Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1169
Revised: September 3, 2024
Accepted: September 23, 2024
Published online: October 27, 2024
Processing time: 143 Days and 3.7 Hours
Helicobacter pylori (H. pylori) is associated with the development of gastrointestinal disorders ranging from gastritis to gastric cancer. The evidence of the association between metabolic dysfunction-associated steatohepatitis (MASH) and H. pylori infection in the literature is scarce. Therefore, we aim to evaluate the risk of developing MASH in patients who have had a diagnosis of H. pylori infection independently of any confounding variables.
To evaluate the risk of developing MASH in patients who have had a diagnosis of H. pylori infection.
This study used a validated multicenter research database of over 360 hospitals across 26 healthcare systems across the United States from 1999 to 2022. Multivariate regression analysis assessed the risk of developing MASH, adjusting for confounders including H. pylori infection, obesity, type 2 diabetes, hypertension, dyslipidemia, and male gender. A two-sided P value < 0.05 was considered as statistically significant, and all statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2008).
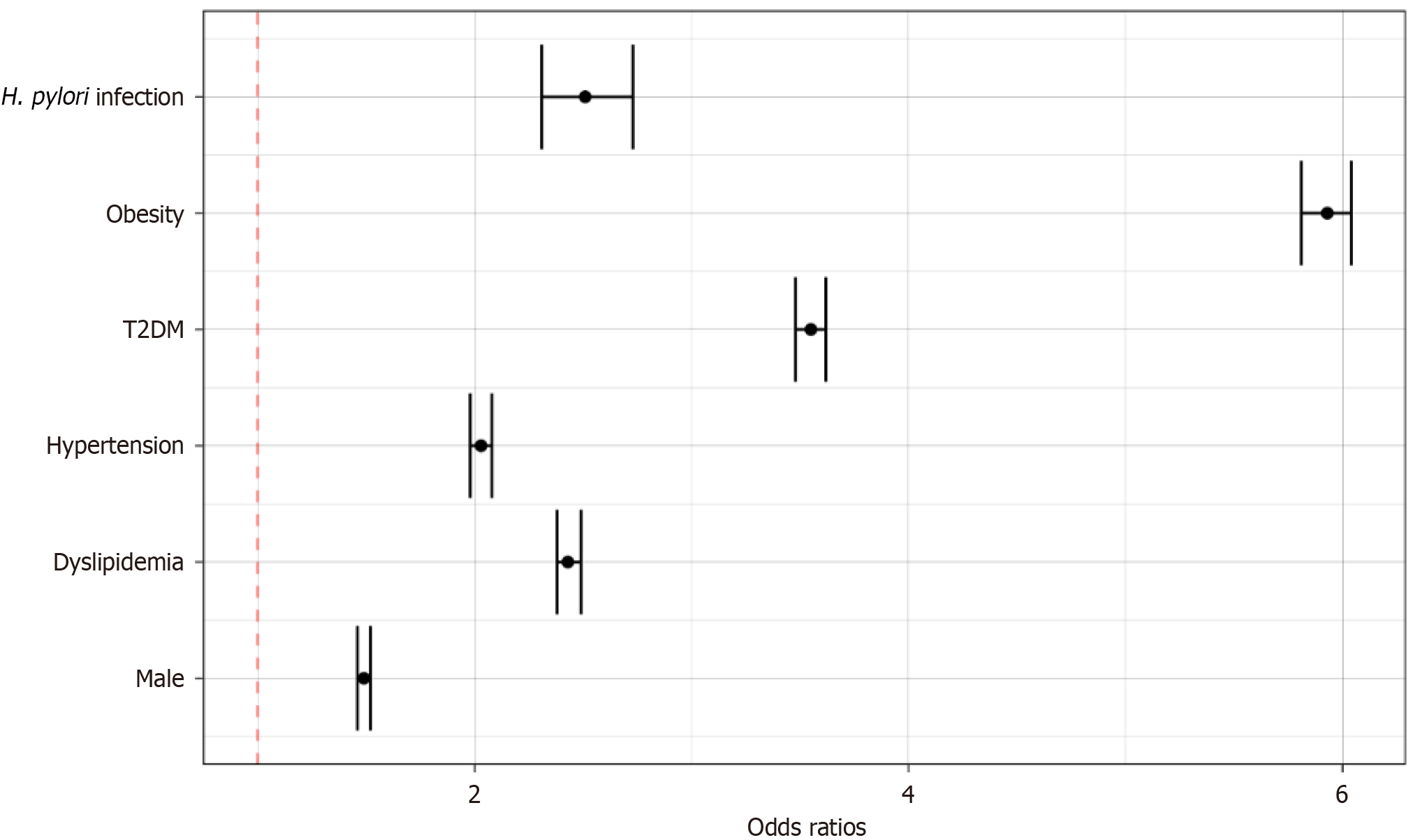
A total of 79476132 individuals were screened in the database and 69232620 were selected in the final analysis after accounting for inclusion and exclusion criteria. Smokers (14.30%), patients with hyperlipidemia (70.35%), hypertension (73.86%), diabetes mellitus type 2 (56.46%), and obese patients (58.15%) were more common in patients with MASH compared to control. Using a multivariate regression analysis, the risk of MASH was increased in diabetics [odds ratio (OR): 3.55; 95%CI: 3.48-3.62], obese (OR: 5.93; 95%CI: 5.81-6.04), males (OR: 1.49; 95%CI: 1.46-1.52), individuals with hyperlipidemia (OR: 2.43; 95%CI: 2.38-2.49) and H. pylori infection (OR: 2.51; 95%CI: 2.31-2.73).
This is the largest population-based study in the United States illustrating an increased prevalence and odds of developing MASH in patients with H. pylori infection after adjusting for risk factors.
Core Tip: Helicobacter pylori (H. pylori) is associated with the development of several gastrointestinal disorders ranging from gastritis to gastric cancer. The evidence of the association between metabolic dysfunction-associated steatohepatitis (MASH) and H. pylori infection in the literature is scarce. In this study, we evaluate the risk of developing MASH in patients who have had a diagnosis of H. pylori infection independently of any confounding variables. A validated multicenter and research platform database of more than 360 hospitals from 26 different healthcare systems across the United States from 1999 to September 2022 was utilized to construct this study.
- Citation: Abdel-Razeq R, Bitar L, Bitar ER, Onwuzo C, Abu-Hammour MN, Eren B, Mohamed I, Johnson A, Boustany A, Onwuzo S, Asaad I. Prevalence and risk factors associated with metabolic dysfunction-associated steatohepatitis in patients with Helicobacter pylori infection: A population-based study. World J Hepatol 2024; 16(10): 1169-1176
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1169.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1169
Metabolic dysfunction-associated steatohepatitis (MASH) is considered the most severe form of the metabolic dysfunction-associated steatotic liver disease (MASLD) spectrum and is characterized by the presence of chronic hepatitis (inflammation of the liver), hepatocellular injury, and variable degrees of fibrosis, in addition to the accumulation of fat in the liver that is present in all forms of MASLD[1-3]. In the United States, the prevalence of MASLD is estimated to be around 30%[4,5], while that of MASH is between 3%-5%[5]. The main sequelae of MASH are liver scarring, cirrhosis and liver cancer, and the main causes of death from MASLD/MASH are cardiovascular disease and extrahepatic malignancies. Besides lifestyle modifications, there is currently no approved therapy for MASLD[1,6].
Helicobacter pylori (H. pylori) is a Gram-negative, spiral-shaped, microaerophilic acidophilic bacterium that usually infects the mucinous surface of the stomach, with a prevalence in the United States of about 30%-50%. H. pylori infection is a major risk factor for the development of gastritis, gastric and duodenal ulcers, as well as gastric cancer. In addition, it can also cause manifestations outside the gastrointestinal system, most notably cardiovascular, hematological, respiratory, neurodegenerative, ophthalmological, otorhinolaryngologic, and endocrine diseases[7-9].
Despite the increasing number of studies investigating the association of H. pylori and MASH, conclusive evidence of an actual link between these two entities is scarce. Previous studies have proposed many mechanisms through which H. pylori can contribute to the pathogenesis of MASH; H. pylori can cause an increase in the levels of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-6 by maintaining a chronic low-grade inflammation and can lead to the dysbiosis of the gastrointestinal microbiota with transport of their metabolites into the portal circulation to activate Toll-Like Receptors. These factors promote insulin resistance and may favor the deposition of fat in the liver[10-12]. However, other studies concluded that H. pylori is not an independent risk factor for the development of MASH in many countries such as China, Japan, and Central Europe[13-15].
Many of these previous studies have limited data and fail to provide sufficient information. Moreover, the results are inconsistent among different cohorts and different regions. Therefore, we aim to evaluate the risk of developing MASH in patients who have had a diagnosis of H. pylori infection independently of any confounding variables.
Explorys Inc., Cleveland, OH, United States is a validated multicenter and research platform database of more than 360 hospitals form 26 different healthcare systems across the United States consisting of data from 1999 to September 2022. Explorys was developed and has been prospectively maintained by IBM Corporation, Watson Health[16], including electronic health records from greater than 60 million unique patients and provide a broad regional distribution of the United States representing approximately 15% of the population. It was utilized to construct a retrospective cohort analysis. A systematized Nomenclature of medicine-clinical terms hierarchy[17] was used to select diagnosis, findings, and procedures. Prescription drug orders are mapped into SNOMED and RxNorm[18]. Institutional Review Board was not required as source data are de-identified. To protect patient confidentiality, Explorys rounds population count to the nearest 10 and treats all counts between zero and 10 as equivalent. The study was conducted in accordance to the Declaration of Helsinki (as revised in 2013). Access to the database is granted to participating healthcare systems. Use of Explorys platform has been validated in multiple fields including gastroenterology[19-21].
Patients aged 18 years and above were included in this study. Exclusion criteria included pregnancy, patients with cancer and patients with alcohol use disorder. The individuals included were from different socioeconomic backgrounds and ethnic groups.
Patients with MASH were compared to those who did not. The risk of developing MASH was calculated using a multivariate regression analysis to account for potential cofounders including a history of H. pylori infection, obese patients, those with type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, and male individuals. A 2-sided P value ≤ 0.05 was considered as statistically significant, and all statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2008).
A total of 79476132 individuals were screened in the database and 69232620 patients were selected in the final analysis after applying the inclusion and exclusion criteria. Patients were divided into two subgroups: Patients who had been diagnosed with MASH, whether by ultrasound, fibroscan or liver biopsy, were considered cases; and patients without MASH were considered control. The baseline characteristics of patients with MASH are reported in Table 1. Smokers (14.30%), patients with hyperlipidemia (70.35%), hypertension (73.86%), T2DM (56.46%) and obesity (58.15%) were more common in patients of the case group category.
| MASH | No MASH | |
| Smoker | 8650 (14.03) | 3629290 (5.24) |
| Male | 26020 (42.21) | 31483000 (45.51) |
| Hyperlipidemia | 43360 (70.35) | 11634530 (16.82) |
| Hypertension | 45520 (73.86) | 13997560 (20.23) |
| T2DM | 34800 (56.46) | 5552250 (8.02) |
| Obesity | 35840 (58.15) | 5123940 (7.40) |
| H. pylori infection | 570 (0.92) | 79420 (0.11) |
| Total | 61630 | 69170990 |
Using a multivariate regression analysis, the risk of developing MASH was increased in diabetics [odds ratio (OR): 3.55; 95%CI: 3.48-3.62], obese patients (OR: 5.93; 95%CI: 5.81-6.04), males (OR: 1.49; 95%CI: 1.46-1.52), individuals with hyperlipidemia (OR: 2.43; 95%CI: 2.38-2.49) and those with a H. pylori infection (OR: 2.51; 95%CI: 2.31-2.73) as reported in Figure 1.
MASLD is the most common liver disease worldwide, and the leading cause of liver-related morbidity and mortality[22]. In 20%-30% of cases, MASLD can progress to MASH[6], which is the most severe and aggressive form of the disease, marked by chronic inflammation of the liver with possible progression to advanced scarring (cirrhosis) and hepatic failure[23]. It is estimated that 3% to 6% of Americans are affected by MASH with a rising prevalence, and a strong association with obesity, dyslipidemia, type 2 diabetes, as well as metabolic syndrome[24].
As for the prevalence of H. pylori, it also differs amongst nations globally. In industrialized countries, it is roughly 30%, but it can reach as high as 80% in developing nations[25,26]. Given the evidence that H. pylori infection has been associated with a wide spectrum of gastrointestinal tract disorders, such as peptic ulcer, mucosa associated lymphoid tissue lymphoma, gastric adenocarcinoma and others, there has been speculation regarding the possible association of H. pylori infection with the development of non-alcoholic fatty liver disease[27].
In our present study, we examined whether there is a link between the incidence of MASH and H. pylori infection. We found that the risk of developing MASH was indeed increased in people infected with H. pylori. Our results were statisti
The proportion of subjects infected with H. pylori was significantly higher in our group of MASH positive patients (0.92%) than in the MASH-negative patients (0.11%). This is in accordance with Chen et al’s study[28] conducted in China, which noted a prevalence of 26.6% of MASH among 2263 subjects, 53% of which were also diagnosed with H. pylori infection, whilst in the 1660 non-MASH subjects, only 43.6% were diagnosed with H. pylori infection (P value = 4.9 × 10-4)[28]. Furthermore, in Sumida et al’s study[29] conducted in Japan, the prevalence of MASH was (80.8%) in the H. pylori-positive subjects and 50.7% (P value = 8 × 10-3) in the H. pylori negative subjects[29,30].
This association was furthermore supported by many studies in different regions in the world[29-32]. Similarly, two other studies from Turkey and Japan respectively pointed to H. pylori infection as one of the independent risk factors for the emergence of MASH[27,30]. These results have been supported by additional findings among Asian populations, and further data from outside Asia has also replicated these findings[31-33].
In Guatemalan people who tested positive for Cytotoxic-associated gene A (CagA) and Vacuolating Toxin (VacA), Alvarez et al[33] showed a positive connection between MASH and H. pylori. CagA and VacA have been found to be more virulent, with a change in the gut microbiota and permeability, an increase in the inflammation state and a critical involvement in the development of gastric cancer[34,35].
However, Kang et al[36] found that in H. pylori-infected individuals, MASH was more common in CagA-negative individuals than in CagA-positive individuals. Additionally, despite adjusting for numerous conventional risk variables, CagA negative H. pylori positivity was strongly related with MASH, demonstrating a clinically important association between these two disorders. They also showed a slight, significant correlation in a recent meta-analysis with an odds ratio of 1.21 (95%CI: 1.07-1.37)[32,36].
In our study, the calculated odds ratio between H. pylori and MASH was 2.51 (95%CI: 2.31-2.73), suggesting a strong association between these two disorders.
Given that there are numerous theories explaining the link between H. pylori infection and MASH, and as no clear-cut evidence for any one pathway has been found, the pathophysiology is likely complex and multifactorial. In a meta-analysis conducted by Mantovani et al[37], H. pylori infection was found to be mildly associated with an increased risk of both prevalent and incident MASLD. The meta-analysis included 13 observational studies involving a total of 81162 middle-aged individuals, predominantly of Asian ethnicity. The results showed that H. pylori infection was linked to a higher risk of prevalent MASLD (random-effects OR 1.20, 95%CI: 1.07-1.35) and incident MASLD (random-effects hazard ratio 1.14, 95%CI: 1.05-1.23). The study suggested that chronic H. pylori infection may play a role in the development of MASLD, possibly through mechanisms involving systemic inflammation, insulin resistance, and changes in gut microbiota. During H. pylori infection, inflammatory cytokines, and vasoactive substances such as IL-6, IL-8, and TNF-α are released. They induce chronic low-grade systemic inflammation which may accelerate the progression to MASH and cause insulin resistance. A comprehensive review of nine studies also examined and confirmed this relationship[36].
The inflammatory process, which is characterized by several kinases including JNK, IKK/NF-kB, upregulates Ser phosphorylation or suppresses the autophosphorylation of the tyrosyl group of the insulin receptor substrate-137 Leading to insulin resistance[36,38]. In addition, TNF-α can increase free fatty acids by accelerating lipolysis resulting in hepatocyte dysfunction, which is a part of the pathway to MASH[38,39].
A hormonal link has also been hypothesized, specifically focusing on the hormones of the adipose tissue such as adiponectin which is decreased in patients with MASH and MASLD. This hormone protects against inflammation and fibrosis by lowering lipid storage in the liver[38,39]. According to this, H. pylori infection may result in decreased adiponectin levels and, eventually, decreased protection against fibrosis and MASH.
Furthermore, many common gene bases were discovered across H. pylori at the genomic level. These 95 genes significantly enriched 108 pathways according to pathway analysis (P value < 0.001), many of which have been linked to both H. pylori and MASH. Examples of these pathways include the response to LPS (lipopolysaccharide), the inflammatory response, aging, the response to hypoxia, and cytokine activity[28].
These findings imply that multiple genetic pathways exist between H. pylori and MASH, through which many genes interact to affect the pathogenic progression of both diseases.
Despite all the positive findings discussed, some studies have reported somewhat conflicting results[30,40-42]. Some findings were made in China, where a sizable cross-sectional study of 21456 people demonstrated no link between H. pylori infection and MASH as identified by ultrasound[42]. According to a cross-sectional study conducted in Japan on 13737 patients, H. pylori infection may not even be a risk factor for MASH[41].
Other investigations have found no correlation between MASH and H. pylori serology positivity in highly endemic locations[41,42]; this was also confirmed in a recent American investigation using data from the Third National Health and Nutrition Examination Survey[34]. Furthermore, after controlling for confounding variables, Fan et al’s findings[42] that the prevalence of MASH was considerably greater in participants with H. pylori infection (36.0% vs 33.3%, P value 0.05) was disproved.
Measurement of these markers at a single time point may not be able to accurately predict the inflammatory condition of the disease, as these negative results must be related to the variation of serum aminotransferases during MASH[33].
Although there have been conflicting results, studies examining the impact of H. pylori eradication on MASH may offer stronger proof that there is, in fact, a link between them. This is consistent with a recent meta-analysis that demonstrated a positive association between H. pylori infection and fatty liver disease with an improvement in the markers of MASLD as well as the MASLD fibrosis score following eradication of Pylori[35,37].
One of the drawbacks of our study was the inclusion of subjects aged 18 years and above. Therefore, using elder subject group alone would be beneficial and could lessen the influence of age on the results, since young subjects are less likely to develop MASLD.
Moreover, our study relied on different methods for the detection of H. pylori infection. Some used the ELISA test which frequently overestimates the number of positive individuals. Its reliability is lower than other diagnostic tools used in other patients such as histology or rapid urease test or urea breath test.
In addition, there was no uniform standard used for diagnosis of MASH in these patients. Some were diagnosed using ultrasonography which has inevitable limitations and may provide an improper diagnosis. Others were assessed using Fibroscan, and others underwent liver biopsy which is the gold standard for evaluation of MASH.
However, our study has several strengths worth noting. This case-control study is a large representative sample from the United States general population. It covered patients from 360 hospitals across the nation for a considerable amount of time-roughly 23 years. The individuals included were from different socioeconomic backgrounds and ethnic/racial groups, and thus, the results are generalizable. The large number of patients with H. pylori infection and MASH has provided high statistical power to assess the magnitude of the relationship between these two common conditions. Additionally, analyses were performed after adjusting potential confounding variables, which increased the validity of our findings and made our results more credible.
H. pylori infection is implicated in several systemic disorders. Based on clinical investigations demonstrating higher rates of MASH in H. pylori positive patients in our study, a link between them has recently been suggested, although it is worth noting that there have been conflicting results in other studies.
Although the pathophysiology underlying this association is still not fully understood, potential mechanisms involve a systemic pro-inflammatory state, which favors a lipogenic profile, as well as an effect on hormonal levels that affects fibrosis and lead to insulin resistance. An implication in common gene pathways was also found. Furthermore, the elimination of H. pylori has demonstrated decreases in markers of MASLD activity as well as liver fat scores, supporting the evidence for this association.
To our knowledge, this is the first and most recent case-control study integrating large sample size population to explore possible association between H. pylori and MASH as previous studies exploring this association have been constrained by several significant limitations, including smaller and regionally restricted sample sizes. Our study could offer new insights into the current field of H. pylori-MASH correlation study and if confirmed, treating H. pylori might be a brand-new, specific therapeutic strategy for MASH. Although our current study does not delve into genetic analyses, future research should aim to explore specific genetic markers, such as single nucleotide polymorphisms or other genomic variations, that could modulate the relationship between H. pylori infection and MASH. Understanding these genetic underpinnings could provide crucial insights into the mechanisms driving this association, particularly in pathways related to inflammation, insulin resistance, and lipid metabolism. This would also complement our findings on hormonal influences and gene enrichment pathways implicated in the progression of MASH.
| 1. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1940] [Article Influence: 388.0] [Reference Citation Analysis (33)] |
| 2. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 727] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 3. | Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M. Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther. 2021;15:3997-4009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7934] [Article Influence: 793.4] [Reference Citation Analysis (8)] |
| 5. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 6. | Fernando DH, Forbes JM, Angus PW, Herath CB. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Kelly DJ. The physiology and metabolism of the human gastric pathogen Helicobacter pylori. Adv Microb Physiol. 1998;40:137-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53:33-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 9. | Franceschi F, Zuccalà G, Roccarina D, Gasbarrini A. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol. 2014;11:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Doulberis M, Srivastava S, Polyzos SA, Kountouras J, Papaefthymiou A, Klukowska-Rötzler J, Blank A, Exadaktylos AK, Srivastava DS. Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Cheng DD, He C, Ai HH, Huang Y, Lu NH. The Possible Role of Helicobacter pylori Infection in Non-alcoholic Fatty Liver Disease. Front Microbiol. 2017;8:743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Heydari K, Yousefi M, Alizadeh-Navaei R, Lotfi P, Sheydaee F, Raei M, Vahdatinia A, Hessami A, Rafati S, Moosazadeh M, Ghasemian R, Salehi F, Massoudi H, Ghaffari-Saravi F, Rismantab S. Helicobacter pylori Infection and Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Turk J Gastroenterol. 2022;33:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wernly S, Wernly B, Semmler G, Völkerer A, Rezar R, Semmler L, Stickel F, Aigner E, Niederseer D, Datz C. Non-alcoholic fatty liver disease is not independently associated with Helicobacter pylori in a central European screening cohort. Minerva Med. 2022;113:936-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Okushin K, Takahashi Y, Yamamichi N, Shimamoto T, Enooku K, Fujinaga H, Tsutsumi T, Shintani Y, Sakaguchi Y, Ono S, Kodashima S, Fujishiro M, Moriya K, Yotsuyanagi H, Mitsushima T, Koike K. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. 2015;15:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Wang W, Fan M, Gong R, Zhang Y, Zeng J, Xu S, Lin R. Helicobacter pylori infection is not an independent risk factor of non-alcoholic fatty liver disease in China. BMC Gastroenterol. 2022;22:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | IBM Corporation. The IBM Explorys platform: liberate your healthcare data. 2024 [cited 24 April 2024]. Available from: https://www.ibm.com/case-studies/smartanalyst-dermatology/. |
| 17. | United States National Library of Medicine. SNOMED CT. Sep 18, 2019 [cited 24 April 2024] Available from: https://www.nlm.nih.gov/healthit/snomedct/index.html. |
| 18. | Gaudet-Blavignac C, Foufi V, Bjelogrlic M, Lovis C. Use of the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) for Processing Free Text in Health Care: Systematic Scoping Review. J Med Internet Res. 2021;23:e24594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Alkhayyat M, Saleh MA, Coronado W, Abureesh M, Zmaili M, Qapaja T, Almomani A, Khoudari G, Mansoor E, Cooper G. Epidemiology of neuroendocrine tumors of the appendix in the USA: a population-based national study (2014-2019). Ann Gastroenterol. 2021;34:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Alkhayyat M, Qapaja T, Aggarwal M, Almomani A, Abureesh M, Al-Otoom O, Zmaili M, Mansoor E, Abou Saleh M. Epidemiology and risk of psychiatric disorders among patients with celiac disease: A population-based national study. J Gastroenterol Hepatol. 2021;36:2165-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Kubihal S, Gupta Y, Shalimar, Kandasamy D, Goyal A, Kalaivani M, Goyal A, Kedia S, Kachhawa G, Ambekar S, Bhatia D, Garg V, Gupta N, Tandon N. Prevalence of non-alcoholic fatty liver disease and factors associated with it in Indian women with a history of gestational diabetes mellitus. J Diabetes Investig. 2021;12:877-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 22. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1463] [Article Influence: 365.8] [Reference Citation Analysis (1)] |
| 23. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1375] [Article Influence: 196.4] [Reference Citation Analysis (0)] |
| 24. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1083] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 25. | Salama RI, Emara MH, Mostafa HM, Abd-Elsalam S, Alnabawy SM, Elshweikh SA, Zaghloul MS. Helicobacter pylori infection and risk of salmonella infection. Medicine (Baltimore). 2019;98:e14335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Khoshbaten M, Baghaei K, Bafandeh Y, Saeidi GR, Gachkar L, Al Dulaimi D, Lamouki RM, Rostami Nejad M, Bonyadi MR. The role of Helicobacter pylori and CagA in response to treatment in Iranian Gastroesophageal Reflux Diseases patients. Gastroenterol Hepatol Bed Bench. 2013;6:S93-S98. [PubMed] |
| 27. | Santos MLC, de Brito BB, da Silva FAF, Sampaio MM, Marques HS, Oliveira E Silva N, de Magalhães Queiroz DM, de Melo FF. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol. 2020;26:4076-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 28. | Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. 2017;42:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 29. | Sumida Y, Kanemasa K, Imai S, Mori K, Tanaka S, Shimokobe H, Kitamura Y, Fukumoto K, Kakutani A, Ohno T, Taketani H, Seko Y, Ishiba H, Hara T, Okajima A, Yamaguchi K, Moriguchi M, Mitsuyoshi H, Yasui K, Minami M, Itoh Y. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J Gastroenterol. 2015;50:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Abo-Amer YE, Sabal A, Ahmed R, Hasan NFE, Refaie R, Mostafa SM, Mohamed AA, Khalil M, Elagawy W, Abd-Elsalam S. Relationship Between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease (NAFLD) in a Developing Country: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020;13:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Liu R, Liu Q, He Y, Shi W, Xu Q, Yuan Q, Lin Q, Li B, Ye L, Min Y, Zhu P, Shao Y. Association between Helicobacter pylori infection and nonalcoholic fatty liver: A meta-analysis. Medicine (Baltimore). 2019;98:e17781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Manatsathit W, Jaruvongvanich V, Ungprasert P. Helicobacter pylori and Risk of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018;52:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Alvarez CS, Florio AA, Butt J, Rivera-Andrade A, Kroker-Lobos MF, Waterboer T, Camargo MC, Freedman ND, Graubard BI, Lazo M, Guallar E, Groopman JD, Ramírez-Zea M, McGlynn KA. Associations between Helicobacter pylori with nonalcoholic fatty liver disease and other metabolic conditions in Guatemala. Helicobacter. 2020;25:e12756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter Pylori CagA and VacA Modulate Host Pathways that Impact Disease. Front Microbiol. 2010;1:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Jamali A, Karbalai S, Tefagh G, Jamali R, Ahmadi A. The Effects of Helicobacter Pylori Eradication on Liver Function and Metabolic Profile in Non-diabetic Non-alcoholic Steatohepatitis: A 5-year Randomized Clinical Trial. Middle East J Dig Dis. 2022;14:85-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Kang SJ, Kim HJ, Kim D, Ahmed A. Association between cagA negative Helicobacter pylori status and nonalcoholic fatty liver disease among adults in the United States. PLoS One. 2018;13:e0202325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Mantovani A, Turino T, Altomari A, Lonardo A, Zoppini G, Valenti L, Tilg H, Byrne CD, Targher G. Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: An updated meta-analysis. Metabolism. 2019;96:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr. 2011;53:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 39. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 734] [Article Influence: 52.4] [Reference Citation Analysis (1)] |
| 40. | Okushin K, Tsutsumi T, Ikeuchi K, Kado A, Enooku K, Fujinaga H, Moriya K, Yotsuyanagi H, Koike K. Helicobacter pylori infection and liver diseases: Epidemiology and insights into pathogenesis. World J Gastroenterol. 2018;24:3617-3625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Baeg MK, Yoon SK, Ko SH, Noh YS, Lee IS, Choi MG. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:2592-2600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Front Microbiol. 2018;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/