Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.699
Peer-review started: December 16, 2022
First decision: December 31, 2022
Revised: January 7, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: May 27, 2023
Processing time: 158 Days and 11.8 Hours
Methotrexate (MTX) is the usual first-line treatment for rheumatoid arthritis (RA). Long-term use of MTX has been associated with liver steatosis (LS) and liver fibrosis (LF).
To determine if LS in patients treated with MTX for RA is associated with MTX cumulative dose (MTX-CD), metabolic syndrome (MtS), body mass index (BMI), the male sex, or LF.
A single-center, prospective study of patients receiving MTX for RA was per
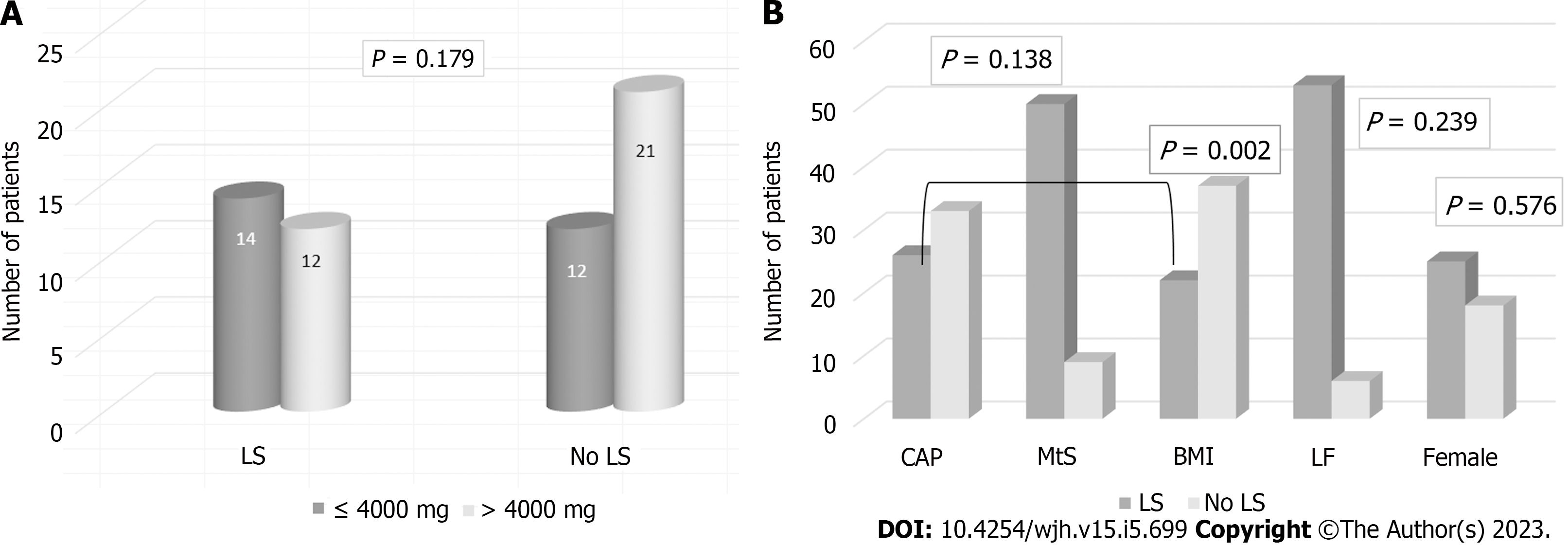
Fifty-nine patients were included. Forty-three were female (72.88%), and the mean age was 61.52 years (standard deviation: 11.73). When we compared MTX-CD ≤ 4000 mg (26 patients; 14 with LS and 12 without) with > 4000 mg (33 patients; 12 with LS and 21 without), no statistical differences were found (P = 0.179). We compared CAP scores stratified by MtS, BMI, sex, and LF. There were no significant differences in CAP scores based on the presence of MtS [CAP/MtS: 50 no MtS (84.75%); 9 MtS (15.25%); P = 0.138], the male sex (CAP/sex: 8 male/18 female LS; 8 male/25 female no LS; P = 0.576), or LF [CAP/fibrosis: 53 no LF (89.83%); 6 LF (10.17%); P = 0.239]. LS determined by CAP was significantly associated with BMI > 25 (CAP/BMI: 22 BMI ≤ 25 (37.29%); 37 BMI > 25 (62.71%); P = 0.002].
LS in patients with RA treated with MTX was not associated with MTX-CD, LF, the male sex, or MtS. However, BMI was significantly related to LS in these patients.
Core Tip: Methotrexate (MTX) is the cornerstone of treatment for rheumatoid arthritis and has been associated with the development of liver fibrosis (LF) and liver steatosis (LS). The objective of this work was to study if LS in patients with rheumatoid arthritis treated with MTX and determine the association with body mass index, MTX cumulative dose, sex, LF, and metabolic syndrome. We concluded that LS in patients with rheumatoid arthritis on MTX treatment was not related to MTX-cumulative dose, LF, the male sex, or metabolic syndrome. In our study, body mass index was significantly associated with LS in these patients.
- Citation: Castiella A, Lopez-Dominguez L, Sanchez-Iturri MJ, Urreta I, De Diego A, Belzunegui J, Zapata E. Liver steatosis in patients with rheumatoid arthritis treated with methotrexate is associated with body mass index. World J Hepatol 2023; 15(5): 699-706
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/699.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.699
Methotrexate (MTX) has been used in the treatment of oncological and chronic inflammatory diseases. It is also the cornerstone of treatment for rheumatoid arthritis (RA). The most concerning long-term adverse effect of this treatment is the development of liver fibrosis (LF)[1-5]. Liver steatosis (LS) has been associated with RA and with MTX treatment[6]. Liver biopsy has been the gold standard for the study of LF and LS, but it has several limitations[3]. There is a disparity of fibrosis values between biopsy samples, and it is an invasive technique accompanied by risks[3].
Recent studies have been carried out on non-invasive measurements of LF. Transient elastography (TE) is a non-invasive method without side effects that also allows the sequential determination of liver fibrosis measurements over time, which makes it of great interest for the follow-up of these patients[2-4].
MTX, as a risk factor for secondary LS, has been studied recently. RA has been associated with moderate to severe LS; predisposing factors such as higher body mass index (BMI), the male sex, and MTX cumulative dose (MTX-CD) have been published[7]. However, there have been conflicting results, and the impact of MTX on nonalcoholic fatty liver disease (NAFLD) is still unclear[6-11].
The computer attenuation parameter (CAP) measures carried out at the time of TE correlates with the histological LS[12]. The CAP algorithm calculates the ultrasound signal attenuation[12]. LS has been evaluated recently using CAP in chronic MTX users and was common with moderate and severe LS predicting moderate to severe LF[13].
The objective of our work was to determine if LS in patients with RA treated with MTX is associated with BMI, MTX-CD, sex, LF, and metabolic syndrome (MtS).
We performed a single-center, prospective study of patients receiving MTX for RA. The principle objective of this work was to study the presence of LF by TE and aspartate aminotransferase to platelet ratio index (APRI)[14] as well as the detection of LS by ultrasonography and CAP. Fibroscan® (FS) (Fibroscan®402, Echosens, France, www.echosens.com) was used for fibrosis determination (LF > 7 KpA). CAP was used for LS (CAP > 248 dB/m)[11]. Demographic variables, laboratory data, MTX-CD (> 4000 mg), MtS criteria, BMI (> 25), TE, and CAP scores were collected from all patients.
Patients were recruited between February 1, 2019 and January 31, 2020 from the Gastroenterology-Rheumatology clinics of our hospital. The inclusion criteria were patients aged 18 years or older diagnosed with RA by a rheumatologist, and being treatment with MTX (without limitation on the duration of treatment). The exclusion criteria were previous diagnosis of liver disease (hepatitis B or C virus infection, known NAFLD), alcohol consumption greater than 60 g/d for males or 40 g/d for females, HIV infection on antiretroviral therapy, diabetes mellitus, chronic renal failure, congestive heart failure, or BMI greater than 30 kg/m². Patients receiving leflunomide in the 3 years prior to the study were also excluded.
Demographic data analysis, treatment history, and MTX-CD were collected through computerized medical records. LF was defined by FS (measurement greater than 7 Kpa) and by APRI score (result greater than 0.7). The FS assessment was performed by a trained nurse. At the time of inclusion in the study, a blood test was performed to calculate the APRI score [aspartate aminotransferase level (upper limit of normal)/platelet level × 100]. High transaminase levels were defined as results above 33 U/L. Finally, disease activity was defined by a rheumatologist using the Disease Activity Score in 28 joints-c-reactive protein score. Data were collected by means of a questionnaire, a review of the computerized clinical history, and a visit to the gastroenterology clinic.
Initially, a descriptive analysis was performed by calculating the mean and standard deviation (SD) (or median and interquartile range) for quantitative variables. For qualitative variables, absolute and relative frequencies were calculated as percentages. To compare the distribution of qualitative variables, the χ2 test or Fisher’s exact test was used. Similarly, the Student’s t-test or the Mann-Whitney U test was used to compare quantitative variables. STATA 16.1 software was used for all the analyses. Statistical review of the study was performed by a biomedical statistician (IU).
The clinical research ethics committee of the Gipuzkoa health area (Código de Protocolo: ACLFSC-2018-01; Acta 01/2019) approved this study, and participants signed an informed consent form prior to inclusion.
We included 59 patients in the study. There were 43 females (72.88%), and 61.52 years (SD: 11.73) was the mean age. Clinical characteristics are presented in Table 1 and laboratory data in Table 2 (Supple
| Clinical characteristics | Value |
| Female/male | 43 (73%); 16 (27%) |
| Age in yr | 61.52 (11.73) |
| Height in cm | 162.02 (7.66) |
| Weight in kg | 67.33 (10.52) |
| Waist circumference in cm | 88.81 (10.92) |
| BMI in kg/m² | 25.55 (3.05) |
| BMI < 25 score | 22 (37.29%) |
| BMI > 25 score | 37 (62.71%) |
| Metabolic syndrome | 9 (15.25%) |
| Type 2 diabetes | 2 (3.57%) |
| DAS28 score | 2.36 (1.14) |
| Treatment duration MTX in mo | 82.43 (65.08) |
| MTX-CD in mg | 5214.5 (4031.9) |
| FibroScan in kPa | 5.02 (2.24) |
| APRI in score | 0.32 (0.15) |
| CAP in dB/m | 251.33 (51.13) |
| Classification | Value |
| AST in U/L | 24.52 (12.56) |
| ALT in U/L | 22.23 (12.15) |
| GGTP in U/L | 23.42 (13.43) |
| AP in U/L | 76.05 (23.72) |
| Bilirubin in mg/dL | 0.50 (0.25) |
| Albumin in g/dL | 4.39 (0.29) |
| Glucose in mg/dL | 101.50 (16.08) |
| Triglycerides in mg/dL | 99.43 (45.87) |
| Cholesterol in mg/dL | 205.42 (43.57) |
| HDL-cholesterol in mg/dL | 62.85 (14.74) |
Treatment duration and times of disease progression were longer in the MTX-CD > 4000 mg group. MTX monotherapy was used in 46 patients (77.90%). Only 7 patients (11.80%) were on nonsteroidal anti-inflammatory drug therapy in association with MTX.
Ultrasonography was performed in 56 patients, of whom 39 presented no LS (69.64%), and 17 (30.36%) had LS. CAP was determined in all 59 patients, categorizing 33 patients without LS and 26 patients with LS.
We then compared both methods (56 patients in total). Ultrasonography presented a positive predictive value of 88.2% [95% confidence interval (CI): 63.6%-98.5%] and a negative predictive value of 76.9% (95%CI: 60.7%-88.9%), with a sensitivity of 62.5% (95%CI: 40.6%-81.2%) and a specificity of 93.8% (95%CI: 79.2%-99.2%) compared to CAP. When comparing MTX-CD ≤ 4000 mg (26 patients, 14 with LS and 12 without) with > 4000 mg (33 patients; 12 with LS and 21 without), we found no statistical differences in LS between low and high MTX-CD (P = 0.179) (Figure 1A). CAP scores were compared stratified by BMI, sex, LF, or MtS. No significant differences were observed based on the the male sex (CAP/sex: 8 males/18 females LS; 8 males/25 females no LS; P = 0.576), LF [CAP/Fibrosis: 53 no LF (89.83%); 6 LF (10.17%); P = 0.239], or MtS [CAP/MtS: 50 no MtS (84.75%); 9 MtS (15.25%); P = 0.138]. Nonetheless, LS measured by CAP was significantly related with BMI > 25 [CAP/BMI: 22 BMI ≤ 25 (37.29%); 37 BMI > 25 (62.71%); P = 0.002] (Figure 1B).
MTX is the gold standard of RA treatment, both in monotherapy and associated with biological therapies[15]. LF has been associated with chronic MTX use in this disease. There is increasing evidence that LF is broadly affected by other factors: Alcohol, other associated drugs, and MtS are directly related with the development of LF[16-19].
Drugs can affect LS development. The possible effect of MTX in the presence of LS in patients with RA is currently being studied. According to laboratory research, folate deficiency produced by chronic MTX treatment could promote liver fat accumulation[20], but folic acid supplementation has been recommended and is currently being used in treatment regimens. Studies have shown conflicting results, and the impact of MTX on LS is still unclear[6].
Choi et al[6] investigated whether MTX-CD in 368 RA patients led to LS determined by ultrasound, but they did not detect a significant association between LS development and MTX administration, suggesting that to adjust for individualized risk factors for NAFLD may be more efficient than MTX discontinuation in LS detection/management. Hypertriglyceridemia and higher BMI were associated with an increased risk of LS.
Erre et al[7] recently studied the independent association of LS and RA. In 223 patients with RA, they found that RA is independently associated with LS (moderate to severe), scored by ultrasound, and male sex, higher BMI, and MTX-CD are independent risk factors for the development of LS[7].
Mori et al[8] studied the association between NAFLD and liver injury during MTX treatment in 846 patients with RA. They did not observe a significant impact of MTX dose and duration on histological severity. On the other hand, Sakthiswary et al[9] concluded, in a retrospective study, that the MTX-CD was the only independent predictor of MTX-associated LS with transaminitis in a cohort of 978 patients with RA.
Recently, detection of LS by CAP in chronic MTX users was published for the first time. Tomaszewski et al[13] studied 172 patients on MTX (45 with RA). Diabetes mellitus, hypertension, and BMI ≥ 30 were predictors of LS. LS determined by CAP was frequent. Moderate and severe LS in this study predicted moderate to severe fibrosis of the liver.
Our prospective study was designed to determine in patients with RA treated with MTX if LS, as measured by CAP, was associated with BMI, sex, LF, or MTX-CD. When we compared MTX-CD ≤ 4000 mg with > 4000 mg, no statistical differences were found. There were no significant differences between the presence and absence of MtS, the male sex, or LF, but LS determined by CAP was significatively associated with BMI > 25 (P = 0.002).
Our study had limitations. The sample size was relatively small, and we included all the patients with RA on MTX treatment, without a treatment duration limitation. More females than males were included in this study, and given the limited sample size, it is difficult to conclude that there is no relationship between sex and LS. The strengths of the study were that it was a prospective study and that LS was determined as measured by the CAP.
We concluded that in our series of patients treated with MTX for RA, LS is not associated with MTX-CD, LF, the male sex, or MtS. In our study, BMI is significantly associated with LS. It seems that other factors, apart from MTX-CD or treatment duration, are more important for the development of LS in these patients.
Methotrexate (MTX) remains the cornerstone of treatment for rheumatoid arthritis (RA), both in monotherapy and in association with other treatments. The most concerning adverse effect of this treatment, in the long term, is liver fibrosis (LF). Liver steatosis (LS) has been associated with RA and with MTX.
MTX, as a risk factor for secondary LS, has been studied recently. RA has been independently associated with moderate to severe LS. Sex, higher body mass index (BMI), and MTX cumulative dose (MTX-CD) are predisposing factors. However, the studies have shown conflicting results, and the impact of MTX on LS is still unclear.
The objective of our work was to study if LS in RA patients treated with MTX was related to BMI, MTX-CD, metabolic syndrome (MtS), sex, or LF.
We performed a prospective study of RA patients treated with MTX. The principal objective of this work was to study the presence of LF by transient elastography and aspartate aminotransferase to platelet ratio index as well as the detection of LS by ultrasonography and computer attenuation parameter (CAP).
Fifty-nine patients were included in the study. When comparing MTX-CD ≤ 4000 mg with > 4000 mg, we found no statistical differences in LS between low and high MTX-CD. We compared CAP scores with MtS, BMI, sex, and LF. There were no significant differences based on the presence or absence of MtS, the male sex, or LF. LS determined by CAP was significantly associated with BMI > 25.
We concluded that, in our series, LS in RA patients treated with MTX is not related to sex, MTX-CD, MtS, or LF. BMI > 25 is significatively associated with LS in our study. Other factors, apart from MTX-CD or time in treatment, are more important for the development of LS in these patients.
The routine incorporation of FS for the study of LF and LS in RA patients with MTX treatment is critical and will aid in understanding the real impact of MTX on LS. More studies (larger and multicentric) are recommended to validate these results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tanaka N, Japan; Zhang Y, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Wade SD, Yoshida EM, Carruthers MN, Weinblatt ME. Transient Elastography for Monitoring for Hepatotoxicity in Rheumatoid Arthritis Patients on Long-term Methotrexate. J Clin Rheumatol. 2021;27:e131-e134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Arena U, Stasi C, Mannoni A, Benucci M, Maddali-Bongi S, Cammelli D, Assarat A, Marra F, Pinzani M. Liver stiffness correlates with methotrexate cumulative dose in patients with rheumatoid arthritis. Dig Liver Dis. 2012;44:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Barbero-Villares A, Mendoza J, Trapero-Marugan M, Gonzalez-Alvaro I, Daudén E, Gisbert JP, Moreno-Otero R. Evaluation of liver fibrosis by transient elastography in methotrexate treated patients. Med Clin (Barc). 2011;137:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Park SH, Choe JY, Kim SK. Assessment of liver fibrosis by transient elastography in rheumatoid arthritis patients treated with methotrexate. Joint Bone Spine. 2010;77:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Olsson-White DA, Olynyk JK, Ayonrinde OT, Paramalingam S, Keen HI. Assessment of liver fibrosis markers in people with rheumatoid arthritis on methotrexate. Intern Med J. 2022;52:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Choi Y, Lee CH, Kim IH, Park EH, Park S, Yoo WH. Methotrexate use does not increase the prevalence of hepatic steatosis: a real-world retrospective nested case-control study. Clin Rheumatol. 2021;40:2037-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Erre GL, Castagna F, Sauchella A, Meloni P, Mangoni AA, Farina G, Woodman R, Dore MP, Vidili G. Prevalence and risk factors of moderate to severe hepatic steatosis in patients with rheumatoid arthritis: an ultrasonography cross-sectional case-control study. Ther Adv Musculoskelet Dis. 2021;13:1759720X211042739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Mori S, Arima N, Ito M, Fujiyama S, Kamo Y, Ueki Y. Non-alcoholic steatohepatitis-like pattern in liver biopsy of rheumatoid arthritis patients with persistent transaminitis during low-dose methotrexate treatment. PLoS One. 2018;13:e0203084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Sakthiswary R, Chan GY, Koh ET, Leong KP, Thong BY. Methotrexate-associated nonalcoholic fatty liver disease with transaminitis in rheumatoid arthritis. ScientificWorldJournal. 2014;2014:823763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (3)] |
| 10. | García DS, Saturansky EI, Poncino D, Martínez-Artola Y, Rosenberg S, Abritta G, Ascimani-Peña C, Cravero A. "Hepatic toxicity by methotrexate with weekly single doses associated with folic acid in rheumatoid and psoriatic arthritis. What is its real frequency? Ann Hepatol. 2019;18:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate Hepatotoxicity and the Impact of Nonalcoholic Fatty Liver Disease. Am J Med Sci. 2017;354:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 914] [Article Influence: 101.6] [Reference Citation Analysis (2)] |
| 13. | Tomaszewski M, Dahiya M, Mohajerani SA, Punja H, Ko HH, Sun M, Ramji A. Hepatic steatosis as measured by the computed attenuation parameter predicts fibrosis in long-term methotrexate use. Can Liver J. 2021;4:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Castiella Eguzkiza A, de Diego A, Lopez Dominguez L, Sanchez Iturri MJ, Urreta I, Vaamonde M, Belzunegui J, Zapata E. Evaluation of liver fibrosis in patients with rheumatoid arthritis treated with methotrexate. Utility of fibroscan and biomarkers in clinical practice. UEG Journal. 2021;9 (suppl):655-656. |
| 15. | Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, Kremer J, Nakamura MC, Russell LA, Singh JA, Smith BJ, Sparks JA, Venkatachalam S, Weinblatt ME, Al-Gibbawi M, Baker JF, Barbour KE, Barton JL, Cappelli L, Chamseddine F, George M, Johnson SR, Kahale L, Karam BS, Khamis AM, Navarro-Millán I, Mirza R, Schwab P, Singh N, Turgunbaev M, Turner AS, Yaacoub S, Akl EA. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 649] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 16. | Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 17. | Park JS, Park MC, Park YB, Lee SK, Lee SW. Concurrent use of methotrexate and celecoxib increases risk of silent liver fibrosis in rheumatoid arthritis patients with subclinical reduced kidney function. Clin Rheumatol. 2014;33:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lee SW, Park HJ, Kim BK, Han KH, Lee SK, Kim SU, Park YB. Leflunomide increases the risk of silent liver fibrosis in patients with rheumatoid arthritis receiving methotrexate. Arthritis Res Ther. 2012;14:R232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Cervoni JP, Alby-Lepresle B, Weil D, Zhong P, Aubin F, Wendling D, Toussirot E, Vuitton L, Carbonnel F, Blondet R, Thévenot T, Calès P, Monnet E, Di Martino V. A pragmatic non-invasive assessment of liver fibrosis in patients with psoriasis, rheumatoid arthritis or Crohn's disease receiving methotrexate therapy. Clin Res Hepatol Gastroenterol. 2020;44S:100003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Sid V, Siow YL, O K. Role of folate in nonalcoholic fatty liver disease. Can J Physiol Pharmacol. 2017;95:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |