deaths in 1990 to 3% in 2019[1]. Patients with advanced liver disease who are not eligible for transplant frequently need palliative care due to their high risk of death, high burden of symptoms, poor quality of life, and frequent hospitalizations. Early provision of palliative care can lead to improvements in quality of life and a reduction of the physical and psychological symptom burden, with the potential for reduced utilisation of healthcare resources and even improved survival for patients with serious illnesses[2]. Similarly, timely palliative care can improve health-related quality of life and reduce the need for hospitalisation of patients with advanced liver cirrhosis[3-5]. Ascites remains the most common complication in cirrhosis that necessitates hospitalisation, and progresses to refractory ascites (RA) in up to 30% of cases[6]. As many as 20% of patients presenting with ascites die within the first year of diagnosis[7]. RA is classified as either diuretic resistant or diuretic intractable and, following the onset of RA, patients have a median lifespan of 6-12 months in the absence of liver transplantation[8]. The current guidelines for the management of RA recommend large volume paracentesis (LVP)[8] with intravenous albumin infusion to decrease the risk of paracentesis-induced circulatory dysfunction[9]. Although LVP is considered safe, it requires patient-hospital contact as often as weekly and is associated with poor quality of life and malnutrition which, together, increase morbidity and mortality[8,10,11].
In selected patients with RA, transjugular intrahepatic portosystemic shunt (TIPS) and Automated Low-Flow Ascites Pump System [alfapump Ò (AP) system] are therapeutic alternatives to repeated LVP[10,11]. However, TIPS is contraindicated in patients with marked pulmonary arterial hypertension, heart failure, hepatic encephalopathy, coagulopathy, and elevated right or left heart pressures[12], whereas the alfapump® system is contraindicated in patients with obstructive uropathy, advanced sarcopenia, bed confinement and abdominal skin infections[13]. Clinical trials are still being conducted to determine the best candidates for the alfapump® device and its cost effectiveness[14].
Individuals with RA who are not eligible for TIPS or liver transplantation, in particular those with a limited life expectancy, should be considered for palliative care. Repeated LVP is the conventional main treatment in these cases[8].
Long-term abdominal drains (LTAD) are tunnelled drains inserted under local anaesthesia, that enable community nurses or trained caregivers to drain small amounts (1-2 L) of ascitic fluid at home, up to three times a week, thus reducing hospital visits and the use of healthcare resources[15,16]. They represent a reliable and cost-effective strategic option in the palliative management of recurrent malignant ascites and are currently being studied as a palliative measure in RA[16-19].
Absolute contraindications to the insertion of LTAD include loculated or chylous ascites, candidacy for liver trans
There are currently two types of LTAD available in the United Kingdom: PleurXTM, recently rebranded as PeriXTM (United Kingdom Medical, Basingstoke, United Kingdom) and Rocket® (Rocket Medical plc, Watford, United Kingdom)[20].
In 2022, the British Association for the Study of the Liver/British Society of Gastroenterology End of Life Special Interest Group published a consensus to help standardise the use of long-term abdominal drains in cirrhosis, including patient selection and community management[20]. A recent feasibility trial conducted in the United Kingdom compared palliative LTAD with LVP in refractory ascites secondary to advanced liver disease[18]. The trial yielded favourable results of LTAD in terms of efficacy, safety, acceptability by patients and clinical staff, and decreased healthcare resource utilisation[18]. However, pending the results of a national multicentre randomised controlled trial (REDUCe2, ISRCTN26993825), LTAD are currently not used as standard of care in advanced decompensated cirrhosis.
To contribute real-world data to the available scarce evidence, our study aimed to further investigate this subject by retrospectively evaluating the effectiveness and safety of LTAD in comparison with recurrent LVP, which is the current standard of care, in palliated patients with end-stage liver disease and RA followed-up at a United Kingdom tertiary centre.
This is a retrospective, single-centre, observational cohort study aimed at analysing the effectiveness and safety of palliative LTAD in comparison with repeat palliative LVP in patients with end-stage liver disease and RA followed-up at the Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom, between January 2018 and December 2022.
All consecutive patients above 18 years of age referred to palliative care owing to end-stage liver disease of any aetiology and RA defined according to the International Ascites Club criteria[21] (but without loculated, chylous, or malignant ascites), who were not eligible for TIPS and liver transplantation and had undergone palliative treatment of ascites at our centre during the 5-year study period with either repeat LVP or LTAD, were included.
Data was retrospectively collected from electronic patient records to avoid recollection bias, and included: age at diagnosis of RA, aetiology of liver disease, Child-Pugh score at the time of diagnosis of RA, ascites proteins (as a protein concentration of ≤ 15 g/L in ascitic fluid has been associated with an increased risk of developing spontaneous bacterial peritonitis[8]), use of diuretics, comorbidities, presence of hepatocellular carcinoma, presence of hepatic encephalopathy, date of LTAD insertion, perioperative complications, baseline creatinine, eGFR and sodium, date of referral to palliative care, use of prophylactic antibiotics, occurrence and date of cellulitis, peritonitis, other localised infections, sepsis, bacteria identified in the case of infection, leakage and bleeding on the site of the abdominal drain, drain displacement, blockage, hypotension, acute kidney injury (AKI), date and reason for hospital admissions, total number of hospital admissions, frequency of ascitic drainage per week, litres of ascites drained each time, need for additional LVP, date and cause of death. The presence of shortness of breath, abdominal pain/discomfort, anorexia and poor mobility before and after the insertion of LTAD were also evaluated.
All paracenteses were undertaken in our dedicated Hepatology Day Case Unit by two Hepatology Advanced Clinical Practitioners (ACPs). In preparation for the paracentesis drain insertion, patients received appropriate advice regarding withholding current anticoagulant treatment, according to the local protocols. Bloods, including full blood count and clotting, were taken within 5 d of the drain insertion. An international normalized ratio (INR) > 2 and/or a platelet count < 50 × 109 were considered contraindications to drain insertion requiring correction.
A safe insertion site was confirmed using bedside ultrasound and usually chosen slightly above the iliac crest, avoiding the inferior epigastric vessels and any visible vessels. Local anaesthetic (5-10 mL of 2% lidocaine) was injected and a Bonanno Safe-T-centesis 18G catheter (Becton, Dickinson and Company, Franklin Lakes, New Jersey, United States) was inserted with aseptic technique following a small incision with a sterile scalpel. Human albumin was administered (8-10 g/L of ascitic fluid removed)[8] to prevent paracentesis-induced circulatory dysfunction.
At the time of the first paracentesis, the ascitic fluid was tested for cell count, bacterial cultures, proteins, amylase, triglycerides and cytology. Ascitic neutrophil count was routinely tested at every subsequent LVP.
The drain was left in situ for up to six hours. Long-term antibiotic use was not routinely administered unless a history of spontaneous bacterial peritonitis (SBP) was present. The frequency of LVP varied depending on clinical need and patients’ symptoms.
Rocket® (Rocket Medical) LTAD insertion was performed in Interventional Radiology under local anaesthesia using ultrasound guidance, as previously described[22]. Bloods, including full blood count and clotting, were taken within 5 d of the drain insertion. Correction of clotting parameters was considered necessary prior to the procedure if INR > 2 and/or a platelet count < 50 × 109. Active anticoagulation was withheld before drain insertion according to the local protocols. At the time of LTAD insertion, ascitic cell count, bacterial cultures and proteins were assessed. Until 2020, the decision to commence prophylactic antibiotics was made on a case-by-case basis. Thereafter, all patients with a LTAD were prescribed long-term prophylactic ciprofloxacin 250 mg twice a day. The Hepatology ACPs arranged referral to district nurses for ascitic drainage of 1-2 litres twice a week in the community. Further follow-up in the Hepatology Day Case Unit was decided on a case-by-case basis.
The primary endpoint was the difference in overall survival between patients with LTAD and patients undergoing repeat LVP. Secondary endpoints were differences in the incidence of drain-related complications in the two groups and endpoint-free survival for first ascites/drain-related hospitalisation, time to AKI (defined as an absolute increase in serum creatinine of at least 26.5 micromol/L within 48 h or by a > 50% increase in serum creatinine from baseline within 7 d, or a urinary output of less than 0.5 mL/kg/h over > 6 h[23]) and time to drain-related complications between the two groups.
This study was conducted in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practice and approved by the Clinical Audit Division at Oxford University Hospitals NHS Foundation Trust (REC:8587). No ethical approval and informed consent were required for this study, as the information used was collected as part of the normal clinical care and data were collected retrospectively by the care team involved, and were anonymised.
Categorical variables were expressed as number and percentage. In the LTAD group, the percentage of patient-related outcomes was calculated using the total number of patients with LTAD as a denominator, while the percentage of drain-related complications was computed using the total number of drains inserted as a denominator. Time 0 of follow-up was considered the time of LTAD insertion (for the LTAD group) or the time of the first LVP since deemed palliative/referred to palliative care (for the LVP group). A complete-case analysis approach was used.
Kolmogorov-Smirnov and Shapiro-Wilk test of normality were used to assess the distribution of quantitative variables, which were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Fisher’s Exact test and Mann-Whitney U test were used to compare qualitative and quantitative variables, respectively.
Kaplan-Meier survival estimate curves were generated to stratify outcomes according to the type of drainage. Patients were censored at death or at the time of last encounter, in case they were alive on 31/12/2022 or lost to follow-up. Statistical analysis was performed using SPSS (v.29.0; IMB® SPSS®, Inc, Chicago, IL, United States). A two-sided P value < 0.05 was considered statistically significant.
Forty-nine patients met the criteria for this study. Thirty (61%) had LTAD and 19 (39%) were treated with repeated LVP only. The median individual follow-up after the decision to provide palliative care was 165 (IQR 360) d, for the whole cohort. Median follow-up with LTAD in place or undergoing LVP, age, Child-Pugh score, liver disease aetiology, baseline renal function, ascitic protein, and the presence of hepatocellular carcinoma were not significantly different between the two cohorts (Table 1).
| Baseline characteristics | LTAD (n = 30) | LVP (n = 19) | P value |
| Age, yr (SD) | 71 (11) | 66 (12) | 0.07 |
| Male sex | 18 (60) | 15 (79) | 0.22 |
| Child-Pugh score (IQR) | 9 (2) | 9 (2) | 0.48 |
| Child-Pugh class B/C | 24/11 (69/31) | 12/7 (63/37) | 0.76 |
| Aetiology (MASLD/ArLD/Viral/Other) | 9/12/2/7 (30/40/7/23) | 3/10/1/5 (16/53/5/26) | 0.69 |
| HCC | 5 (17) | 4 (21) | 0.46 |
| Proteins in ascites ≤ 15 g/L | 14 (47) | 9 (47) | 0.76 |
| Prophylactic antibiotics | 25/311 (81) | 8/19 (42) | 0.012 |
| Previous peritonitis | 2 (7) | 5 (26) | 0.86 |
| T2DM | 12 (40) | 8 (42) | 1.00 |
| Use of metformin | 3 (10) | 3 (16) | 0.66 |
| Use of diuretics | 18 (60) | 12 (63) | 1.00 |
| Use of NSBBs | 13 (43) | 4 (21) | 0.13 |
| Use of antihypertensive | 2 (7) | 2 (10) | 0.66 |
| Use of lactulose | 13 (43) | 13 (68) | 0.14 |
| Baseline creatinine (IQR) | 104 (68) | 84 (143) | 0.44 |
A total of 35 drains were placed in 30 patients. The amount of ascites drained at each home visit was 1-2 litres. The median time with drain in place was 135 (IQR 226) d. This group had a mean age of 71 ± 11 years; 18 (60%) patients were male. The most common aetiology of liver cirrhosis was alcohol (40%), followed by metabolic dysfunction-associated steatotic liver disease (MASLD, 30%). At the time of insertion of the indwelling drains, 9 (30%) patients were classified as Child-Pugh B8, 10 (33%) patients were classified as B9, and 9 (30%) patients were classified as Child-Pugh C. The insertion of LTAD was successful in all cases, with no procedure-related deaths or perioperative complications.
Among the 30 patients in the LTAD group, shortness of breath, abdominal discomfort, anorexia and poor mobility were present in 11 (37%), 21 (70%), 13 (43%), and 24 (80%), respectively. Following LTAD insertion, symptomatic relief of shortness of breath and abdominal pain was seen in 71% and 69% of cases, respectively, while anorexia and poor mobility resolved in 46% and 37% of cases, respectively.
Data on prophylactic antibiotics was available for 31 out of the 35 cases of LTAD insertion. Prophylactic antibiotics were prescribed in 25 (81%) cases (Table 1). Ciprofloxacin was the most common choice (88% of cases), while trime
Hospital admission due to ascites or drain-related complications occurred in 11 (37%) patients with LTAD. The median time to first admission following insertion of the LTAD was 44 (IQR 93) d.
Drain displacement occurred in 4 (11%) cases and prompted drain removal in 3 patients; catheter blockage occurred in 2 (5%) cases, requiring drain removal in 1. Two patients (5%) had self-limiting bleed at the drain site, which did not require hospitalisation or removal of the indwelling catheter. Four (11%) patients developed abdominal cellulitis, one of which was also diagnosed with concurrent bacterial peritonitis. Blood and ascitic cultures yielded multisensitive Gram-positive S. aureus for this patient. These infections were treated successfully with antibiotics and resolved without removal of the catheter. Five out of 30 (17%) patients developed bacterial peritonitis (total number of peritonitis episodes 10; 3 patients had a single episode, one patient had 3 episodes and one patient had 4 episodes), despite 2 of them receiving prophylaxis with ciprofloxacin and 1 with trimethoprim/sulfamethoxazole. Among these 5 patients, ascitic fluid cultures detected multisensitive E. coli, multisensitive S. aureus, multi-resistant coagulase negative staphylococci, E. cloacae and Pseudoglutamicibacter cumminsii. None of these cases resulted in death.
The 19 patients in the LVP group had a mean age of 66 ± 12 years, and 15 (79%) were male. Alcohol-related liver disease (53%) and MASLD (16%) were again the most common causes of chronic liver disease. Five (26%) patients were classified as Child-Pugh B8 and 4 as B7 (21%), while 7 (37%) patients were in Child-Pugh class C. The median drain frequency was 21 (IQR 7) d. The median follow-up time for these patients was 80 d (IQR 239).
Twelve (63%) of the 19 patients in this group were on diuretic treatment, and 8 (42%) were prescribed prophylactic antibiotics (Table 1). In particular, 4 (21%) patients were prescribed ciprofloxacin and 3 (16%) trimethoprim/sulfamethoxazole. One (5%) patient developed peritonitis whilst on ciprofloxacin and was then switched to trimethoprim/sulfa
Hospital admission due to ascites or drain-related complications occurred in 13 (68%) patients undergoing LVP, with a median time to first admission of 7.5 (IQR 35) d. Two (11%) patients had drain-related cellulitis, 1 of which required hospitalisation for concurrent confusion. One (5%) LVP was complicated by abdominal wall hematoma requiring interventional radiology-guided embolisation of the bleeding vessel. Five (28%) patients developed bacterial peritonitis despite receiving antibiotic prophylaxis, i.e., 4 patients with ciprofloxacin and 1 with trimethoprim/sulfamethoxazole. In 2 cases, these infections resulted in death. Ascitic cultures identified E. coli in one case, while in another case there was no growth despite elevated white cell count on the ascitic fluid and the presence of symptoms compatible with peritonitis. Streptococcus species (S. orallis, S. gordonii and S. anginosus) were isolated in the remaining 3 cases.
The comparison of the outcomes of interest in the two cohorts is reported in Table 2. Long-term prophylactic antibiotics were more frequently prescribed in the LTAD group compared to the LVP group (81% vs 42%; P = 0.012). The incidence of peritonitis did not differ between the two groups (P = 0.46).
| Outcomes | LTAD (n = 30) | LVP (n = 19) | P value |
| Median survival, d | 124 (330) | 297 (438) | 0.06 |
| Median follow-up (with drain in place/undergoing LVP), d | 135 (226) | 80 (239) | 0.98 |
| Ascites/drain related admissions | 11 (37) | 17 (89) | 0.004 |
| Time to first hospitalisation, (IQR), d | 44 (93) | 10 (35) | 0.002 |
| AKI | 8 (27) | 11 (58) | 0.014 |
| Drain-related complications | 14 (47) | 11 (58) | 0.06 |
| Patients with peritonitis | 5 (17) | 5 (26) | 0.46 |
| Total No. of peritonitis episodes | 10 (33) | 5 (26) | 0.98 |
| Cellulitis | 4 (13) | 2 (10) | 1.00 |
| Site leakage | 12 (40) | 2 (10) | 0.10 |
| Bleeding of drain site | 2 (7) | 1 (5) | 1.00 |
| Hypotension | 6 (20) | 4 (21) | 0.71 |
Despite a similar use of diuretics, non-selective beta-blockers, antihypertensive, metformin and laxatives in the two groups (Table 1; concomitant pharmacological treatments for individual patients are listed in Supplementary Table 1), the incidence of AKI was significantly lower in patients with LTAD (P = 0.014). Furthermore, ascites/drain-related hospital admissions occurred less frequently in the LTAD cohort (P = 0.004) (Table 2). Median time to first hospitalisation was also significantly longer in these patients, compared to the LVP cohort (44 vs 10 d, respectively; P = 0.002).
Other clinical endpoints, such as cellulitis, peritonitis, site leakage, bleeding at drain site and hypotension were not significantly different between the groups (Table 2).
The overall survival (since palliation) was not significantly different between the two groups (log-rank P = 0.26), Figure 1. Nevertheless, endpoint-free survival was significantly shorter in the LVP group for time to first ascites/drain-related hospitalisation (P = 0.003), time to AKI (P < 0.001) and time to the development of drain-related complications (P = 0.018) (Figure 2).
A “safety” composite endpoint including (1) Death secondary to drain-related complications; (2) Bleeding at the insertion site; (3) Bacterial peritonitis; and (4) Cellulitis was also compared between the two cohorts. Again, this was significantly shorter for the LVP group (log-rank P = 0.018) (data not shown).
In our single-centre retrospective evaluation of the use of palliative LTAD in comparison with repeat palliative LVP for the management of RA in patients with end-stage liver disease, LTAD was associated with a reduced incidence of AKI, as well as a reduced number of ascites- or drain-related hospital admissions and time to first hospitalisation. Time to the development of AKI and of drain-related complications was also significantly shorter in patients with LTAD.
The scarcity of real-world data on indwelling abdominal drains precludes international societies from making strong recommendations on their use. In its guidelines on the outpatient therapy of cirrhosis, the British Society of Gastroenterology (BSG) has mentioned long-term abdominal drains as an experimental approach that may be considered for patients with advanced liver disease in palliative care[24].
Following promising results from the REDUCe trial[18], a 12-wk feasibility randomised controlled trial comparing the use of LVP (19 patients) vs LTAD (17 patients) in RA due to end-stage liver disease, which showed preliminary evidence that LTAD are acceptable and safe in end-stage liver disease and lead to a reduction in healthcare resource utilisation, the use of LTAD is currently being evaluated in the REDUCe2 study, a United Kingdom multicentre randomised controlled clinical trial. To our knowledge, our study represents the largest set of real-world data comparing the use of LVP vs Rocket® indwelling peritoneal catheters in a cohort of palliated cirrhotic patients with RA.
We found no significant difference in the incidence of peritonitis between the 2 groups. All the microorganisms identified were typical for SBP. This is likely the consequence of the more frequent administration of prophylactic antibiotics in patients with indwelling catheters compared to those undergoing LVP (83% vs 42%, P = 0.012). In a systematic review from 2019 assessing the use of LTAD in end-stage liver disease[25], the rates of bacterial peritonitis (BP) varied from 0% to 42% across individual studies, with an overall combined rate of 17%, similarly to our study findings. However, it is unclear whether all reported cases of BP in this systemic review were true BP or there were cases of positive bacterial cultures secondary to colonisation. The more regular follow-up schedule in the setting of a clinical trial and the universal treatment with prophylactic antibiotics in both groups are likely accountable for the lower rates of peritonitis recorded in the REDUCe study (6% vs 11% in the LTAD vs LVP group, respectively)[18], compared to real-world data. In the trial, the LTAD group did not show an increased rate of peritonitis compared to the LVP group. The incidence of peritonitis reported in our study may further decrease in the future, as since 2020, antibiotic prophylaxis is prescribed to all palliated patients with RA undergoing LTAD insertion at our centre, as per BSG recommendation[20].
When comparing the occurrence of complications between the two treatment modalities, there was a significantly lower rate of AKI in the LTAD group (P = 0.014) despite similar use of diuretics between the two cohorts. Previous studies have focused on changes in creatinine over time, which hinders a direct comparison between our findings and other published reports[25]. Contributing factors to the higher incidence of AKI in the LVP group are likely a higher rate of circulatory dysfunction following drainage of larger quantities of ascites (despite regular administration of intravenous albumin), as well as the higher rate of ascites and drain-related admissions seen in this group, underlining the multi
Episodes of leakage and cellulitis were comparable in both groups. These were typically managed with minimal medical intervention and did not require LTAD removal in any of the cases. Though higher rates of site leakage and cellulitis were noted in the LTAD group in our study (34% and 11%, respectively) compared to the aforementioned systematic review (8% and 6%, respectively)[25], a comparable incidence of cellulitis/leakage (41% collectively) was observed in the REDUCe study[18].
There was no significant difference in the overall survival between the LVP and LTAD groups. However, the endpoint-free survival for all other time-related events (time to first ascites/drain-related hospitalisation, time to AKI, and time to drain-related complications) was significantly longer for patients with LTAD.
Symptomatic relief of shortness of breath and abdominal discomfort was seen in 70% of cases following LTAD placement, while anorexia resolved in 50% of patients. These findings corroborate the results of the REDUCe trial, showing that LTAD improves quality of life for patients with RA. Furthermore, the trial has shown that indwelling drains are also cost-effective, as they reduce healthcare resource utilisation and inpatient burden. In fact, median fortnightly total costs were about 15% lower in the LTAD group, as the overall hospital costs were higher in the LVP group[18]. We did not undertake a cost analysis, as our hospital and community databases are not merged and tariffs for community support workers and community costs were not available. As the REDUCe trial was also undertaken in the United Kingdom setting, we would not expect significant differences with regards to costs, in our study.
A consensus on the palliative management of patients with decompensated cirrhosis and RA was published only in 2023[24]. Until then, the treatment of these patients exclusively relied upon local standard operating protocols and the discretion of the individual specialist teams. Accordingly, despite our cohort coming from a single centre, the lack of a unified approach may have resulted in differences in antibiotic prophylaxis, time of referral for LTAD and/or specialist palliative treatment, and management of complications associated with RA. Timing and duration of follow-up might have also led to differences in patients’ management, as new technologies and evidence arose between 2018 and 2022. Moreover, the type and dose of diuretics might have changed over time for each individual patient (according to symptoms, creatinine and electrolyte levels), and this may represent a confounding factor. The variable frequency of LVP and amount of ascites removed on each occasion, as well as the concomitant use of other medications (such as non-selective beta-blockers, metformin, antihypertensive and laxatives, although these were not significantly different between the two groups), or possible episodes of hepatic encephalopathy, all of which can favour the occurrence of AKI, are further potential confounding factors. Given the limited sample size, multivariate regression analysis was deemed unsuitable.
The single-centre observational design and the relatively small sample size are limitations of our study that should be taken into consideration in interpreting the results. Larger, more heterogeneous cohorts and randomised controlled trials are needed to validate our findings.
In conclusion, our study demonstrates that the use of palliative LTAD is effective and overall safe for the management of RA in patients with end-stage liver disease. Compared to LVP, LTAD may reduce the incidence of renal dysfunction, hospital admissions and healthcare resource utilisation. Results are eagerly awaited from a randomised controlled trial currently recruiting in the United Kingdom, comparing LVP and LTAD.
Repeat large volume paracentesis (LVP) with albumin infusion is currently the standard treatment for the management of refractory ascites (RA) in patients with end-stage liver disease who are not eligible for transjugular intrahepatic portosystemic shunt or liver transplant, including those on a palliative care pathway. This treatment requires frequent patient-hospital contact and is associated with poor quality of life. Long-term abdominal drains (LTAD) are a reliable and cost-effective strategic option in the palliative management of recurrent malignant ascites, but are currently not routine practice in patients with end-stage liver disease and RA. The safety and cost-effectiveness of LTAD are currently being studied in this setting, with preliminary encouraging results.
As the use of LTAD may improve the quality of life of palliated patients with end-stage liver disease and RA, it is important to assess their utility and safety in this setting. We aimed to provide real-world data from our own experience to the available scarce evidence.
The objective of this study was to retrospectively assess the effectiveness and safety of LTAD in comparison with recurrent LVP for the management of ascites in palliated patients with end-stage liver disease and RA.
This observational study included 49 consecutive patients with end-stage liver disease and RA requiring palliative drainage of ascites. Overall survival, the incidence of drain-related complications and endpoint-free survival for first ascites/drain-related hospitalisation, time to acute kidney injury and time to drain-related complications were compared between 30 patients who were managed with LTAD and 19 patients who underwent LVP.
The study found similar incidence of peritonitis between the two groups, although prophylactic antibiotics were more frequently prescribed in patients with LTAD. However, the incidence of acute kidney injury, ascites- and drain-related hospital admissions was lower in the LTAD group. There was no difference in the overall survival between the two groups, but time to acute kidney injury, first ascites/drain-related hospital admission and drain-related complications were shorter in the LTAD group.
The use of palliative LTAD for the management of RA in patients with end-stage liver disease appears to be effective and overall safe. Compared to LVP, the use of LTAD in this setting may reduce the incidence of renal dysfunction, hospital admissions and healthcare resource utilisation.
Larger, more heterogeneous cohorts and randomised controlled trials are needed to validate the findings of this study.
| 1. | Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, Pryke R, Hutchinson SJ, Sangro B, Martin NK, Cecchini M, Dirac MA, Belloni A, Serra-Burriel M, Ponsioen CY, Sheena B, Lerouge A, Devaux M, Scott N, Hellard M, Verkade HJ, Sturm E, Marchesini G, Yki-Järvinen H, Byrne CD, Targher G, Tur-Sinai A, Barrett D, Ninburg M, Reic T, Taylor A, Rhodes T, Treloar C, Petersen C, Schramm C, Flisiak R, Simonova MY, Pares A, Johnson P, Cucchetti A, Graupera I, Lionis C, Pose E, Fabrellas N, Ma AT, Mendive JM, Mazzaferro V, Rutter H, Cortez-Pinto H, Kelly D, Burton R, Lazarus JV, Ginès P, Buti M, Newsome PN, Burra P, Manns MP. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 448] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 2. | Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5180] [Article Influence: 323.8] [Reference Citation Analysis (0)] |
| 3. | Baumann AJ, Wheeler DS, James M, Turner R, Siegel A, Navarro VJ. Benefit of Early Palliative Care Intervention in End-Stage Liver Disease Patients Awaiting Liver Transplantation. J Pain Symptom Manage. 2015;50:882-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Ufere NN, Robinson B, Donlan J, Indriolo T, Bloom J, Scherrer A, Mason NM, Patel A, Lai JC, Chung RT, Volandes A, El-Jawahri A. Pilot Randomized Controlled Trial of an Advance Care Planning Video Decision Tool for Patients With Advanced Liver Disease. Clin Gastroenterol Hepatol. 2022;20:2287-2295.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Shinall MC Jr, Karlekar M, Martin S, Gatto CL, Misra S, Chung CY, Porayko MK, Scanga AE, Schneider NJ, Ely EW, Pulley JM, Jerome RN, Dear ML, Conway D, Buie R, Liu D, Lindsell CJ, Bernard GR. COMPASS: A Pilot Trial of an Early Palliative Care Intervention for Patients With End-Stage Liver Disease. J Pain Symptom Manage. 2019;58:614-622.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Salerno F, Borroni G, Moser P, Badalamenti S, Cassarà L, Maggi A, Fusini M, Cesana B. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol. 1993;88:514-519. [PubMed] |
| 7. | Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1990] [Article Influence: 248.8] [Reference Citation Analysis (2)] |
| 9. | Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 415] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rosi S, MacDonald S, Malago M, Stepanova M, Younossi ZM, Trepte C, Watson R, Borisenko O, Sun S, Inhaber N, Jalan R. Alfapump® system vs. large volume paracentesis for refractory ascites: A multicenter randomized controlled study. J Hepatol. 2017;67:940-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Hudson B, Round J, Georgeson B, Pring A, Forbes K, McCune CA, Verne J. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. Lancet Gastroenterol Hepatol. 2018;3:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | American College of Radiology, Society for Pediatric Radiology, and Society for Interventional Radiology. ACR-SIR-SPR practice parameter for the creation of a transjugular intrahepatic portosystemic shunt (TIPS), Amended 2014 (Resolution 39). Practice Parameters and Technical Standards, 2014: 1-22. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Aagaard NK, Malago M, De Gottardi A, Thomas M, Sauter G, Engelmann C, Aranovich D, Cohen M, Thévenot T, Ehmann T, Capel J, Angeli P, Jalan R, Stirnimann G. Consensus care recommendations for alfapump(®) in cirrhotic patients with refractory or recurrent ascites. BMC Gastroenterol. 2022;22:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Weil-Verhoeven D, Di Martino V, Stirnimann G, Cervoni JP, Nguyen-Khac E, Thévenot T. Alfapump(®) implantable device in management of refractory ascites: An update. World J Hepatol. 2022;14:1344-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 15. | Fleming ND, Alvarez-Secord A, Von Gruenigen V, Miller MJ, Abernethy AP. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J Pain Symptom Manage. 2009;38:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | White J, Carolan-Rees G. PleurX peritoneal catheter drainage system for vacuum-assisted drainage of treatment-resistant, recurrent malignant ascites: a NICE Medical Technology Guidance. Appl Health Econ Health Policy. 2012;10:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Rocket Medical PLC. Indwelling Peritoneal Catheter (IPC) Insertion Kit R54400-16-40 (ZDOCK249 IFU). [DOI] [Full Text] |
| 18. | Macken L, Bremner S, Gage H, Touray M, Williams P, Crook D, Mason L, Lambert D, Evans CJ, Cooper M, Timeyin J, Steer S, Austin M, Parnell N, Thomson SJ, Sheridan D, Wright M, Isaacs P, Hashim A, Verma S. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther. 2020;52:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Cooper M, Pollard A, Pandey A, Bremner S, Macken L, Evans CJ, Austin M, Parnell N, Steer S, Thomson S, Hashim A, Mason L, Verma S. Palliative Long-Term Abdominal Drains Versus Large Volume Paracentesis in Refractory Ascites Due to Cirrhosis (REDUCe Study): Qualitative Outcomes. J Pain Symptom Manage. 2021;62:312-325.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Macken L, Corrigan M, Prentice W, Finlay F, McDonagh J, Rajoriya N, Salmon C, Donnelly M, Evans C, Ganai B, Bedlington J, Steer S, Wright M, Hudson B, Verma S; British Association for the Study of the Liver/British Society of Gastroenterology (BASL/BSG) End of Life Special Interest Group. Palliative long-term abdominal drains for the management of refractory ascites due to cirrhosis: a consensus document. Frontline Gastroenterol. 2022;13:e116-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1022] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 22. | Macken L, Mason L, Evans C, Gage H, Jordan J, Austin M, Parnell N, Cooper M, Steer S, Boles J, Bremner S, Lambert D, Crook D, Earl G, Timeyin J, Verma S. Palliative long-term abdominal drains versus repeated drainage in individuals with untreatable ascites due to advanced cirrhosis: study protocol for a feasibility randomised controlled trial. Trials. 2018;19:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3717] [Article Influence: 265.5] [Reference Citation Analysis (0)] |
| 24. | Mansour D, Masson S, Corless L, Douds AC, Shawcross DL, Johnson J, Leithead JA, Heneghan MA, Rahim MN, Tripathi D, Ross V, Hammond J, Grapes A, Hollywood C, Botterill G, Bonner E, Donnelly M, McPherson S, West R. British Society of Gastroenterology Best Practice Guidance: outpatient management of cirrhosis - part 2: decompensated cirrhosis. Frontline Gastroenterol. 2023;14:462-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Macken L, Hashim A, Mason L, Verma S. Permanent indwelling peritoneal catheters for palliation of refractory ascites in end-stage liver disease: A systematic review. Liver Int. 2019;39:1594-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu M, China S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM