Published online Dec 27, 2024. doi: 10.4254/wjh.v16.i12.1417
Revised: October 2, 2024
Accepted: October 29, 2024
Published online: December 27, 2024
Processing time: 225 Days and 20.3 Hours
Hepatic abscesses represent infections of the liver parenchyma from bacteria, fungi, and parasitic organisms. Trends in both abscess microbiology and manage
To evaluate the clinical presentations of liver abscesses and hydatid liver disease at two South African tertiary-level hospitals.
Information accessed from electronic discharge summaries of patients from two South African referral hospitals in Johannesburg, South Africa from January 2016 to December 2020 were reviewed and analyzed. All patients older than 13 years presenting with infective liver collections (pyogenic, amoebic) and hydatid di
In total, 222 patients were included. There were 123 males (55.41%) and 99 females (44.59%), with a median age of 48 years. Comorbidities included HIV (24.23%), hypertension (20.57%), and diabetes mellitus (16.83%). The majority (74.77%) of abscesses were pyogenic, while amoebic and hydatid abscesses represented 16.22% and 9.01%, respectively. The predominant etiology of the pyogenic liver abscesses (PLA) was biliary-related disease. WBC and C-reactive protein were significantly higher in the pyogenic group (P < 0.0002 and P < 0.007, respectively) when compared to the amoebic and hydatid groups. In patients with PLAs, organisms were cultured on blood in 17.58% and abscess fluid in 56.60%. Klebsiella, Escherichia coli and Streptococci were the most cultured organisms. Sixteen percent of the cultures were polymicrobial. In the overall group, 76.00% (n = 169) of patients requiring drainage had a percutaneous transhepatic catheter drain placed, while 8.76% (n = 19) had open surgery. The median length of hospital stay was 13 days. The mortality rate was 3.02%.
In this study, the most common type of liver abscess was PLAs of biliary origin in middle-aged males. The microbiology was similar to those described in Asian populations, and non-surgical management via percutaneous drainage was sufficient in the majority of cases with acceptable morbidity and mortality.
Core Tip: The incidence, etiology, and microbiology of liver abscesses vary across geographical areas. There is a paucity of published data evaluating liver abscesses and hydatid liver disease in Sub Saharan Africa. HIV and diabetes mellitus were major comorbidities, highlighting immunosuppression as an important factor in the pathogenesis of infective liver co
- Citation: Pillay K, Khan ZA, Nweke EE, Omoshoro-Jones J. Clinicopathological presentation of liver abscesses and hydatid liver disease from two South African tertiary hospitals. World J Hepatol 2024; 16(12): 1417-1428
- URL: https://www.wjgnet.com/1948-5182/full/v16/i12/1417.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i12.1417
Liver abscesses represent a suppurative infection of the hepatic parenchyma from bacteria (pyogenic), parasites, and rarely fungi[1,2]. Mixed etiology can occur with bacterial superinfection of a parasitic abscess. Pyogenic liver abscesses (PLAs) remain the most common in the developed world[1]. The incidence differs across population groups and geo
There are definite distinctions in the clinicopathologic manifestations of these infective liver collections in different geographical areas and population groups[9]. The differences between geographical areas can be attributed to socioe
Data from low-income and middle-income countries (LMICs) in this area are grossly lacking. González-Alcaide et al[8] found that the United States, Japan, and Taiwan were the largest contributors to research in this field. Most papers on amoebic abscesses were from India and Mexico[8]. This retrospective analysis evaluated demographic, clinical, radio
A descriptive analysis of patients managed for infective liver collections at the study sites from January 2016-December 2020 was conducted. Study approval was granted by the University of the Witwatersrand Human Research Ethics Committee (Medical) (M200245).
Patients presenting with pyogenic and amoebic liver abscesses and hydatid liver disease at the Charlotte Maxeke Johannesburg Academic Hospital and Chris Hani Baragwanath Academic Hospital were included. These two hospitals are tertiary-level referral centers and serve primary and secondary-level patients. The Chris Hani Baragwanath Academic Hospital is one of the ten largest hospitals in the world. It has approximately 3200 beds and notes 150000 inpatient admissions per year. It services the population of Soweto and receives referrals from multiple hospitals within its cluster in Gauteng as well as from neighboring provinces (Northwest province, Limpopo and Mpumalanga). The Charlotte Maxeke Johannesburg Academic Hospital has 1088 beds and provides services to the northern suburbs of Johannesburg.
The study analyzed data from 5 years from January 1, 2016 to December 31, 2020. Eligible patients’ data were retrieved from the hospitals’ electronic discharge summary database using the International Classification of Diseases 10 codes: K75.0 (abscess of the liver), A06.4 (amoebic liver abscess), and K77.0 (liver disorders in infectious and parasitic diseases classified elsewhere). Patients under 13 years of age, patients with recurrent admission, and abscesses secondary to trauma were excluded. In our institution, adult surgical units manage patients above the age of 13. All patients older than 13 diagnosed with liver abscesses were managed in the hepatobiliary units and were therefore included in the study.
The above information was cross-referenced with the National Health Laboratory Service and participating hospitals’ radiology records. Relevant datasets were captured on an Excel data sheet. This included demographic characteristics, comorbidities, clinical presentation, laboratory results, radiology findings, treatment modalities, complications, and mortalities. Information regarding the socioeconomic status of patients was not available. The results of blood tests (including blood cultures) were from specimens obtained on admission. Abscess culture results were from specimens obtained at the time of percutaneous or surgical drainage. Clinical details, amoebic and hydatid serologies, histology, and imaging characteristics were used to differentiate types of infective liver collections.
PLA etiological agents were divided into groups according to the presumed source of infection: Biliary (cholecystitis, cholangitis from benign or malignant biliary tract disease); portal pyemia (hematogenous spread via the portal vein from an infectious intra-abdominal process); hematogenous (from a distant infective process); cryptogenic (where no cause could be identified); and other (e.g., intra-abdominal malignancy). The management modalities evaluated were the type of antibiotic used, the need and method of drainage (percutaneous, surgical), and the use of endoscopic retrograde cholangiopancreatography (ERCP). The decision for drainage of the abscess was determined by the size of the abscess (> 5 cm) and failure to respond to antibiotic treatment. Mortality was defined as death during hospital admission.
Data analysis was performed using STATA statistical software version 16. Continuous variables are presented as descriptive data and expressed as means with standard deviations or median and interquartile ranges for variables that were not normally distributed. Categorical variables were expressed as percentages. The differences between groups and mortality data were evaluated by the Mann-Whitney U test and one-way analysis of variance, with P values < 0.05 considered statistically significant.
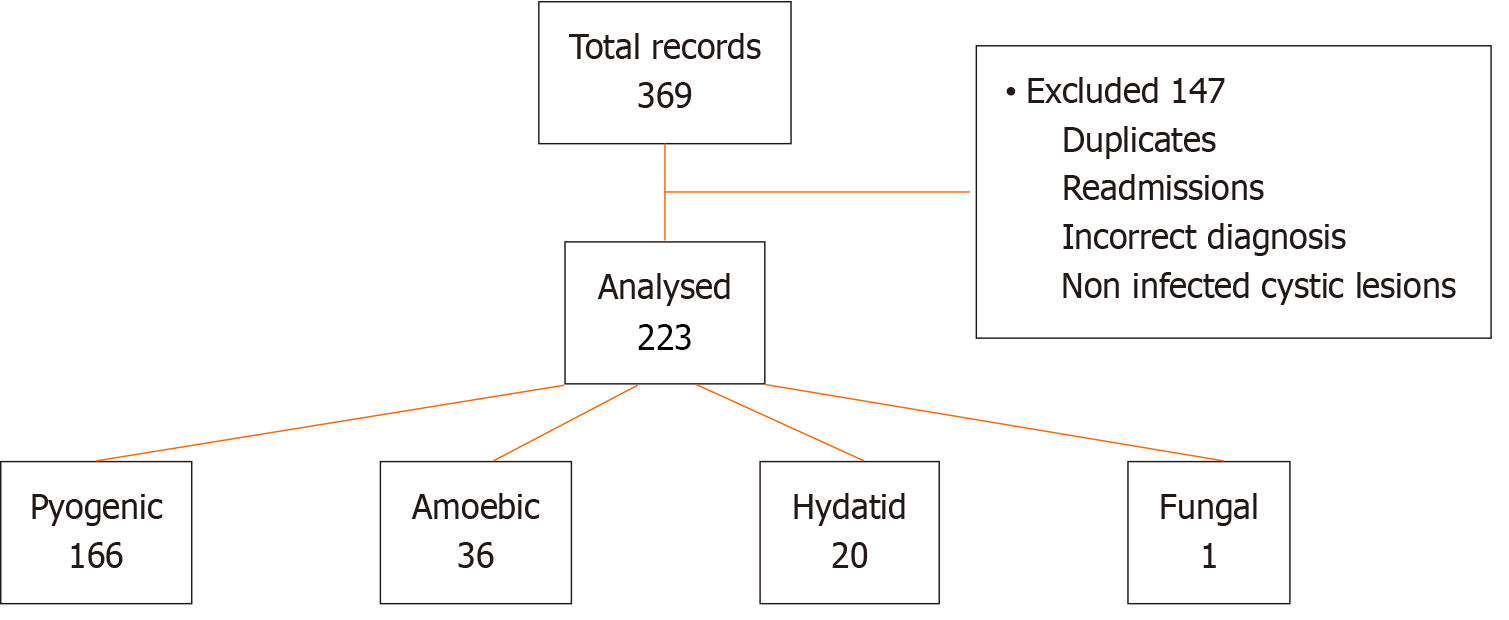
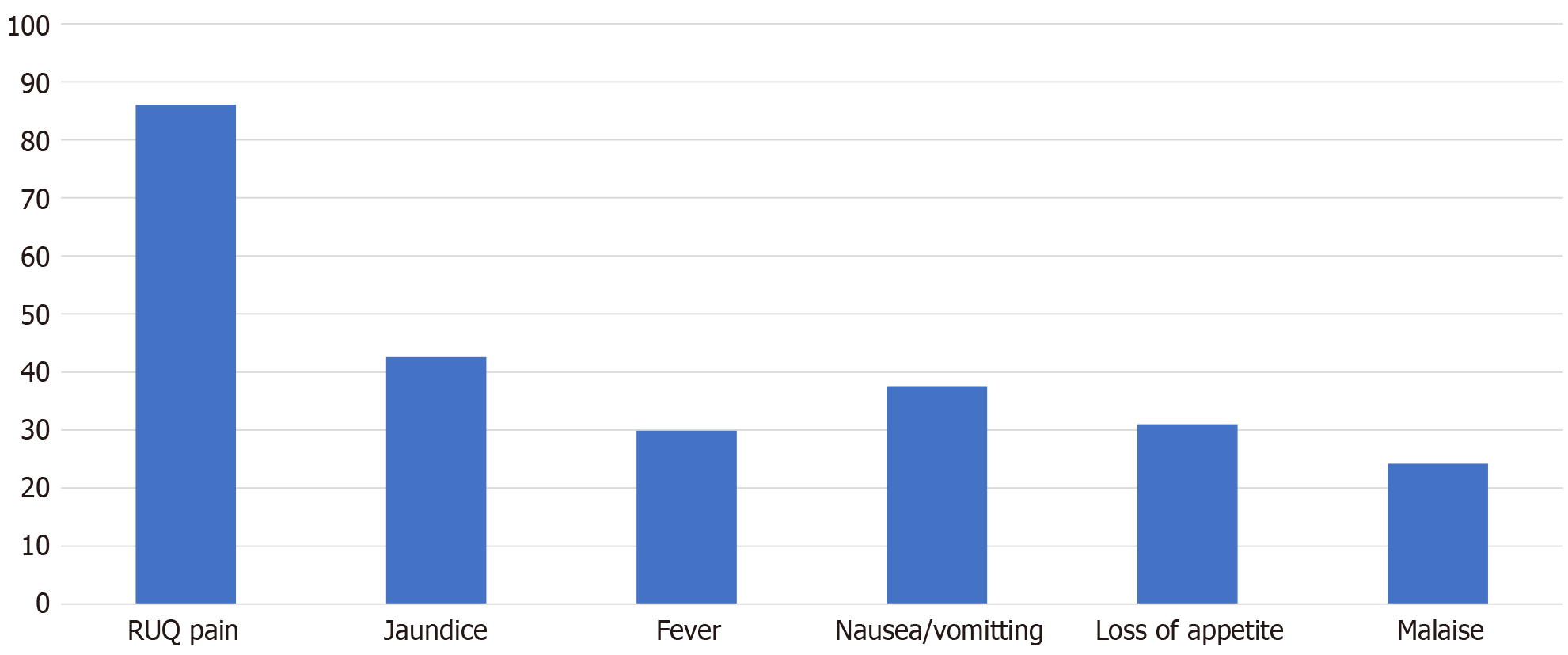
Two hundred and twenty-two patients met the inclusion criteria for the study (Figure 1). The median age was 48 years (range: 15-87 years). Fifty-five percent (n = 123) of patients were males. The most common comorbidities in the cohort were HIV (24.60%), followed by hypertension (22.70%) and diabetes mellitus (17.30%). One patient had documented cirrhosis, and one had chronic kidney disease. Table 1 illustrates the demographic differences between the types of liver abscesses. Presenting symptoms were right upper quadrant pain (86.1%), jaundice (42.60%), nausea and vomiting (38.00%), and loss of appetite (32.20%) (Figure 2). Fever was present in 28.89% of patients and more frequently in the pyogenic and amoebic groups than in the hydatid group.
| Feature | Pyogenic (n = 166) | Amoebic (n = 36) | Hydatid (n = 20) | P value |
| Age | 52 (41-60) | 45.5 (35.5-55.5) | 38.5 (26-44) | 0.0005 |
| Sex | ||||
| Male | 90 (54.22) | 28 (77.78) | 5 (25) | 0.001 |
| Female | 76 (45.78) | 8 (22.76) | 15 (75) | 0.001 |
| HIV-positive | 27 (17.88) | 15 (45.45) | 8 (42.11) | 0.001 |
| Diabetes mellitus | 35 (21.34) | 2 (5.56) | 1 (5) | 0.024 |
PLA comprised 74.80% (n = 166) of the cohort. Sixteen percent (n = 36) of patients were diagnosed with amoebic abscesses and 9.00% (n = 20) with hydatid disease. One patient with an actinomycosis-related liver abscess confirmed on liver biopsy was excluded from further statistical analysis. The etiology of PLA was biliary disease in 48.80% (n = 81) of patients. These were related to malignancy in 13.25% (n = 22), benign biliary disease in 25.30% (n = 42), and biliary interventions in 10.25% (n = 17) of patients. Choledocholithiasis was the most common benign biliary cause diagnosed in 23 patients (54.80%). Intra-abdominal causes via portal seeding contributed to 8.40% of cases, with appendicitis noted most frequently (n = 7). In 29.20% (n = 65) of cases, there was no identifiable cause (cryptogenic abscess) (Table 2).
| Disease | n = 166 | % |
| Biliary | 81 | 48.80 |
| Benign | 42 | 25.30 |
| Benign CBD/CHD stricture | 8 | 4.82 |
| Cholecystitis/complicated cholecystitis | 6 | 3.61 |
| Choledocholithiasis | 23 | 13.86 |
| Mirrizi syndrome | 3 | 1.81 |
| HIV cholangiopathy | 1 | 0.60 |
| Ruptured gallbladder (sickle cell crisis) | 1 | 0.60 |
| Malignant | 22 | 13.25 |
| Cholangiocarcinoma | 7 | 4.22 |
| Gallbladder cancer | 6 | 3.61 |
| Pancreatic cancer | 5 | 3.01 |
| Periampullary tumor | 4 | 2.41 |
| Iatrogenic injury/biliary intervention | 17 | 10.24 |
| Bile duct injury (laparoscopic cholecystectomy) | 2 | 1.20 |
| Bile duct stricture (laparoscopic cholecystectomy) | 2 | 1.20 |
| Bile duct stricture (post pancreaticoduodenectomy) | 1 | 0.60 |
| Benign stricture (blocked stent) | 2 | 1.20 |
| Choledocholithiasis (blocked biliary stent) | 2 | 1.20 |
| Biliary malignancy (blocked stent) | 7 | 4.22 |
| Post portal vein embolization (cholangiocarcinoma) | 1 | 0.60 |
| Portal pyemia | 14 | 8.43 |
| Appendicitis | 7 | 4.22 |
| Colitis | 2 | 1.20 |
| Diverticular disease | 3 | 1.81 |
| Perforated peptic ulcer | 1 | 0.60 |
| Jejunal perforation | 1 | 0.60 |
| Other | 5 | 3.01 |
| Locally advanced colon cancer | 1 | 0.60 |
| Metastatic colon cancer | 2 | 1.20 |
| Metastatic adenocarcinoma (unknown primary) | 1 | 0.60 |
| Rectosigmoid stricture | 1 | 0.60 |
| Hematogenous | 1 | 0.60 |
| Cryptogenic | 65 | 39.16 |
The laboratory results differed across the groups by pathology (Table 3). PLA had a raised median WBC and C-reactive protein (CRP), which was significantly (P < 0.0002 and P < 0.007) higher than the amoebic and hydatid groups. Seventy-five percent of patients with PLA had leukocytosis, 98.10% had an increased CRP and 21.00% had a CRP level above 300 mg/L (Table 4). The median total bilirubin was elevated in the pyogenic group and within the normal range for amoebic and hydatid collections. Regarding liver biochemistry alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) were raised in all groups. In patients with PLA larger than 10 cm, there was a trend towards a higher WBC, CRP, total bilirubin, ALP, and GGT when compared to the smaller abscesses. This was not statistically significant.
| Pyogenic | Amoebic | Hydatid | |||||
| Laboratory test | Median | IQR | Median | IQR | Median | IQR | P value |
| WBC (4-10 × 109/L) | 15.76 | 9.90-21.20 | 13.18 | 9.16-16.02 | 8.90 | 6.28-12.76 | 0.0002 |
| CRP (< 10 mg/L) | 211.0 | 110.0-289.0 | 181.0 | 60.5-246.5 | 90.0 | 28.5-215.5 | 0.0070 |
| Hemoglobin (12-15 g/dL) | 10.50 | 8.80-12.10 | 10.50 | 8.80-11.90 | 11.35 | 8.35-12.35 | 0.8300 |
| Platelets (150-400 × 109/L) | 391 | 242-575 | 495 | 401-615.5 | 483.5 | 322.5-562.5 | 0.0200 |
| Albumin (35-50 g/L) | 29.0 | 25.0-34.0 | 28.0 | 24.0-32.0 | 32.5 | 26.5-40.5 | 0.1100 |
| Total bilirubin (5-21 μmol/L) | 23.0 | 11.0-80.0 | 9.0 | 5.0-19.0 | 7.5 | 6.0-29.5 | 0.0001 |
| Conjugated bilirubin (< 3 μmol/L) | 16.0 | 6.0-52.0 | 4.0 | 3.0-14.0 | 4.5 | 3.0-21.5 | 0.0001 |
| ALT (7-35 U/L) | 39.0 | 25.0-89.0 | 26.0 | 12.0-44.0 | 20.5 | 14.0-32.0 | 0.0002 |
| AST (13-35 U/L) | 48.0 | 27.0-87.0 | 32.0 | 20.0-63.0 | 31.5 | 23.5-40.5 | 0.0080 |
| ALP (42-98 U/L) | 221 | 169-425 | 179 | 124-243 | 215 | 120-421 | 0.0100 |
| GGT (< 40 U/L) | 201.0 | 110.0-416.0 | 157.0 | 82.0-208.0 | 130.0 | 93.0-315.5 | 0.0300 |
| Urea (2.1-7.1 mmol/L) | 5.2 | 3.2-9.8 | 3.6 | 2.7-5.0 | 3.7 | 3.1-6.1 | 0.0200 |
| Creatinine (49-90 mmol/L) | 79.0 | 59.0-117.5 | 67.0 | 58.0-88.0 | 66.5 | 50.0-74.0 | 0.0200 |
| INR (0.9-1.2) | 1.31 | 1.18-1.49 | 1.27 | 1.21-1.46 | 1.20 | 1.14-1.43 | 0.5000 |
| Parameter | n (%) |
| Leukocytosis | 121 (74.69) |
| Raised CRP (> 10 mg/L) | 154 (98.09) |
| CRP (> 300 mg/L) | 33 (21.02) |
| Thrombocytopenia | 18 (12.02) |
| HB (< 10 g/dL) | 65 (40.63) |
| Hypoalbuminemia (< 30 g/L) | 89 (40.63) |
| Bilirubin (> 21 μmol/L) | 85 (53.46) |
| ALT (> 2 normal) | 44 (28.03) |
| AST (> 2 normal) | 53 (33.76) |
| ALP (> 2 normal) | 94 (58.75) |
| GGT (> 2 normal) | 132 (82.50) |
| INR > 1.5 | 29 (22.83) |
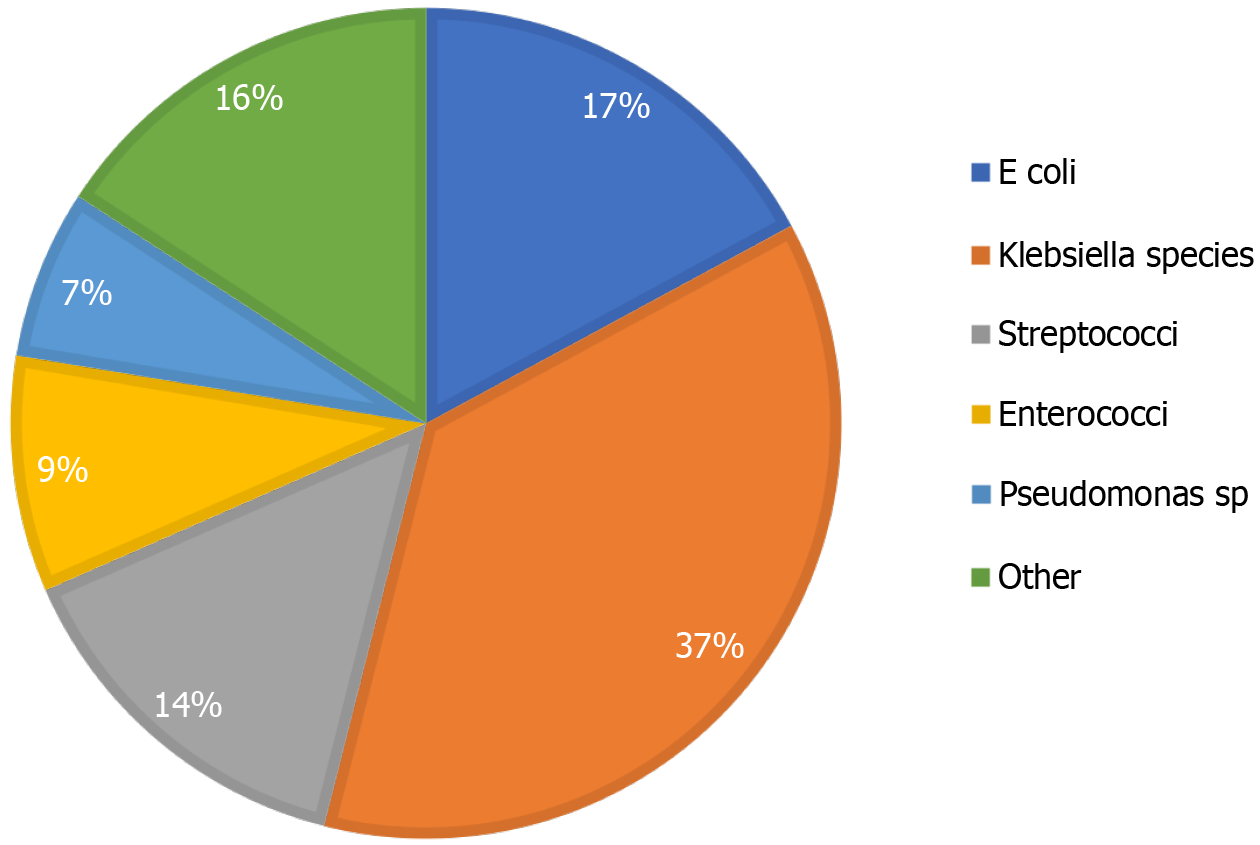
Blood cultures were obtained in 54.80% (n = 91) of patients presenting with PLA, and 17.60% (n = 16) were positive. The most commonly cultured organism was Escherichia coli (E. coli) (n = 7) followed by Klebsiella pneumoniae (K. pn
Transabdominal ultrasonography and triple phased contrast-enhanced abdominal CT were main imaging modalities used for patient evaluations. Magnetic resonance imaging was used to further characterize lesions of suspicious/indeterminate nature, ascertain etiology, and determine communication with the biliary tree. The median size of PLA was 8 cm, with 76.79% being larger than 5 cm. The predominant radiological presentation for all groups was multiple collections on the right side of the liver. Amoebic and hydatic collections were significantly larger (P = 0.0001) than PLA (Table 5).
| Item | Pyogenic | Amoebic | Hydatid | P value |
| Ultrasound | 125 (79.11) | 25 (78.13) | 15 (72.22) | |
| CT | 146 (90.12) | 30 (90.91) | 17 (94.44) | |
| MRI | 13 (8.13) | 0 | 2 (11.11) | |
| Size (median; interquartile range) | 8.00 (5.85-10.00) | 11.00 (9.00-14.00) | 14.50 (12.00-19.30) | 0.0001 |
| Range (cm) | 1.5-25.0 | 4.5-25.0 | 3.0-22.0 | |
| Size > 5 cm | 86 (76.79) | 16 (84.21) | 9 (90.00) | |
| Multiple | 80 (51.28) | 15 (46.88) | 8 (47.06) | |
| Right | 54.89 | 52.17 | 50.00 | |
| Left | 25.56 | 13.04 | 21.43 | |
| Bilobar | 19.55 | 34.78 | 28.57 | |
| Percutaneous drainage | 120 (74.53) | 30 (90.91) | 8 (42.11) | |
| ERCP | 50 (31.25) | 4 (12.12) | 9 (45.00) | |
| Surgery | 6 (3.73) | 5 (13.89) | 9 (45.00) | |
| Puncture, aspiration, injection, re-aspiration | 2 (10.53) |
In addition to appropriate fluid therapy, various antimicrobial regimens were used depending on the type of abscess. In the pyogenic group, amoxicillin-clavulanic acid and piperacillin-tazobactam were the empiric antibiotics administered in most instances and then tailored to the culture results based on susceptibility testing. Metronidazole was the principal antimicrobial administered for amoebic collections, and albendazole was administered at a dose of 15-20 mg/kg for hydatid disease. The majority (n = 123; 74.10%) of PLA resolved with percutaneous catheter drainage (PCD), whilst surgery was necessary in 3.61% (n = 6). Of these, percutaneous interventions by aspiration alone (n = 2, 0.94%) or catheter drainage (n = 156, 73.23%) were definitive therapy methods.
Thirty-seven patients (22.29%) with PLA were treated with antibiotics alone and required no intervention. The median size of PLA not requiring drainage was 5.15 cm, and the median size of abscesses requiring drainage was 8.20 cm. The median size of amoebic abscesses that required percutaneous drainage was 11.60 cm. Thirty-one percent of patients underwent ERCP (n = 50). ERCP was necessary in PLA to treat benign and malignant biliary tract obstruction. Four amoebic abscess cases required ERCP and endobiliary stent placement for a fistulous connection between the biliary tracts and the abscess cavity. Seven patients required ERCP in the hydatid group: One had biliary stricture; one for compression at hepatic ducts confluence; three for biliary fistulae; and two had diagnostic ERCP.
The indications for surgical management in patients presenting with PLA was rupture (n = 3), and 2 patients had an aspiration of their liver abscesses at the time of cholecystectomy. Three of the patients with amoebic disease underwent surgery: Three laparotomies for rupture and two laparoscopic drainage. In the hydatid disease cases, 5 patients were admitted for elective surgical procedures, and 4 patients had surgical procedures for symptoms unresponsive to medical treatment. The surgical procedures included three open cystectomies, three pericystectomies, one laparoscopic drainage of an infected hydatid cyst, and two partial hepatectomies. Two patients had a procedure-puncture, aspiration, injection, re-aspiration (PAIR).
The median length of stay was 13 days (interquartile range: 8-21). Overall, 12.70% (n = 28) of patients had complications (Table 6). Complications associated with amoebic liver abscesses were rupture in 3 patients and post-ERCP pancreatitis in 1 patient. In patients with cystic echinococcosis, three complications were noted: Two deep vein thrombosis and one intrathoracic rupture of the hydatid cyst. There were seven documented deaths with a mortality rate of 3.2%. Six deaths occurred in the pyogenic group: 2 patients had biliary tract malignancies; 1 patient died postoperatively after the repair of a bile duct injury; 1 patient had complicated appendicitis; and 2 patients with cryptogenic abscess died from septic shock. Patients that died tended to be older, but this did not reach statistical significance. The presence of diabetes mellitus in patients who died was not significantly higher than in patients that lived. No statistically significant differences were noted in WBC, CRP, bilirubin levels, ALP, GGT, cultures, or length of stay in patients who died.
| General complications | n | Complications of liver abscess | n |
| AKI | 3 | Lung abscess (pleuro-biliary fistula) | 1 |
| Aspiration pneumonia | 1 | Ruptured liver abscess | 6 |
| Clostridium difficile infection | 1 | Procedure related complications | |
| DVT | 2 | Cecal stump blowout | 1 |
| DVT/PE | 1 | Contrast induced nephropathy | 1 |
| PE | 2 | Post-ERCP bleed | 1 |
| Pneumonia | 1 | Pneumothorax | 2 |
| Pleural effusion | 2 | Post-ERCP pancreatitis | 1 |
| Small bowel injury | 1 |
There are varied incidences and demographics of liver abscesses geographically with a paucity of data in the South African population. This study was consistent with other studies reporting a male predominance[5]. However, a higher mean age of 60 years was observed for PLA in Asian[5], Australian[9], and European populations[11,12]. Meddings et al[3] reported a higher risk of developing PLA in patients older than 50 years of age. PLA are likely to occur in middle-aged and older patients due to the higher incidence of comorbidities such as diabetes mellitus, pancreaticobiliary, and other malignancies[13].
About 10% of the world’s population is infected with E. histolytica[14]. The distribution of the disease varies. However, it is frequently encountered in LMICs[2] and more prevalent in tropical and subtropical climates[14]. Amoebic liver abscesses are associated with low socioeconomic status as well as chronic alcoholism[2,7]. Indian studies demonstrated a higher incidence of amoebic liver abscesses compared to PLA, with up to two-thirds of cases being due to amoebic-related abscesses[2]. Amoebic abscesses presented at a younger age (40-43 years) than PLA[2,15]. In South Africa, a median age of 39 was previously documented[16]. This study also found patients with amoebic abscesses to be sig
Cystic echinococcosis, caused by the larval stages of Echinococcus granulosis (E. granulosis) infection, is the most co
The symptoms of pyogenic and amoebic liver abscesses are nonspecific, and diagnosis requires a high index of suspicion. Liver abscesses usually present with abdominal pain and fever and less commonly nausea, vomiting, and weight loss[1,21]. Infection with E. granulosis is initially asymptomatic. Most patients in this study presented with right upper quadrant pain, nausea, vomiting, and jaundice. Symptoms such as epigastric and right upper quadrant pain, nausea, malaise, vomiting, and jaundice tended to develop from the increasing size and number of cysts within the liver and pressure effects on surrounding organs[17]. Forty percent of patients in the current study had multiple abscesses and a median abscess size of 8 cm in PLA and 11 cm in amoebic abscesses.
Numerous studies have confirmed a shift in the etiology of PLA in the last decade. Biliary diseases[5,9] have superseded enteric causes. Cryptogenic abscesses, where no exact cause can be identified, are still the most common in many series[9,13,22]. In contrast, biliary etiologies occurred in 48.8% of patients, whilst ‘cryptogenic’ collections were in 39.1% of cases in this study. This is similar to the work of Losie et al[23] in a Canadian population. In the 15-year review, calculus disease (and its complications) as well as biliary malignancy increased. This study similarly revealed a predominance of benign biliary disease, including choledocholithiasis and other complications, and cholangiocarcinoma. This may reflect the increasing incidence of gallstone disease in the population and the long waiting times for surgery in patients with symptomatic gallstones in the public hospital setting.
In agreement with other studies, all PLA cases in this study had increased WBC and CRP[9]. Hypoalbuminemia (< 30 g/dL), likely due to an acute phase response, was evident in all groups. Though anemia (hemoglobin < 10 g/dL) was present in all the groups, it was more pronounced in PLA with over one-third of patients having hemoglobin of less than 10 g/dL. Anemia is a common finding in the literature[5,6,9] in patients with PLA. Derangements in liver chemistry were observed in the various groups. A larger proportion of patients had increased ALP and GGT as opposed to transaminases in PLA (Table 4). The median ALP and GGT were increased across the groups. Other studies of PLA and amoebic liver abscesses have reported similar findings[5,15,24]. Pang et al[9] showed that liver chemistry was deranged in 70% of patients, and the mean values for ductal enzymes were higher. Bilirubin levels were elevated in 50% of their patients (corroborating the findings of this study), with 13% of patients having clinically significant hyperbilirubinemia.
As noted in the literature[12,17,24-26] and in this study, clinical and laboratory findings were nonspecific. Ultrasound remains an important diagnostic tool and with the addition of a CT scan, the diagnosis can be confirmed in 90% of cases[1]. The combination of imaging modalities allows for the characterization of liver abscesses and cystic echinococcosis. In addition, it may help with determining the specific etiology in the case of PLA[5,12]. Larger collections were noted in the hydatid disease subgroup. This may reflect bias as a fair proportion of patients were admitted for elective surgery due to cyst sizes larger than 10 cm or due to pressure symptoms and pain. In all three subgroups, the collections were located on the right side of the liver. This observation has been reported in other studies[11,22]. In the case of pyogenic collections, the literature suggests that this is due to portal vein anatomy and a dense network of biliary canaliculi in the right lobe. Hydatid disease has also been found to predominate on the right side of the liver[19], and ultrasonographic features of amoebic abscesses are described as a solitary, well-defined cystic, intrahepatic cavity, most commonly found in the posterior part of the right lobe[2,14].
The cultures from aspirates had a higher positive bacterial yield than blood culture. This is similar to data from Pang et al[9], where a higher rate of positive culture was noted from abscess aspirates. Forty-eight percent of the blood cultures were positive in their study, which is higher than noted in this study. Klebsiella has emerged as the most common pathogen isolated from PLA in studies from Asia[5,13,25,26] and is associated with diabetes mellitus[4,25] and cryptogenic abscesses. In Central Europe, the causative agent is more likely to be Staphylococcus, Streptococcus, or E. coli. North and South American studies show a preponderance of E. coli[3,6,25] and Streptococcus[3,25]. In the United States, the most common organisms identified are Streptococcus species and E. coli, with 16.3% being polymicrobial[3]. The predominance of gram-negative bacteria is consistent with Asian studies, which report a high prevalence of Klebsiella species and E. coli in PLA[5,13].
The management of PLAs includes broad-spectrum antibiotic therapy to cover commonly isolated organisms. Antibiotics are used as standalone treatments for patients with small abscesses (< 5 cm diameter). Drainage is advised in PLA greater than 5 cm[9]. In our study, sizes greater than 5 cm and failure to respond to antibiotics alone was an indication for drainage. Percutaneous drainage, either ultrasound-guided or CT scan-guided, has become the most common approach[22,27]. Surgery is reserved for failed PCD or to manage complications and rupture[9]. Greater success rates are noted with PCD compared to aspiration alone[27,28], which is the favored management option in this and other studies[11]. A large proportion of amoebic liver abscesses, if uncomplicated, will respond to metronidazole alone[16]. However, if the abscess is greater than 10 cm or there is a failure to respond clinically to metronidazole, it should be drained per
There is a grey area for the need for intervention in amoebic abscesses between 5 cm and 10 cm in size. Bammigatti et al[28] found no significant difference in outcomes in patients randomized to metronidazole alone or percutaneous drainage in uncomplicated amoebic abscesses greater than 5 cm. The large median size of PLA and amoebic liver abscesses and the finding that 76.8% of PLA were greater than 5 cm and 63.7% of amoebic abscesses were greater than 10 cm accounted for the large proportion of patients requiring image-guided percutaneous drainage in our institutions. Failure of percu
The World Health Organization treatment guidelines for hydatid disease advocate for single, asymptomatic cysts less than 5 cm to be treated with albendazole alone. Surgical treatment is reserved for symptomatic cysts, cysts greater than 10 cm, and complicated cysts[29,30]. The options are a pericystectomy, open cystectomy, partial hepatectomy, or lobectomy. Percutaneous options include drainage of infected cysts or the PAIR procedure. In patients with hydatid disease, 45% required surgical intervention. Albendazole was the agent used as chemotherapy preoperatively and postoperatively in our setting. These agents can also be used as a stand-alone treatment in cysts less than 5 cm in size or patients unfit for surgery[17]. Surgery offers a high cure rate and is advised in patients with large active cysts with multiple daughter cysts or superficially located cysts with an increased risk of rupture[17]. PAIR procedure is effective in solitary, active cysts greater than 5 cm[30]. It is an option for patients who require drainage but are unfit for surgery or refuse surgery. It is also a management option for recurrences after surgical intervention. Eleven percent of patients underwent PAIR procedures in our institutions, which is comparable to other studies[30].
The mortality rate from PLA is notably high if not promptly and adequately treated. Mortality has decreased in recent years due to the effective use of broad-spectrum antibiotics and percutaneous drainage. The mortality rate was 3.00%, with no deaths occurring in the amoebic and hydatid groups. The mortality rate of PLA reported in the literature has a wide range (3%-30%)[3]. Ruiz-Hernández et al[11] evaluated factors contributing to mortality in PLAs and found that older age, sepsis, higher bilirubin levels, biliary and cryptogenic origin, and the development of pneumonia were related to mortality. Only septic shock was noted to be a predictor of mortality, a finding noted in other studies too[3]. This study did not find any statistically significant difference in patients who died when age, septic markers, bilirubin levels, and comorbidities were analyzed. Due to the small number of deaths, logistic regression analysis could not be done to as
Uncomplicated amoebic abscesses have a mortality rate of less than 1%[16]. A South African study showed a mortality rate of 0% in uncomplicated disease, which rose sharply to 25% when complications arose[16]. In a study by Ghosh et al[2], where amoebic abscesses predominated, a mortality rate of 2.5% was reported. All deaths in their study occurred in patients who had surgical intervention[2].
The limitations of this study are that the validity of the data depend on the accuracy of the discharge records as it was an administrative database review rather than a review of clinical medical records. This was partially alleviated by cross-referencing laboratory and radiology records. International Classification of Diseases 10 and procedure codes were used to identify patients in this study. Misclassification may have led to the underrepresentation of data. This study noted low numbers of patients with hepatic echinococcus and did not necessarily reflect the number of patients treated in our institutions. Some patients were diagnosed and managed in outpatient clinics and would therefore be missed. This affected the ability to draw significant conclusions in this subgroup of patients. The radiology reports had missing abscess size data in 38% of cases, and scans before 2018 were not stored for images to be reviewed. This was a source of bias for radiology data interpretation. The in-hospital mortality data may also be underrepresented as it relies on this data being updated in the discharge records.
Nevertheless, the findings of this study are an important contribution to the body of knowledge regarding these pathologies in South Africa and other LMICs. Additionally, this study forms the basis for more detailed and prospective research.
Liver abscesses pose a diagnostic challenge due to the nonspecific clinical findings and in the context of PLA require prompt and accurate diagnosis due to high mortality rates. In this study representing a Sub-Saharan African population, the most common presentation was PLA in middle-aged males. HIV and diabetes mellitus were comorbidities noted in a significant proportion of patients. In PLA, biliary etiology predominated, and the organism most often cultured was Klebsiella species. In most cases, nonoperative management via percutaneous drainage was sufficient, with surgery used to treat complications.
The authors would like to thank the hepatobiliary units of Chris Hani-Baragwanath Academic Hospital and Charlotte Maxeke Johannesburg Academic Hospital for allowing access to their administrative databases. We want to acknowledge and thank the National Health Laboratory Service for providing us with the blood and microbiology investigation results and the University of Witwatersrand Radiology Department for access to the radiology reports. The authors would also like to thank Professor Deirdre Kruger for her assistance with the statistical analysis.
| 1. | Lardière-Deguelte S, Ragot E, Amroun K, Piardi T, Dokmak S, Bruno O, Appere F, Sibert A, Hoeffel C, Sommacale D, Kianmanesh R. Hepatic abscess: Diagnosis and management. J Visc Surg. 2015;152:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Ghosh S, Sharma S, Gadpayle AK, Gupta HK, Mahajan RK, Sahoo R, Kumar N. Clinical, laboratory, and management profile in patients of liver abscess from northern India. J Trop Med. 2014;2014:142382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, Coffin C, Kaplan GG. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Liu L, Chen W, Lu X, Zhang K, Zhu C. Pyogenic Liver Abscess: A Retrospective Study of 105 Cases in an Emergency Department from East China. J Emerg Med. 2017;52:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Santos-Rosa OM, Lunardelli HS, Ribeiro-Junior MA. Pyogenic Liver Abscess: Diagnostic and Therapeutic Management. Arq Bras Cir Dig. 2016;29:194-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Mukhopadhyay M, Saha AK, Sarkar A, Mukherjee S. Amoebic liver abscess: presentation and complications. Indian J Surg. 2010;72:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | González-Alcaide G, Peris J, Ramos JM. Areas of research and clinical approaches to the study of liver abscess. World J Gastroenterol. 2017;23:357-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Pang TC, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: an audit of 10 years' experience. World J Gastroenterol. 2011;17:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 123] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (3)] |
| 10. | Yoo JJ, Lee TK, Kyoung DS, Park MA, Kim SG, Kim YS. A population-based study of pyogenic liver abscess in Korea: Incidence, mortality and temporal trends during 2007-2017. Liver Int. 2021;41:2747-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Ruiz-Hernández JJ, León-Mazorra M, Conde-Martel A, Marchena-Gómez J, Hemmersbach-Miller M, Betancor-León P. Pyogenic liver abscesses: mortality-related factors. Eur J Gastroenterol Hepatol. 2007;19:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Serraino C, Elia C, Bracco C, Rinaldi G, Pomero F, Silvestri A, Melchio R, Fenoglio LM. Characteristics and management of pyogenic liver abscess: A European experience. Medicine (Baltimore). 2018;97:e0628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Shi S, Xia W, Guo H, Kong H, Zheng S. Unique characteristics of pyogenic liver abscesses of biliary origin. Surgery. 2016;159:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Krige JE, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system. BMJ. 2001;322:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | McGarr PL, Madiba TE, Thomson SR, Corr P. Amoebic liver abscess--results of a conservative management policy. S Afr Med J. 2003;93:132-136. [PubMed] |
| 16. | Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, Pavone P, Cappellani A, Cacopardo B. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 241] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 17. | Petrone L, Cuzzi G, Colace L, Ettorre GM, Busi-Rizzi E, Schininà V, Pucillo L, Angeletti C, Pane S, Di Caro A, Bordi E, Girardi E, Pozio E, Corpolongo A, Teggi A, Brunetti E, Goletti D. Cystic echinococcosis in a single tertiary care center in Rome, Italy. Biomed Res Int. 2013;2013:978146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kayal A, Hussain A. A comprehensive prospective clinical study of hydatid disease. ISRN Gastroenterol. 2014;2014:514757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kloppers C, Couzens-bohlin K, Bernon M, Burmeister S, Beningfield S, Kotze U, Krige J, Jonas E. Hydatid disease in South-Africa – Is it a different disease in patients with HIV co-infection? HPB. 2019;21:S593. [DOI] [Full Text] |
| 20. | Maria Kozielewicz D, Sikorska K, Stalke P. Liver abscesses – from diagnosis to treatment. Clin Exp Hepatol. 2021;7:329-336. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 21. | Mezhir JJ, Fong Y, Jacks LM, Getrajdman GI, Brody LA, Covey AM, Thornton RH, Jarnagin WR, Solomon SB, Brown KT. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Losie JA, Lam JC, Gregson DB, Parkins MD. Epidemiology and risk factors for pyogenic liver abscess in the Calgary Health Zone revisited: a population-based study. BMC Infect Dis. 2021;21:939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Wong WM, Wong BC, Hui CK, Ng M, Lai KC, Tso WK, Lam SK, Lai CL. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol. 2010;16:2458-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Chan KS, Chen CM, Cheng KC, Hou CC, Lin HJ, Yu WL. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;58:366-368. [PubMed] [DOI] [Full Text] |
| 26. | Chung YF, Tan YM, Lui HF, Tay KH, Lo RH, Kurup A, Tan BH. Management of pyogenic liver abscesses - percutaneous or open drainage? Singapore Med J. 2007;48:1158-65; quiz 1165. [PubMed] |
| 27. | Cai YL, Xiong XZ, Lu J, Cheng Y, Yang C, Lin YX, Zhang J, Cheng NS. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: a systematic review and meta-analysis. HPB (Oxford). 2015;17:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Bammigatti C, Ramasubramanian NS, Kadhiravan T, Das AK. Percutaneous needle aspiration in uncomplicated amebic liver abscess: a randomized trial. Trop Doct. 2013;43:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 737] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 30. | Secchi MA, Pettinari R, Mercapide C, Bracco R, Castilla C, Cassone E, Sisco P, Andriani O, Rossi L, Grondona J, Quadrelli L, Cabral R, Rodríguez León N, Ledesma C. Surgical management of liver hydatidosis: a multicentre series of 1412 patients. Liver Int. 2010;30:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/