Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1321
Revised: September 9, 2024
Accepted: October 10, 2024
Published online: November 27, 2024
Processing time: 220 Days and 2.2 Hours
Whether patients with compensated cirrhosis and low-level viremia (LLV) of hepatitis B should receive antiviral therapy (AVT) is still controversial, and published results are inconsistent.
To investigate the link between LLV in compensated cirrhosis and prognosis concerning hepatocellular carcinoma (HCC), decompensation, and liver-related events.
The PubMed, EMBASE, and Cochrane Library databases were searched up to March 5, 2023. Outcomes of interest were assessed by pooled hazard ratios (HRs). The study was registered with PROSPERO (CRD42023405345).
Six cohort studies representing 3155 patients were included. Compared with patients with undetectable HBV DNA, patients with LLV was associated with increased risk of HCC (HR: 2.06, 95%CI: 1.36-3.13; Q-statistic-P = 0.07, I2 = 51%) regardless of receiving AVT or not (AVT group: HR: 3.14; 95%CI: 1.73-5.69; Q-statistic-P = 0.60, I2 = 0%; un-AVT group: HR: 1.73, 95%CI: 1.09-2.76; Q-statistic-P = 0.11, I2 = 50%). The pooled results showed no statistical association between LLV and decompensation of cirrhosis (HR: 2.06, 95%CI: 0.89-4.76; Q-statistic-P = 0.04, I2 = 69%), and liver-related events (HR: 1.84, 95%CI: 0.92-3.67; Q-statistic-P = 0.03, I2 = 72%), respectively. Grading of Recommendations Assessment, Development and Evaluation assessment indicated moderate certainty for HCC, very low certainty for decompensation of cirrhosis and liver-related clinical events.
LLV in compensated cirrhotic patients is associated with increased risk of HCC, higher tendency for hepatic decompensation and liver-related events. Closer screening of HCC should be conducted in this population.
Core Tip: The need for antiviral therapy in patients with compensated cirrhosis and low-level hepatitis B viremia remains debated. This meta-analysis analyzed data from six cohort studies, revealing that low-level viremia in compensated cirrhosis is linked to a higher risk of hepatocellular carcinoma and an increased likelihood of cirrhosis decompensation and liver-related events. These findings support current guidelines for antiviral treatment, underscore the importance of vigilant cancer screening, and highlight the need for regular monitoring of viral levels.
- Citation: Lin WC, Lin K, Li MK, Liu X, Huang YF, Wang X, Wu B. Low level of hepatitis B viremia is associated with increased risk of hepatocellular carcinoma in compensated cirrhotic patients. World J Hepatol 2024; 16(11): 1321-1330
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1321.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1321
Chronic hepatitis B is a leading cause of hepatocellular carcinoma (HCC) and the second most common cause of cancer-related mortality. The global prevalence of hepatitis B surface antigen in the general population was 3.8% in 2019, with approximately 1.5 million new hepatitis B virus (HBV) infections, 296 million chronic infections, and 820000 deaths resulting from HBV-related cirrhosis, HCC, or liver failure[1,2]. Given the severity of this situation, the treatment and management of patients with hepatitis B are particularly important. Antiviral therapy (AVT) has improved the prognosis of infected patients through the suppression of viral replication, especially at an early stage of chronic liver disease and even early cirrhosis[3]. However, many patients with compensated cirrhosis with low-level viremia (LLV) remain. Currently, there is no consensus among different guidelines on whether this population should receive AVT. The American Association for the Study of the Liver Diseases and the European Association for the Study of the Liver recommend AVT for these patients[4,5]. Moreover, both China and Japan recommend initiating AVT for cirrhotic patients with detectable HBV DNA[2,6]. In contrast, the Asia-Pacific Association for the Study of the Liver guidelines recommend AVT only for patients with HBV-DNA > 2000 IU/mL[7]. The Korean Association for the Study of the Liver also noted that there is a lack of strong evidence for AVT in this population. To date, no randomized controlled trial (RCT) has evaluated the effect of AVT in patients with LLV and compensated cirrhosis[8]. There is also a subset of the population with LLV even under potent AVT, and whether the risk of HCC in this population is different from that in the population with LLV and without AVT remains unclear. Recent retrospective studies have identified a positive correlation between LLV in compensated cirrhosis and the development of HCC[9,10] while several other studies have reported contradictory findings[11,12]. Currently, there are no prospective studies or meta-analyses that conclusively clarify the relationship between LLV in patients with compensated cirrhosis and HCC.
Considering the inconsistent international guideline recommendations, contradictory observational studies, and the scarce studies on the natural history of patients with compensated cirrhosis and LLV, a systematic review and meta-analysis are needed. We aimed to investigate the association between LLV and HCC risk in compensated cirrhosis patients and to explore whether there is a difference in HCC risk between treated and untreated patients with LLV. Additionally, potential associations between LLV and hepatic decompensation, as well as between LLV and liver-related clinical events, were investigated.
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis 2009 guidelines. The protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/PROSPERO/ - registration number CRD42023405345).
Two authors (Lin WC and Lin K) independently searched the PubMed, EMBASE, and Cochrane Library databases for published articles without language restrictions, from inception to March 5, 2023. PubMed is a widely used biomedical database that primarily focuses on studies from the United States. EMBASE complements PubMed by offering broader coverage of European and international journals, particularly in the fields of pharmacology and drug-related research. The Cochrane Library, including the Cochrane Central Register of Controlled Trials, is a key resource for accessing RCTs. The keywords used were as follows: “low-level viremia”, “LLV”, and “cirrhosis”. Additionally, conference abstracts and bibliographies of related literature were screened to identify other articles that might meet the inclusion requirements.
The inclusion and exclusion criteria were established according to the PICOS framework (population, intervention, comparison, outcome, and study design). The criteria for considering studies for this review were as follows: (1) Studies that investigated the association of LLV with HCC, hepatic decompensation and liver-related events in patients with compensated cirrhosis; (2) Studies that reported the associated adjusted relative risk (RR)/hazard ratio (HR)/odds ratio (OR) with corresponding 95%CIs or other measures that can be used to compute these values; and (3) Were designed as clinical trials, cohort studies, or case-control studies. The exclusion criteria were as follows: (1) Studies focused on children or adolescents; (2) Certain publication types (animal studies, editorials, letters, and reviews); and (3) Studies with unavailable data. If multiple studies used the same population, the article with the most information or the largest sample size was included. For the definition of outcomes in this study, HCC diagnosis was confirmed by histological evidence or radiological findings determined by dynamic computed tomography and/or magnetic resonance imaging (nodule > 1 cm with arterial hypervascularity and portal/delayed-phase washout)[13]. Decompensated cirrhosis is defined as cirrhosis with severe complications such as ascites, esophagogastric variceal bleeding, or hepatic encephalopathy, with liver function mostly classified as Child-Pugh class B or C[14]. Liver-related events include decompensation of cirrhosis, HCC, liver transplantation, and death[15].
For each article, the study information and basic characteristics were extracted, including the first author, publication year, region, type of study, participants (sample size, sex ratio, age), definition of LLV, baseline ALT (U/L), number of hepatitis B e antigen (HBeAg) positive cases, duration of follow-up, outcomes reported, associated adjusted RR/HR/OR with corresponding 95%CIs, or other measures that could be used to compute these values, and adjustments for confounders. The Newcastle-Ottawa Scale (NOS), a risk of bias assessment tool for observational studies recommended by the Cochrane Collaboration, was used to assess the quality of the included observational studies. Studies with an NOS score > 6 were considered high quality[16]. The overall strength of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method, a widely recognized method for eva
Aggregate data from the included studies were evaluated via meta-analysis, and the random-effects model was applied, considering the potential heterogeneity. The heterogeneity was measured using the Q test (P < 0.10 was considered statistically significant) and the I2 test (I2 < 30%, = 30%-50%, and > 50% represented low, moderate, and high heterogeneity, respectively)[18]. Funnel plots were used to detect publication bias. For studies where HRs were not provided but Kaplan-Meier curves were available, HRs were calculated according to previous practical guidelines[19]. All statistical analyses were conducted using Review Manager (RevMan) software (version 5.4) (Cochrane Collaboration). A P value for the interaction of less than 0.1 indicated a statistically significant subgroup effect. A two-tailed P < 0.05 was considered statistically significant in other analyses.
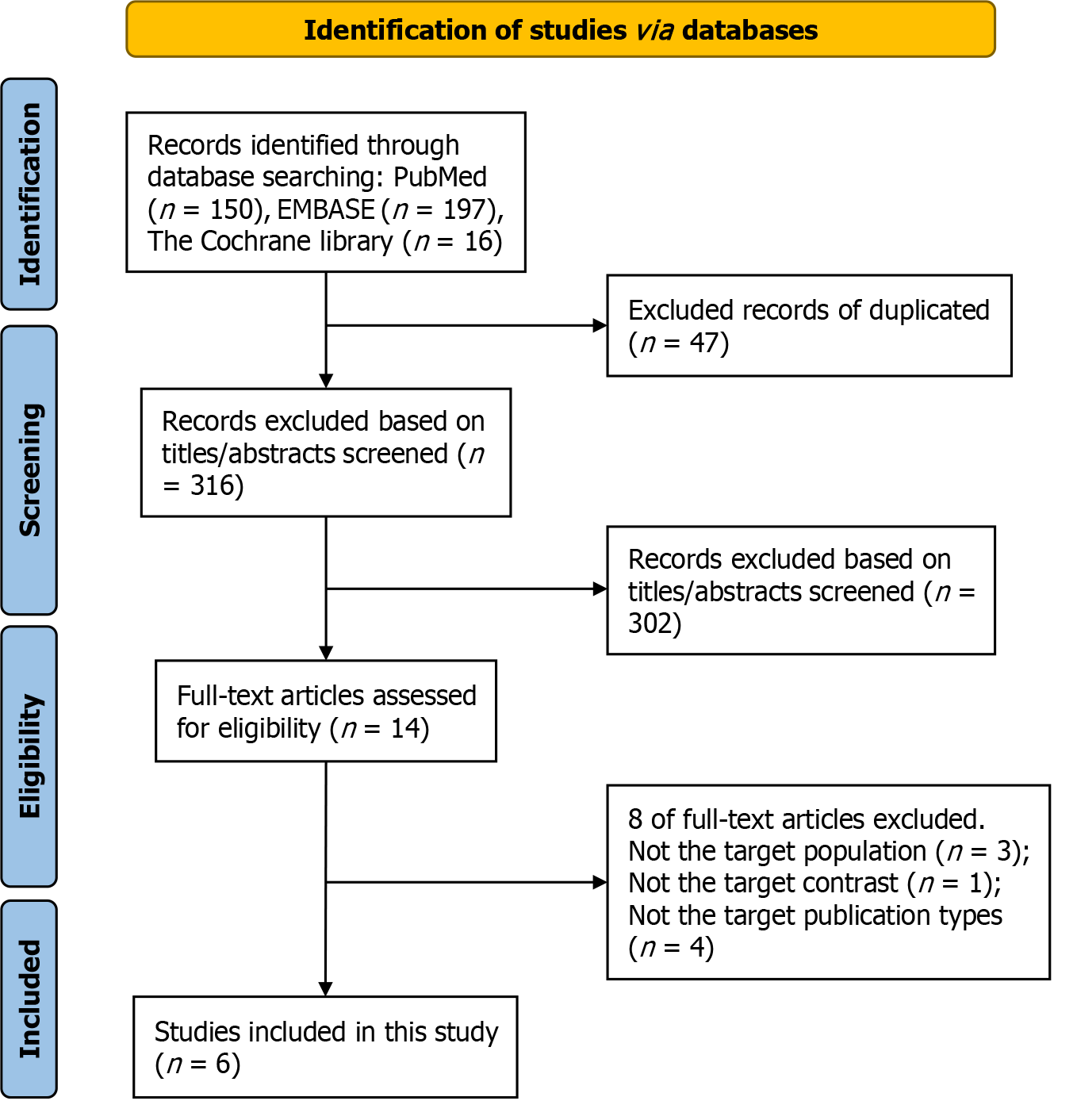
The initial systematic search of the electronic databases identified 363 articles (PubMed: 150; EMBASE: 197; Cochrane Library: 16). After excluding duplicates and screening titles and abstracts, 14 articles underwent a more detailed full-text assessment, of which 8 were further excluded for the following reasons: (1) Not the target population (n = 3); (2) Not the target contrast (n = 1); or (3) Certain publication types for which data were not available (two were editorials; one was review; one was an abstract, the full article of which has been included in this meta-analysis) (the specific reasons for the excluded studies can be found in Supplementary Table 1). Finally, a total of six studies, including 3155 people, were included in this meta-analysis[9-12,20,21]. The flow chart of the literature search is depicted in Figure 1.
In general, the included studies were retrospective cohort studies with moderate to very low certainty for estimating HCC risk. The pooled results revealed that patients with LLV were associated with an increased risk of HCC regardless of whether they received AVT, but there was no significant association between LLV and decompensation of cirrhosis or liver-related events.
All six included studies were retrospective cohort studies from Asia, with a total of 3155 patients. Sample sizes ranged from 200 to 1075, with a mean age between 44 and 55.1 years, and follow-up duration ranged from 42 months to 132 months. The prevalence of diabetes varies from 14.8% to 23.7%. With respect to the definition of LLV, three articles defined LLV as HBV-DNA quantification between 20 and 2000 IU/mL[9,11,12], two defined as between 12 and 2000 IU/mL[20,21] and one defined it as between 100 and 2000 IU/mL[10]. All studies reported HCC[9-12,20,21], three reported on hepatic decompensation[10,11,20], and three reported liver-related clinical events[9,10,12]. Additionally, HRs in the two articles were calculated by extracting data from Kaplan-Meier curves, so there were no adjustment factors[10,20] for these studies. Sinn et al[21] only provided HRs adjusted for age, whereas the other three studies adjusted for multiple factors, including HBeAg, albumin, and renal function (creatinine or estimated glomerular filtration rate)[9,11,12]. The basic characteristics of the included studies are summarized in Table 1.
| Ref. (first author, year) | Country/region | Type of study | Sample size/LLV/MVR | Age (year)/sex (male%) | Definition of LLV | Baseline ALT (U/L) | HBeAg positive (n) | Diabetes (%) | Duration of follow-up | Outcomes reported (HR, 95%CI) | Adjustments for confounders |
| Huang et al[11], 2023 | Korea, Singapore, Japan | Retrospective cohort study | 1075/742/333 | LLV: 55.1/67.8%; MVR: 59.1/72.1% | With ≥ 1 detectable serum HBV-DNA (20-2000 IU/mL) | LLV: 38.5; MVR: 31.2 | LLV: 109; MVR: 46 | LLV: 16.3; MVR: 19.2 | LLV: 62.8 months; MVR: 64.2 months | HCC: MVR Ref LLV 1.20 (0.79, 1.82); Hepatic decompensation: MVR Ref LLV 1.10 (0.66, 1.82) | Sex, diabetes, positive HBeAg, FIB-4 index, Albumin, eGFR, recruitment centers |
| Lee et al[20], 2020 | Korea | Retrospective cohort study | 440/110/330 | LLV: 51.1/80.6%; MVR: 52.9/61.5% | With ≥ 1 detectable serum HBV-DNA (12-2000 IU/mL) | 138.31 | 6.4%1 | NA | 132 months | HCC: MVR Ref LLV 2.64 (1.10, 6.34); Hepatic decompensation: MVR Ref LLV 2.75 (1.39, 5.45) | NA |
| Lee et al[23], 2022 | Korea | Retrospective cohort study | 567/391/176 | 54.8/67.9% (LLV: 54.0/66.5%; MVR: 56.7/71.0%) | With ≥ 1 detectable serum HBV-DNA (20-2000 IU/mL) | 30.0 (LLV: 30.3; MVR: 29.3) | 81 (LLV: 50; MVR: 31) | LLV: 15.1; MVR: 14.8 | 71.9 months | HCC: MVR Ref LLV 1.42 (0.69, 2.91); Liver-related clinical events: MVR Ref LLV 1.82 (0.84, 3.91) | Age, sex, alcohol consumption, HBeAg, AST, ALT, total bilirubin, albumin, platelet count, and eGFR no. of LLV episodes |
| Sinn et al[21], 2015 | Korea | Retrospective cohort study | 246/175/71 | 51.8/66%1 | With ≥ 1 detectable serum HBV-DNA (12-2000 IU/mL) | 24 (median)1 | 111 | NA | 5.6 years (median) | HCC: MVR Ref LLV 4.79 (1.11, 20.50) | Age |
| Yang et al[9], 2023 | Korea | Retrospective cohort study | 627/386/241 | 54.7/64.4% (LLV: 52.2/65.8%; MVR: 58.7/62.2%) | With ≥ 1 detectable serum HBV-DNA (20-2000 IU/mL) | 24 (LLV: 25; MVR: 23) | 22 (LLV: 19; MVR: 3) | LLV: 16.4; MVR: 23.7 | 3.9 years | HCC: MVR Ref LLV 2.36 (1.36, 4.11); liver-related clinical events: MVR Ref LLV 1.14 (0.77, 1.70) | Age, AST, ALT, albumin, platelet, total cholesterol, total bilirubin, creatinine, PT, INR, HBeAg, family history, diabetes, hypertension, alcohol drinking, fatty liver |
| Zhang et al[10], 2021 | China | Retrospective cohort study | 200/60/140 | 44/72.4%1 | With ≥ 1 detectable serum HBV-DNA (100-2000 IU/mL) | 801 | 56.7%1 | NA | 42 months | HCC: MVR Ref LLV 3.64 (1.61, 8.20); Hepatic decompensation: MVR Ref LLV 5.11 (1.02, 25.55); Liver-related clinical events: MVR Ref LLV 3.59 (1.66, 7.63) | NA |
All the observational studies had NOS scores greater than 6, indicating that all the studies were of acceptable quality (Supplementary Table 2 for the specific NOS scoring table). A funnel plot for the associations between outcomes suggested that there was no evidence of publication bias (a funnel plot for the outcomes is shown in Supplementary Figures), although a limited number of included studies is not recommended according to the guidelines. Sensitivity analysis was not conducted due to the small number of articles included in this meta-analysis. The GRADE assessment indicated moderate certainty for the estimates of LLV and increased risk of HCC, very low certainty for the estimates of LLV and decompensation of cirrhosis and liver-related clinical events (the GRADE assessment is shown in Supplementary Table 3).
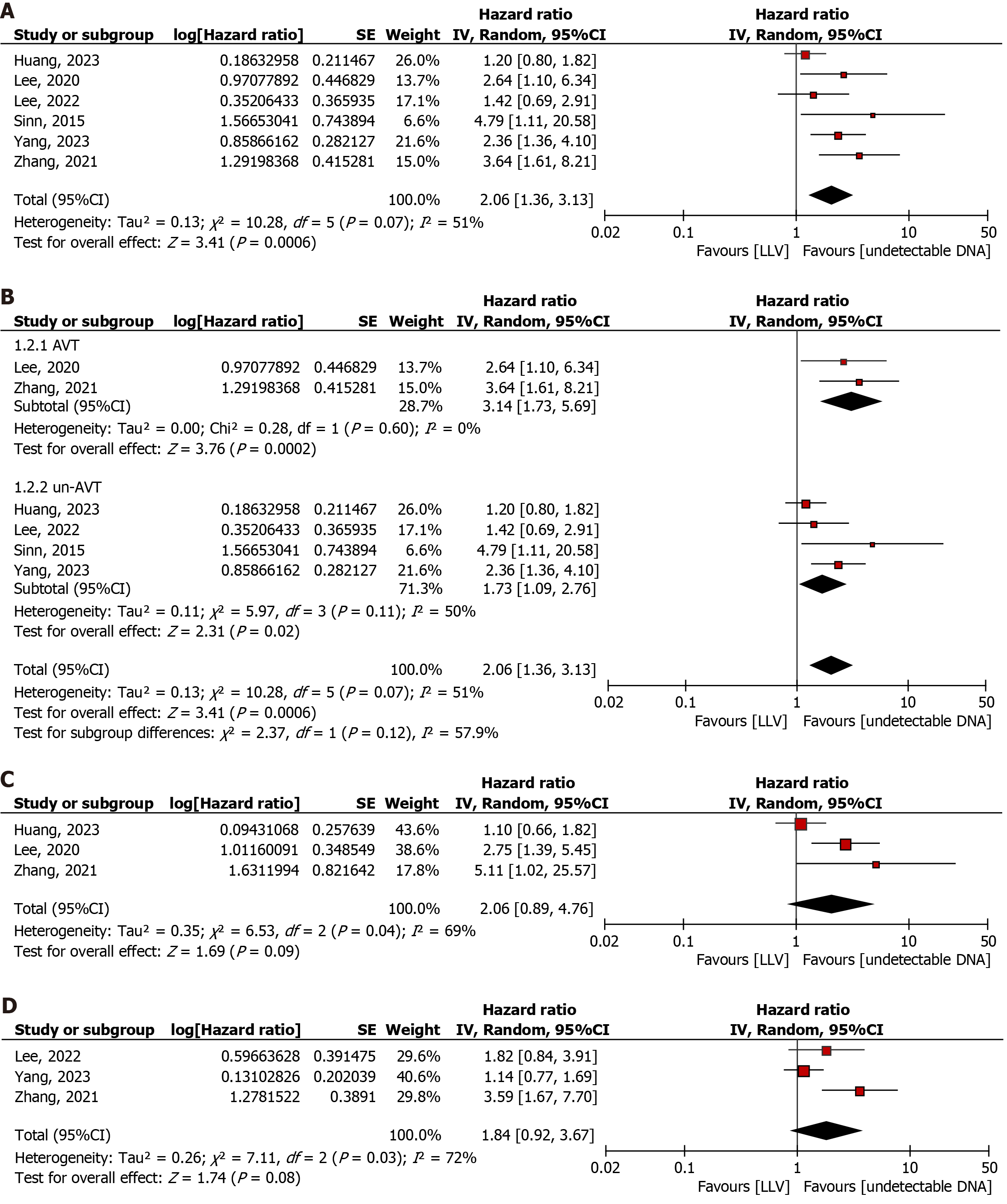
A total of 6 articles were included to explore the association between LLV and the risk of HCC in patients with compensated cirrhosis (LLV: 1864; undetectable HBV DNA: 1291)[9-12,20,21]. The pooled results revealed that LLV was significantly positively associated with an increased risk of HCC (HR: 2.06, 95%CI: 1.36-3.13; P = 0.0006; Q statistic, P = 0.07; I2 = 51%), with high heterogeneity (Figure 2A).
Stratified subgroup analysis based on the presence or absence of AVT showed that LLV was significantly associated with an increased risk of HCC regardless of AVT status (AVT: HR: 3.14; 95%CI: 1.73-5.69; P = 0.0002; Q statistic, P = 0.60, I2 = 0%; un-AVT: HR: 1.73, 95%CI: 1.09-2.76; P = 0.02; Q statistic, P = 0.11, I2 = 50%). There was no significant difference between the two groups [test for subgroup differences: χ² = 2.37, df = 1 (P = 0.12), I² = 57.9%; Figure 2B].
Three articles were included to investigate the association between LLV and the risk of decompensation of cirrhosis (LLV: 912; undetectable HBV DNA: 803)[10,11,20]. The pooled results revealed no significant association between LLV and the risk of decompensation of cirrhosis (HR: 2.06, 95%CI: 0.89-4.76; P = 0.09; Q statistic, P = 0.04; I2 = 69%), with high heterogeneity (Figure 2C).
Three studies investigated liver-related clinical events (LLV: 837; undetectable HBV DNA: 557)[9,10,12]. The pooled results revealed no significant association between LLV and liver-related events (HR: 1.84, 95%CI: 0.92-3.67; P = 0.08; Q statistic, P = 0.03, I2 = 72%), with high heterogeneity (Figure 2D).
In this meta-analysis, we found that patients with LLV and compensated cirrhosis had a significantly greater risk of HCC compared to patients with undetected HBV DNA, regardless of AVT. Additionally, patients in the LLV group had a greater tendency for decompensation and liver-related clinical events.
To the best of our knowledge, this is the first systematic review and meta-analysis to explore the associations between LLV and HCC in patients with compensated cirrhosis. In the absence of RCTs, our results support for the guidelines for AVT in LLV patients. Consistent with our study, several observational studies have emphasized the importance of AVT in LLV patients. A meta-analysis and systematic review published in 2016, which included results of 10 observational studies, found that AVT was associated with a reduced risk of HCC, decompensation, and all-cause mortality in patients with elevated HBV DNA levels of ≥ 4 Log copies/mL and compensated cirrhosis[22]. Furthermore, Lee et al[23] recently compared the cost and effectiveness (quality-adjusted life years) of AVT in a virtual cohort of 10000 patients aged 54 years who received AVT vs those who did not. Their baseline case analysis simulation found that AVT in LLV patients with compensated cirrhosis contributed positively to clinical benefit and the national health care budget[23].
Regarding the association between LLV after treatment and HCC risk, few relevant studies exist. An observational study from Wong et al[24] in 2013 revealed no significant association between AVT and the 3-year risk of hepatitis events, (defined as any cirrhotic complications, HCC, and/or liver-related mortality), when the HBV DNA concentration was less than 2000 IU/L among patients with liver cirrhosis (entecavir cohort: n = 482; control cohort: n = 69; HR 0.80, 95%CI: 0.20-2.10; P = 0.91). However, it is important to note that in this observational study, ALT levels and the HBeAg-positive rate were higher in the control group than in the experimental group, and the sample size of the control group was much smaller than that of the experimental group. The results need to be further validated in future large-scale cohort studies and RCTs.
The mechanisms linking low-level hepatitis B viremia (LLV) to HCC are complex and not fully understood. One key mechanism involves the integration of HBV DNA into the host genome, which may activate proto-oncogenes through insertional mutagenesis. Additionally, HBV proteins interact with host proteins to regulate cell signaling pathways associated with HCC[25,26]. Even at low viremia levels, HBV can induce persistent, low-grade inflammation, leading to sustained immune activation. Over time, this inflammation contributes to liver damage, fibrosis, and cirrhosis, all of which are significant risk factors for HCC[27,28]. Additionally, accumulating evidence from both human and mouse models demonstrates that inflammation facilitates HCC progression by promoting proliferative and survival signaling, inducing angiogenesis, evading immune surveillance, supporting cancer stem cell survival, activating invasion and metastasis, and inducing genomic instability[29]. Interestingly, LLV is associated with a non-significantly increased risk of liver decompensation and liver-related events. Previous studies have shown that LLV is related to persistent low-grade inflammation, which may affect the dynamic changes in liver fibrosis[30,31]. Whether these changes may increase portal venous pressure, which is known to be highly related to hepatic decompensation events[32] remains unknown. Moreover, a recent Korean study revealed that impaired liver function, rather than LLV, may have a greater impact on liver decompensation[9]. The association between LLV and liver decompensation should be further validated in future investigations.
Given our finding that LLV in patients with compensated cirrhosis is associated with an increased risk of HCC compared with undetectable HBV DNA, regardless of AVT, close monitoring of HBV DNA levels should be emphasized in clinical practice. Recent studies also suggest some solutions for managing patients with LLV. In a prospective study of 211 patients with LLV and chronic hepatitis or compensated cirrhosis, Li et al[33] reported that after 12 and 24 weeks of conversion to tenofovir alafenamide fumarate treatment, the virological response rates were 54.7% and 62.7%, significantly higher than those in the control group that continued entecavir treatment (6.7% and 9.3%, respectively)[33]. Furthermore, combination therapy with nucleoside analogs and interferon or pegylated interferon may have better efficacy in achieving a virological response[34].
Our study has several limitations, and the results should be interpreted with caution. First, the included articles were observational studies, which were not sufficient to establish causality. The heterogeneity among studies may compromise the accuracy of the pooled results of our meta-analysis. Second, despite our comprehensive search of databases and related resources, the number of eligible studies remains limited. In addition to studies concerning HCC outcomes, research on hepatic decompensation and liver-related events is sparse. As a result, there are too few studies to conduct a detailed subgroup analysis, and a multifactorial analysis of prognostic factors of LLV is precluded. Prospective studies are needed for further exploration. Third, due to variations in medical practices and measurement equipment across regions, the HBV DNA quantification interval for LLV varied slightly, although every study defined LLV as HBV DNA less than 2000 IU/mL, as the guideline[5]. Fourth, some studies included in the analysis did not adjust for influencing factors, such as HBeAg, which is associated with an increased risk of liver cancer[35]. This lack of adjustment may have introduced errors in the results. Approximately 75% of annual hepatitis B cases are diagnosed in Asia, where the disease is a leading cause of chronic hepatitis, cirrhosis, and HCC[36]. This regional prevalence accounts for the predominance of Asian studies in the literature, but regional variations may effect the generalizability of these findings to other parts of the world.
This meta-analysis highlights the elevated risk of HCC in patients with LLV and compensated cirrhosis compared to those with undetectable HBV DNA, regardless of AVT. Clinicians should prioritize vigilant monitoring of these high-risk patients, employing enhanced surveillance strategies such as more frequent imaging and biomarker testing to enable early detection of HCC. The increased risk of decompensation and liver-related events in the LLV group underscores the need for close management to prevent disease progression. Additionally, further research is needed to elucidate the mechanisms underlying the increased risk of HCC and liver-related events in LLV patients. Future studies should incorporate diverse geographical regions to improve generalizability. Key variables, including baseline HBV DNA levels and HBeAg seropositivity, which are associated with persistent HBV DNA posttreatment and an increased risk of HCC, should be considered. Given that age is a known risk factor for HCC, future studies should also explore the potential differential impact of LLV on prognosis across age groups. Finally, inconsistencies in LLV diagnosis across studies necessitate further investigation to determine whether varying definitions of LLV influence prognosis, ultimately refining hepatitis B surveillance strategies.
In summary, LLV in patients with compensated cirrhosis is associated with an elevated risk of HCC. Additionally, patients in the LLV group are more likely to progress to cirrhosis decompensation and other liver-related clinical events. These findings support current guidelines recommending AVT for patients with compensated cirrhosis and LLV. They also highlight the need for vigilant HCC screening in compensated cirrhotic patients with LLV and underscore the importance of monitoring HBV DNA levels in clinical practice. Future prospective studies and randomized trials are essential to further elucidate the relationship between LLV and HCC in patients with compensated cirrhosis.
We thank all other staff from the Department of Gastroenterology, The Third Affiliated Hospital of Sun Yat-sen Unive
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56700] [Article Influence: 7087.5] [Reference Citation Analysis (135)] |
| 2. | Chinese Society of Hepatology; Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. [Guidelines for the prevention and treatment of chronic hepatitis B (version 2022)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:1309-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 3. | Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4014] [Article Influence: 446.0] [Reference Citation Analysis (1)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin Liver Dis (Hoboken). 2018;12:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 6. | Drafting Committee for Hepatitis Management Guidelines; the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol Res. 2020;50:892-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, Sinn DH, Lee SH, Lee JH, Lee HW. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin Mol Hepatol. 2020;26:411-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Yang J, Choi WM, Shim JH, Lee D, Kim KM, Lim YS, Lee HC, Choi J. Low Level of Hepatitis B Viremia Compared With Undetectable Viremia Increases the Risk of Hepatocellular Carcinoma in Patients With Untreated Compensated Cirrhosis. Am J Gastroenterol. 2023;118:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Zhang Q, Peng H, Liu X, Wang H, Du J, Luo X, Ren H, Hu P. Chronic Hepatitis B Infection with Low Level Viremia Correlates with the Progression of the Liver Disease. J Clin Transl Hepatol. 2021;9:850-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Huang DQ, Tamaki N, Lee HW, Park SY, Lee YR, Lee HW, Lim SG, Lim TS, Kurosaki M, Marusawa H, Mashiba T, Kondo M, Uchida Y, Kobashi H, Furuta K, Izumi N, Kim BK, Sinn DH. Outcome of untreated low-level viremia versus antiviral therapy-induced or spontaneous undetectable HBV-DNA in compensated cirrhosis. Hepatology. 2023;77:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Lee HW, Park SY, Lee YR, Lee H, Lee JS, Kim SU, Park JY, Kim DY, Ahn SH, Kim BK. Episodic Detectable Viremia Does Not Affect Prognosis in Untreated Compensated Cirrhosis With Serum Hepatitis B Virus DNA <2,000 IU/mL. Am J Gastroenterol. 2022;117:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6431] [Article Influence: 803.9] [Reference Citation Analysis (10)] |
| 14. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2216] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 15. | Wang X, Wu B. Endoscopic sequential therapy for portal hypertension: Concept and clinical efficacy. Liver Res. 2021;5:7-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 17. | Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 640] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 18. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48659] [Article Influence: 2115.6] [Reference Citation Analysis (4)] |
| 19. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 5109] [Article Influence: 268.9] [Reference Citation Analysis (1)] |
| 20. | Lee SB, Jeong J, Park JH, Jung SW, Jeong ID, Bang SJ, Shin JW, Park BR, Park EJ, Park NH. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin Mol Hepatol. 2020;26:364-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 23. | Lee H, Jang S, Ahn SH, Kim BK. Cost-effectiveness of antiviral therapy in untreated compensated cirrhosis patient with serum HBV-DNA level < 2000 IU/mL. Hepatol Int. 2022;16:294-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, Chan HY, Wong VW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 403] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 25. | Abu-Amara M, Feld JJ. Does antiviral therapy for chronic hepatitis B reduce the risk of hepatocellular carcinoma? Semin Liver Dis. 2013;33:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Kar A, Samanta A, Mukherjee S, Barik S, Biswas A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J Med Virol. 2023;95:e28436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 27. | Carr BI, Rui F, Ince V, Yilmaz S, Zhao X, Feng Y, Li J. Comparison of patients with hepatitis B virus-associated hepatocellular carcinoma: Data from two hospitals from Turkey and China. Portal Hypertens Cirrhosis. 2023;2:165-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 28. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 989] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 29. | Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 30. | Sun Y, Wu X, Zhou J, Meng T, Wang B, Chen S, Liu H, Wang T, Zhao X, Wu S, Kong Y, Ou X, Wee A, Theise ND, Qiu C, Zhang W, Lu F, Jia J, You H. Persistent Low Level of Hepatitis B Virus Promotes Fibrosis Progression During Therapy. Clin Gastroenterol Hepatol. 2020;18:2582-2591.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 31. | Lok AS, Hadziyannis SJ, Weller IV, Karvountzis MG, Monjardino J, Karayiannis P, Montano L, Thomas HC. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut. 1984;25:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Yang Y, Li M, Luo J, Yu H, Tian H, Wang X. Non‐hemodynamic effects: Repurposing of nonselective beta‐blockers in cirrhosis? Portal Hypertens Cirrhosis. 2022;1:153-156. [DOI] [Full Text] |
| 33. | Li ZB, Li L, Niu XX, Chen SH, Fu YM, Wang CY, Liu Y, Shao Q, Chen G, Ji D. Switching from entecavir to tenofovir alafenamide for chronic hepatitis B patients with low-level viraemia. Liver Int. 2021;41:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, Peng ML, Ren H, Hu P. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther. 2018;47:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ; Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 36. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH, Sjalfoellah Noer HM, Sollano J, Sun HS, Xu DZ. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 345] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/