Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.707
Peer-review started: September 1, 2022
First decision: November 14, 2022
Revised: December 3, 2022
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 27, 2023
Processing time: 258 Days and 21.6 Hours
Giant hepatic cavernous hemangioma with multiple satellite nodules is a rare subtype of hepatic cavernous hemangioma, the most common vascular liver tumor. We report on a tumor with unusual histologic features: (1) Finger-like infiltration pattern; (2) lack of encapsulation; (3) blurred tumor/liver interface; and (4) massive satellitosis-referring to the article “Hepatic cavernous hemangioma: underrecognized associated histologic features”.
A 60-year-old man presented with increasing uncharacteristic abdominal discomfort and mildly elevated blood parameters of acute inflammation. Imaging revealed an unclear, giant liver tumor of the left liver lobe. A massive vascular tumor with extensive satellitosis broadly infiltrating the adjacent liver parenchyma was resected via hemihepatectomy of segments II/III. Histopathological diagnosis was giant hepatic cavernous hemangioma with multiple satellite nodules, featuring unusual characteristics hardly portrayed in the literature. Retrospectively, this particular morphology can explain the difficult pre- and perioperative diagnosis of a vascular liver tumor that is usually readily identifiable by modern imaging methods.
This case emphasizes the exact histological workup of tumor and tumor-induced parenchyma changes in radiologically unclassifiable liver tumors.
Core Tip: This case highlights that attention to tumour/tissue boarders and knowledge about unusual perilesional parenchyma changes is not only of academic pathological interest, but has an important role in unclear preoperative imaging to discriminate between benign and malignant entities in interdisciplinary hepato-oncology and highly precise modern imaging techniques.
- Citation: Fischer AK, Beckurts KTE, Büttner R, Drebber U. Giant cavernous hemangioma of the liver with satellite nodules: Aspects on tumour/tissue interface: A case report. World J Hepatol 2023; 15(5): 707-714
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/707.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.707
Giant hepatic cavernous hemangioma with multiple satellite nodules is a rare subtype of hepatic cavernous hemangioma, the most common vascular liver tumor. We report on a tumor with unusual histologic features: (1) Finger-like infiltration pattern; (2) lack of encapsulation; (3) blurred tumor/liver interface; and (4) massive satellitosis-referring to the article “Hepatic cavernous hemangioma: underrecognized associated histologic features” by Kim et al[1], 2005, in Liver International.
We report on a 60-year-old man with an unclear liver tumour of the left liver lobe. Increasing abdominal pressure, finally emanating to the left thorax, indigestion, and night sweat.
Symptoms increased for two months.
No special notes.
Sixty-year-old man with an unclear liver tumor of the left liver lobe.
Symptoms comprised increasing abdominal pressure, finally emanating to the left thorax, indigestion, and night sweat over two months. Weight loss or exhaustion were not perceived.
Leukocytes, aspartate transaminase, alanine transaminase, and lactate dehydrogenase were mildly elevated, liver enzymes in the normal range. C reactive protein was mildly elevated at first but increased significantly with aggravation of symptoms.
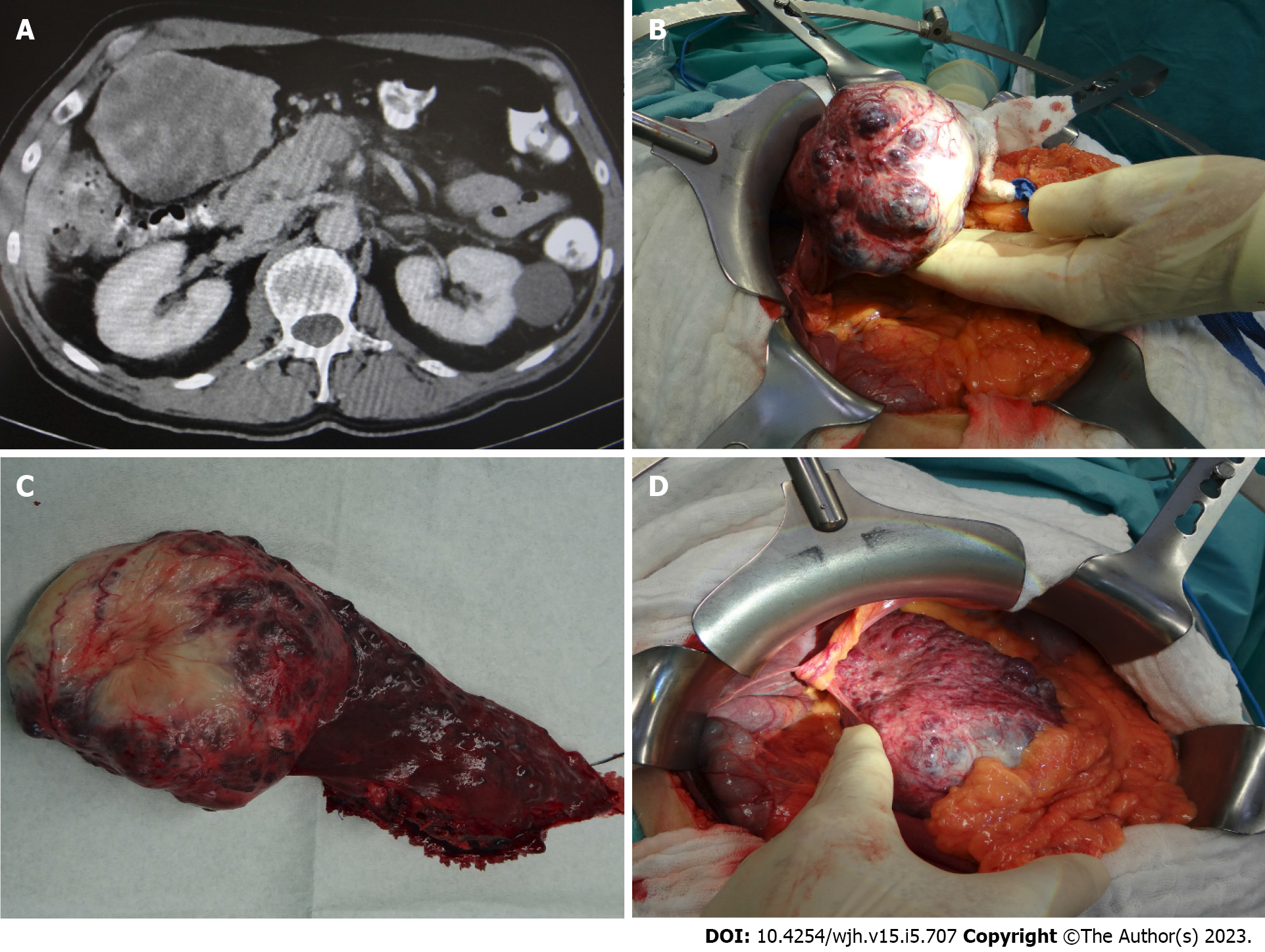
Contrast enhanced computed tomography (CT) showed a voluminous exophytic tumor arising from liver segment III, with atrophy of the left liver lobe, compression of the liver hilus and the left colon flexur (Figure 1A). Radiological diagnosis of hemangioma remained unclear because of blurred tumour boarders and multiple tumour satellites in the adjacent liver parenchyma.
Intraoperatively a solid and spongy dark red tumour measuring 10.5 cm was detected, with multiple small satellite nodules (0.1-0.9 cm) in the neighbouring liver parenchyma (Figure 1B + hypen + D), reminding of metastasis.
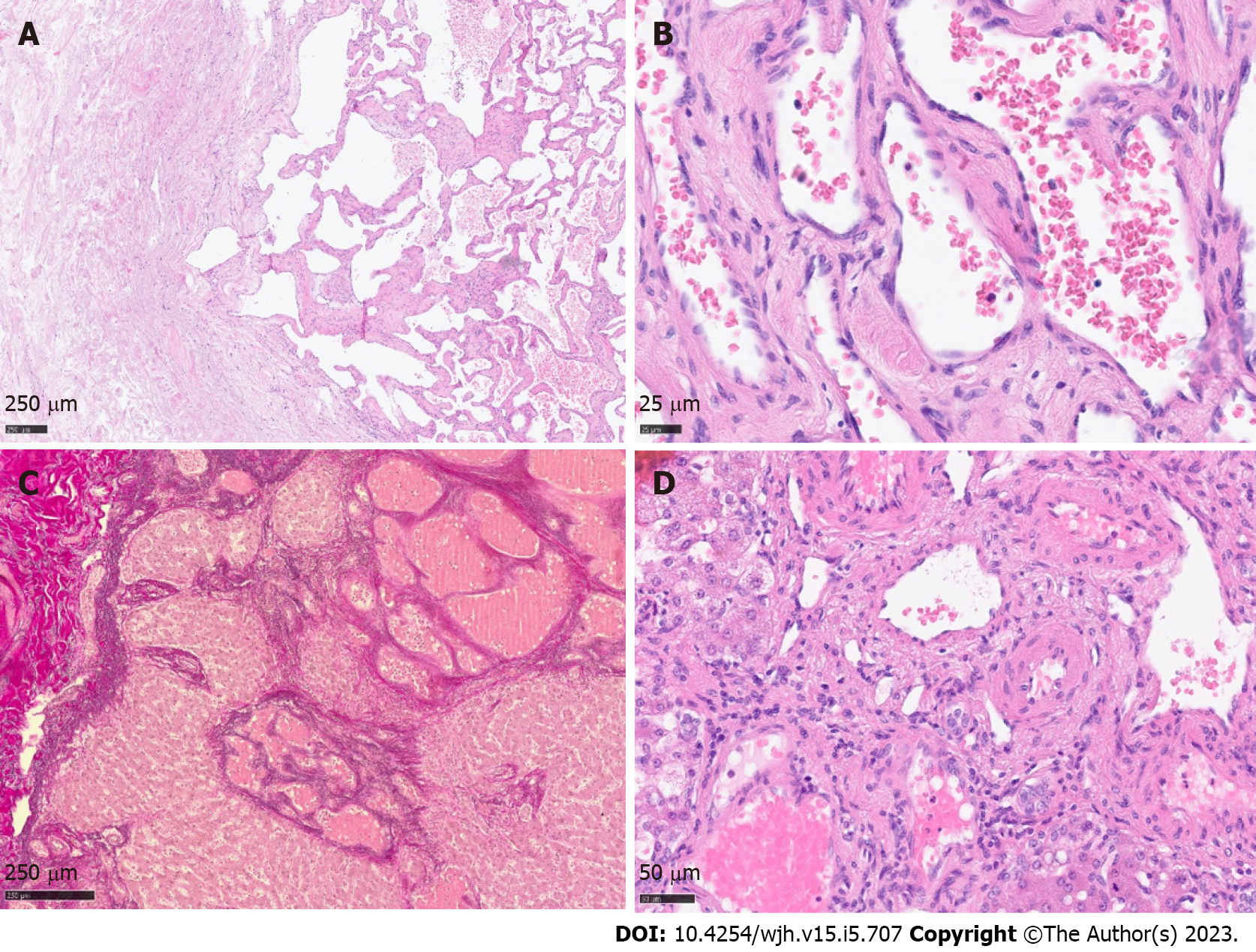
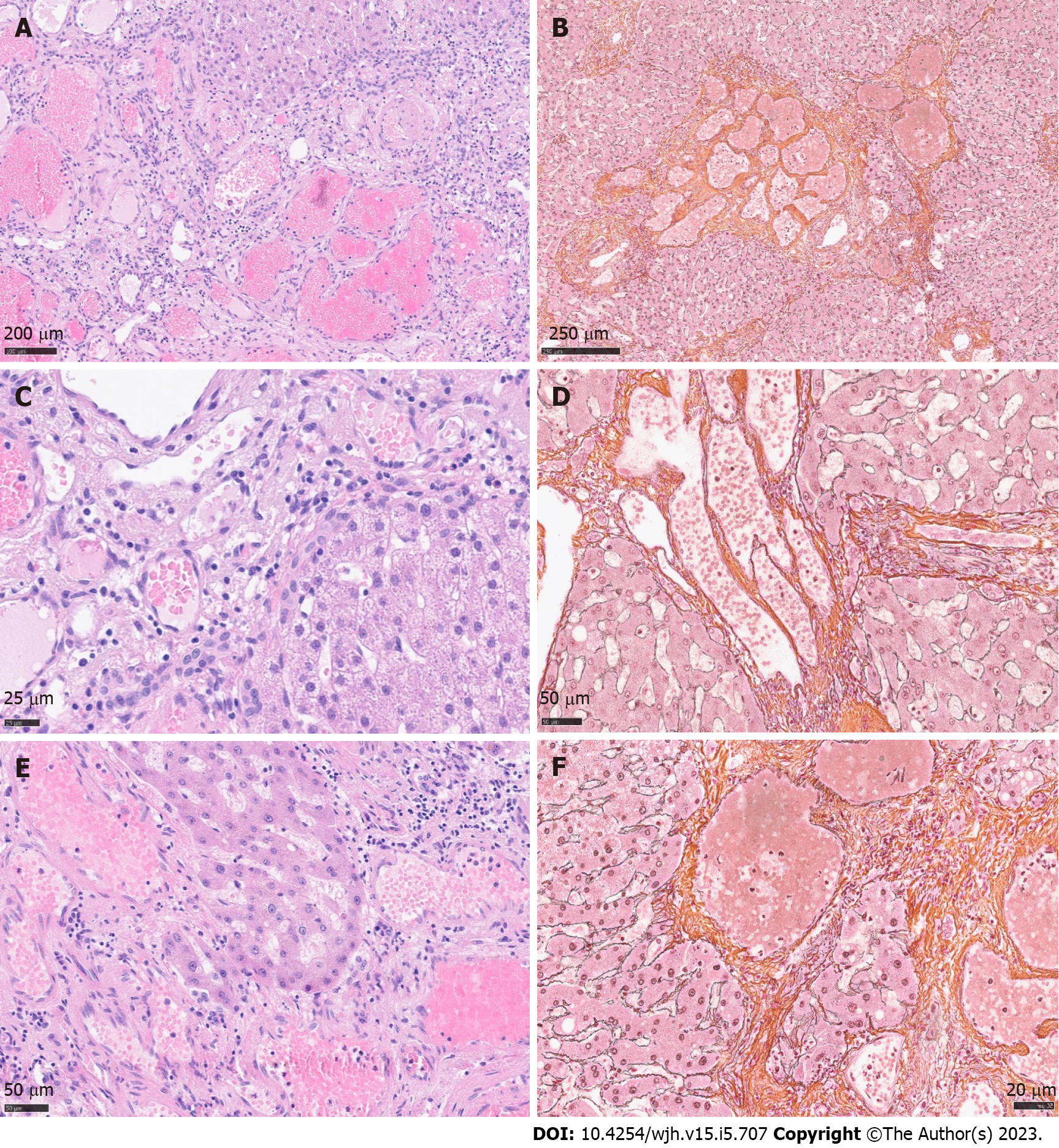
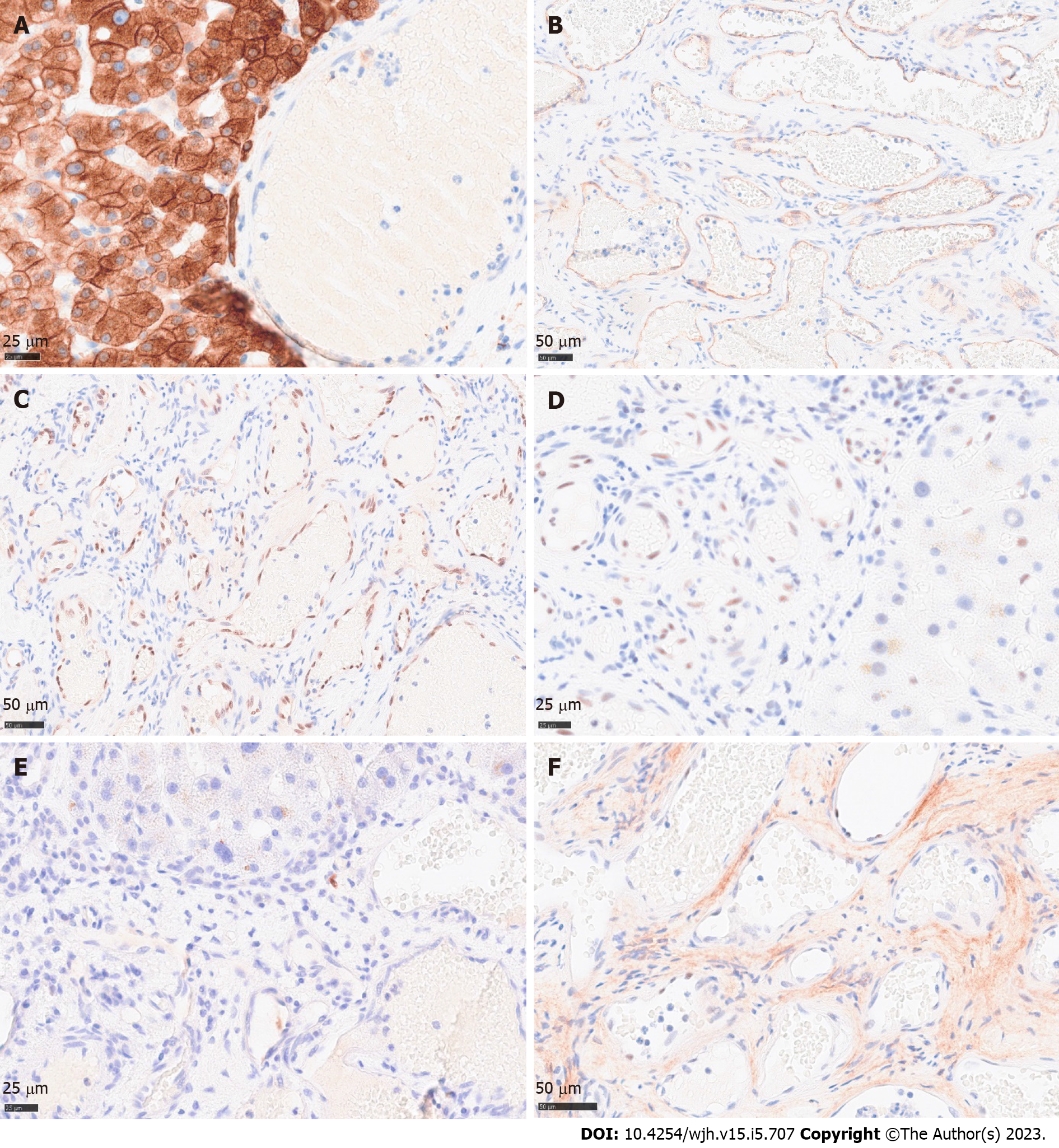
Histology revealed a main vascular tumour with a fibrous capsule (Figure 2A), with multiple diffusely spreading unencapsulated capillary tumour foci at the periphery (Figure 2C, Figure 3A + hypen + F), readily identifiable through dilatated vessels with intravascular hemocongestion and capillaries in a retiform pattern, with interposed trabecula of liver parenchyma (Figure 3C + hypen + F, Figure 4A). The vessels were lined by flat inconspicious endothelial cells without mitotic activity. Immunohistochemistry (CD34, ERG and Fli1) highlighted the vascular nature (Figure 4B + hypen + D). No pathological nuclear TP53 accumulation or proliferative activity was found (Figure 4E and F). Capsule-like fibrosis was only observed around the main tumour, whereas satellite nodules lacked a fibrous interface with the adjacent liver parenchyma. Sometimes, small bile ducts and sparse lympho-plasmocytic inflammatory infiltration surrounded the main tumour.
Giant hepatic vascular hemangioma with satellite nodules.
Left lateral hemihepatectomy of segments II/III with construction of a bladder fistula was performed.
After resection the patient recovered well and was devoid of symptoms.
Hepatic cavernous hemangioma (CH) is a well-known entity, the most common benign vascular liver tumor with an incidence of 0.4% to 20% in autopsies[1-4]. The term “giant cavernous hemangioma” should be applied for tumors greater than 4 cm[2,5,6], 5 cm[1,7], or even 10 cm[6], depending on the literature. It occurs more often and has greater dimensions in (young) women than in men[1-7]. Etiology and pathogenesis are still unknown, though hormonal influence is discussed as a possible trigger[1,2,7]. Some tumors express estrogen receptors, and growth during puberty, pregnancy, and under medication with oral contraceptives is observed[1,2,7,8-11]. However, single studies also negate a correlation between hormonal influence, sex, and tumor size[5]. A solitary lesion under 3 cm is typical, classically seen in the right posterior liver lobe[2,12], although tumors can occur anywhere in the liver[2,5,12]. In up to 10% of cases, multifocal tumors arise and seldom diffuse hemangiomatosis is found, both much more often in women than in men[1,2,5]. Rarely are hemangiomas associated with focal nodular hyperplasia[2]. They are also observed in hereditary hemorrhagic telangiectasia (HHT; Rendu-Osler-Weber disease)[1,2].
Spontaneous involution by intratumoral thrombosis and vascular obliteration, as well as secondary fibrosis and calcification with phleboliths can occur[1,2,5,7], rarely resulting in a so-called “solitary necrotic nodule” as an end-stage form of completely sclerosed hemangioma[2]. Most tumors are asymptomatic and only detected by incidence. If the hemangioma lies directly under the liver capsule and starts to expand, capsule stretching can cause abdominal pain, and the tumor can even be palpable by clinical examination. Small CH only require surgery if symptomatic, extended tumors should be resected because of the elevated risk of rupture, acute thrombosis and tumor bleeding[1,2,7,13]. Alternatively, transarterial embolization or percutaneous radiofrequency ablation can be an option[2]. Rarely is liver transplantation necessary[1,5]. A rare complication in giant hemangioma in the liver or in extremities is Kasabach Merritt syndrome[2,5], a form of disseminated intravascular coagulopathy in convoluted tumor vessels with coagulopathy, thrombocythemia, and hypofibrinogenemia, triggered by intravascular aggregation of thrombocytes, strong activation of coagulation, and consumption of fibrinogen, with extensive bleeding[14,15].
In most cases highly precise contrast enhanced ultrasound of the liver or contrast-enhanced CT or magnetic resonance imaging does not require histological confirmation of the diagnosis, sparing invasive liver biopsy with the risk of bleeding. Typical imaging reveals peripherical nodular enhancement in the arterial phase, resulting from tumor feeding via liver arteries, with progressive centripetal partial or complete fill-in in the portal venous phase, and washout in the late phase[16-18]. However, classical radio-morphological features can be lost with increasing tumor size and morphological variations like multinodularity[1,2,5] or rarely even liver infiltration, then referred to as diffuse hemangiomatosis1. Differential diagnosis of hepatic hemangiosarcoma must be considered. Other (vascular) disorders like peliosis hepatis, Budd Chiari syndrome, or venous occlusive disease/sinus obstruction syndrome can mostly be excluded by anatomic distribution in the liver, lacking zonal growth and filling phenomenon[2].
Cavernous hemangioma endothelial cells, so-called “CHECs” by Zhang et al[19], 2006, show an enhanced angiogenic activity compared with normal liver sinusoidal endothelial cells or “LSECs”. They express elevated levels of vascular endothelial growth factor (VEGF), metalloproteinases, and angiopoietins[19,20]. The VEGF influence on vascular proliferation of liver hemangioma was also clinically noted. Shrinkage of incidentally detected liver hemangioma was observed during antiangiogenic therapy in patients with colon carcinoma who were treated with bevacicumab[21,22], a recombinant humanized monoclonal anti-VEGF-antibody hampering neoangiogenesis in various tumors or diabetic retinopathy. In hypoxic conditions, (neo) angiogenesis is promoted by autocrine and paracrine secretion of VEGF, which activates the PI3-Kinase/Akt-pathway and the Ras-dependent signaling pathway through Mitogen-activated protein kinases extracellular signal-regulated protein kinase 1 (ERK1) and ERK2. Hypoxia leads to ERK1 and ERK2 activation by phosphorylation, which then hamper degradation of hypoxia inducible factor 1α (HIF1α). This factor consecutively binds the hypoxia-responsible element of the VEGF promotor in the nucleus, enhancing VEGF expression, and resulting finally in the proliferation of endothelial cells[21,22]. Hu et al[23], 2006, found an aberrantly enlarged endoplasmic reticulum (ER) in “CHECs” by electron microscopy and a downregulation of the protein Derlin-1 that plays a role in the transport of misfolded proteins from the ER to the cytosol for degradation. A shrinkage of the ER to normal size again was observed when Derlin-1 was overexpressed[19], implying a possible error in protein degradation in consecutive storage in ER in “CHECs”.
In our case, the tumor displayed massive satellitosis, but not the diffuse small cystic infiltration pattern of hemangiomatosis. Apart from the main tumor, we did not find the classical histomorphological criteria for CH (“well demarcated”, “fibrous capsule-like border”)[1,2,5,7] in the satellite nodules. However, we recognized several atypical features reported by Zimmermann et al[24], 1996, and Kim et al[5], 2005, in their series of giant cavernous liver hemangiomas with unusual features, like the so-described “interdigitating pattern”[24] where tumor parts have finger-like expansion into the liver parenchyma, without formation of a typical fibrous interface (Figure 2C + hypen + F). Considering these particular features, together with the classical morphology of the main tumor, other differential diagnosis like peliosis hepatis, hereditary hemorrhagic telangiectasia, or hemangiosarcoma could be readily excluded.
Spreading pattern also evoked the question of primarily multiple solitary hemangiomas in one liver lobe, of which one nodule started to expand massively, perhaps because of benefited localization next to greater arteria, or general arterial supply only sufficient for the expansion of one nodule. However, a review by Bioulac-Sage et al[2], 2008, describes a similar extension pattern of dilatated vessels in the close periphery of giant hemangiomas (0.1-2.0 cm beyond tumor borders), so-called hemangioma-like vessels (HLV)[2], discussing the “HLVs” as a process of expansion. In our case, we found satellite nodules infiltrating the whole resected liver lobe, up to 10 cm away from the main tumor (Figure 1C). The extremely low proliferation index and lack of TP53 accumulation in satellite nodules contradicted a rapid tumor expansion.
Blurred tumor borders and satellite nodules were a challenging aspect in preoperative imaging and, together with the untypical clinical setting (age, sex), did not permit a firm preoperative radiological diagnosis or definite exclusion of malignancy.
Giant cavernous hemangioma of the liver with unusual features is a challenging preoperative diagnosis. It requires thorough combined radiological and histomorphological workup with special regard to (1) finger-like infiltration pattern; (2) lack of encapsulation; (3) blurred tumor/liver interface; and (4) massive satellitosis. Moreover, attention must be paid to areas with diffuse and dense vascular spreading pattern, so that hemangiomatosis is not overlooked. Considering these rarely described features is essential in preoperative imaging and liver biopsy, to not prematurely drop the diagnosis of cavernous hemangioma, as well as to enlarge the portfolio of (malignant) differential diagnosis. Cases like this enhance the importance of interdisciplinary collaboration of radiology, hepatology, and hepatopathology, and the correlation of rare histomorphological aspects with modern imaging methods.
We thank Dr. Jochen Müller, MD, who greatly supported this work.
| 1. | Kim GE, Thung SN, Tsui WM, Ferrell LD. Hepatic cavernous hemangioma: underrecognized associated histologic features. Liver Int. 2006;26:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Torbenson M, Zen Y, The, MM. Tumors of the liver. AFIP Atlas of Tumor Pathology Series 4 2018. ISBN-10. 1933477415. |
| 3. | Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis. 2008;28:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Adam YG, Huvos AG, Fortner JG. Giant hemangiomas of the liver. Ann Surg. 1970;172:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 5. | Sandulescu LD, Urhut CM, Sandulescu SM, Ciurea AM, Cazacu SM, Iordache S. One stop shop approach for the diagnosis of liver hemangioma. World J Hepatol. 2021;13:1892-1908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (4)] |
| 6. | Di Carlo I, Koshy R, Al Mudares S, Ardiri A, Bertino G, Toro A. Giant cavernous liver hemangiomas: is it the time to change the size categories? Hepatobiliary Pancreat Dis Int. 2016;15:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Lerut J, Iesari S. Vascular tumours of the liver: a particular story. Transl Gastroenterol Hepatol. 2018;3:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Conter RL, Longmire WP Jr. Recurrent hepatic hemangiomas. Possible association with estrogen therapy. Ann Surg. 1988;207:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 357] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Christopherson WM, Mays ET, Barrows GH. Liver tumors in women on contraceptive steroids. Obstet Gynecol. 1975;46:221-223. [PubMed] |
| 11. | Nissen ED, Kent DR, Nissen SE, McRae DM. Association of liver tumors with oral contraceptives. Obstet Gynecol. 1976;48:49-55. [PubMed] |
| 12. | Kamyab AA, Rezaei-Kalantari K. Hepatic Hemangioma in a Cluster of Iranian Population. J Med Ultrasound. 2019;27:97-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yang YG, Chen WF, Mai WH, Li XF, Zhou HL, Liu LJ, Li MY. Spontaneous intracapsular hemorrhage of a giant hepatic cavernous hemangioma: a rare case report and literature review. BMC Gastroenterol. 2021;21:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Osman NM. Kasabach - Merritt syndrome: A case report. Sudan J Paediatr. 2013;13:49-52. [PubMed] |
| 15. | Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 276] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Doyle DJ, Khalili K, Guindi M, Atri M. Imaging features of sclerosed hemangioma. AJR Am J Roentgenol. 2007;189:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Song JS, Kim YN, Moon WS. A sclerosing hemangioma of the liver. Clin Mol Hepatol. 2013;19:426-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Shin YM. Sclerosing hemangioma in the liver. Korean J Hepatol. 2011;17:242-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zhang WJ, Ye LY, Wu LQ, Xin YL, Gu F, Niu JX, Yang ZH, Zhu GJ, Grau GE, Lou JN. Morphologic, phenotypic and functional characteristics of endothelial cells derived from human hepatic cavernous hemangioma. J Vasc Res. 2006;43:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Mahajan D, Miller C, Hirose K, McCullough A, Yerian L. Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol. 2008;49:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Hu D, Ran YL, Zhong X, Hu H, Yu L, Lou JN, Sun LX, Yang ZH. Overexpressed Derlin-1 inhibits ER expansion in the endothelial cells derived from human hepatic cavernous hemangioma. J Biochem Mol Biol. 2006;39:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Zimmermann A, Baer HU. Fibrous tumor-liver interface in large hepatic neoplasms: its significance for tumor resection and enucleation. Liver Transpl Surg. 1996;2:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chae HB, South Korea; D'Alessio V, Italy; Zharikov YO, Russia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH