Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.304
Peer-review started: October 20, 2023
First decision: December 25, 2023
Revised: January 11, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 27, 2024
Processing time: 159 Days and 1.4 Hours
Studies have shown that non-alcoholic fatty liver disease (NAFLD) may be associated with sleep disorders. In order to explore the explicit relationship between the two, we systematically reviewed the effects of sleep disorders, especially obstructive sleep apnea (OSA), on the incidence of NAFLD, and analyzed the possible mechanisms after adjusting for confounding factors. NAFLD is independently associated with sleep disorders. Different sleep dis
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is independently associated with sleep disorders. Different sleep dis
- Citation: Bu LF, Xiong CY, Zhong JY, Xiong Y, Li DM, Hong FF, Yang SL. Non-alcoholic fatty liver disease and sleep disorders. World J Hepatol 2024; 16(3): 304-315
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/304.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.304
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases, with an estimated global prevalence of 25%[1]. Its epidemiological and demographic characteristics vary around the world, and are positively correlated with obesity prevalence[2]. Due to unhealthy lifestyle behaviors among the population in China, the prevalence of NAFLD has risen sharply from 23.8% in 2001 to 32.9% in 2018, gradually replacing hepatitis B as the main cause of chronic liver disease[3]. NAFLD is a systemic disease characterized by steatosis and abnormal accumulation of fat in hepatic parenchymal cells, metabolically stressed liver damage closely related to insulin resistance (IR), as well as certain genetic factors, possessing a complex multifactorial pathogenesis and heterogeneous clinical manifestations[4,5]. Non-alcoholic steatohepatitis (NASH), a subtype of NAFLD, is a potential progressive liver disease that may lead to cirrhosis, hepatocellular carcinoma, and even death[6]. Various extrahepatic manifestations such as chronic kidney disease, cardiovascular disease and obstructive sleep apnea (OSA), is also associated with NAFLD, imposing a substantial burden and economic impact on patients and society[7]. In the past decades, studies have found that sleep disorders might facilitate the development of NAFLD accompanied by obesity, inflammation, IR, as well as glucose or lipid metabolic disorders[8]. The underlying mechanism may be related to the increased secretion of stress hormones (such as cortisol and catecholamines) by activating the hypothalamic-pituitary-adrenal axis, thereby increasing the risk of the metabolic syndrome[9]. Nowadays, there is an increasing interest in understanding whether different sleep patterns can serve as causative factors for NAFLD. Current research on sleep stage changes in NAFLD patients shows inconsistent findings. Some studies indicate a possible decrease in the percentage of rapid-eye-movement sleep in NAFLD patients[10]. Additionally, other studies suggest changes in non-rapid eye movement sleep structure, such as a potential decrease in the proportion of slow wave sleep. Further large-scale research is needed to gain a better understanding of these sleep characteristics in NAFLD patients[11]. In this review, the association between different sleep traits and NAFLD is investigated, the recent advances concerning the correlations between NAFLD and sleep disorders are summed up, the complicated and interrelated relationship between OSA and NAFLD are elucidated, and their identical and different mechanisms and clinical features are discussed. Furthermore, the effect of continuous positive airway pressure (CPAP) treatment on OSA is also summarized, aiming to provide current and future therapeutic implications for NAFLD.
The pathogenesis of NAFLD is complex and multi-factorial. Previous studies have confirmed its positive correlations with metabolic diseases such as obesity, IR, metabolic syndrome, and type 2 diabetes. The pathogenesis of NAFLD has frequently been probed and two hypotheses were successively proposed, namely the early proposed "two-hit" model and the current "multiple-hit theory". The "two-hit" model believes that IR and abnormal hepatic lipid accumulation is the first hit, while the oxidative stress and inflammation is the second hit[12]; however, because other alternative factors including glucose and lipid metabolism disorders, intestinal flora disorder and epigenetic regulation were confirmed to be involved in NAFLD development, the "multiple-hit theory" has been widely accepted nowadays[13]. In addition, a dysregulated circadian rhythm due to sleep mode changes have been implicated in the pathogenesis of NAFLD[14,15]. As one of the most reliable markers of the circadian rhythm, melatonin (MT) is also involved in the pathogenesis of NAFLD. It is known that MT promotes sleep, circadian rhythms, and neuroendocrine processes. Current evidence suggests that MT protects against liver damage by inhibiting oxidation, inflammation, hepatic stellate cell proliferation, and hepatocyte apoptosis, thus inhibiting the progression of NAFLD[16]. Ren et al[17] observed that MT could ameliorate high-fat diet/chronic intermittent hypoxia-induced hepatocellular damage by activating sirtuin 1-mediated autophagy signaling.
In this review, we see sleep duration, daytime napping, daytime sleepiness, sleep quality and sleep habits as sleep-related traits (Table 1). A randomized controlled trial indicates a causal relationship between sleep characteristics and NAFLD. The onset of NAFLD is the result of changes in sleep patterns, whereas alterations in sleep characteristics are not the cause of NAFLD. The causal relationship between the two is unidirectional[18]. Recent studies concerning the relationship between sleep duration and NAFLD suggest that short sleep duration and long daytime naps are risk factors for NAFLD[19-21]. A cohort study has shown that in young adults, short sleep duration is independently associated with an increased risk of incident NAFLD, regardless of the presence of intermediate/high fibrosis scores[22]. Furthermore, a cross-sectional study found a decreasing trend in the proportion of NAFLD in pace with increased sleep duration in men, whereas in women, the proportion of NAFLD displayed a U-shaped distribution, with the lowest in the group (6-7 h of sleep) and the highest in the group (≤ 6 h or ≥ 8 h of sleep)[23]. Similarly, a meta-analysis of the relationship between sleep duration (or quality) and NAFLD incidence showed that both short sleep duration (≤ 6 h) and long sleep duration (≥ 8 h) may increase the risk of NAFLD, and the incidence of NAFLD increases as the sleep duration decreased[24,25]. Accordingly, a case-control study on NAFLD demonstrated that optimal sleep duration (7-9 h/d) is negatively associated with IR and liver stiffness in patients with NAFLD[26]. Taken together, too short or too long sleep duration may both increase the risk of NAFLD in both men and women.
| Items | Correlations | |
| Sleep | Sleep duration | Short sleep duration and long daytime naps are risk factors for NAFLD[19-22] |
| Moderate sleep duration reduces the risk of NAFLD[23-26] | ||
| Excessive sleep duration increases the risk of NAFLD[23,25,30,31] | ||
| Sleep quality | Poor sleep quality was significantly associated with an increased risk of NAFLD[19,25] | |
| Sleep disorders | Insomnia and daytime sleepiness | Increases the risk of NAFLD in participants with insomnia or daytime sleepiness[35,38,39] |
| Sleep-wake disturbance | Raises the risk of NAFLD through obesity, IR, disorder of glucose-lipid metabolism and inflammation[40,42-45] | |
In addition, there were differences in the association between sleep duration and NAFLD in different populations: (1) Taking gender into account, a community-based longitudinal cohort study concluded that short sleep duration reduced the risk of NAFLD in men but had no risk in women[27]. Liu et al[28] found that sleep duration is an independent influencing factor for male NAFLD. The risk of NAFLD decreases with an increase in sleep duration in males, but there is also no significant correlation observed in females. A cross-sectional survey involving 4828 participants suggested that sleep quality was associated with NAFLD, and there were also gender differences[29]; and (2) Taking age into account, excessive nighttime sleep duration was associated with a moderately increased risk of NAFLD in a retrospective study targeted at middle-aged or elderly men in China[30]. In addition, in another cohort study of middle-aged or elderly people in South Korea, a positive correlation was also found between excessive sleep duration and elevated NAFLD scores[31].
A population-based study showed that NAFLD is independently associated with sleep disorders after the adjustment of age, gender, and ethnicity[32]. Sleep disorders are present in NAFLD regardless of underlying cirrhosis[33]. The prevalence of sleep disorders was significantly higher in individuals with NAFLD compared to controls; while the prevalence of NAFLD was higher in individuals with sleep disorders compared to good sleepers[34]. Common sleep disorders associated with NAFLD include insomnia, daytime sleepiness, sleep-wake disorders and sleep-disordered breathing such as OSA (Table 2).
| Confounders | OR/HR (95%CI) | OR/HR (95%CI) adjustments for BMI | Ref. |
| Age, Alcohol, Smoking, Physical activity, Blood pressure, BMI, Marriage, Education level, Presence of job, Loud snoring, and Sleep apnea | M 1.28 (1.13-1.44) | M 1.03 (0.90-1.19) | Kim et al[19], 2013 |
| W 1.71 (1.38-2.13) | W 1.59 (1.23-2.05) | ||
| Age, BMI, METs, and IR | 1.31 (1.10-1.56) | 1.29 (1.04-1.60) | Peng et al[20], 2017 |
| Age, BMI, Alcohol, Smoking, ALT, HDL-C, TG, Diabetes, Blood pressure, Physical activity | M 1.39 (1.13-1.72) | M 2.57 (1.88-3.52) | Okamura et al[21], 2019 |
| W 1.46 (1.05-2.04) | W 9.38 (5.84-15.1) | ||
| Age, BMI, Smoking and Physical activity | M 0.98 (0.62-1.54) | M 1.18 (0.67-2.08) | Imaizumi et al[23], 2015 |
| W 1.44 (1.06-1.96) | W 1.38 (0.95-2.01) | ||
| Age, Sex, BMI, Smoking, Adiponectin, and TNF-α | 1.66 (Not available) | 1.62 (Not available) | Katsagoni et al[26], 2017 |
| Age, Sex, BMI, HDL, Smoking, and Physical activity | 2.230 (1.304-3.813) | 1.462 (1.029-2.077) | Kim et al[31], 2019 |
| Age, Smoking, BMI and Physical activity | 1.13 (0.58–2.19) | 0.93 (0.41–2.10) | Takahashi et al[29], 2020 |
| BMI, Salt intake, Physical activity, and MetS | 2.83 (2.63-3.05) | 1.64 (1.35-2.00) | Wang et al[50], 2020 |
| BMI and Abdominal obesity | 2.42 (2.36-2.48) | 1.21 (1.17-1.26) | Chung et al[51], 2021 |
| BMI, Abdominal obesity, METs, and IR | 4.89 (3.08–5.98) | 1.78 (1.11–6.82) | Nobili et al[79], 2014 |
| Sex, Age, BMI, IR and METs | 4.20 (1.88-9.37) | 3.85 (1.35-10.94) | Fu et al[55], 2022 |
| BMI and Abdominal obesity | 1.45 (1.03-1.98) | 1.22 (1.02-1.45) | Krolow et al[62], 2021 |
A meta-analysis of seven studies showed that people with insomnia or excessive daytime sleepiness have an increased risk of NAFLD[35]. Moreover, patients with NAFLD may have more severe daytime sleepiness and shorter sleep duration[36]. A mendelian randomization demonstrated that trouble getting up in the morning and insomnia were associated with an increased risk of NAFLD[37]. Similarly, a case-control study found that nearly 30% of patients with biopsy-proven NAFLD confirmed insomnia, and the prevalence of NAFLD in insomnia patients was significantly higher than that in non-insomnia patients[38]. Furthermore, daytime sleepiness is significantly linked to the biochemical and histologic surrogates of NAFLD severity. It is not only positively correlated with liver enzymes and IR, independent of cirrhosis, but also positively correlated with the degree of fibrosis[39].
Sleep-wake disorder, also known as non-24-h sleep-wake rhythm disorder, is a circadian rhythm sleep-wake disorder characterized by an inability to entrain to the 24-h environment. Sleep-wake disorders may increase the risk of NAFLD in patients suffered from obesity, IR, inflammation, and disorders in glucose or lipid metabolism, resulting in weight gain by increasing the food-sensitive dopaminergic activity[40] and the circulating concentration of growth hormone-releasing peptide[41]. It is well-known that IR plays a central role in the progression of hepatic steatosis and fibrosis. Therefore, IR may be a major intersection between sleep-wake disorders and NAFLD[42]. In addition, sleep-wake disorders can also facilitate glycometabolism, promote lipid mobilization in adipose tissue by increasing cortisol hormone concentrations and weakening the tissue response to insulin, and accelerate the transport of free fatty acids to the liver[43]. Increased sympathetic nervous system and adrenal cortical activity may lead to the adverse metabolic effects of sleep-wake disorders. In a comparative study, the sleep of healthy volunteers was experimentally fragmented at all stages using auditory and mechanical stimuli. After two nights of sleep fragmentation, the results indicated that insulin sensitivity and glucose effectiveness, i.e., the ability of glucose to mobilize itself was independent of the insulin response, were both decreased. In addition, morning cortisol levels were elevated, and the sympathetic nervous system was excited[44]. Sleep-wake disorders are also associated with elevated pro-inflammatory factors such as interleukin (IL)-1β, which are involved in the development of liver inflammation promoting NAFLD[45].
OSA is the most common sleep breathing disorder. A general population-based polysomnography study showed that the incidence of mild OSA was estimated to be 59% in men but 33% in women, while the incidence of moderate to severe OSA was estimated to be 30% in men but 13% in women[46]. It is characterized by episodes of apnea, hypopnea and sleep fragmentation (SF) due to restricted airflow in the collapsed upper airway during sleep[47]. It has been shown that SF-induced intermittent hypoxia (IH) and sleep deprivation are associated with IR and metabolic dysfunction, as well as adipose tissue dysfunction which are thought to play key roles in the metabolic abnormalities of OSA[48,49]. Snoring is the direct consequence of airway collapse in OSA patients, which is independently and positively associated with a higher incidence of NAFLD[50].
There is growing evidence that OSA is involved in the development of NAFLD with IH acting as the most important connecting factor[51,52]. The IH of OSA may also be involved in the progression of NAFLD by affecting the level of liver enzymes. It increased hepatic production of lysyl oxidase, an enzyme that cross-links collagen, and may serve as a biomarker of liver fibrosis in patients with severe obesity and NAFLD[53]. In animal models, IH can directly induce hepatic steatosis by repeating brief hypoxia and reoxygenation simulating OSA[54]. Fu et al[55] found that IH caused by OSA may aggravate NAFLD and lead to a higher risk of NASH in patients with obesity.
There are many studies on the aspects of OSA affecting NAFLD. Severe OSA is more likely to be associated with significant liver disease, one possible reason being its independent correlation with increased liver stiffness[56]. A systematic review and meta-analysis demonstrated that OSA is associated with an increased risk of NAFLD, NASH and fibrosis[57]. Jin et al[58] found significant correlations between OSA and NAFLD in terms of hepatic steatosis, lobular inflammation and fibrosis, suggesting that OSA may be involved in the progression of NAFLD through elevated liver enzyme levels and hepatic histological changes. In the presence of obesity, patients with OSA may potentially contribute to liver injury in NAFLD through IR and systemic inflammation[59]. Another case-control study showed that in the absence of considering obesity and metabolic syndrome, patients with OSA have a significantly high incidence of NAFLD and exhibit notable hepatic fibrosis[60]. After excluding the confounding factor of obesity, the severity of OSA emerges as an independent risk factor for both NAFLD and liver fibrosis[61]. Krolow et al[62] found that patients with moderate to severe OSA had an increased risk of hepatic fibrosis after adjusting for obesity level. Kim et al[63] demonstrated that the severity of OSA increased with the prevalence of NAFLD regardless of the gender. Also, compared to non-obese OSA patients, obese patients with OSA were more prone to developing NAFLD. In addition, regarding hepatic steatosis, there was no association between liver fibrosis and the severity of OSA. A retrospective analysis suggested that age and obesity predicted high liver fibrosis risk as assessed by noninvasive scoring systems, but not OSA severity[64]. In a cross-sectional study of human subjects, the risk of hepatic steatosis increased along with the severity of OSA and sleep-related hypoxemia after the adjustment of confounding factors including centripetal obesity[65].
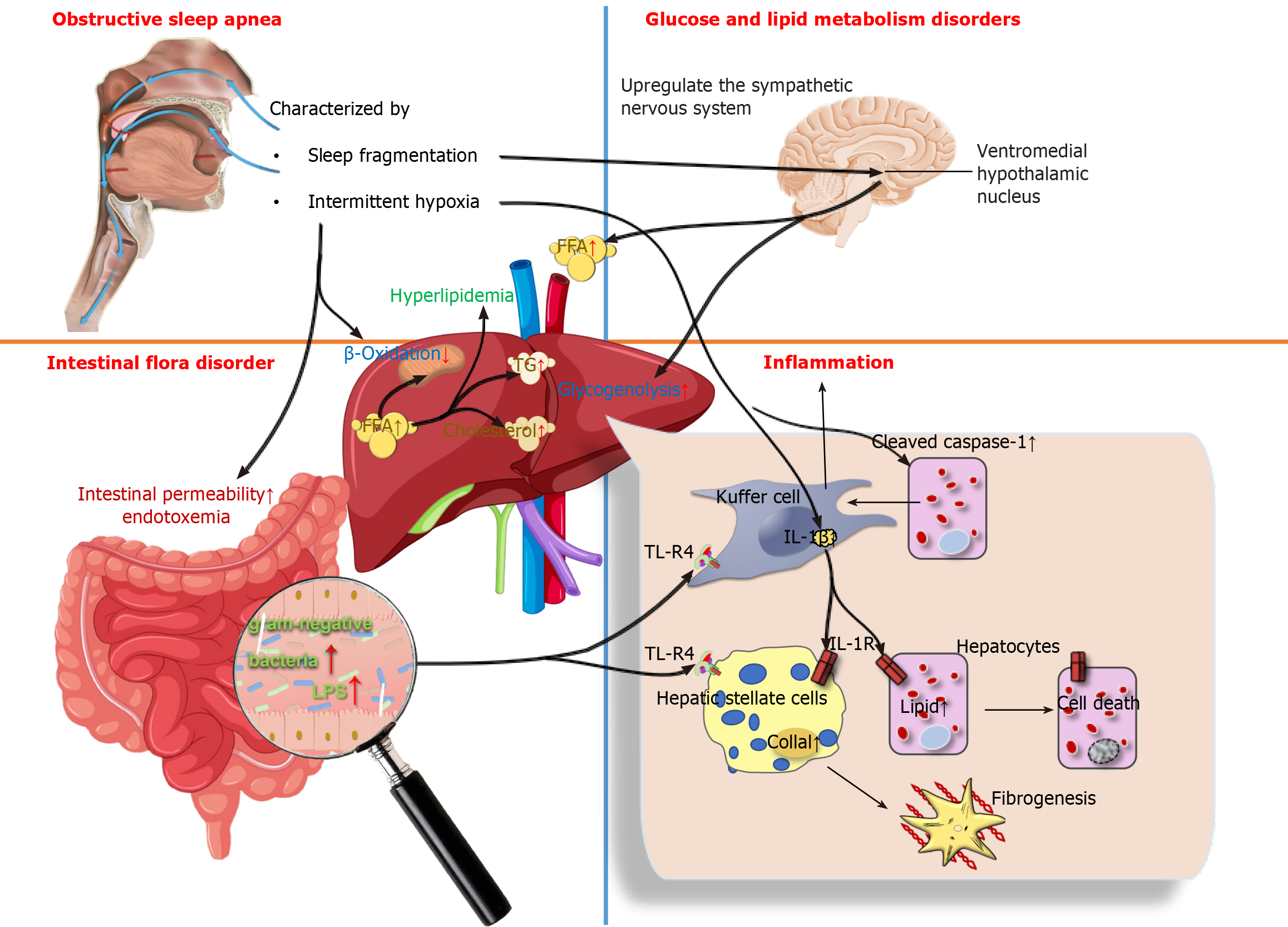
Recent studies have been devoted to determining the influence of IH and OSA-related parameters on NAFLD severity. A multivariate analysis showed that the apnea-hypopnea index (AHI), oxygen desaturation index (ODI), lowest desaturation values, and percentage of sleep duration with mean nocturnal oxygen saturation (SpO2) were independent predictors of NAFLD after adjustment for body mass index (BMI), weight, and IR (it was found that the most correlated parameter for the severity of NAFLD was the duration of IH during sleep)[66]. Furthermore, decreasing SpO2 during sleep was also associated independently with a higher risk of liver cytolysis[65]. Benotti et al[67] found that OSA severity (as measured by the AHI) and hypoxia parameters were positively correlated with NAFLD severity in subjects without metabolic syndrome. Cakmak et al[68] reported that AHI and ODI values were significantly higher in the moderate and severe NAFLD groups compared to counterparts in the non-NAFLD group, SpO2 and lowest O2 saturation (LaSO2) were significantly lower in the mild and severe NAFLD groups. These results revealed that the parameters AHI, ODI, LaSO2, and SpO2 levels play pivotal roles in the association between NAFLD and OSA. The severity of OSA was also associated with a decrease in high-density lipoprotein-cholesterol and an increase in BMI, triglycerides (TG), homeostasis model assessment IR index, transaminases, and FIB-4 index (a noninvasive score for liver fibrosis)[69]. Human subjects with OSA had significantly higher levels of alanine transaminase (ALT) and aspartate transaminase (AST) than those without OSA[70]. A single-center, cross-sectional study indicated that OSA may be an independent risk factor for dyslipidemia, and that OSA and obesity have a synergistic effect on ALT elevation[71]. A cross-sectional study showed that the risk of developing NAFLD increases in older patients with OSA, and high TG is an important factor leading to the development of liver injury[72]. Given that the pathological mechanism of OSA promotes the development of NAFLD, there are three aspects included, as shown in Figure 1.
Metabolism disorders in glucose and lipid: OSA is independently associated with metabolic dysfunction, including dyslipidemia and IR. Yokoe et al[73] found that IH impaired glucose homeostasis and stimulated pancreatic β-cell replication only during periods of hypoxic exposure, but the presence of hyperglycemia may increase the hypoxic susceptibility of β-cells. The mechanism of systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus is also involved in the process of IH inducing the occurrence of IR by up-regulating the sympathetic nervous system, increasing circulating free fatty acids (FFAs) and hepatic glycogenolysis[74]. In addition, IH induces the occurrence of hyperlipidemia by inhibiting the clearance of TG-rich lipoproteins. Drager et al[75] observed that, in male C57BL/6J mice on a high-cholesterol diet under exposure to IH air for 4 weeks, the clearance of lipoprotein lipase, a key enzyme for lipoprotein clearance, was inhibited; resulting in a significant increase in total cholesterol and TG levels. IH-induced hyperlipidemia is also associated with the up-regulation of sterol regulatory element binding protein-1 and the over-expression of stearoyl coenzyme A desaturase 1[76,77]. In conclusion, the mechanism by which OSA promotes the development of NAFLD may be IH-reduced utilization of FFAs by limiting β-oxidation in mitochondria, and excessive FFAs are diverted to the synthesis of TG and cholesterol to trigger hyperlipidemia, which ultimately leads to the development of NAFLD.
Inflammation: The roles of IH in the progression of NAFLD are related to inflammation[78]. IH in OSA patients affects liver histology and activation of inflammatory cells in NAFLD regardless of obesity or IR[79]. In NAFLD animal models, IH has been shown to modulate inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 to produce pro-inflammatory effects[80,81]. Savransky et al[82] found that the levels of IL-1β, IL-6 and TNF-α were elevated in mice following exposure to IH, lobular inflammation and fibrosis were documented in the liver. Similarly, comparable results were observed in humans. Schaefer et al[83] used in vitro models of NASH to study the impacts of IH on the liver, they found that IH contributed to a significant induction of IL-6 expression in both hepatocytes and macrophages. Furthermore, in vitro and in vivo models of NAFLD have shown that IH promotes the production of inflammatory signals by activating inflammatory bodies or caspase-1 in fat-laden hepatocytes, as well as promoting crosstalk between hepatocytes and Kupffer cells by releasing extracellular vesicles to induce hepatocellular damage. This is followed by increased cell mortality through a variety of mechanisms, including apoptosis and pyroptosis[84]. Notably, Taylor et al[85] discovered that human adipocytes are highly sensitive to IH, which enhances inflammatory gene expression in adipose tissue and the release of inflammatory cytokines involved in the development of NAFLD.
Intestinal flora disorder: There is a wide range of microorganisms in the human intestine, in which various microorganisms interact with each other to form a dynamic ecosystem called the gut microbial ecology. It has been shown that IH in OSA may affect the ecology of the gut microbiota and mediate a variety of cardiovascular diseases that coexist with OSA[86]. OSA is a risk factor for intestinal injury. Regardless of the metabolic status, intestinal permeability may be a possible factor leading to the susceptibility of OSA patients to NAFLD[87]. For example, Nobili et al[88] found that a novel correlation exists between OSA and NAFLD, namely that IH may disrupt the intestinal-liver axis in pediatric NAFLD by increasing the number of gram-negative bacteria in the intestine and intestinal permeability, with increased endotoxemia coupled with toll-like receptor-4 (TLR-4) up-regulation in hepatocytes, Kupffer cells and hepatic stellate cells[88,89]. In addition, one of the characteristic manifestations of OSA-SF, induces metabolic alterations in the organism that may be mediated in part by concurrent changes in gut microbiota, which was confirmed using SF-derived microbiota routinized in germ-free mice[90]. Chronic SF-induced reversible gut microbiota changes led to systemic and visceral white adipose tissue inflammation in addition to altered insulin sensitivity in mice, most likely via enhanced colonic epithelium barrier disruption.
Currently, CPAP is the globally accepted gold standard for the treatment of OSA. It can keep the airway open and reduce daytime sleepiness, improving cognition and sleep quality in OSA patients[91]. There have been many studies performed to explore the effects of CPAP therapy on OSA patients suffering from NAFLD, but the results obtained were varied. Some observational data suggested that CPAP treatment improves hepatic biochemistry of NAFLD in OSA patients; and that CPAP treatment is statistically significantly associated with improvement of hepatic injury in OSA patients, but a sufficiently long duration of treatment (greater than or equal to 3 months) may be required to achieve a positive effect. Chen et al[92] enrolled 160 patients with OSA and measured serum transaminases before and after CPAP treatment. After 3 months of treatment, both ALT and AST levels decreased significantly. A recent meta-analysis also showed that, compared to controls, ALT and AST levels were significantly lower in OSA patients after CPAP treatment, and was more effective in OSA patients treated with CPAP for more than 3 months[93]. Hirono et al[94] found a significant reduction in AST and ALT levels and significant improvement in liver injury after 6 months of CPAP treatment in 50 patients with OSA suffering from NAFLD. In addition, the effect of CPAP treatment on NAFLD in OSA patients was also related to OSA patients’ adherence. Patients with good adherence to CPAP showed significantly decreased levels in AST and ALT than those with poor adherence[95]. Sundaram et al[96] also found that treatment of OSA with CPAP may reverse liver injury parameters and reduce oxidative stress, indicating that CPAP could be a new therapy for preventing NAFLD progression in obese children with OSA.
However, some randomized controlled trials did not show a benefit of CPAP treatment on liver injury in OSA patients. For instance, Jullian-Desayes et al[97] found that six to twelve weeks of effective CPAP did not show any impact on reducing steatosis, NASH or liver fibrosis even after adjustment for gender, BMI, baseline AHI and severity of liver injury. Also, in the randomized controlled trial by Kohler et al[98], 94 patients with moderate to severe OSA were randomized to therapeutic or subtherapeutic CPAP treatment. Plasma ALT and AST levels were measured before and after treatment. The results showed that 4 wk of active CPAP treatment did not show any beneficial effect on transaminase levels compared to subtherapeutic CPAP in patients with OSA. Ng et al[99] showed that 6 months of CPAP treatment did not lead to improvement in hepatic steatosis and liver fibrosis, despite a significant correlation between hepatic steatosis and markers of OSA severity. Labarca et al[100] performed a systematic evaluation and meta-analysis of 5 randomized controlled trials involving patients with OSA and NASH who were treated with CPAP, but did not find obvious changes in hepatic steatosis, liver fibrosis and transaminase levels (ALT and AST) in OSA patients. Differences regarding the effect of CPAP treatment in OSA patients on NAFLD may be related to the duration of CPAP treatment, compliance of OSA patients and the severity of NAFLD progression.
The effects of NAFLD on sleep can be observed from some observational studies, although there are no animal experiments to explain the specific mechanism by which NAFLD affects sleep. NAFLD patients have altered sleep status, namely in NAFLD, sleep duration was shortened, sleep onset was delayed and sleep quality poorer[39,101]. Moreover, NAFLD may increase the risk of developing OSA. A study showed that OSA is common in adults who have biopsy-proven NAFLD[102]. Similarly, in a 6-month prospective study, Romdhane et al[103] found that the incidence of OSA was relatively higher in patients with NAFLD in comparison with controls. In a nationwide population-based study, Chung et al[51] found that NAFLD was significantly associated with an increased risk of OSA after adjusting for multiple metabolic variables. Specifically, in younger, male or obese patients with NAFLD, there is a higher risk of OSA than that in older, female or non-obese patients.
The mechanism by which NAFLD affects OSA may be related to MT metabolism disorder. It is known that sleep is closely related to the metabolism of MT, which is metabolized by the liver. Liver metabolic dysfunction in NAFLD patients increases escalates as disease progresses. Currently, it has been found that key factors in NAFLD-induced sleep disorders include hepatic encephalopathy and circadian rhythm imbalance due to altered MT metabolism. Moreover, in the advanced stages of NAFLD, cirrhosis has an effect on circadian sleep regulation by a delay in the 24-h MT rhythm, which is likely to be related to reduced sensitivity to light signals[104]. The core feature of NAFLD is a discoordination between central and peripheral circadian rhythms[105]. This phenomenon has also been observed in db/db (hereditary obesity) mice[106], and the main circadian rhythm defect lies in the peripheral liver oscillator rather than the behavioral rhythm or master clock, but the mechanism by which peripheral circadian rhythm disorder affects the central circadian rhythm remains to be explored.
This paper provides some significant insights into the correlations between sleep disorders and the occurrence or development of NAFLD. Excessive or short sleep duration and poor sleep quality may increase the risk of NAFLD. Similarly, insomnia, daytime sleepiness, sleep-wake disorders and OSA have been associated with the development of NAFLD. NAFLD is also a risk factor for OSA; thus, it is necessary to screen and monitor the occurrence and development of NAFLD in OSA patients. Moreover, CPAP treatment can stabilize and slow down the progression of NAFLD under certain circumstances. Sleep factors can be added to the list of changeable lifestyle behaviors to reduce the risk of NAFLD. This includes maintaining proper sleep duration, improving sleep quality, and addressing sleep disorders.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7943] [Article Influence: 794.3] [Reference Citation Analysis (8)] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4027] [Article Influence: 503.4] [Reference Citation Analysis (2)] |
| 3. | Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 4. | Kumar R, Priyadarshi RN, Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J Clin Transl Hepatol. 2020;8:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 727] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 6. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1377] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 7. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1570] [Article Influence: 224.3] [Reference Citation Analysis (3)] |
| 8. | Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 9. | Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Zarean E, Looha MA, Amini P, Ahmadi A, Dugué PA. Sleep characteristics of middle-aged adults with non-alcoholic fatty liver disease: findings from the Shahrekord PERSIAN cohort study. BMC Public Health. 2023;23:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 11. | Masi D, Spoltore ME, Rossetti R, Watanabe M, Tozzi R, Caputi A, Risi R, Balena A, Gandini O, Mariani S, Spera G, Gnessi L, Lubrano C. The Influence of Ketone Bodies on Circadian Processes Regarding Appetite, Sleep and Hormone Release: A Systematic Review of the Literature. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3166] [Article Influence: 113.1] [Reference Citation Analysis (36)] |
| 13. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2308] [Article Influence: 230.8] [Reference Citation Analysis (1)] |
| 14. | Ji Y, Elkin K, Yip J, Guan L, Han W, Ding Y. From circadian clocks to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Shetty A, Hsu JW, Manka PP, Syn WK. Role of the Circadian Clock in the Metabolic Syndrome and Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2018;63:3187-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Hu C, Zhao L, Tao J, Li L. Protective role of melatonin in early-stage and end-stage liver cirrhosis. J Cell Mol Med. 2019;23:7151-7162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Ren J, Jin M, You ZX, Luo M, Han Y, Li GC, Liu HG. Melatonin prevents chronic intermittent hypoxia-induced injury by inducing sirtuin 1-mediated autophagy in steatotic liver of mice. Sleep Breath. 2019;23:825-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Sun Z, Ji J, Zuo L, Hu Y, Wang K, Xu T, Wang Q, Cheng F. Causal relationship between nonalcoholic fatty liver disease and different sleep traits: a bidirectional Mendelian randomized study. Front Endocrinol (Lausanne). 2023;14:1159258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H, Ryu S. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Peng K, Lin L, Wang Z, Ding L, Huang Y, Wang P, Xu Y, Lu J, Xu M, Bi Y, Wang W, Chen Y, Ning G. Short sleep duration and longer daytime napping are associated with non-alcoholic fatty liver disease in Chinese adults. J Diabetes. 2017;9:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Short sleep duration is a risk of incident nonalcoholic fatty liver disease: a population-based longitudinal study. J Gastrointestin Liver Dis. 2019;28:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Um YJ, Chang Y, Jung HS, Cho IY, Shin JH, Shin H, Wild SH, Byrne CD, Ryu S. Sleep Duration, Sleep Quality, and the Development of Nonalcoholic Fatty Liver Disease: A Cohort Study. Clin Transl Gastroenterol. 2021;12:e00417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 23. | Imaizumi H, Takahashi A, Tanji N, Abe K, Sato Y, Anzai Y, Watanabe H, Ohira H. The Association between Sleep Duration and Non-Alcoholic Fatty Liver Disease among Japanese Men and Women. Obes Facts. 2015;8:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Yang J, Zhang K, Xi Z, Ma Y, Shao C, Wang W, Tang YD. Short sleep duration and the risk of nonalcoholic fatty liver disease/metabolic associated fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2023;27:1985-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Wu C, Zhang C, Xiao L, Tang S. Relationship between Sleep Duration or Sleep Quality and Non-alcoholic Fatty Liver Disease: a Me-ta-analysis. Chinese General Practice. 2020;23:4619-4625. [DOI] [Full Text] |
| 26. | Katsagoni CN, Papatheodoridis GV, Papageorgiou MV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, Fragopoulou E, Kontogianni MD. A "healthy diet-optimal sleep" lifestyle pattern is inversely associated with liver stiffness and insulin resistance in patients with nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. 2017;42:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Miyake T, Kumagi T, Furukawa S, Hirooka M, Kawasaki K, Koizumi M, Todo Y, Yamamoto S, Tokumoto Y, Ikeda Y, Abe M, Kitai K, Matsuura B, Hiasa Y. Short sleep duration reduces the risk of nonalcoholic fatty liver disease onset in men: a community-based longitudinal cohort study. J Gastroenterol. 2015;50:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Liu H, Huang S, Xu M, Zhao D, Wang X, Zhang L, Chen D, Du J, Yu R, Li H, Ye H. The association between sleep duration, quality, and nonalcoholic fatty liver disease: A cross-sectional study. Open Med (Wars). 2023;18:20230670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 29. | Takahashi A, Anzai Y, Kuroda M, Kokubun M, Kondo Y, Ogata T, Fujita M, Hayashi M, Imaizumi H, Abe K, Tanji N, Ohira H. Effects of sleep quality on non-alcoholic fatty liver disease: a cross-sectional survey. BMJ Open. 2020;10:e039947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Liu C, Zhong R, Lou J, Pan A, Tang Y, Chang J, Ke J, Li J, Yuan J, Wang Y, Chen W, Guo H, Wei S, Liang Y, Zhang X, He M, Hu FB, Wu T, Yao P, Miao X. Nighttime sleep duration and risk of nonalcoholic fatty liver disease: the Dongfeng-Tongji prospective study. Ann Med. 2016;48:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Kim JH, Jung DH, Kwon YJ, Lee JI, Shim JY. The impact of the sleep duration on NAFLD score in Korean middle-aged adults: a community-based cohort study. Sleep Med. 2019;57:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of Sleep Disorders with Nonalcoholic Fatty Liver Disease (NAFLD): A Population-based Study. J Clin Exp Hepatol. 2013;3:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Akram ST, Ewy MW, Said A. Sleep disruption in nonalcoholic fatty liver disease: What is the role of lifestyle and diet? Eur J Gastroenterol Hepatol. 2021;33:e308-e312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Wei YT, Lee PY, Lin CY, Chen HJ, Lin CC, Wu JS, Chang YF, Wu CL, Guo HR. Non-alcoholic fatty liver disease among patients with sleep disorders: a Nationwide study of Taiwan. BMC Gastroenterol. 2020;20:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Insomnia and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J Postgrad Med. 2017;63:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Yu L, Lin C, Chen X, Teng Y, Zhou S, Liang Y. A Meta-Analysis of Sleep Disorders and Nonalcoholic Fatty Liver Disease: Potential Causality and Symptom Management. Gastroenterol Nurs. 2022;45:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Fan H, Liu Z, Zhang X, Yuan H, Zhao X, Zhao R, Shi T, Wu S, Xu Y, Suo C, Chen X, Zhang T. Investigating the Association Between Seven Sleep Traits and Nonalcoholic Fatty Liver Disease: Observational and Mendelian Randomization Study. Front Genet. 2022;13:792558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Taketani H, Sumida Y, Tanaka S, Imajo K, Yoneda M, Hyogo H, Ono M, Fujii H, Eguchi Y, Kanemasa K, Chayama K, Itoh Y, Yoshikawa T, Saibara T, Fujimoto K, Nakajima A; Japan Study Group of NAFLD. The association of insomnia with gastroesophageal reflux symptoms in biopsy-proven nonalcoholic fatty liver disease. J Gastroenterol. 2014;49:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Bernsmeier C, Weisskopf DM, Pflueger MO, Mosimann J, Campana B, Terracciano L, Beglinger C, Heim MH, Cajochen C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS One. 2015;10:e0143293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Benedict C, Brooks SJ, O'Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, Larsson EM, Schiöth HB. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443-E447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 41. | Broussard JL, Kilkus JM, Delebecque F, Abraham V, Day A, Whitmore HR, Tasali E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring). 2016;24:132-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 43. | Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 44. | Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 450] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 45. | Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, Gläser S, Glos M, Schmidt CO, Stubbe B, Völzke H, Zimmermann S, Penzel T. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - Results of SHIP-Trend. J Sleep Res. 2019;28:e12770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 47. | Almendros I, Basoglu ÖK, Conde SV, Liguori C, Saaresranta T. Metabolic dysfunction in OSA: Is there something new under the sun? J Sleep Res. 2022;31:e13418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Sacramento JF, Ribeiro MJ, Rodrigues T, Guarino MP, Diogo LN, Seiça R, Monteiro EC, Matafome P, Conde SV. Insulin resistance is associated with tissue-specific regulation of HIF-1α and HIF-2α during mild chronic intermittent hypoxia. Respir Physiol Neurobiol. 2016;228:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Ryan S, Arnaud C, Fitzpatrick SF, Gaucher J, Tamisier R, Pépin JL. Adipose tissue as a key player in obstructive sleep apnoea. Eur Respir Rev. 2019;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Wang H, Gao Q, He S, Bao Y, Sun H, Meng L, Liang J, Sun C, Chen S, Cao L, Huang W, Zhang Y, Huang J, Wu S, Wang T. Self-reported snoring is associated with nonalcoholic fatty liver disease. Sci Rep. 2020;10:9267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Chung GE, Cho EJ, Yoo JJ, Chang Y, Cho Y, Park SH, Shin DW, Han K, Yu SJ. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci Rep. 2021;11:13473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Jullian-Desayes I, Trzepizur W, Boursier J, Joyeux-Faure M, Bailly S, Benmerad M, Le Vaillant M, Jaffre S, Pigeanne T, Bizieux-Thaminy A, Humeau MP, Alizon C, Goupil F, Costentin C, Gaucher J, Tamisier R, Gagnadoux F, Pépin JL. Obstructive sleep apnea, chronic obstructive pulmonary disease and NAFLD: an individual participant data meta-analysis. Sleep Med. 2021;77:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP, Lorenzi-Filho G, Steele KE, Schweitzer MA, Magnuson TH, Lidor AO, Schwartz AR, Polotsky VY. Lysyl Oxidase as a Serum Biomarker of Liver Fibrosis in Patients with Severe Obesity and Obstructive Sleep Apnea. Sleep. 2015;38:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Mesarwi OA, Loomba R, Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med. 2019;199:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 55. | Fu Y, Zhang N, Tang W, Bi Y, Zhu D, Chu X, Shan X, Shen Y, Sun X, Feng W. Chronic intermittent hypoxia contributes to non-alcoholic steatohepatitis progression in patients with obesity. Hepatol Int. 2022;16:824-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 56. | Trzepizur W, Boursier J, Le Vaillant M, Ducluzeau PH, Dubois S, Henni S, Abraham P, Aubé C, Calès P, Gagnadoux F; on the behalf of the METABOL group. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 57. | Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 58. | Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Zhang L, Zhang X, Meng H, Li Y, Han T, Wang C. Obstructive sleep apnea and liver injury in severely obese patients with nonalcoholic fatty liver disease. Sleep Breath. 2020;24:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Agrawal S, Duseja A, Aggarwal A, Das A, Mehta M, Dhiman RK, Chawla Y. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 61. | Lian N, Wu J, Wang B, Lin S, Huang J, Chen J, Lin Q. Risk Factors of Nonalcoholic Fatty Liver Disease and Liver Fibrosis in Non-Obese Patients with Obstructive Sleep Apnea. Nat Sci Sleep. 2022;14:2143-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 62. | Krolow GK, Garcia E, Schoor F, Araujo FBS, Coral GP. Obstructive sleep apnea and severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2021;33:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Kim T, Choi H, Lee J, Kim J. Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Jawa HA, Khatib H, Alzahrani N, Alawi A, Al-Gamdi M, Abuljadayel A, Altayyari S, Alhejaili F, Mosli M, Wali SO. Nonalcoholic Fatty Liver Disease and Fibrosis Risk in Patients With Obstructive Sleep Apnea: A Retrospective Analysis. Cureus. 2021;13:e13623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Trzepizur W, Boursier J, Mansour Y, Le Vaillant M, Chollet S, Pigeanne T, Bizieux-Thaminy A, Humeau MP, Alizon C, Goupil F, Meslier N, Priou P, Calès P, Gagnadoux F; Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group. Association Between Severity of Obstructive Sleep Apnea and Blood Markers of Liver Injury. Clin Gastroenterol Hepatol. 2016;14:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Türkay C, Ozol D, Kasapoğlu B, Kirbas I, Yıldırım Z, Yiğitoğlu R. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care. 2012;57:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, Gerhard G, Still C. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring). 2016;24:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, Yilmaz A. Association Between the Severity of Nocturnal Hypoxia in Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Damage. Hepat Mon. 2015;15:e32655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Bettini S, Serra R, Fabris R, Dal Prà C, Favaretto F, Dassie F, Duso C, Vettor R, Busetto L. Association of obstructive sleep apnea with non-alcoholic fatty liver disease in patients with obesity: an observational study. Eat Weight Disord. 2022;27:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Corey KE, Misdraji J, Gelrud L, King LY, Zheng H, Malhotra A, Chung RT. Obstructive Sleep Apnea Is Associated with Nonalcoholic Steatohepatitis and Advanced Liver Histology. Dig Dis Sci. 2015;60:2523-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Kang EK, Jang MJ, Kim KD, Ahn YM. The association of obstructive sleep apnea with dyslipidemia in Korean children and adolescents: a single-center, cross-sectional study. J Clin Sleep Med. 2021;17:1599-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Jin YX, Wang BY, Wang XL, Yu X, Chen LD, Yang YS, Huang JF. Relationship between Obstructive Sleep Apnea and Liver Abnormalities in Older Patients: A Cross-Sectional Study. Int J Clin Pract. 2023;2023:9310588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008;586:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Shimazu T, Minokoshi Y. Systemic Glucoregulation by Glucose-Sensing Neurons in the Ventromedial Hypothalamic Nucleus (VMH). J Endocr Soc. 2017;1:449-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O'Byrne SM, Kroupa O, Olivecrona G, Blaner WS, Polotsky VY. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics. 2007;31:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Schwenger KJP, Ghorbani Y, Li C, Fischer SE, Jackson TD, Okrainec A, Allard JP. Obstructive Sleep Apnea and Non-alcoholic Fatty Liver Disease in Obese Patients Undergoing Bariatric Surgery. Obes Surg. 2020;30:2572-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V, Verrillo E, Baviera G, Musso G. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 80. | Briançon-Marjollet A, Monneret D, Henri M, Joyeux-Faure M, Totoson P, Cachot S, Faure P, Godin-Ribuot D. Intermittent hypoxia in obese Zucker rats: cardiometabolic and inflammatory effects. Exp Physiol. 2016;101:1432-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Kang HH, Kim IK, Lee HI, Joo H, Lim JU, Lee J, Lee SH, Moon HS. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem Biophys Res Commun. 2017;490:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871-G877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Schaefer E, Wu W, Mark C, Yang A, DiGiacomo E, Carlton-Smith C, Salloum S, Brisac C, Lin W, Corey KE, Chung RT. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol Commun. 2017;1:326-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Hernández A, Geng Y, Sepúlveda R, Solís N, Torres J, Arab JP, Barrera F, Cabrera D, Moshage H, Arrese M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Tripathi A, Melnik AV, Xue J, Poulsen O, Meehan MJ, Humphrey G, Jiang L, Ackermann G, McDonald D, Zhou D, Knight R, Dorrestein PC, Haddad GG. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 87. | Barceló A, Esquinas C, Robles J, Piérola J, De la Peña M, Aguilar I, Morell-Garcia D, Alonso A, Toledo N, Sánchez-de la Torre M, Barbé F. Gut epithelial barrier markers in patients with obstructive sleep apnea. Sleep Med. 2016;26:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Nobili V, Alisi A, Cutrera R, Carpino G, De Stefanis C, D'Oria V, De Vito R, Cucchiara S, Gaudio E, Musso G. Altered gut-liver axis and hepatic adiponectin expression in OSAS: novel mediators of liver injury in paediatric non-alcoholic fatty liver. Thorax. 2015;70:769-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 90. | Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert N, Farré R, Chang EB, Gozal D. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci Rep. 2016;6:35405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 358] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 91. | Bajaj JS, Thacker LR, Leszczyszyn D, Taylor SA, Heuman DM, Raman S, Sterling RK, Siddiqui MS, Stravitz RT, Sanyal AJ, Puri P, Luketic V, Matherly S, Fuchs M, White MB, Noble NA, Unser AB, Wade JB. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:390-397.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Chen LD, Zhang LJ, Lin XJ, Qi JC, Li H, Wu Z, Xu QZ, Huang YP, Lin L. Association between continuous positive airway pressure and serum aminotransferases in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2018;275:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Chen LD, Lin L, Zhang LJ, Zeng HX, Wu QY, Hu MF, Xie JJ, Liu JN. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin Respir J. 2018;12:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Hirono H, Watanabe K, Hasegawa K, Kohno M, Terai S, Ohkoshi S. Impact of continuous positive airway pressure therapy for nonalcoholic fatty liver disease in patients with obstructive sleep apnea. World J Clin Cases. 2021;9:5112-5125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Kim D, Ahmed A, Kushida C. Continuous Positive Airway Pressure Therapy on Nonalcoholic Fatty Liver Disease in Patients With Obstructive Sleep Apnea. J Clin Sleep Med. 2018;14:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Sundaram SS, Halbower AC, Klawitter J, Pan Z, Robbins K, Capocelli KE, Sokol RJ. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. J Pediatr. 2018;198:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Jullian-Desayes I, Tamisier R, Zarski JP, Aron-Wisnewsky J, Launois-Rollinat SH, Trocme C, Levy P, Joyeux-Faure M, Pepin JL. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: Data from randomized trials. Respirology. 2016;21:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 98. | Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Ng SSS, Wong VWS, Wong GLH, Chu WCW, Chan TO, To KW, Ko FWS, Chan KP, Hui DS. Continuous Positive Airway Pressure Does Not Improve Nonalcoholic Fatty Liver Disease in Patients with Obstructive Sleep Apnea. A Randomized Clinical Trial. Am J Respir Crit Care Med. 2021;203:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 100. | Labarca G, Cruz R, Jorquera J. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Non-Alcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. J Clin Sleep Med. 2018;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Marin-Alejandre BA, Abete I, Cantero I, Riezu-Boj JI, Milagro FI, Monreal JI, Elorz M, Herrero JI, Benito-Boillos A, Quiroga J, Martinez-Echeverria A, Uriz-Otano JI, Huarte-Muniesa MP, Tur JA, Martínez JA, Zulet MA. Association between Sleep Disturbances and Liver Status in Obese Subjects with Nonalcoholic Fatty Liver Disease: A Comparison with Healthy Controls. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Wiscombe S, Newton J, Day C, Gibson J, West S. Obstructive sleep apnoea is common in adults with biopsy-proven non-alcoholic fatty liver disease. Sleep Med. 2015;16:1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Romdhane H, Ayadi S, Cheikh M, Bouchabou B, Ben Nejma H, Ennaifer R. Estimation of the prevalence of obstructive sleep apnea in non alcoholic fatty liver disease. Tunis Med. 2018;96:171-176. [PubMed] |
| 104. | Montagnese S, De Pittà C, De Rui M, Corrias M, Turco M, Merkel C, Amodio P, Costa R, Skene DJ, Gatta A. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2014;59:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 105. | Saran AR, Dave S, Zarrinpar A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology. 2020;158:1948-1966.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 106. | Larion S, Padgett CA, Butcher JT, Mintz JD, Fulton DJ, Stepp DW. The biological clock enhancer nobiletin ameliorates steatosis in genetically obese mice by restoring aberrant hepatic circadian rhythm. Am J Physiol Gastrointest Liver Physiol. 2022;323:G387-G400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inoue K, Japan S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zheng XM