Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1132
Revised: August 24, 2024
Accepted: September 20, 2024
Published online: October 27, 2024
Processing time: 117 Days and 22.1 Hours
Hepatocellular carcinoma (HCC) is a disease of public health concern in Nigeria, with chronic hepatitis B and C infections contributing most to the disease burden. Despite the increasing incidence of HCC, surveillance practices for early diagnosis and possible cure are not deeply rooted in the country. This article aims to review the current status of HCC surveillance in Nigeria, stressing the encounters, breaches, and potential prospects. Several factors, such as limited tools for screening and diagnostics, insufficient infrastructure, and low cognizance among the doctors, and the general public affect the surveillance practices for HCC in Nigeria. Moreover, the lack of standardized guidelines and protocols for HCC surveillance further intensifies the suboptimal diagnosis and treatment. Nevertheless, there are opportunities for refining surveillance practices in the country. This would be achieved through boosted public health sensitization campaigns, integrating HCC screening into routine clinical services, and leveraging technological developments for early detection and monitoring. Furthermore, collaboration between government agencies, healthcare providers, and international organizations can facilitate the development of comprehensive HCC surveillance programs personalized to the Nigerian setting. Thus, HCC surveillance practice faces substantial challenges. By addressing the drawbacks and leveraging prospects, Nigeria can improve HCC surveillance, with subsequent improved outcomes for individuals at risk of developing the disease.
Core Tip: Hepatocellular cancer (HCC) is the 6th most common cancer worldwide and the 5th most common cause of global cancer-related mortality. However, in Nigeria and other sub-Saharan African countries, most HCC patients present late with high mortality. Therefore, the best way to deal with such a situation is prevention and surveillance for early detection of cases when a cure is achievable. This editorial aims to shed light on current HCC surveillance practices, and challenges in Nigeria and propose strategies for improvement.
- Citation: Musa Y, Ifeorah IM, Maiyaki AS, Almustapha RM, Maisuna YA, Saleh HT, Yakubu A. Liver cell cancer surveillance practice in Nigeria: Pitfalls and future prospects. World J Hepatol 2024; 16(10): 1132-1141
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1132.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1132
Hepatocellular cancer (HCC) constitutes over 75% of primary liver cell cancers which are malignancies originating from the primary histological cells of the liver[1,2]. It is the 6th most common cause of global cancer morbidity and the 3rd most common cause of global cancer-related mortality after lung and colorectal cancers according to 2022 GLOBOCAN statistics with an annual incidence of 866136 and mortality of 758725[2,3]. HCC mostly affects regions known to be endemic for viral hepatitis especially South-Eastern Asia, and sub-Saharan Africa (SSA)[3].
In the Eastern and Southeast Asian sub-continent, there was a progressive decline in hepatitis B and C due to various control measures with significantly reduced consumption of aflatoxin. This reduced HCC endemicity in those areas. Such achievements were imposed through campaigns on immunization, and other preventive measures. Conversely, some low-prevalence countries in Asia, Europe, Australia, and North America are beginning to show a progressive increase in HCC cases. This development is mostly attributable to the emergence of obesity-related fatty liver disease, alcohol consumption, and other metabolic disorders associated with liver disease[3,4].
In Africa, HCC is ranked as the 4th most common cancer and the second most common cause of cancer-related mortality[4]. Similarly, it is ranked the second and third most common malignancy in men and women, respectively, in SSA[5]. The endemic nature of HCC in SSA is mainly linked to hyperendemicity in the region for hepatitis B and C virus infection which accounts for about half the etiological factors for this disease[3,4]. Variability exists between different parts of Africa concerning HCC etiology with hepatitis C predominantly seen in North Africa and hepatitis B in SSA[3,4]. Other etiological factors are also implicated in HCC development in the continent including emerging metabolic diseases associated with fatty liver, and alcoholic liver diseases, food contamination with fungal aflatoxins, diabetes mellitus, and smoking[4].
The average overall survival of HCC patients after diagnosis in SSA is only 3 months[4]. The endemic nature of HCC and its consequent case fatality record in SSA pose a serious public health concern. Thus, it is imperative to explore all possible avenues to curtail this disease[2].
The Nigerian context of HCC is similar to most SSA countries with relatively higher cases of 11-20 per 100000 population[6,7]. According to the GLOBOCAN registry, the total incidence and mortality of HCC in Nigeria were 4382 and 4252, respectively, making it the 6th commonest malignancy and 5th commonest cause of cancer-related mortality[8]. HCC is reported to be the 3rd most common malignancy among the Nigerian male population with a male-to-female incidence ratio of > 3.5:1[1,8]. Hepatitis B virus (HBV) constitutes close to 60% of HCC etiology in Nigeria[6,7]. Other etiological factors such as hepatitis C virus (HCV), alcohol, and aflatoxin were found to play some role in the pathogenesis of HCC among Nigerians. However, conditions such as fatty liver disease and alpha 1 antitrypsin deficiency are not well established among Nigerian cohorts with HCC. Moreover, studies have documented a 20-fold homozygous alpha 1 antitrypsin deficiency among HCC cohorts[6,7]. Late presentation (Barcelona stage D; a state mainly treated with hospice care) is often the case among HCC patients in Nigeria with few individuals presenting in the B or C stage which may benefit from available interventional radiologic or other chemotherapeutic treatments[6,7].
Risk factors for HCC include both infectious and non-infectious agents. These agents include chronic infection with HBV, HCV, hepatitis delta virus (HDV), and Human immunodeficiency viruses (HIV). Nigeria is hyperendemic for HBV with resultant HBV-associated morbidity and mortality. Factors such as poor preventive measures for HBV among pregnant women, and vaccine uptake drive childhood acquisition of HBV with a high propensity to chronicity and consequently HCC. Other factors such as co-infection with HIV, HCV, and certain HBV genotypes especially sub-types A1, C, and D are more associated with early HCC development in Nigeria before the age of 45[5,9–11].
Non-infectious etiological factors for HCC include aflatoxin exposure, alcohol usage, iron overload (African Dietary Iron Overload and Hemochromatosis), diabetes mellitus, and metabolic dysfunction-associated steatotic liver disease (MASLD)[5,9–11]. Aflatoxin B1 is a class 1 carcinogen and exerts a significant role in the pathogenesis of HCC[9]. Due to climatic conditions in SSA, aflatoxin-producing fungus thrives well in the region[9]. In Nigeria, significant concentrations of aflatoxin in foods such as nuts, grains, rice, sorghum, peanuts, cow milk, and locally brewed alcohol have been documented[5,9,10,12]. Due to the global pandemic of obesity, there is a resultant rise in obesity-associated co-morbidities such as MASLD. MASLD has assumed a significant role in the etiology of HCC in Nigeria due to changing dietary culture, and sedentary lifestyles[5,9,10,12].
The origin of cancer cells in HCC still is not well understood. There is the interplay of primary liver cells such as hepatocytes, lymphocytes, Kupffer cells, activated stellate cells, and endothelial cells. This results in complex T-cell-mediated immunological interaction leading to carcinogenesis[12–14]. HCC mainly develops via multiple pathways comprising inflammation-fibrosis-cirrhosis-carcinoma. Development of HCC follows pathways that may or may not be related to etiological agents[5,12,15,16].
Etiological factors such as HBV, HCV, HDV, HIV, aflatoxin, iron overload, MASLD, and alcohol among others are found to have direct relationships with HCC development. HBV and HDV cause mutation of many oncogenes following DNA integration, regulatory protein accumulations, epigenetic transformation, and alteration in some chromosomes. There is an alteration in cell growth expression via genes regulating transcriptional activation and induction of oxidative stress[15,17,18]. Together with aflatoxin it may result in genomic instability, deactivation of p53, progenitor cell gene overexpression, and activation of signaling pathways responsible for oncogenesis[5,9,12]. Due to impaired T-cell response in HIV, the presence of HBV or HCV would progress unchecked causing activation of hepatic stellate cells, and Kupffer cells leading to fibrosis with consequent accelerated cirrhosis and HCC development. Similarly, direct transformation to HCC without passing through cirrhosis was also reported especially among those with low CD4+ cells and high viremia[5,9,12,15].
The non-etiologically related pathways are reported in cases of chronic alcohol use leading to increased hepatocyte sensitivity with stellate cell activation, fibrogenesis, cirrhosis, and consequent malignant transformation[5,9,12,15]. Carcinogenesis in MASLD may result directly without cirrhosis or through steatohepatitis, fibrosis, and cirrhosis with malignant transformation. Carcinogenic processes include metabolic/endocrine, immune, and genetic alteration mechanisms. It occurs via activated nuclear factor-kappa B, tumor necrosis factor, and micro RNAs[5,12,15].
HCC carcinogenesis is genetically categorized into 2 subtypes; The HBV related type, with alpha-fetoprotein overproduction, P53 mutation, chromosomal instability, PI3K-AKT-mTOR, RAS-MAPK, and mesenchymal epithelial transition factor (MET) alterations. This is the ‘’Proliferative type’’ with a poor prognosis. Conversely, another sub-type called non-proliferative HCC follows a benign course, mostly seen among patients with alcoholic liver disease, and chronic HCV. Mutations identified are that of the TERT promoter gene and CTNNB1 activation[12–14].
Early cases of HCC are mostly seen in developed countries of Western Europe, and North America where active surveillance is fully in place. However, in developing high-burden countries advanced disease is the most common presentation[19]. Some of the advanced features include the frequently encountered right upper abdominal pain and swellings or feeling of dragging sensation in the same region, significant weight loss, and occasional jaundice. Some cases present with generalized abdominal distension, yellowish discoloration of the eye and urine, and other features of hepatic decompensation such as hematemesis, melena, encephalopathy, and coagulopathy[2]. Clinically, cachexia is usually evident in most patients with advanced disease, stigmata of liver cirrhosis may also be found. Other clinical findings are a hard, craggy, nodular liver mass on abdominal examination with or without bruit. Diagnostic paracentesis may reveal bloody aspirate in some cases[2,19].
HCC diagnosis is achieved with characteristic imaging features of a tumor nodule of ≥ 1 cm, plus clinical and biochemical surrogate markers in the presence of liver cirrhosis. However, in the case of suspected HCC de-novo, a histo-pathological confirmation may be needed based on international consensus guidelines[20].
Due to the arterial nature of HCC cells’ blood supply, contrast-enhanced triphasic computed tomographic (CT) scans, contrast-enhanced magnetic resonance imaging (MRI), or contrast-enhanced ultrasound scan are used for HCC diagnosis, thereby giving the characteristic contrast uptake and washout during arterial and venous phases, respectively[20]. The characteristic enhancement and washout pattern during the respective phases confirm HCC in the presence of background liver cirrhosis. The presence or absence of possible metastasis to a portal vein in the form of thrombosis or other contiguous organs such as the lungs and biliary tract can also be assessed through imaging[6,7,20,21].
Liver biopsy is considered when imaging modalities are inconclusive or when dealing with small nodules < 2 cm[7,20]. However, the risk of metastasis via the needle tract is often encountered in a small percentage, but narrow-gauge needles eliminate such risks[7,20]. The biopsy would further establish the actual etiological condition causing the HCC, background presence or absence of cirrhosis, and evidence of metastatic deposit of other tumors to the liver. The histologic characterization into trabecular, pseudo acinar, scirrhous, or fibrolamellar sub-type could also be assessed from the biopsy specimen. Similarly, the cytological grading can be categorized into poorly, moderate, or well-differentiated HCC. Special stains for glypican-3, heat shock protein 70, and glutamine synthetase may be necessary to confirm HCC[6,7,22].
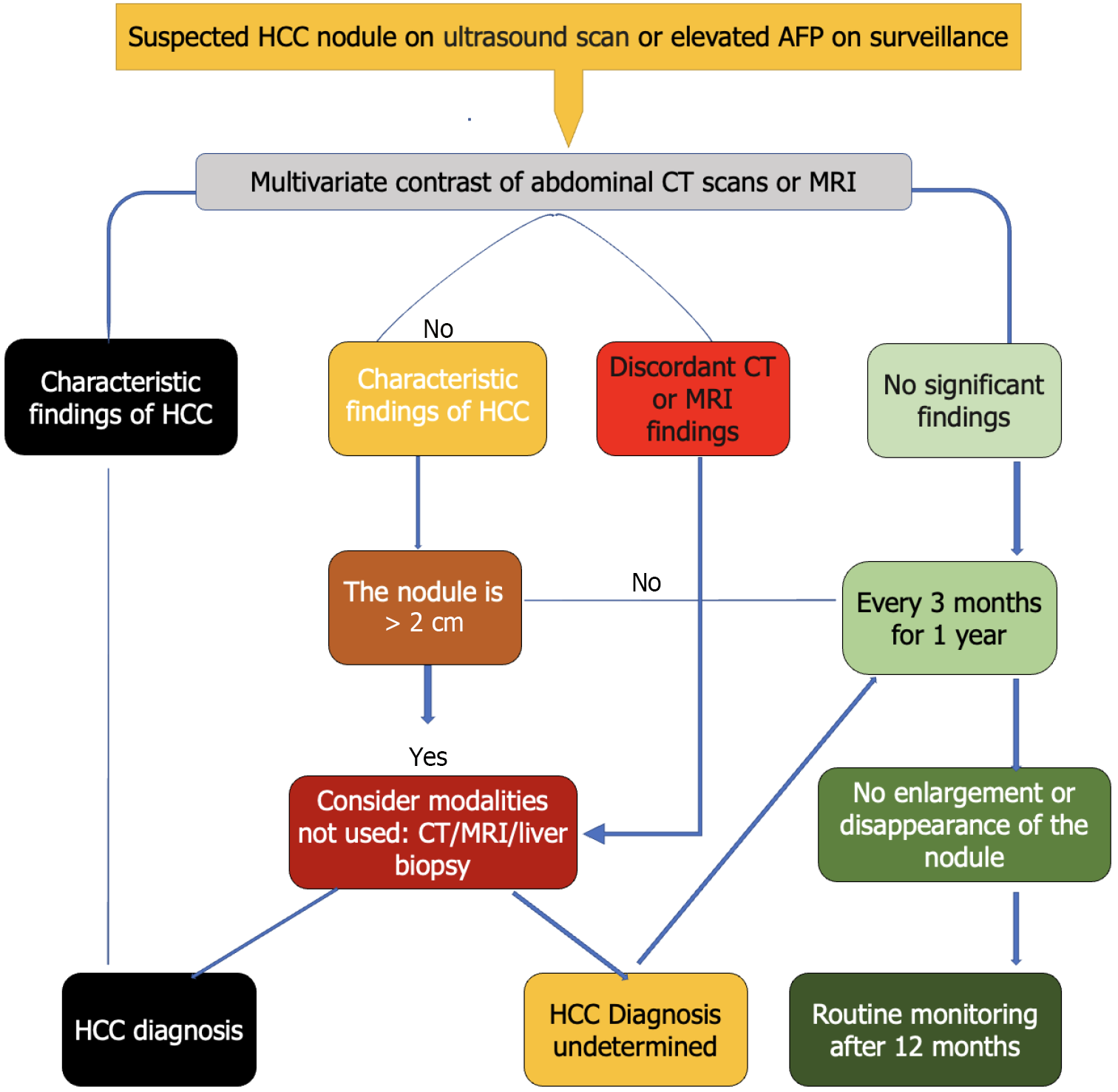
Fine-needle aspiration cytology may be employed in advanced disease, especially in SSA where most patients present with huge fungating tumor masses. Similarly, ascitic fluid cytology may also play a role in HCC diagnosis. Figure 1 shows surveillance and diagnosis algorithms for suspected HCC among at-risk patients[6,7,22].
Various staging systems are used to classify HCC for treatment purposes and prognosis. Therefore, multiple factors such as symptoms, tumor size, metastasis, performance status, and Child-Pugh classification of the background liver disease are considered when choosing an ideal staging system. Such a system needs to consider a possible method of assessing the degree of severity and overall survival before and after interventions.
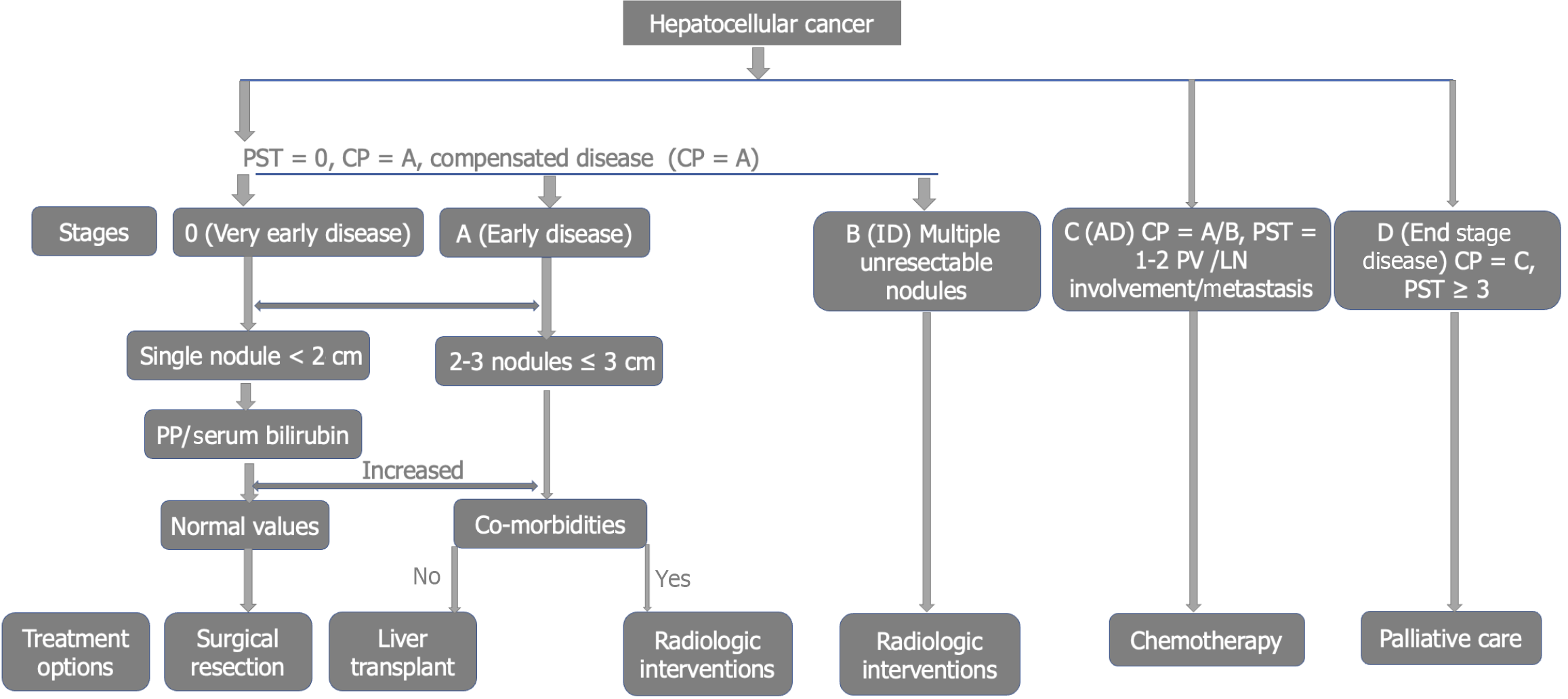
The Barcelona Clinic Liver Cancer (BCLC) classification is a staging system adopted by the Nigerian Society of Gastroenterology and Hepatology (SOGHIN) and the European Association for the Study of the Liver (EASL). This is because it encompasses the tumor size, liver symptoms, performance status, treatment, and other prognostic indicators. It includes 5 stages, from 0 to stage D as shown in Figure 2[6,7,20].
Another system is TNM staging which assesses tumor size (T), Lymph node status (N), and Metastasis (M). However, assessment of actual tumor size, symptoms of liver decompensation, performance status, and prognostic indicators are not captured in this system. Other staging systems include the Chinese University Prognostic Index, Japan Integrated Staging, Hong Kong Liver Cancer Classification, and Cancer of the Liver Italian Program classification as shown in Figure 2[6,7,20].
The treatment strategy in HCC is mainly determined by the clinical stage using the BCLC staging system. Very early disease (stage 0) can be treated by tumor ablative therapy or surgical resection[4,6,7,20]. Early disease (stage A) can be considered for tumor ablation, surgical resection, or orthotropic liver transplant[4,6,7,20]. Intermediate HCC (stage B) is usually managed using interventional radiologic methods such as trans-arterial chemoembolization, radiofrequency ablation, and alcohol injection. This stage can be down-staged following radiologic intervention and be considered for surgery later[4,6,7,20]. Advanced stage (stage C) HCC is usually limited to systemic chemotherapy with various agents. Drugs such as tyrosine kinase inhibitor (Sorafenib), and multi-kinase inhibitor (Regorafenib) are used. Lenvatinib is a combined inhibitor of vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet-derived growth factor. Cabozantinib inhibits many targets including VEGF, MET, and Receptor Tyrosine Kinase/growth factor signaling (RET). Immunotherapy combines Atezolizumab and Bevacizumab, a newer promising treatment option in HCC management[4,6,7,20,23]. End-stage (stage D) HCC is mainly treated using palliative care to improve quality of life and alleviate symptoms[4,6,7,20].
In Nigeria and other SSA countries, HCC is predominantly caused by chronic HBV infection. Thus, prevention of HCC begins with HBV/HCV control and treatment. This includes universal screening and vaccination of all subjects in endemic regions such as Nigeria and other SSA countries. More importantly, prevention of mother-to-child transmission among HBV-positive mothers is another preventive strategy imposed in hyperendemic regions like Nigeria to halt the spread of HBV perinatally. This involves the use of zero-dose vaccine, HBV immunoglobulin at birth, and the incorporation of HBV vaccine in the expanded program on immunization as advocated by the World Health Organization (WHO). According to the 2024 WHO report on HBV vaccination coverage, the African continent is still behind the global target with zero and three doses of HBV vaccination among under 5 years of age of 17% and 74%, respectively, in 2023[24]. However, in Nigeria, WHO reported HBV vaccination coverage of 52% and 62% for zero and three doses among children under 5 years of age, respectively, for 2023[24]. These figures are below the global target set by WHO for HBV elimination by 2030[25]. Thus, any child, adolescent, and young adult Nigerian identified to be HBV negative during pre-school or employment screening is advised to get vaccinated. Those found to be HBV positive are directed to see a doctor for appropriate HBV care enrollment. Similarly, visitors to Nigeria and other endemic countries as well as health workers, and intravenous drug users should be vaccinated[20].
Prompt diagnosis of HBV and HCV with thorough evaluation and treatment of eligible patients prevents the consequences of developing advanced fibrosis, cirrhosis, and ultimate development of HCC. Among the oral and injectable agents used for HBV and HCV therapy, only Pegylated Interferon, Tenofovir Disoproxil Fumarate, and Entecavir were shown to change the course of HCC development among chronic HBV and HCV patients. Moreover, the introduction of direct-acting anti-viral agents revolutionizes the treatment of HCV with better outcomes and an overall decrease in HCC occurrence. However, some studies reported the persistence of HCC risk despite HCV cure[20].
Another HCC preventive measure among at-risk individuals is daily coffee consumption, a finding that has been proven beneficial among Asians, Europeans, and Americans. However, the actual protective dose is yet to be elucidated[20].
HCC is a preventable disease with a poor prognosis and high mortality especially when presented late. The importance of implementing a strong preventive strategy cannot be overstated. This may be the only benefit, as late tumors cannot be completely cured and are associated with poorer outcomes[7,20]. The preventive strategy is designed to regularly screen the targeted population at risk, facilitating the early detection of tumors for potential curative intervention. HCC surveillance utilizes available and affordable investigations at 6-month intervals. Such investigations include abdominal ultrasonography, serum alpha-fetoprotein, and des-gamma carboxy prothrombin (DCP). These investigations can aid early HCC detection, and treatment would be promptly instituted thereby halting the danger associated with advanced disease. Thus, the associated morbidity and mortality could be prevented among the populace. The only proven beneficial time interval for the HCC surveillance program is 6 monthly as long as the risk exists. It has been shown to curb mortality, especially among those without liver cirrhosis[7,20]. Thus, HCC surveillance is an independent determinant of survival among patients at risk. The choice of surveillance time interval is solely based on the presumed doubling time of the cancer cells estimated to be 6 months[10]. Some authorities proposed a shorter time to factor in such issues. However, the survival benefit of the shorter interval is yet to be proven[6,7,10,20,21].
Individuals at risk of developing HCC include any patient with hepatic cirrhosis irrespective of etiology, Africans aged 20 years and above with chronic HBV infection and evidence of viremia of ≥ 10000 copies/mL, and positive HBeAg, evidence of active HCV infection, history of HCC in the family members, chronic alcoholics, established diagnosis of hemochromatosis or primary biliary cholangitis, and evidence of > 20 ng/mL of serum alpha-fetoprotein (AFP)[6,7,10,20,21].
This method is user-friendly and easily accessible for the HCC surveillance program. It has received endorsement from SOGHIN, EASL, the American Association for the Study of Liver Diseases(AASLD), and the Asian-Pacific Association for the Study of the Liver(APASL). This is due to its non-invasiveness, availability, and safety on repeated exposure. It has a pooled sensitivity of 58%-89% in detecting any stage of HCC and a specificity of over 90%[7,20]. Liver ultrasound scans are readily available in Nigerian urban communities[6,7,10,20,21,26].
Challenges: Low sensitivity for early cancers (an average of 47%) especially in the presence of cirrhosis, it is operator dependent, and precision is attenuated in obese individuals. Moreover, the scarcity of radiologists to make the appropriate diagnosis by prompt early detection of cancer nodules may be a major setback in many semi-urban communities in Nigeria[6,7,10,20,21,26].
These challenges may be overcome with the use of contrast-enhanced CT scans and MRI for screening purposes. However, cost, availability, affordability especially in Nigeria, and radiation risk to the patient with the associated lack of superiority of such imaging modalities make ultrasonography the novel imaging modality for HCC surveillance[6,7,10,20,21].
Alpha fetoprotein is the most widely researched serum tumor marker linked to HCC. Its utilization is further reinforced when combined with other screening tools. It is a complex protein produced during embryogenesis by fetal hepatocytes yolk sac. However, the concentration regresses post-delivery, making an acceptable serum concentration at about 10 ng/mL of blood. It has been observed by some authorities that the sensitivity of AFP was not strong enough for routine surveillance; thus, lectin-bound percentage of AFP (AFP L3%) is used[20]. There was an incorporation of DCP as well. For HCC detection, AFP has a sensitivity and specificity of 58% to 62% and 84%, respectively, when the cutoff of ≥ 20 ng/mL was used. However, the sensitivity dropped to 36% while specificity rose to 99% when a cutoff value of ≥ 200 ng/mL was used[27]. However, values of 400 to 1000 ng/mL are considered diagnostic of HCC in most instances. Similarly, a rising value may be the only indicator of malignancy[6,7,10,21].
Challenges: Tumors of the pancreas, ovary, testes, other germ cells, neural tube defects, ataxia telangiectasia, and gestational trophoblastic diseases are all associated with elevated AFP[28]. Similarly, some cases of acute hepatic inflammation, fibrosis from chronic hepatitis, and liver cirrhosis may be associated with elevated AFP. The non-proliferative HCC subtypes that constitute about a quarter of cases liberate no AFP, hence, this may be difficult to detect. In Nigeria, the AFP assay is not easily accessible in many semi-urban communities[6,7,10,21].
This is a defective prothrombin generated from malignant hepatocytes carboxylation due to vitamin K deficiency in such abnormal cells. It is more peculiar to HCC, and increment is proportionate to tumor development. An amount of 40 mAU/mL is considered high, while a value of > 150 mAU/mL may be diagnostic of HCC. DCP is more specific than AFP in detecting HCC once the tumor is > 3 cm in size. At 140 mAU/mL, DCP was found to have higher sensitivity and specificity of 98.5% in Nigerian HCC cohorts[6,7,10,21].
Challenges: DCP is mostly seen in relatively larger tumors > 3 cm, thus, detection of very early tumors is a problem. Similarly, DCP is not easily accessible in numerous secondary health centers across Nigeria. This may bring about a significant challenge to the overall goal of the surveillance program[6,7,10,21].
By employing serological and imaging techniques, the sensitivity of HCC detection during surveillance increased to approximately 84%. The AFP was monitored serially for possible rising levels to rule out other causes. Thus, a 6-monthly practice of combined 2-dimensional sonography of the abdomen by an expert with AFP was adopted as one of the most cost-effective methods of HCC surveillance among the population at risk[6,7,10,21].
Many drawbacks were identified regarding the routine surveillance program, especially in developing countries like Nigeria. These include; poor acceptability by most clients, cost of procedures, incorrect results and their associated consequences, increased HCC diagnostic threshold, recurrent radiation exposure, contrast nephropathy, claustrophobia, risk of invasive procedures such as liver biopsy in highly suspicious lesions with associated false negative histology, bleeding from biopsy site, and false inconclusive results could also be a factor. Similarly, an early tumor may metastasize during biopsy. However, the risk associated with abandoning the regular surveillance program could be very serious when HCC develops in such individuals[10].
Despite numerous recommendations from various international regulatory bodies in hepatology, HCC surveillance has remained suboptimal in many regions. Only about a quarter of patients with liver cirrhosis were reported to be enrolled in this program in developed economies[6,7,10].
A nationwide survey was conducted among physicians practicing in all the geo-political zones of Nigeria. The respective percentage distributions of participants were 24.07% for North-Central, 23.15% for South-South, 18.06 for North-West, 17.13 for South-West, 11.11 for South-East, and 6.48 for the North-Eastern part of Nigeria. It revealed that approximately 74% of the doctors from various fields of medicine were managing hepatitis B, with 2/3 of them managing HCV. Gastroenterologists were managing about 29%, while Family Physicians were managing over 15%[29].
Most doctors utilized abdominal sonography, serum AFP, and clinical features to diagnose HCC. However, despite the late presentation in most instances, over 40% of contacted doctors do not practice any surveillance for HCC among the at-risk patients. Only 25% of the participants offer routine HCC surveillance services among at-risk patients. Moreover, most of the surveillance tests conducted were abdominal sonography and AFP. The low surveillance percentage may be related to the number of Gastroenterologists among the participants[29].
The main reason for the low enrollment into the HCC surveillance program by most doctors was poor awareness of any existing protocol. Other reasons for low HCC surveillance among doctors were the cost of services, and non-acceptability due to cultural or religious beliefs, especially in the Northern part of the country. Some doctors have very tight schedules, while others do not have reliable means of conducting such basic investigations. Others do not have a clear idea regarding the current status of the patients, while some do not believe in the overall process[29].
Another factor was the lack of universal coverage for the National Health Insurance program. Similarly, most patients have routine clinic follow-ups with family physicians, with gastroenterologists having less than 30% of the respondents. Some of the patients were lost to follow-up during the surveillance program. Another factor was the ignorance of some doctors regarding the HCC surveillance program, especially the general practitioners among the participating doctors. Some doctors report logistic issues as a barrier, especially transport to urban areas where facilities such as ultrasound scanning machines are available among others. However, the stated barriers can be eradicated with appropriate education, public awareness efforts, and advocacy directed at various stakeholders[10,29].
HCC surveillance in high-risk individuals is necessary for early detection to increase the chances of curative treatment and survival. Emerging blood-based biomarkers such as the Gender, Age, L3 fraction of AFP and DCP score, liquid biopsy techniques, and the rapidly developing Artificial Intelligence technology are considered and promoted in the developed world. These may greatly improve individualized HCC risk prediction and interpretation of imaging techniques[30]. The reliability and challenges of various HCC surveillance methods should be improved with enhanced sensitivity and specificity to provide a lasting solution for poor uptake. Given the current context, integrating internationally recognized surveillance methods tailored to Nigeria's unique healthcare landscape is paramount for addressing this challenge. Globally recognized guidelines advocate regular imaging and serum marker monitoring e.g. AFP testing. However, this practice is not fully consolidated in Nigeria due to poor funding and inconsistent policies. To pave the way for effective HCC surveillance in Nigeria, it is vital to adapt international best practices to the country’s specific contexts thereby integrating global best practices with Nigeria’s local realities[10,29]. Some biochemical methods such as DCP and AFP-L3 are promising. Others include tumor genome, a process of utilizing free circulating DNA liberated by the malignant cell. However, all these tests need to undergo rigorous checks through trials at various stages to prove their efficacy or otherwise[10,29].
Targeted screening: EASL and SOGHIN guidelines recommend regular surveillance for high-risk groups; chronic HBV, HCV, and those with cirrhosis, and should be employed all over Nigeria[7,10,20,29].
Community education: This is achieved through awareness campaigns in places of worship, social gatherings, and social media platforms on liver health and HCC risk factors in local languages that may improve understanding and adherence[7,10,20,29].
Use of information technology: With the advent of information technology globally, facilities such as telemedicine and mobile health technologies may play a significant role in easy access to quality healthcare, especially in rural communities of Nigeria where specialists are not available[7,10,20,29].
Healthcare infrastructural development: Standard healthcare infrastructures are essential in establishing specialized liver care using modern HCC diagnostic and surveillance tools[7,10,20,29].
Capacity building: The international and local societies of professionals such as EASL, AASLD, APASL, and SOGHIN can collaborate in training physicians at primary and secondary healthcare levels to bridge the gaps in quality healthcare[7,10,20,29].
Health policy development and advocacy for incorporating HCC surveillance and treatment access into the National Health Insurance Schemes can alleviate financial burdens associated with screenings and treatment of HCC in Nigeria[7,10,20,29].
Development of research and surveillance systems: Establishing a national liver disease registry in Nigeria can facilitate continuous monitoring, epidemiological research, and robust HCC surveillance[7,10,20,29].
Some of the existing barriers can be addressed via the following: Economic barriers can be tackled by implementing subsidized or low-cost screening initiatives for most patients that increase participation in HCC surveillance programs[7,10,20,29].
Cultural sensitivity: As Nigeria is a diverse country with over 200 tribes and many religions, understanding and integ
Continuous education and training for healthcare providers, including those in primary care settings, are necessary to facilitate early detection and proper referral of high-risk patients.
Additional measures to be adopted include establishing action networks that involve primary care doctors, the affected patients, and other healthcare professionals. Patient education should be prioritized through various channels, utilizing trained personnel, audiovisual materials, social media platforms, radio and television stations, and mobile applications. Similarly, the guidelines for HBV, HCV, alcoholic liver disease and MASLD should integrate the surveillance program as a routine line of care for every patient diagnosed with such conditions[7,10,20,29].
HCC is a serious health concern in Nigeria, with common etiologies being chronic infection with hepatitis B and C, as well as aflatoxin exposure from contaminated food. Patients often present late with HCC in Nigeria, with symptoms such as abdominal pain, weight loss, jaundice, and ascites. This results in poor prognosis with consequent limited treatment options. Despite the availability of many treatment options such as surgical resection, liver transplantation, and local ablative therapies in Nigeria, limited access to these interventions due to high cost and inadequate healthcare infrastructure is still an issue. Moreover, many physicians are not aware of these treatment options, and many patients lack access to specialized care centers where these treatments are available.
Furthermore, there is poor awareness of the infinite benefit of HCC surveillance among physicians in Nigeria for high-risk populations. This can lead to missed opportunities for early detection of HCC through regular monitoring and screening tests, such as imaging and biochemical tests. The lack of necessary infrastructure for surveillance is also a significant challenge. Limited access to imaging facilities, laboratory tests, and trained healthcare providers can impede efforts to implement effective surveillance programs for HCC in the country. As a result, many individuals at high risk for HCC may not receive timely screening and monitoring, leading to delays in diagnosis and treatment initiation.
The future of HCC surveillance in Nigeria requires a concerted effort to integrate global best practices with local contexts. A focus on risk stratification, community involvement, technological advancements, and enhancing healthcare infrastructure is vital. Furthermore, efforts should be made to increase awareness among healthcare providers, improve access to screening and treatment services, and strengthen healthcare systems to support effective HCC surveillance and management. By employing these strategies and addressing the barriers present, Nigeria can improve the early detection and management of HCC. Collaborative initiatives among healthcare stakeholders will be essential to ensure effective surveillance systems, ultimately reducing the burden of liver cancer across the nation.
| 1. | Manko M, Mohammed MF, Ahmed MS, Bello AK, Egbegbedia PO, Abdullahi U, Jamoh YB, Mustapha SK. Demographic Profile and Etiology of Hepatocellular Carcinoma in Zaria, Northern Nigeria. Niger Med J. 2022;63:282-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Ladep NG, Lesi OA, Mark P, Lemoine M, Onyekwere C, Afihene M, Crossey MM, Taylor-Robinson SD. Problem of hepatocellular carcinoma in West Africa. World J Hepatol. 2014;6:783-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12761] [Article Influence: 6380.5] [Reference Citation Analysis (8)] |
| 4. | Abou-Alfa GK, Afihene M, Capanu M, Li Y, Chou JF, Asombang A, Alatise OI, Bounedjar A, Cunha L, Mekonnen HD, Diop PS, Elwakil R, Ali MM, Ndlovu N, Ndumbalo J, Makondi PT, Tzeuton C, Biachi de Castria T, Agyei-Nkansah AA, Balogun F, Bougouma A, Atipo Ibara BI, Jonas E, Kimani S, Kingham P, Kurrimbukus R, Hammad N, Fouad M, El Baghdady N, Servais Albert Fiacre EB, Sewram V, Spearman CW, Yang JD, Roberts LR, Abdelaziz AO. Africa Guidelines for Hepatocellular Carcinoma Buildup Process. JCO Glob Oncol. 2023;9:e2300159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Okeke E, Davwar PM, Roberts L, Sartorius K, Spearman W, Malu A, Duguru M. Epidemiology of Liver Cancer in Africa: Current and Future Trends. Semin Liver Dis. 2020;40:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Ndububa D, Ojo O, Lesi O, Samaila A, Ngim O. SOGHIN Clinical Practice Guidelines for Management of Hepatocellular Carcinoma. Niger J Gastroenterol Hepatol. 2015;7. |
| 7. | Amojuoyi Ndububa D, Biade Abdulkareem F, Kagu Mustapha S, Ekwunife CN, Erhunmwun Omuemu C. SOGHIN clinical practice guidelines for hepatocellular carcinoma. Niger J Gastroenterol Hepatol. 2023;15:1-15. |
| 8. | World Health Organization. Data visualization tools for exploring the global cancer burden in 2022. Available from: https://gco.iarc.who.int/today. |
| 9. | Mak D, Kramvis A. Epidemiology and aetiology of hepatocellular carcinoma in Sub-Saharan Africa. Hepatoma Res. 2021;7:1-26. [DOI] [Full Text] |
| 10. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 852] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 11. | Malu AO, Ajayi AO, Okeke EN, Samaila AA, Ndububa DA UR. Guidelines for the Management of Chronic Hepatitis B and C. Nigeria. 2021. |
| 12. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1576] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 13. | Cucarull B, Tutusaus A, Rider P, Hernáez-Alsina T, Cuño C, García de Frutos P, Colell A, Marí M, Morales A. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 14. | Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Choi JH, Thung SN. Advances in Histological and Molecular Classification of Hepatocellular Carcinoma. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 16. | Kouroumalis E, Tsomidis I, Voumvouraki A. Pathogenesis of Hepatocellular Carcinoma: The Interplay of Apoptosis and Autophagy. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 762] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 18. | Jiang Y, Han Q, Zhao H, Zhang J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:435-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 19. | Kedar Mukthinuthalapati VVP, Sewram V, Ndlovu N, Kimani S, Abdelaziz AO, Chiao EY, Abou-Alfa GK. Hepatocellular Carcinoma in Sub-Saharan Africa. JCO Glob Oncol. 2021;7:756-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6423] [Article Influence: 802.9] [Reference Citation Analysis (9)] |
| 21. | Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Habib AG. Public health aspects of snakebite care in West Africa: perspectives from Nigeria. J Venom Anim Toxins Incl Trop Dis. 2013;19:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Ntellas P, Chau I. Updates on Systemic Therapy for Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2024;44:e430028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 24. | World Health Organization. Hepatitis B Vaccination Coverage. Available from: https://immunizationdata.who.int/global/wiise-detail-page/hepatitis-b-vaccination-coverage. |
| 25. | Freeland C, Kanu F, Mohammed Y, Nwokoro UU, Sandhu H, Ikwe H, Uba B, Asekun A, Akataobi C, Adewole A, Fadahunsi R, Wisdom M, Akudo OL, Ugbenyo G, Simple E, Waziri N, Vasumu JJ, Bahuli AU, Bashir SS, Isa A, Ugwu GO, Obi EI, Binta H, Bassey BO, Shuaib F, Bolu O, Tohme RA. Barriers and facilitators to hepatitis B birth dose vaccination: Perspectives from healthcare providers and pregnant women accessing antenatal care in Nigeria. PLOS Glob Public Health. 2023;3:e0001332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Hu H, Zhao Y, He C, Qian L, Huang P. Ultrasonography of Hepatocellular Carcinoma: From Diagnosis to Prognosis. J Clin Transl Hepatol. 2024;12:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Colli A, Nadarevic T, Miletic D, Giljaca V, Fraquelli M, Štimac D, Casazza G. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev. 2021;4:CD013346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Adigun OO, Yarrarapu SNS, Zubair M, Khetarpal S. Alpha-Fetoprotein Analysis. 2024 May 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 29. | Davwar PM, Ewelike ID, Owoseni O, Musa Y, Manko, M, Eboikpomwen, JO et al. The Practice of Hepatocellular Cancer Surveillance in Nigeria. Jos J Med. 2019;13:36-42. |
| 30. | Ahn JC, Lee YT, Agopian VG, Zhu Y, You S, Tseng HR, Yang JD. Hepatocellular carcinoma surveillance: current practice and future directions. Hepatoma Res. 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/