Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1225
Revised: September 2, 2024
Accepted: October 11, 2024
Published online: November 27, 2024
Processing time: 133 Days and 10.3 Hours
Autoimmune hepatitis is an uncommon condition that affects both adults and children and is characterized by chronic and recurrent inflammatory activity in the liver. This inflammation is accompanied by elevated IgG and autoantibody levels. Historically, treatment consists of steroids with the addition of azathi
Core Tip: Autoimmune hepatitis is a serious condition that affects both adults and children and has a poor prognosis if left untreated. Historically, treatment has involved high-dose steroids to induce remission and maintenance therapy with azathioprine, which achieves remission in 80% of cases but requires alternative therapies for the remaining 20%. While there have been advancements in immunosuppressive therapies for other diseases, treatment protocols for autoimmune hepatitis have remained largely unchanged. In this article, we explored alternative treatment options for patients for whom the standard protocol is not suitable.
- Citation: Costaguta A, Costaguta G, Álvarez F. Autoimmune hepatitis: Towards a personalized treatment. World J Hepatol 2024; 16(11): 1225-1242
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1225.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1225
Autoimmune hepatitis (AIH) is a chronic inflammatory disease of the liver with a fluctuating course that inevitably progresses to cirrhosis and liver failure if not treated promptly[1]. Up to half of the patients present with complications such as cirrhosis, portal hypertension, and decreased liver function[2-4]. AIH should be considered in the differential diagnosis of any liver disease regardless of severity. It was first described by Waldenström[5] in adult females with abnormal liver function tests, increased circulating IgG levels, and specific autoantibodies[6,7]. It can be classified into two types: AIH type 1 (AIH-1), with positive antinuclear antibodies and/or anti-smooth-muscle antibodies; and AIH type 2 (AIH-2), with anti-liver-kidney microsomal type 1 and/or anti-liver cytosol type 1 antibodies. While AIH-1 affects all ages, AIH-2 is predominantly diagnosed in female pediatric patients and often follows a more severe clinical course; thus, timely and appropriate treatment is critical for improving outcomes[8,9].
In 1999, the International AIH Group established diagnostic criteria[10-12], which were later validated for pediatric patients[13,14], encompassing clinical, biochemical, and histological features such as interface hepatitis, a hallmark of AIH, characterized by lymphoplasmacytic infiltration of the limiting plate of the liver[15,16]. To confirm the diagnosis, exclusion of viral or toxic hepatitis, alpha-1-antitrypsine deficiency, and Wilson’s disease is necessary. When left untrea
The primary goal of AIH treatment is to normalize transaminase levels, IgG levels, and autoantibody titers and achieve histological remission[11,17]. Treatment typically involves high-dose steroids during the induction phase followed by gradual tapering over several weeks. Azathioprine is subsequently introduced as a maintenance therapy, leading to remission rates of up to 80%[11,18]. For those patients who do not respond to or relapse during steroid tapering, alterna
This article evaluated the current treatment options for AIH, including conventional and unconventional methods, and investigated promising therapies that have not yet been widely implemented in clinical practice as well as potential future treatments.
AIH is histologically characterized by a dense portal infiltrate, primarily composed of lymphocytes and plasma cells. Although autoantibodies are diagnostic markers, AIH is mainly considered a T cell-driven disease[20-22].
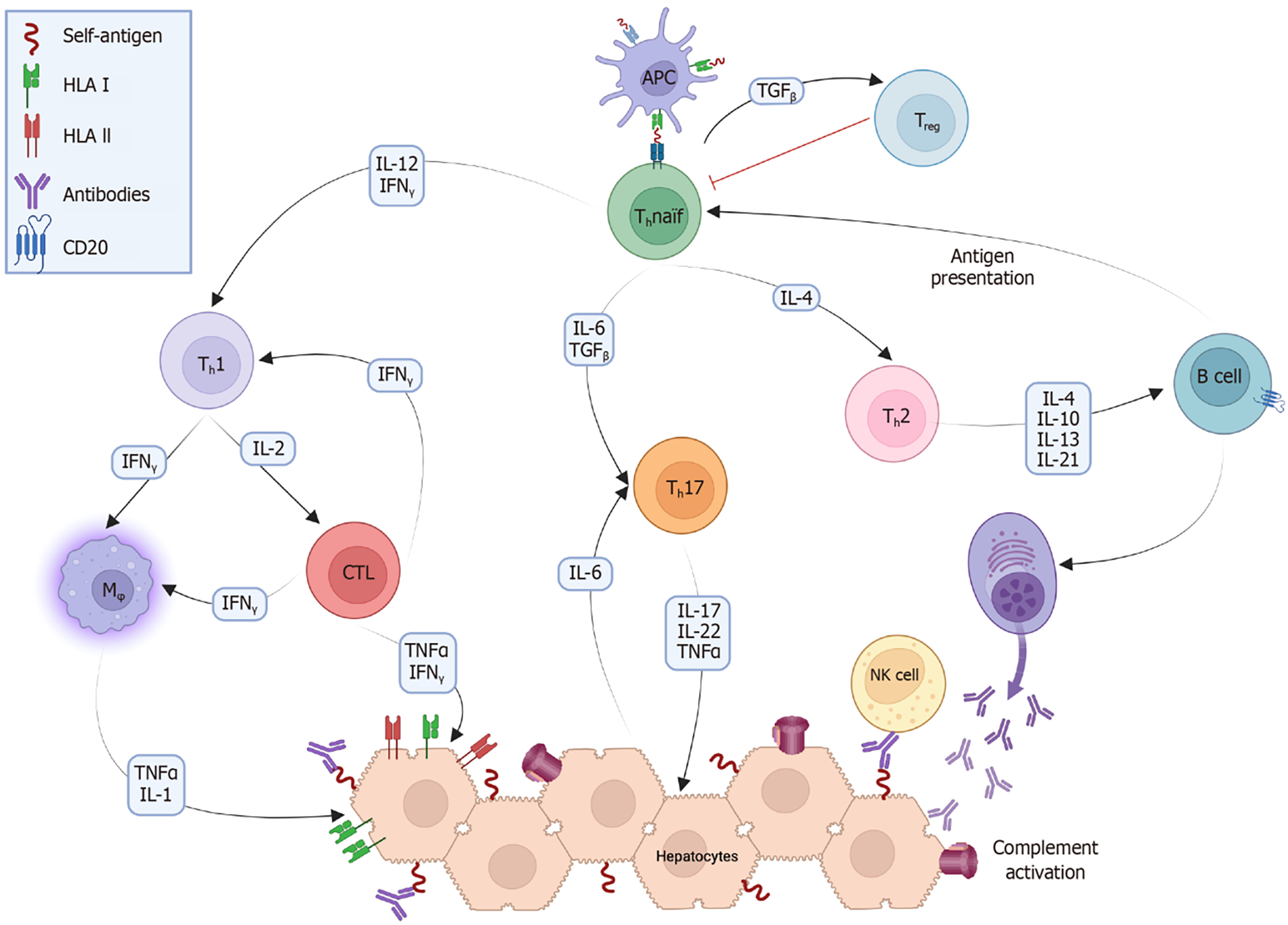
AIH is a complex disease influenced by both genetic predisposition and environmental triggers. Molecular mimicry and epitope spreading contribute to the activation of cluster of differentiation (CD) 4 + [T helper (Th) cells, mainly Th1] and CD8 + (cytotoxic T cells) cells within a proinflammatory microenvironment, leading to the breakdown of immunological tolerance against self-antigens[23,24]. Activated B cells acquire the necessary costimulatory signals to function as professional antigen-presenting cells (APCs), such as macrophages and dendritic cells, further stimulating T cell activation. Once activated, Th cells mature into different phenotypes that produce cytokines essential for orchestrating the immune response[25]. Regulatory T cells (Tregs) are important in the development of AIH. When activated within the liver microenvironment by specific cytokines such as transforming growth factor beta and low levels of interleukin (IL)-2, they secrete anti-inflammatory interleukins to dampen excessive immune responses. Tregs may be deficient in either the number or function in patients with AIH, although findings can be contradictory across studies[21,26]. Nonetheless, prevailing evidence suggests that Tregs in patients with AIH fail to effectively control inflammation, allowing effector T cells, especially Th1 and Th17, to perpetuate the autoimmune attack on the liver tissue (Figure 1).
Treatment of AIH should focus on halting the necroinflammatory process, preventing progression, and ideally reversing fibrosis. A panel of experts proposed the definitions for response criteria in AIH including “complete biochemical response” as normalization of serum transaminases and IgG within 6 months of starting treatment, “insufficient response” as the lack of complete response by 6 months, and “non-response” as less than a 50% decrease in transaminase levels within the first 4 weeks[27] (Table 1).
| Drug family | Medication | Remission rates | Mechanism of action | Advantages | Disadvantages | Ref. |
| Steroids | Prednisone | 80% | CD4 + T cell sequestration in reticuloendothelial system; Inhibition of IL-1 and IL-6 synthesis; Inhibition of cell-cell adhesion molecules | Inexpensive; Widely available; Extensive experience in its use | Weight-gain; Growth stunting; Hyperglycemia; Psychosis; Osteoporosis; Arterial hypertension | Mieli-Vergani et al[17]; Mack et al[29] |
| Budesonide | 70%-80% | Theoretically less side-effects than prednisone | Expensive; Contraindicated in cirrhosis and liver failure; May not control autoreactive cells in peripheric lymphoid tissue | Mack et al[29]; Olivas et al[36]; Manns et al[37]; Woynarowski et al[38] | ||
| Antimetabolites | Azathioprine | 80% | Purine synthesis inhibition | Extensive experience in its use | Not for induction; Bone marrow toxicity; Vomiting and gastrointestinal symptoms; Hepato-splenic T cell lymphoma | Mack et al[29]; Olivas et al[36]; Muratori et al[39] |
| Mycophenolate mofetil | 72%-84% | GMP, GTP, dGTP synthesis inhibition halting lymphocyte proliferation | Excellent alternative in azathioprine intolerance; Better tolerated than azathioprine; Less side effects | Not for induction; Diarrhea; Abdominal pain; Dizziness | Inductivo-Yu et al[45]; Santiago et al[50]; Snijders et al[53] | |
| Methotrexate | 55% | Purine and pyrimidines through folate depletion | May be an alternative in azathioprine intolerance | Not for induction; Hepatotoxicity; Limited experience; Low tolerability | Haridy et al[56] | |
| Calcineurin inhibitors | Cyclosporin A | 80%-94% | Binding of immunophilins; Proinflammatory cytokine synthesis inhibition | Useful as an alternative to steroids for induction; Useful for steroid-sparing (liver failure) | Monitoring of trough levels; Nephrotoxicity; Gingival hyperplasia; Hypertrichosis; Not ideal for chronic use | Alvarez et al[67]; Malekzadeh et al[68]; Cuarterolo et al[72] |
| Tacrolimus | 69%-75% | Good alternative in anti-metabolite refractory patients | Same as cyclosporin A; Diabetes | Abdollahi et al[78]; Hanouneh et al[79]; Efe et al[80]; Efe et al[81] | ||
| mTOR inhibitors | Sirolimus | 0% (response in 50%) | Impair dendritic cells and T cell proliferation and differentiation | Alternatives in difficult-to-treat patients; Sparing of Tregs | Anecdotical experience; Not effective in monotherapy | Kurowski et al[90]; Chatrath et al[91] |
| Everolimus | 0% (response in 83%) | Jannone et al[92] | ||||
| B cell depletion | Anti-CD 20 (rituximab) | 75%-100% | Direct decrease in antigen-presenting cells; Indirect decrease in autoreactive plasmacytes; Decrease of B cell-mediated T cell activation; Direct decrease in autoreactive T cells (belimumab only) | Useful for induction and maintenance; Ensures compliance; Steroid-sparing | Expensive; Lack of long-term safety data | D’Agostino et al[102]; Than et al[108]; Costaguta et al[110] |
| Anti-BAFF (belimumab) | Unknown | Better response in other diseases than with rituximab | Ongoing trial for AIH | ClinicalTrials.gov [124]; Wise and Stohl[125] | ||
| Biologics | Anti-TNF (infliximab) | 61% | Inhibits dendritic cell activation; Inhibits T helper differentiation; Inhibits cytotoxic T cells | Useful for induction and/or maintenance; Extensive experience in other diseases; Good safety profile | Anti-TNF may trigger AIH; Hepatotoxicity | Weiler-Normann et al[129]; Rajanayagam and Lewindon[130]; Weiler-Normann et al[131] |
| Anti-IL1 (anakinra) | Unknown | Inhibits monocyte/macrophage activation; Inhibits fibroblast proliferation; Inhibits ROS synthesis | Experimental benefits in other forms of hepatitis; Theoretical benefits on fibrosis | Unknown efficacy in AIH; Possible hepatotoxicity | Iracheta-Vellve et al[137]; Petrasek et al[138] | |
| Anti-IL17 (secukinumab) | Unknown | Inhibits macrophage cytokine secretion; Inhibit endothelial cell expression of integrins and selectins; Inhibit neutrophil recruitment and decrease survival | Effective in murine models; Experimental benefits since IL-17 is central in many autoimmune disorders | Unknown efficacy in human AIH; Th17/Treg disbalance may paradoxically be detrimental in some autoimmune disorders | Zhao et al[144]; Yu et al[145] | |
| JAK inhibitors | Tofacitinib | Unknown | Inhibition of proinflammatory cytokine synthesis | Effective in murine models; Effective in STAT gain-of-function-related AIH | Unknown efficacy in human AIH; Relatively new medications | Hadžić et al[157]; Kaneko et al[158] |
| Cell therapy | Chimeric antigen receptor-T cell therapy | Unknown | Selective targeting of autoreactive CD19 + cells; Cell therapy-mediated B cell depletion | Effective in B cell malignancies; Excellent safety profile; Targeted immunosuppression | Expensive therapy; Not easily available | Schett et al[169] |
| Treg cell transfer | Unknown | Induction of peripherical tolerance to self-antigens; Dampening of the inflammatory response | Effective in murine models; Theoretical possibility of tolerance “resetting”; Excellent safety profile | Lack of specific self-antigens in AIH-1; Expensive therapy; Not easily available | Lapierre et al[172]; Sánchez-Fueyo et al[173] |
Since its first description by Waldenström[5] in 1951, prednisone has formed the backbone of treatment, making AIH the first liver disease for which effective medical therapy was available[28]. Doses are adapted throughout the treatment, being higher during induction at 2 mg/kg/day (maximum 60 mg/day), usually for the 1st month, and tapering according to biochemical response, aiming to stop them as soon as possible, usually within 6 months to 12 months. Occasionally, a low daily dose (3-5 mg) is required to maintain transaminase levels in the normal range[29]. Oral prednisone effectively achieves AIH remission, but this comes at the cost of myriad side effects, some of which are irreversible, including cataracts or cutaneous striae. Additionally, severe side effects, such as vertebral collapse in osteopenia, psychosis, and suicidal ideation may occur[30-32].
Budesonide: Budesonide, a synthetic steroid, has been demonstrated to be effective in several inflammatory conditions, particularly when used topically. Although it has been suggested as an alternative to prednisone in naïve patients[29], a recent publication indicated that in real-life scenarios it is typically considered a second-line option when the side effects of prednisone are to be avoided[33]. Furthermore, budesonide acts through the same glucocorticoid receptor and employs the same pathway as prednisone to suppress the inflammatory process[34]. Therefore, it is probably not useful for prednisone-refractory patients[35].
The appeal of budesonide comes from its safety profile derived from its high first-pass effect, which inactivates 90% of the drug, reaching the liver through portal circulation, resulting in high intrahepatic but low systemic levels. As expected, budesonide is associated with fewer and milder side effects than prednisone; however, its lower systemic levels may be insufficient to control activated T cells in secondary lymphoid organs, compromising the therapeutic effect[35]. Never
Two recent randomized controlled trials favored budesonide over prednisone in both adults and children, suggesting budesonide as the first choice in the treatment of patients with AIH during induction and maintenance. However, the results should be interpreted with caution as they were criticized because of the unusually high rate of treatment failure observed in the prednisone group[37,38]. Considering all these factors, budesonide is a viable option for stable patients without cirrhosis experiencing unbearable side effects from systemic corticosteroids[35].
Azathioprine: Azathioprine is used to maintain remission, while steroids are tapered and withdrawn. It has demonstra
Mofetil mycophenolate: Mofetil mycophenolate (MMF) is the prodrug of mycophenolic acid (MPA), which was the first application of human genetics to define a therapeutic target[43]. MPA functions by inhibiting the enzymes critical for guanosine monophosphate, guanosine triphosphate, and deoxyguanosine triphosphate synthesis, which are essential for lymphocyte proliferation[43,44]. MMF was developed to improve the oral bioavailability of MPA. Its effectiveness was first documented in the treatment of 15 adult patients who were either refractory or intolerant to standard therapy. These patients received 1 g of MMF twice daily for an average of 41 months, either alone or in combination with prednisone. Remarkably, all patients showed significant improvement, with 11 patients achieving remission. No hematological side effects were observed[45]. Subsequent studies corroborated these findings in 29 adult patients, where MMF was effective in inducing remission in 84% of the patients who tolerated the treatment. Although one-third of the patients experienced side effects such as vomiting, pancreatitis, hair loss, and deep venous thrombosis, hematological complications were notably absent[46].
Pharmacokinetic studies are essential for monitoring the therapeutic threshold of MMF, as blood levels vary signi
Other studies have consistently demonstrated that MMF is more tolerable than azathioprine in patients with AIH[51,52]. In a recent multicenter prospective trial, MMF and azathioprine were compared, associated to prednisolone for treating newly diagnosed adults with AIH. After 24 weeks, 56.4% of patients in the MMF group achieved remission, while only 29% in the azathioprine group achieved remission. MMF also showed significantly higher biochemical response than azathioprine (72.2%) to (32.3%). Notably, MMF had a good safety profile, with no serious adverse events reported, whereas four such events occurred in the azathioprine group. Drug tolerance was better for MMF, with only 2 patients discontinuing treatment due to side effects, compared to 8 patients in the azathioprine group[53]. These findings suggest that the combination of prednisolone and MMF could be a suitable alternative, considering the potential risks associated with azathioprine. However, further research and extensive clinical experience are needed to establish the role of MMF in AIH management.
Methotrexate: Methotrexate is commonly used in oncology and rheumatology because of its anti-inflammatory and immunosuppressive properties. It is a folate analog that inhibits dihydrofolate reductase, which is necessary for the production of purines and pyrimidines that are essential components of DNA synthesis and cellular proliferation[54]. Despite its broad application in other conditions, its use in AIH is limited and generally discouraged due to several factors, such as low tolerability, possible hepatotoxicity, and contraindication in patients with cirrhosis[55]. A 2018 case series revealed that only 54.5% of 11 adult patients with AIH achieved remission within 12 months on methotrexate. However, liver biochemical deterioration led to discontinuation, and 2 patients experienced drug-induced liver injury[56]. Experience with methotrexate in pediatric AIH cases is even more limited and primarily consists of sporadic case reports[57].
Calcineurin inhibitors (CNI) such as cyclosporine A (CsA) and tacrolimus have revolutionized immunosuppression in post-transplant settings, as first described by Calne et al[58] and Starzl et al[59]. These drugs inhibit calcineurin, an enzyme critical for activating nuclear factor of activated T cells, which is crucial for the synthesis of the cytokines necessary for the development and survival of activated T cells[60-62]. CNI binds to cytoplasmic receptors known as immunophilins, cyclophilin (for CsA), and FK-binding protein (for tacrolimus), which inhibits the function of calcineurin, leading to reduced cytokine transcription[63]. While CNI are widely used in transplant medicine and other autoimmune conditions[64], their role in AIH has been limited and is typically reserved as a second-line or third-line option[4,17].
The use of CsA in the treatment of AIH has shown promising results, particularly in cases resistant to steroids, as initially reported in the treatment of a 14-year-old patient[65]. This early success made CsA an option for treating steroid-refractory AIH, with a success rate of approximately 80%[66]. In 1999, a study investigating the use of CsA as first-line treatment for pediatric AIH was conducted. This study included 32 children diagnosed with AIH who were administered CsA with a target trough concentration of 250 ng/mL for the first 3 months. Responders then reduced their CsA doses to maintain a trough level of 200 ng/mL. After 6 months of treatment, the patients transitioned to a low dose of prednisone combined with azathioprine for 3 months, followed by prednisone tapering. The results showed that 25 of 30 patients who completed the study (83%) achieved normal liver function tests after CsA induction, and this improvement was sustained in all patients after 12 months. The protocol was well tolerated, with gingival hyperplasia being the main side effect, which resolved after discontinuation of CsA. Importantly, none of the patients developed renal dysfunction or arterial hypertension, likely because of careful CsA dosing and monitoring. Furthermore, patients with extrahepatic immune diseases, such as atopic dermatitis, psoriasis, and ulcerative colitis, showed resolution of their conditions[67].
Despite other promising results with CsA in AIH treatment[68], its use has been limited by adverse side effects. In one series, 15 children received CsA for 6 years, and significant side effects were reported, with drug withdrawal in 3 patients and dose reductions in another 3 patients due to hypertension, tremors, or glomerulopathy[69]. CsA is associated with a high incidence of adverse events, but most cases are mild and temporary, such as gingival hyperplasia and hypertrichosis[70]. A study of 84 pediatric patients showed a more favorable outcome, with 94% achieving remission over a mean follow-up period of 29 months, many within the first 12 months, and 72% within the first 6 months. Adverse effects were mostly mild, sporadic, and transient, with hypertrichosis affecting 55% and gingival hyperplasia affecting 39% of patients. Additionally, 8 patients had a single instance of abnormal serum creatinine levels, and 3 patients experienced one episode of arterial hypertension. All adverse events resolved quickly[71].
CsA was also effective in treating patients with liver failure, as determining the optimal timing is challenging due to the uncertainty of patient response and the possible need for liver transplantation. High-dose steroids are associated with poor postoperative outcomes and high complication rates. In a retrospective study of 237 pediatric patients with AIH, 37 children with liver failure and no prior treatment received 1 mg/kg prednisone with CsA targeting a trough level of 200 ng/mL, while 13 received standard of care. After a median of 24 days, 45 patients normalized their internation nor
Since its introduction in the early 1990s, tacrolimus has become a nearly universal immunosuppressor for post-liver transplantation because of its higher affinity for immunophilins[73]. In 1995, the use of tacrolimus in 21 treatment-naïve patients with AIH resulted in a mean transaminase level decrease of 80% after 3 months and only a mild increase in serum creatinine[74]. Similar outcomes have been reported previously[75-77]. A recent systematic review analyzed the use of tacrolimus vs MMF as second-line treatment. The study found biochemical remission rates of 68.9% and 73.5% for tacrolimus and MMF, respectively. Notably, most studies have focused on patients who were intolerant to first-line treatments, with remission rates of 25%-32% for first-line non-responders. The rates of side effects were similar for tacrolimus (25.5%) and MMF (24.2%)[78].
Another meta-analysis reviewed seven studies involving 162 adult patients who were either refractory or intolerant to standard treatment. The analysis found a biochemical response rate of 74.7%, with an 85% histological remission rate in patients who underwent a control biopsy[79]. Two additional systematic reviews compared tacrolimus and MMF as second-line therapies in adults[80] and children[81]. The remission rates for MMF and tacrolimus were 69.4% and 72.5%, respectively, with side effects of 8.3% and 12.5%, respectively. In the pediatric cohort, tacrolimus appeared to be more effective than MMF in patients refractory to treatment, although the difference was not statistically significant. These findings have been corroborated by other authors[82], and an open trial is currently underway to further clarify the role of tacrolimus in AIH treatment[83].
Mammalian target of rapamycin (mTOR) is a protein kinase that activates anabolic cellular pathways that increase protein, nucleotide, and lipid synthesis and inhibits catabolic pathways associated with autophagy[84-86]. Targeted immunosuppression by blocking mTOR with sirolimus or everolimus impairs dendritic cell maturation and inhibits T cell proliferation and differentiation[87]. Evidence suggests that mTOR inhibition does not affect CD4 + T cells with FOXP3 upregulation in the thymus, indicating that these drugs do not affect natural Tregs[88,89]. Unfortunately, practical evi
In 2014, a study of 127 children with AIH found that only two of the four children treated with sirolimus showed a biochemical response after 6 months albeit with continued low-dose prednisone[90]. Simultaneously, 5 adult patients with steroid-refractory AIH were treated with sirolimus for 4-72 months. Four of these patients had a biochemical response, but only two achieved remission. All patients reduced their steroid doses, and sirolimus was well tolerated, with only two experiencing increased cholesterol and triglyceride levels[91]. A study on 6 difficult-to-treat patients who received everolimus reported reduced transaminase levels in 5 patients, although none achieved complete remission. No negative side effects were observed when therapeutic trough levels were maintained[92]. Recently, researchers have compared lymphocyte populations in patients with AIH under standard and non-standard therapy. They found a pro
AIH is primarily a T cell-mediated disease, but B cell activation and stimulation are necessary to maintain the immune response. The two main mechanisms that contribute to this process are B cell expression of specific antigen receptors that act as APCs for T cells and B cell costimulation of T cells[94]. AIH is thought to be triggered by the presentation of self-antigens by professional APCs to unstimulated Th0 cells, which then mature into various Th subtypes (Figure 1). Activated B cells differentiate into plasma cells, which produce antibodies, and into APCs, which stimulate Th0 cells to sustain the immune response[95-97]. B cell and T cell interactions are essential for adapting and directing immune responses and involve membrane proteins such as CD40/CD40 L and CD28/cytotoxic T-lymphocyte-associated protein 4. These interactions not only contribute to the production of autoantibodies but also play a critical role in Th1 activation observed in AIH[98-101].
B cells play a crucial role in the development of AIH, as initially demonstrated in a murine model of AIH-2, in which B cell depletion via anti-CD-20 antibodies resulted in significant improvement in liver inflammation and reduction in the number of memory B cells[24]. The successful treatment of the first pediatric patient with refractory AIH using rituximab, an anti-CD20 antibody, provided evidence of B cell depletion efficacy and demonstrated a direct link between B cell activation and inflammatory activity in AIH[102]. Remarkably, this patient was able to discontinue all immunosuppressive medications, maintained normal liver function and histology for 14 years, and recently gave birth to a healthy child (personal communication).
Since then, numerous case reports have documented the efficacy of rituximab in the treatment of AIH[103-107]. A study from 2019 by the International AIH Group involving 22 adults with refractory AIH found that 71% achieved flare-free remission after 2 years, with only 5 patients experiencing flares over an 11-year follow-up. Furthermore, a 61% reduction in the prednisolone dose in the first 12 months of treatment and no serious adverse events related to rituximab were reported[108].
The first pediatric series successfully treated with rituximab was published in 2021[109]. Recently, we reported 6 patients treated with rituximab, all of whom normalized liver enzymes and IgG levels and achieved negative auto
Rituximab is not yet a standard treatment for AIH despite successful reports of B cell depletion and is only considered a potential rescue therapy without formal recommendations in guidelines[17]. Moreover, no governmental health authorities have approved rituximab for the treatment of AIH[111]. Concerns about long-term safety, including B cell depletion and infection risk, may account for this hesitation. However, information on rituximab comes mostly from hematologic malignancies, which may differ from AIH risk[112,113].
Recently, alternative methods for reducing B cell survival have been suggested, such as inhibition of B-cell activating factor (BAFF), a protein belonging to the tumor necrosis factor (TNF) family[114]. BAFF can be either a membrane-bound or soluble protein that binds to B cell receptors to extend survival and promote maturation, and elevated BAFF levels are found in the sera of patients with autoimmune disease[115-118]. Belimumab, a humanized monoclonal antibody targeting BAFF, is effective in treating various autoimmune diseases, including systemic lupus erythematosus in both adults and children[119] and rheumatoid arthritis[120] and is currently being studied in clinical trials for multiple sclerosis[121].
The rationale for using BAFF inhibitors in AIH is to reduce B cell activity and thereby dampen the autoimmune response. However, its efficacy in AIH remains to be established[122,123]. An ongoing clinical trial is expected to provide more insights and is projected to be completed between 2028 and 2029[124]. The efficacy of depleting B cells using monoclonal antibodies targeting the high-affinity BAFF receptor has not been established in AIH, although it has already been proven superior in other B cell-mediated diseases[125].
Antibody-mediated cytokine neutralization has become the primary therapy for various autoimmune diseases including rheumatoid arthritis, ulcerative colitis, Crohn’s disease, and psoriasis[126,127]. Despite these successes, its use in AIH has been limited, partly due to the complex pathogenesis of AIH involving a multitude of cytokines and mechanisms. Anti-TNF therapy for AIH remains controversial despite its potential benefits due to sporadic cases of anti-TNF-triggered AIH[128]. Nevertheless, infliximab, an antibody against TNF, has been used to treat refractory AIH with some success[129], including some pediatric cases[130]. To date, a case series involving 11 patients with refractory AIH is the largest to date. Researchers have used infliximab to induce remission, with the aim of discontinuing the drug after enzyme normalization. After the induction phase, 8 of the 11 patients exhibited significant improvement in liver function tests. Three of these patients were able to discontinue infliximab within the first 12 months of therapy, while two maintained remission on antimetabolite monotherapy. One patient experienced recurrent flares during treatment, leading to discontinuation, and another required intermittent infusions to control the flares. Two patients were unable to discontinue infliximab, and one remained on treatment for over 12 years[131]. Seven patients experienced adverse effects, with five developing infliximab-related infections. These included hepatic abscesses, urinary tract infections, recurrent shingles, and pneumo
Recently, anti-TNF-related AIH cases have emerged, likely resulting from the widespread use of anti-TNF therapies for other conditions[133-135]. Although liver injury in these cases resembles drug-induced liver injury, further research and experience are necessary to provide formal recommendations on using anti-TNF agents in AIH.
Figure 1 shows the various theoretical targets for cytokine inhibition. Recently, the IL-1 superfamily has attracted the attention of researchers owing to its involvement in other autoimmune diseases and its potential role in liver diseases[136]. Two studies have shown that IL-1 inhibitors, such as anakinra or canakinumab, can significantly reduce inflammation in animal models of ethanol-induced hepatitis[137,138]. However, there is currently no real-life experience with the use of these inhibitors in AIH, and reports of anakinra-related hepatotoxicity complicate their potential application[139,140]. Consequently, the use of IL-1 inhibitors in AIH remains theoretical at this time.
Another cytokine, IL-17, is important in many autoimmune diseases and merits a mention[141-143]. Over the past decade, evidence has suggested that Th17 cells and IL-17 are central to AIH pathogenesis. Patients with AIH have significantly higher circulating levels of IL-17 than patients with other chronic liver diseases, and downstream IL-17-related cytokines are also elevated. Anti-IL-17 administration in murine AIH models improves liver inflammation[144,145]. However, it must be noted that while IL-17 is necessary for the development of inflammatory bowel disease (IBD), the use of anti-IL-17 has led to paradoxical intestinal inflammation in some cases, and the pathogenesis of this effect remains unclear[146-148]. Therefore, more evidence is needed before anti-IL-17 can be considered a viable treatment option for AIH.
Janus kinases (JAK), including JAK1, JAK2, JAK3, and tyrosine kinase 2, are a group of intracellular enzymes that play an essential role in the production of numerous cytokines. When a cytokine binds to its specific receptor, JAK kinases become active, leading to signal transduction through signal transducer and activator of transcription (STAT) transcription factors[149]. Recently, JAK inhibitors have gained popularity in the treatment of various diseases, such as rheumatoid arthritis, IBD, and graft-vs-host disease[150-153]. A study in a murine model of AIH used tofacitinib, a pan-JAK inhibitor. The JAK1/STAT signaling pathway is activated following liver injury. Tofacitinib-treated mice showed improvement, with a decrease in proinflammatory cytokines and an increase in anti-inflammatory cytokines. This study also reported a higher Treg/Th17 ratio and reduced liver fibrosis[154].
A second study found that ruxolitinib, a JAK1/2 inhibitor, reduced serum and liver cytokines, liver fibrosis, hepatocyte apoptosis, and neutrophil infiltration in a similar animal model[155]. Other authors have confirmed similar impacts on most proinflammatory cytokines and a decrease in T cell liver infiltration, along with an increase in the Treg population in murine models[156]. Although there is currently limited research on the use of JAK inhibitors for human AIH, recent cases of STAT1 and STAT3 gain-of-function-related AIH were successfully treated with baricitinib and tofacitinib, respectively[157,158].
Cellular therapy has experienced a resurgence thanks to advancements in stem cell therapy and applied genetics[159-161], including chimeric antigen receptor (CAR)-T cells for treating autoimmune conditions. This approach involves genetically modifying a patient’s cells to target specific antigens, thereby offering a versatile and effective solution[162]. CAR-T cells targeting CD19 have successfully eliminated neoplastic cells in lymphomas, leukemia, and myelomas[163,164]. Additionally, this therapy can be applied to Tregs or dendritic cells to harness their immunomodulatory capabilities in certain autoimmune-related or immune-related diseases[165,166]. The process involves extracting, isolating, expanding, and modifying the patient’s immune cells, then reinfusing them in larger numbers to suppress the autoreactive immune response. However, Tregs alone have not been proven to be sufficient in clinical settings[167,168]. A clinical trial examining the efficiency of CAR-T cells for B cell-mediated autoimmune illnesses, including pemphigus vulgaris and systemic lupus erythematosus, is anticipated to conclude by 2026[169]. The results of this trial, considering the critical role of B cells in AIH, suggest the potential of CAR-T cells for treating AIH[170].
Another approach is to use Tregs isolated from patients with autoimmune disease. These Tregs can be naturally isolated from peripheral blood, expanded before reinfusion, or induced from conventional T cells by stimulating them with transforming growth factor beta or low-dose IL-2. Induced Tregs have shown promise in the treatment of autoimmune diseases in murine models[171,172]. While the exact role of Tregs in the development of AIH is still debated, an experimental study provided major insights. Using a murine model of AIH-2, the study revealed the following: (1) Ectopic expression of the autoantigen formiminotransferase cyclodeaminase in the thymus led to a decreased number of autoreactive T cells, halting the development of AIH; (2) In the absence of central tolerance to formiminotransferase cyclodeaminase, the adoptive transfer of ex vivo expanded Tregs inhibited the proliferation of liver-specific autoreactive T cells; and (3) Once inflammation was resolved, high numbers of Tregs were no longer needed to maintain tolerance, suggesting that although a break in immunotolerance triggers AIH, it can be restored under certain circumstances[173]. The main issue with these therapies is that AIH-1, which accounts for most AIH cases, lacks liver-specific autoantigens. This could limit the effectiveness of the transferred T cells in targeting the liver. However, Treg infusion has shown promise in inducing tolerance after liver transplantation, suggesting that these cells can remain home to the liver during active inflammation[174].
Given the current armamentarium of different immunosuppressive treatments available, tailoring therapy to individual patient needs should become the mainstay (Table 2). Although most patients respond to standard treatments, special considerations may be given to certain subpopulations.
| Population | Less-desirable options | Reasons | Available options |
| Obesity and metabolic syndrome | Steroids | Weight gain; Arterial hypertension; Steroid-induced hyperglycemia | Induction with cyclosporin A: Trough target of 250 ng/mL for 3 months and decrease to 200 ng/mL for the next 3 months. Tapering of cyclosporin with addition of 0.3-0.5 mg/kg daily prednisone for 3 months, and then every other day for another 3 months. Azathioprine at usual dose from the 6th month onwards; Induction with rituximab at 375 mg/m2 once a week for 4 weeks. Maintenance with the same dose every 6 months. Adapt according to lymphocyte (CD20 +) count and IgG levels |
| Adolescents; Eating disorders; Body dysmorphia | Steroids; CsA | Weight gain; Stretch marks; Acne; Growth stunting; Psychosis and suicidal ideation; Hypertrichosis; Gingival hyperplasia | Budesonide at doses of 9 mg for induction, with progressive tapering to 6 mg and then 3 mg. Maintenance with azathioprine: Rituximab induction as previously mentioned. Maintenance with rituximab or azathioprine; Prednisone at 0.5-1 mg/kg daily + CsA targeting 200 ng/mL for 3-6 months as induction |
| Concomitant autoimmune disorders | NA | Treatment should be aimed at controlling all the conditions simultaneously with the smaller number of medications possible | In antibody-mediated diseases: Rituximab at 375 mg/m2 for induction and maintenance |
| Inflammatory bowel disease | NA | Infliximab at 5-10 mg/kg at week 0, 2, and 6 for induction. Maintenance with a dose every 4 weeks to 8 weeks. Doses and frequencies are adapted according to IBD activity; Although not yet proven, in this scenario the use of JAK inhibitors may become useful, although no evidence exists presently | |
| Non-compliance | Steroids; CsA; Tacrolimus | Medications with significant side effects, requiring multiple daily doses, and with a set therapeutic range are less desirable | Rituximab ensures the compliance of patients as doses are administered a few times a year and under healthcare personnel surveillance |
The incidence of obesity and metabolic syndrome is increasing worldwide across all ages, posing significant challenges to patient care[175,176]. High-dose steroids are associated with several side effects, including weight gain, fluid and sodium retention, hypertension, lipid anabolism, and peripheral insulin resistance[177,178]. For patients with obesity, it is challenging to determine the appropriate steroid dosage owing to inconclusive pharmacokinetics[179]. In our experience, these patients benefit from alternate induction protocols using either CsA or rituximab, both of which help avoid steroid-related adverse effects. In some cases, CsA can be combined with low-dose steroids to minimize the side effects while achieving remission[67,72,110].
Patients with diabetes often cannot tolerate steroids owing to their glucocorticoid effects and risk of decompensation[180-182]. High doses of steroids can even induce diabetic ketoacidosis, especially in poorly controlled diabetes[183,184]. Thus, steroids should be avoided, and tacrolimus is not ideal for maintenance therapy due to its impact on carbohydrate metabolism[185,186]. As in the previous subgroup of patients, an induction protocol using either rituximab or CsA can be used to avoid the use of steroids. Thereafter, azathioprine, MMF, and rituximab were all suitable for maintenance therapy.
Adolescent patients with AIH present additional challenges owing to the significant role of body image and self-perception during this developmental stage[187]. Steroid-induced physical changes (weight gain, stretch marks, and acne) can lead to bullying and social exclusion, profoundly impacting mental health, especially in female patients[188,189]. The asymptomatic nature of AIH, coupled with these adverse effects, often leads to poor compliance with long steroid courses, resulting in treatment failure and potential liver decompensation[190,191]. The use of rituximab may be considered in this subgroup of patients when expressing serious concern about the steroid-related side effects. Budesonide may also be a valuable treatment option for mild cases. Induction with a combination of CsA followed by low-dose prednisone may also be effective, as it generally avoids undesired side effects.
Patients with eating disorders and body dysmorphia face similar challenges as the psychological aspects of body composition changes are critical[192,193]. Unexpected weight gain can reduce treatment compliance and complicate the management of eating disorders. In these cases, CsA induction protocols may be less desirable because of transient side effects, such as hypertrichosis and gingival hyperplasia. Therefore, rituximab was successfully used for both induction and maintenance in these patients.
Some patients with AIH may have other immune dysregulation syndromes[194] and may be associated with other immune-mediated diseases[195]. In these cases, close collaboration with an immunology team is warranted. We succe
Patients diagnosed with renal disease warrant mention. Glomerulonephritis, which is sometimes associated with AIH, precludes the use of CNIs. Additionally, although not inherently nephrotoxic, high-dose steroids should be avoided because of their detrimental effects in patients who may already have hypertension. Considering that several cases of glomerulonephritis are secondary to circulating immunocomplexes, rituximab may be the preferred treatment option.
Patients who do not comply with treatment also pose a challenge, as detection is difficult owing to fluctuating liver enzyme levels, which may be only slightly abnormal. Other causes of liver enzyme abnormalities must be investigated until nonadherence to the therapeutic strategy is confirmed. Psychological disorders that affect patient autonomy and reliability can exacerbate these issues. We successfully used rituximab as a second-line treatment in these situations, as it allowed us to ensure adherence through biannual administration in a controlled setting.
AIH is a complex disease that involves various aspects of the immune system. Steroids have been pivotal for the effective management of these patients, yielding excellent results. Nevertheless, not every patient responds adequately, nece
| 1. | Alvarez F. Autoimmune hepatitis and primary sclerosing cholangitis. Clin Liver Dis. 2006;10:89-107, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (35)] |
| 2. | Jiménez-Rivera C, Ling SC, Ahmed N, Yap J, Aglipay M, Barrowman N, Graitson S, Critch J, Rashid M, Ng VL, Roberts EA, Brill H, Dowhaniuk JK, Bruce G, Bax K, Deneau M, Guttman OR, Schreiber RA, Martin S, Alvarez F. Incidence and Characteristics of Autoimmune Hepatitis. Pediatrics. 2015;136:e1237-e1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (35)] |
| 3. | Cuarterolo M, Ciocca M, Álvarez F. [Autoimmune hepatitis in children: current perspectives]. Arch Argent Pediatr. 2014;112:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Ramonet M, Ramirez-Rodriguez N, Álvarez Chávez F, Arregui MC, Boldrini G, Botero Osorio V, Cuarterolo M, Godoy M, Medina Monroy FA, Oropeza G, Pérez Carusi R, Pérez Rodríguez D, Reynoso-Zarzosa FA, Ciocca M. Autoimmune hepatitis in pediatrics, a review by the Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Arch Argent Pediatr. 2022;120:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Waldenström J. Leber. Blutproteine und Nahrungseiweiss. Deutsch Z Verdau Stoffwechselkr. 1950;15:113-119. |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 7. | Maggiore G, Riva S, Sciveres M. Autoimmune diseases of the liver and biliary tract and overlap syndromes in childhood. Minerva Gastroenterol Dietol. 2009;55:53-70. [PubMed] |
| 8. | Longhi MS, Mieli-Vergani G, Vergani D. Autoimmune hepatitis. Curr Pediatr Rev. 2014;10:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lapierre P, Alvarez F. Type 2 autoimmune hepatitis: Genetic susceptibility. Front Immunol. 2022;13:1025343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 10. | Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 670] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 2017] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 12. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1308] [Article Influence: 72.7] [Reference Citation Analysis (1)] |
| 13. | Mileti E, Rosenthal P, Peters MG. Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clin Gastroenterol Hepatol. 2012;10:417-21.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Ferri PM, Ferreira AR, Miranda DM, Simões E Silva AC. Diagnostic criteria for autoimmune hepatitis in children: a challenge for pediatric hepatologists. World J Gastroenterol. 2012;18:4470-4473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Wang MX, Morgan T, Lungo W, Wang L, Sze GZ, French SW. "Piecemeal" necrosis: renamed troxis necrosis. Exp Mol Pathol. 2001;71:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Herzog D, Soglio DB, Fournet JC, Martin S, Marleau D, Alvarez F. Interface hepatitis is associated with a high incidence of late graft fibrosis in a group of tightly monitored pediatric orthotopic liver transplantation patients. Liver Transpl. 2008;14:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Mieli-Vergani G, Vergani D, Baumann U, Czubkowski P, Debray D, Dezsofi A, Fischler B, Gupte G, Hierro L, Indolfi G, Jahnel J, Smets F, Verkade HJ, Hadžić N. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2018;66:345-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 18. | Mieli-Vergani G, Vergani D. Autoimmune paediatric liver disease. World J Gastroenterol. 2008;14:3360-3367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 20. | Sirbe C, Simu G, Szabo I, Grama A, Pop TL. Pathogenesis of Autoimmune Hepatitis-Cellular and Molecular Mechanisms. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol. 2022;19:158-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 22. | Löhr H, Treichel U, Poralla T, Manns M, Meyer zum Büschenfelde KH. Liver-infiltrating T helper cells in autoimmune chronic active hepatitis stimulate the production of autoantibodies against the human asialoglycoprotein receptor in vitro. Clin Exp Immunol. 1992;88:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | John K, Hardtke-Wolenski M, Jaeckel E, Manns MP, Schulze-Osthoff K, Bantel H. Increased apoptosis of regulatory T cells in patients with active autoimmune hepatitis. Cell Death Dis. 2017;8:3219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Béland K, Marceau G, Labardy A, Bourbonnais S, Alvarez F. Depletion of B cells induces remission of autoimmune hepatitis in mice through reduced antigen presentation and help to T cells. Hepatology. 2015;62:1511-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Immunohistochemical features of the portal tract mononuclear cell infiltrate in chronic aggressive hepatitis. Arch Dis Child. 1992;67:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Wang H, Feng X, Yan W, Tian D. Regulatory T Cells in Autoimmune Hepatitis: Unveiling Their Roles in Mouse Models and Patients. Front Immunol. 2020;11:575572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Pape S, Snijders RJALM, Gevers TJG, Chazouilleres O, Dalekos GN, Hirschfield GM, Lenzi M, Trauner M, Manns MP, Vierling JM, Montano-Loza AJ, Lohse AW, Schramm C, Drenth JPH, Heneghan MA; International Autoimmune Hepatitis Group (IAIHG) collaborators(‡). Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J Hepatol. 2022;76:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 28. | Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 29. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 648] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 30. | Ward LM, Ma J, Robinson ME, Scharke M, Ho J, Houghton K, Huber A, Scuccimarri R, Barsalou J, Roth J, Shenouda N, Matzinger MA, Lentle B, Jaremko JL, Koujok K, Watanabe Duffy K, Stein R, Sbrocchi AM, Rodd C, Miettunen PM, LeBlanc CMA, Larche M, Jurencak R, Cummings EA, Couch R, Cabral DA, Atkinson S, Alos N, Sykes E, Konji VN, Rauch F, Siminoski K, Lang B. Osteoporotic Fractures and Vertebral Body Reshaping in Children With Glucocorticoid-treated Rheumatic Disorders. J Clin Endocrinol Metab. 2021;106:e5195-e5207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 356] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | West S, Kenedi C. Strategies to prevent the neuropsychiatric side-effects of corticosteroids: a case report and review of the literature. Curr Opin Organ Transplant. 2014;19:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Díaz-González Á, Hernández-Guerra M, Pérez-Medrano I, Sapena V, Riveiro-Barciela M, Barreira-Díaz A, Gómez E, Morillas RM, Del Barrio M, Escudé L, Mateos B, Horta D, Gómez J, Conde I, Ferre-Aracil C, El Hajra I, Arencibía A, Zamora J, Fernández A, Salcedo M, Molina E, Soria A, Estévez P, López C, Álvarez-Navascúes C, García-Retortillo M, Crespo J, Londoño MC; ColHai Registry. Budesonide as first-line treatment in patients with autoimmune hepatitis seems inferior to standard predniso(lo)ne administration. Hepatology. 2023;77:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 34. | Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 94] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Manns MP, Jaeckel E, Taubert R. Budesonide in Autoimmune Hepatitis: The Right Drug at the Right Time for the Right Patient. Clin Gastroenterol Hepatol. 2018;16:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Olivas I, Cobreros M, Londoño MC, Díaz-González Á. Budesonide in the first line treatment of patients with autoimmune hepatitis. Gastroenterol Hepatol. 2022;45:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E, Bahr MJ, Günther R, Hultcrantz RW, Spengler U, Lohse AW, Szalay F, Färkkilä M, Pröls M, Strassburg CP; European AIH-BUC-Study Group. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 38. | Woynarowski M, Nemeth A, Baruch Y, Koletzko S, Melter M, Rodeck B, Strassburg CP, Pröls M, Woźniak M, Manns MP; European Autoimmune Hepatitis-Budesonide Study Group. Budesonide versus prednisone with azathioprine for the treatment of autoimmune hepatitis in children and adolescents. J Pediatr. 2013;163:1347-53.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 39. | Muratori L, Lohse AW, Lenzi M. Diagnosis and management of autoimmune hepatitis. BMJ. 2023;380:e070201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 40. | Brinkert F, Arrenberg P, Krech T, Grabhorn E, Lohse A, Schramm C. Two Cases of Hepatosplenic T-Cell Lymphoma in Adolescents Treated for Autoimmune Hepatitis. Pediatrics. 2016;138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Miles M, Shivakumar S. A Case of Hepatosplenic Gamma-delta T Cell Lymphoma with Intravascular Lymphoma Like Features in a Young Male With Autoimmune Hepatitis. Am J Gastroenterolo. 2015;110:S333-S334. [DOI] [Full Text] |
| 42. | Steinmann S, Lohse AW. Treatment of autoimmune hepatitis: Budesonide does not solve our problems. Hepatology. 2023;77:1071-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 43. | Allison AC. Two lessons from the interface of genetics and medicine. Genetics. 2004;166:1591-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14 Suppl 1:s2-s8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 45. | Inductivo-Yu I, Adams A, Gish RG, Wakil A, Bzowej NH, Frederick RT, Bonacini M. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Hlivko JT, Shiffman ML, Stravitz RT, Luketic VA, Sanyal AJ, Fuchs M, Sterling RK. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Halac U, Alvarez F. Hepatitis: new hope for difficult cases of autoimmune hepatitis. Nat Rev Gastroenterol Hepatol. 2009;6:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Jothimani D, Cramp ME, Cross TJ. Role of mycophenolate mofetil for the treatment of autoimmune hepatitis-an observational study. J Clin Exp Hepatol. 2014;4:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 50. | Santiago P, Schwartz I, Tamariz L, Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Kolev M, Diem S, Diem L, Rodrigues SG, Berzigotti A, Stirnimann G, Semmo N. Mycophenolate mofetil as second line treatment in autoimmune hepatitis - A retrospective single center analysis. J Transl Autoimmun. 2022;5:100172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 52. | Dalekos GN, Arvaniti P, Gatselis NK, Gabeta S, Samakidou A, Giannoulis G, Rigopoulou E, Koukoulis GK, Zachou K. Long-term results of mycophenolate mofetil vs. azathioprine use in individuals with autoimmune hepatitis. JHEP Rep. 2022;4:100601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 53. | Snijders RJALM, Stoelinga AEC, Gevers TJG, Pape S, Biewenga M, Tushuizen ME, Verdonk RC, de Jonge HJM, Vrolijk JM, Bakker SF, Vanwolleghem T, de Boer YS, Baven Pronk MAMC, Beuers U, van der Meer AJ, Gerven NMFV, Sijtsma MGM, van Eijck BC, van IJzendoorn MC, van Herwaarden M, van den Brand FF, Korkmaz KS, van den Berg AP, Guichelaar MMJ, Levens AD, van Hoek B, Drenth JPH; Dutch Autoimmune Hepatitis Working Group. An open-label randomised-controlled trial of azathioprine vs. mycophenolate mofetil for the induction of remission in treatment-naive autoimmune hepatitis. J Hepatol. 2024;80:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 54. | Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23:739-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Efe C, Ozaslan E, Purnak T. Methotrexate in the Treatment of Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2018;16:149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Haridy J, Nicoll A, Sood S. Methotrexate Therapy for Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2018;16:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Sultan MI, Biank VF, Telega GW. Successful treatment of autoimmune hepatitis with methotrexate. J Pediatr Gastroenterol Nutr. 2011;52:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 58. | Calne RY, Rolles K, White DJ, Thiru S, Evans DB, Mcmaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporin A in organ transplantation. Adv Nephrol Necker Hosp. 1981;10:335-347. |
| 59. | Starzl TE, Iwatsuki S, Klintmalm G, Schröter GP, Weil R 3rd, Koep LJ, Porter KA. Liver transplantation, 1980, with particular reference to cyclosporin-A. Transplant Proc. 1981;13:281-285. [PubMed] |
| 60. | Williams CR, Gooch JL. Calcineurin inhibitors and immunosuppression - a tale of two isoforms. Expert Rev Mol Med. 2012;14:e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Casey MJ, Meier-Kriesche HU. Calcineurin inhibitors in kidney transplantation: friend or foe? Curr Opin Nephrol Hypertens. 2011;20:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin A beta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:9398-9403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Mochizuki M, Masuda K, Sakane T, Ito K, Kogure M, Sugino N, Usui M, Mizushima Y, Ohno S, Inaba G. A clinical trial of FK506 in refractory uveitis. Am J Ophthalmol. 1993;115:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Park YJ, Yoo SA, Kim M, Kim WU. The Role of Calcium-Calcineurin-NFAT Signaling Pathway in Health and Autoimmune Diseases. Front Immunol. 2020;11:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 65. | Hyams JS, Ballow M, Leichtner AM. Cyclosporine treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Alvarez F, Ciocca M, Cañero-Velasco C, Ramonet M, de Davila MT, Cuarterolo M, Gonzalez T, Jara-Vega P, Camarena C, Brochu P, Drut R, Alvarez E. Short-term cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol. 1999;30:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Debray D, Maggiore G, Girardet JP, Mallet E, Bernard O. Efficacy of cyclosporin A in children with type 2 autoimmune hepatitis. J Pediatr. 1999;135:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Zizzo AN, Valentino PL, Shah PS, Kamath BM. Second-line Agents in Pediatric Patients With Autoimmune Hepatitis: A Systematic Review and Meta-analysis. J Pediatr Gastroenterol Nutr. 2017;65:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 71. | Cuarterolo M, Ciocca M, Velasco CC, Ramonet M, González T, López S, Garsd A, Alvarez F. Follow-up of children with autoimmune hepatitis treated with cyclosporine. J Pediatr Gastroenterol Nutr. 2006;43:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Cuarterolo ML, Ciocca ME, López SI, de Dávila MT, Alvarez F. Immunosuppressive therapy allows recovery from liver failure in children with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2011;9:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Åberg F, Sallinen V, Tuominen S, Adam R, Karam V, Mirza D, Heneghan MA, Line PD, Bennet W, Ericzon BG, Grat M, Lodge P, Rasmussen A, Schmelzle M, Thorburn D, Fondevila C, Helanterä I, Nordin A; European Liver and Intestine Transplant Association (ELITA). Cyclosporine vs. tacrolimus after liver transplantation for primary sclerosing cholangitis - a propensity score-matched intention-to-treat analysis. J Hepatol. 2024;80:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Van Thiel DH, Wright H, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, Irish W, Starzl TE. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:771-776. [PubMed] |
| 75. | Hegade V, Tirou K, Davies M. Tacrolimus in steroid refractory autoimmune hepatitis. Gut. 2011;60:A233-A234. [DOI] [Full Text] |
| 76. | Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol. 2007;13:3232-3236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (6)] |
| 78. | Abdollahi M, Ekrami NK, Ghojazadeh M, Boezen HM, Somi M, Alizadeh BZ. Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations? World J Gastroenterol. 2020;26:5896-5910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 79. | Hanouneh M, Ritchie MM, Ascha M, Ascha MS, Chedid A, Sanguankeo A, Zein NN, Hanouneh IA. A review of the utility of tacrolimus in the management of adults with autoimmune hepatitis. Scand J Gastroenterol. 2019;54:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Efe C, Hagström H, Ytting H, Bhanji RA, Müller NF, Wang Q, Purnak T, Muratori L, Werner M, Marschall HU, Muratori P, Gunşar F, Klintman D, Parés A, Heurgué-Berlot A, Schiano TD, Cengiz M, May-Sien Tana M, Ma X, Montano-Loza AJ, Berg T, Verma S, Larsen FS, Ozaslan E, Heneghan MA, Yoshida EM, Wahlin S. Efficacy and Safety of Mycophenolate Mofetil and Tacrolimus as Second-line Therapy for Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2017;15:1950-1956.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Efe C, Taii HA, Ytting H, Aehling N, Bhanji RA, Hagström H, Purnak T, Muratori L, Werner M, Muratori P, Klintman D, Schiano TD, Montano-Loza AJ, Berg T, Larsen FS, Alkhouri N, Ozaslan E, Heneghan MA, Yoshida EM, Wahlin S. Tacrolimus and Mycophenolate Mofetil as Second-Line Therapies for Pediatric Patients with Autoimmune Hepatitis. Dig Dis Sci. 2018;63:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Ferre-Aracil C, Riveiro-Barciela M, Trapero-Marugán M, Rodríguez-Perálvarez M, Llovet LP, Téllez L, Sánchez-Torrijos Y, Díaz-Fontenla F, Salcedo-Plaza M, Álvarez-López P, de la Mata M, Londoño MC, Bañares-Cañizares R, Calleja JL. Tacrolimus as an Effective and Durable Second-Line Treatment for Chronic Autoimmune Hepatitis: A Multicentric Study. Dig Dis Sci. 2021;66:2826-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Stoelinga AEC, Tushuizen ME, van den Hout WB, Girondo MDMR, de Vries ES, Levens AD, Moes DAR, Gevers TJG, van der Meer S, Brouwer HT, de Jonge HJM, de Boer YS, Beuers UHW, van der Meer AJ, van den Berg AP, Guichelaar MMJ, Drenth JPH, van Hoek B; Dutch Autoimmune Hepatitis Group. Tacrolimus versus mycophenolate for AutoImmune hepatitis patients with incompLete response On first-line therapy (TAILOR study): a study protocol for a phase III, open-label, multicentre, randomised controlled trial. Trials. 2024;25:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 909] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 85. | Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1626] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 86. | Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 983] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 87. | Damoiseaux JG, Defresne MP, Reutelingsperger CP, Van Breda Vriesman PJ. Cyclosporin-A differentially affects apoptosis during in vivo rat thymocyte maturation. Scand J Immunol. 2002;56:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, Joosten I. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 89. | Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 623] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 90. | Kurowski J, Melin-Aldana H, Bass L, Alonso EM, Ekong UD. Sirolimus as rescue therapy in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2014;58:e4-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 91. | Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med. 2014;127:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 92. | Jannone G, Scheers I, Smets F, Stephenne X, M Sokal E. Everolimus is Safe as a Second-/Third-Line Therapy in Pediatric Autoimmune Hepatitis. JPGN Rep. 2022;3:e227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 93. | Derben FC, Ytting H, Hartleben B, Bantel H, Wedemeyer H, Willemoe GL, Jaeckel E, Taubert R. Salvage therapies of autoimmune hepatitis limit proinflammatory immune cells while sparing regulatory T cells. Hepatol Commun. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 94. | Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Role of B cells as antigen presenting cells. Front Immunol. 2022;13:954936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 95. | Mathieu M, Cotta-Grand N, Daudelin JF, Boulet S, Lapointe R, Labrecque N. CD40-activated B cells can efficiently prime antigen-specific naïve CD8+ T cells to generate effector but not memory T cells. PLoS One. 2012;7:e30139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q, Zhu B, Tang H, Hua Z, Hou B. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4(+) T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity. 2018;49:695-708.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 97. | Hua Z, Hou B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol Rev. 2020;296:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 98. | Cargill T, Culver EL. The Role of B Cells and B Cell Therapies in Immune-Mediated Liver Diseases. Front Immunol. 2021;12:661196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Schultheiß C, Steinmann S, Lohse AW, Binder M. B cells in autoimmune hepatitis: bystanders or central players? Semin Immunopathol. 2022;44:411-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Cano RLE, Lopera HDE. Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press, 2013. |
| 101. | King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 102. | D'Agostino D, Costaguta A, Álvarez F. Successful treatment of refractory autoimmune hepatitis with rituximab. Pediatrics. 2013;132:e526-e530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Carey EJ, Somaratne K, Rakela J. Successful rituximab therapy in refractory autoimmune hepatitis and Evans syndrome. Rev méd Chile. 2011;139:1484-1487. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 104. | Santos ES, Arosemena LR, Raez LE, O'Brien C, Regev A. Successful treatment of autoimmune hepatitis and idiopathic thrombocytopenic purpura with the monoclonal antibody, rituximab: case report and review of literature. Liver Int. 2006;26:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Barth E, Clawson J. A Case of Autoimmune Hepatitis Treated with Rituximab. Case Rep Gastroenterol. 2010;4:502-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Burak KW, Swain MG, Santodomingo-Garzon T, Lee SS, Urbanski SJ, Aspinall AI, Coffin CS, Myers RP. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol. 2013;27:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 107. | Saul SA, Taylor SA, Mohammad S. Treatment of Refractory Pediatric Autoimmune Hepatitis With Rituximab. JPGN Rep. 2021;2:e069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Than NN, Hodson J, Schmidt-Martin D, Taubert R, Wawman RE, Botter M, Gautam N, Bock K, Jones R, Appanna GD, Godkin A, Montano-Loza AJ, Lammert F, Schramm C, Manns MP, Swain M, Burak KW, Adams DH, Hirschfield GM, Oo YH. Efficacy of rituximab in difficult-to-manage autoimmune hepatitis: Results from the International Autoimmune Hepatitis Group. JHEP Rep. 2019;1:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 109. | Wennmann M, Kathemann S, Kampmann K, Ohlsson S, Büscher A, Holzinger D, Della Marina A, Lainka E. A Retrospective Analysis of Rituximab Treatment for B Cell Depletion in Different Pediatric Indications. Front Pediatr. 2021;9:651323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Costaguta GA, Álvarez F. B cell depletion for autoimmune liver diseases: A retrospective review of indications and outcomes. JPGN Rep. 2024;5:326-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 111. | Hanif N, Anwer F. Rituximab. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 112. | Labrosse R, Barmettler S, Derfalvi B, Blincoe A, Cros G, Lacombe-Barrios J, Barsalou J, Yang N, Alrumayyan N, Sinclair J, Ong MS, Camargo CA Jr, Walter J, Haddad E. Rituximab-induced hypogammaglobulinemia and infection risk in pediatric patients. J Allergy Clin Immunol. 2021;148:523-532.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Randall KL. Rituximab in autoimmune diseases. Aust Prescr. 2016;39:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Boneparth A, Davidson A. B-cell activating factor targeted therapy and lupus. Arthritis Res Ther. 2012;14 Suppl 4:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Shabgah AG, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. The role of BAFF and APRIL in rheumatoid arthritis. J Cell Physiol. 2019;234:17050-17063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 117. | Nocturne G, Mariette X. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol. 2018;14:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 118. | Sanges S, Guerrier T, Launay D, Lefèvre G, Labalette M, Forestier A, Sobanski V, Corli J, Hauspie C, Jendoubi M, Yakoub-Agha I, Hatron PY, Hachulla E, Dubucquoi S. Role of B cells in the pathogenesis of systemic sclerosis. Rev Med Interne. 2017;38:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Joy A, Muralidharan A, Alfaraj M, Shantharam D, Cherukuri ASS, Muthukumar A. The Role of Belimumab in Systemic Lupus Erythematosis: A Systematic Review. Cureus. 2022;14:e25887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Cohen SB. Updates from B Cell Trials: Efficacy. J Rheumatol Suppl. 2006;77:12-17. [PubMed] |
| 121. | Clinical Trial Finder. Addition of belimumab to B-cell depletion in relapsing-remitting multiple sclerosis. [cited 26 September 2024]. Available from: https://nationalmssociety-org.clinicaltrialconnect.com/trials/NCT04767698. |
| 122. | Kolev M, Sarbu AC, Möller B, Maurer B, Kollert F, Semmo N. Belimumab treatment in autoimmune hepatitis and primary biliary cholangitis - a case series. J Transl Autoimmun. 2023;6:100189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 123. | Arvaniti P, Giannoulis G, Gabeta S, Zachou K, Koukoulis GK, Dalekos GN. Belimumab is a promising third-line treatment option for refractory autoimmune hepatitis. JHEP Rep. 2020;2:100123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | ClinicalTrials.gov. Belimumab in Autoimmune Hepatitis (BELief). [cited 26 September 2024]. Available from: https://classic.clinicaltrials.gov/ct2/show/study/NCT06381453. |
| 125. | Wise LM, Stohl W. Belimumab and Rituximab in Systemic Lupus Erythematosus: A Tale of Two B Cell-Targeting Agents. Front Med (Lausanne). 2020;7:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 126. | Karampetsou MP, Liossis SN, Sfikakis PP. TNF-α antagonists beyond approved indications: stories of success and prospects for the future. QJM. 2010;103:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 128. | Cassim S, Bilodeau M, Vincent C, Lapierre P. Novel Immunotherapies for Autoimmune Hepatitis. Front Pediatr. 2017;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Weiler-Normann C, Wiegard C, Schramm C, Lohse AW. A case of difficult-to-treat autoimmune hepatitis successfully managed by TNF-alpha blockade. Am J Gastroenterol. 2009;104:2877-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Rajanayagam J, Lewindon PJ. Infliximab as rescue therapy in paediatric autoimmune hepatitis. J Hepatol. 2013;59:908-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 131. | Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Möller S, Lohse AW. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 132. | Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 133. | Nakayama S. Autoimmune Hepatitis Triggered by Anti-TNF-α Therapy. Case Rep Med. 2013;2013:561748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Vollmer O, Felten R, Mertz P, Lebrun-Vignes B, Salem JE, Arnaud L. Characterization of auto-immune hepatitis associated with the use of anti-TNFα agents: An analysis of 389 cases in VigiBase. Autoimmun Rev. 2020;19:102460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, Mariz E, Bernardes M, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: A single center report of 8 cases. World J Gastroenterol. 2015;21:7584-7588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 136. | Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1402] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 137. | Iracheta-Vellve A, Petrasek J, Gyogyosi B, Bala S, Csak T, Kodys K, Szabo G. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017;37:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 138. | Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 139. | Ahmed O, Brahmania M, Alsahafi M, Alkhowaiter S, Erb S. Anakinra Hepatotoxicity in a Patient With Adult-Onset Still's Disease. ACG Case Rep J. 2015;2:173-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 140. | Murray GM, Ng SK, Beasley D, Johansen L, Ramanan AV. Severe hepatotoxicity as a rare side effect of anakinra in a patient with systemic JIA. Rheumatology (Oxford). 2021;60:e307-e308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 141. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3875] [Article Influence: 227.9] [Reference Citation Analysis (0)] |
| 142. | Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, Carman JA, Erzurum SC, Lipshitz HD, Fox PL, Hamilton TA, Li X. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol. 2013;191:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Mino T, Murakawa Y, Fukao A, Vandenbon A, Wessels HH, Ori D, Uehata T, Tartey S, Akira S, Suzuki Y, Vinuesa CG, Ohler U, Standley DM, Landthaler M, Fujiwara T, Takeuchi O. Regnase-1 and Roquin Regulate a Common Element in Inflammatory mRNAs by Spatiotemporally Distinct Mechanisms. Cell. 2015;161:1058-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 309] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 144. | Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, Qiu D, Wei J, Liu Y, Shen L, Chen X, Peng Y, Li Z, Ma X. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One. 2011;6:e18909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 145. | Yu H, Huang J, Liu Y, Ai G, Yan W, Wang X, Ning Q. IL-17 contributes to autoimmune hepatitis. J Huazhong Univ Sci Technolog Med Sci. 2010;30:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Magyari L, Kovesdi E, Sarlos P, Javorhazy A, Sumegi K, Melegh B. Interleukin and interleukin receptor gene polymorphisms in inflammatory bowel diseases susceptibility. World J Gastroenterol. 2014;20:3208-3222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 147. | Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 413] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 148. | Deng Z, Wang S, Wu C, Wang C. IL-17 inhibitor-associated inflammatory bowel disease: A study based on literature and database analysis. Front Pharmacol. 2023;14:1124628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 149. | Gadina M, Gazaniga N, Vian L, Furumoto Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J Autoimmun. 2017;85:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 150. | Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 151. | Herrera-deGuise C, Serra-Ruiz X, Lastiri E, Borruel N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne). 2023;10:1089099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 152. | Braun LM, Zeiser R. Kinase Inhibition as Treatment for Acute and Chronic Graft-Versus-Host Disease. Front Immunol. 2021;12:760199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 153. | Harrington R, Al Nokhatha SA, Conway R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J Inflamm Res. 2020;13:519-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 154. | Wang H, Feng X, Han P, Lei Y, Xia Y, Tian D, Yan W. The JAK inhibitor tofacitinib ameliorates immunemediated liver injury in mice. Mol Med Rep. 2019;20:4883-4892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Shaker ME, Hendawy OM, El-Mesery M, Hazem SH. The JAK inhibitor ruxolitinib abrogates immune hepatitis instigated by concanavalin A in mice. Int Immunopharmacol. 2022;103:108463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 156. | Shao T, Leung PSC, Zhang W, Tsuneyama K, Ridgway WM, Young HA, Shuai Z, Ansari AA, Gershwin ME. Treatment with a JAK1/2 inhibitor ameliorates murine autoimmune cholangitis induced by IFN overexpression. Cell Mol Immunol. 2022;19:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 157. | Hadžić N, Deheragoda M, Worth A, Bansal S, Samyn M, Kusters M. JAK Inhibition in STAT1 Gain-of-Function-Mediated Treatment-Resistant Autoimmune Hepatitis. N Engl J Med. 2024;390:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 158. | Kaneko S, Sakura F, Tanita K, Shimbo A, Nambu R, Yoshida M, Umetsu S, Inui A, Okada C, Tsumura M, Yamada M, Suzuki H, Kosaki K, Ohara O, Shimizu M, Morio T, Okada S, Kanegane H. Janus kinase inhibitors ameliorate clinical symptoms in patients with STAT3 gain-of-function. Immunother Adv. 2023;3:ltad027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 159. | Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos Trans R Soc Lond B Biol Sci. 2015;370:20150017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 160. | Lefrère JJ, Berche P. [Doctor Brown-Sequard's therapy]. Ann Endocrinol (Paris). 2010;71:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Chu DT, Nguyen TT, Tien NLB, Tran DK, Jeong JH, Anh PG, Thanh VV, Truong DT, Dinh TC. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 162. | Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 163. | Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 471] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 164. | Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 828] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 165. | Eggenhuizen PJ, Ng BH, Ooi JD. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 166. | Passeri L, Marta F, Bassi V, Gregori S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 167. | Bender E. Creating T cells to guard against autoimmune disease. Nature. 2021;595:S60-S62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 168. | ClinicalTrials. gov. CAR-T Cells Targeting B Cell Related Autoimmune Diseases. [cited 26 September 2024]. Available from: https://clinicaltrials.gov/study/NCT05459870. |
| 169. | Schett G, Mackensen A, Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023;402:2034-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 252] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 170. | Akamatsu M, Mikami N, Ohkura N, Kawakami R, Kitagawa Y, Sugimoto A, Hirota K, Nakamura N, Ujihara S, Kurosaki T, Hamaguchi H, Harada H, Xia G, Morita Y, Aramori I, Narumiya S, Sakaguchi S. Conversion of antigen-specific effector/memory T cells into Foxp3-expressing T(reg) cells by inhibition of CDK8/19. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 171. | Safinia N, Grageda N, Scottà C, Thirkell S, Fry LJ, Vaikunthanathan T, Lechler RI, Lombardi G. Cell Therapy in Organ Transplantation: Our Experience on the Clinical Translation of Regulatory T Cells. Front Immunol. 2018;9:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 172. | Lapierre P, Béland K, Yang R, Alvarez F. Adoptive transfer of ex vivo expanded regulatory T cells in an autoimmune hepatitis murine model restores peripheral tolerance. Hepatology. 2013;57:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 173. | Sánchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, Thirkell S, Lowe K, Fry L, Heward J, Kerr A, Ali J, Fisher C, Lewis G, Hope A, Kodela E, Lyne M, Farzaneh F, Kordasti S, Rebollo-Mesa I, Jose Lozano J, Safinia N, Heaton N, Lechler R, Martínez-Llordella M, Lombardi G. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 174. | Amer OE, Sabico S, Khattak MNK, Alnaami AM, Aljohani NJ, Alfawaz H, AlHameidi A, Al-Daghri NM. Increasing Prevalence of Pediatric Metabolic Syndrome and Its Components among Arab Youth: A Time-Series Study from 2010-2019. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 175. | Li W, Qiu X, Ma H, Geng Q. Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front Endocrinol (Lausanne). 2022;13:1029736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 176. | Alexander TH, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, Hammerschlag PE, Hannley M, Hughes GB, Moscicki R, Nelson RA, Niparko JK, Rauch SD, Telian SA, Brookhouser PE, Harris JP. Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol Neurotol. 2009;30:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 177. | Kulkarni S, Durham H, Glover L, Ather O, Phillips V, Nemes S, Cousens L, Blomgran P, Ambery P. Metabolic adverse events associated with systemic corticosteroid therapy-a systematic review and meta-analysis. BMJ Open. 2022;12:e061476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 178. | Delaleu J, Destere A, Hachon L, Declèves X, Lloret-Linares C. Glucocorticoids dosing in obese subjects: A systematic review. Therapie. 2019;74:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 179. | Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 180. | Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 181. | Tufton N, Ahmad S, Rolfe C, Rajkariar R, Byrne C, Chowdhury TA. New-onset diabetes after renal transplantation. Diabet Med. 2014;31:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 182. | Tiwari A, Al-robeh H, Sharma H, Ammari Z, Khan MS, Jaume JC. Steroid-Induced Diabetic Ketoacidosis in a Patient with Type 2 Diabetes Mellitus. AACE Clin Case Rep. 2018;4:131-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 183. | Alakkas Z, Alzaedi OA, Somannavar SS, Alfaifi A. Steroid-Induced Diabetes Ketoacidosis in an Immune Thrombocytopenia Patient: A Case Report and Literature Review. Am J Case Rep. 2020;21:e923372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 184. | Fuji S, Kim SW, Mori S, Furuta K, Tanosaki R, Heike Y, Takaue Y, Fukuda T. Decreased insulin secretion in patients receiving tacrolimus as GVHD prophylaxis after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45:405-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 185. | Shivaswamy V, Bennett RG, Clure CC, Ottemann B, Davis JS, Larsen JL, Hamel FG. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl Res. 2014;163:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 186. | Toenders YJ, Dorsman H, van der Cruijsen R, Crone EA. Developing body estimation in adolescence is associated with neural regions that support self-concept. Soc Cogn Affect Neurosci. 2024;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 187. | Nazmi S, Nikbakht HA, Behmanesh F, Gholamnia-Shirvani Z, Azizi A. Body image concern and demographic characteristics as predictors of anxiety in adolescent girls. Int J Adolesc Med Health. 2024;36:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 188. | Creese H, Saxena S, Nicholls D, Pascual Sanchez A, Hargreaves D. The role of dieting, happiness with appearance, self-esteem, and bullying in the relationship between mental health and body-mass index among UK adolescents: a longitudinal analysis of the Millennium Cohort Study. EClinicalMedicine. 2023;60:101992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 189. | Wellen BCM, Lin HC, Stellway JE. Psychosocial considerations in pediatric autoimmune liver disease. Clin Liver Dis (Hoboken). 2022;20:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 190. | Kerkar N, Annunziato RA, Foley L, Schmeidler J, Rumbo C, Emre S, Shneider B, Shemesh E. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 191. | Halbeisen G, Braks K, Huber TJ, Paslakis G. Exploring Gender Differences in Early Weight Change and Variability in Adolescents with Anorexia Nervosa during Inpatient Treatment. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 192. | Torres S, Vieira AI, Vieira FM, Miller KM, Guerra MP, Lencastre L, Reis AC, Timóteo S, Nunes P, Barbosa MR. A Comprehensive Study of Positive Body Image as a Predictor of Psychological Well-Being in Anorexia Nervosa. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 193. | Capra AP, Chiara E, Briuglia S. Autoimmune hepatitis in genetic syndromes: A literature review. World J Hepatol. 2021;13:1328-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 194. | Wang CR, Tsai HW. Autoimmune liver diseases in systemic rheumatic diseases. World J Gastroenterol. 2022;28:2527-2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 195. | Carroll MW, Kuenzig ME, Mack DR, Otley AR, Griffiths AM, Kaplan GG, Bernstein CN, Bitton A, Murthy SK, Nguyen GC, Lee K, Cooke-Lauder J, Benchimol EI. The Impact of Inflammatory Bowel Disease in Canada 2018: Children and Adolescents with IBD. J Can Assoc Gastroenterol. 2019;2:S49-S67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 196. | DeFilippis EM, Kumar S. Clinical Presentation and Outcomes of Autoimmune Hepatitis in Inflammatory Bowel Disease. Dig Dis Sci. 2015;60:2873-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/