Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.675
Peer-review started: February 7, 2023
First decision: March 22, 2023
Revised: April 4, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 27, 2023
Processing time: 105 Days and 13.7 Hours
Hereditary hemorrhagic teleangiectasia (HHT), also known as Rendu-Osler-Weber syndrome, is the most common cause of hepatic vascular malformations in adults. Different vascular shunts (arteriovenous, arterioportal or portovenous) lead to different clinical manifestations. Even though no hepatic-related symptoms are reported in the majority of cases, the severity of liver disease could lead to refractory medical conditions, in some cases requiring liver tran
Core Tip: Hereditary hemorrhagic teleangiectasia (HHT) is the most common cause of hepatic vascular malformation in adults. Although liver involvement is common in HHT, most patients do not present any hepatic-related symptoms. Unfortunately, some patients have severe forms of disease with refractory medical conditions related to the hepatic vascular malformations. For those patients the only definitive treatment available at present is liver transplantation.
- Citation: Ielasi L, Tonnini M, Piscaglia F, Serio I. Current guidelines for diagnosis and management of hepatic involvement in hereditary hemorrhagic teleangiectasia. World J Hepatol 2023; 15(5): 675-687
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/675.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.675
Hereditary hemorrhagic teleangiectasia (HHT) or Rendu-Osler-Weber syndrome is a rare autosomal dominant disorder characterized by mucocutaneous teleangiectases and systemic vascular malformations (VMs). HHT can be ruled in by using the Curaçao criteria (recurrent epistaxis, multiple mucosal/cutaneous teleangiectases, visceral VMs and first-degree relative with HHT); if at least 3 of these criteria are met, the diagnosis of HHT is considered to be definite (Table 1)[1,2].
| Curaçao criteria | Description |
| Epistaxis | Spontaneous and recurrent |
| Teleangiectases | Multiple, at characteristic sites: Lips, oral cavity, fingers, nose |
| Visceral lesions | GI telangiectasia, pulmonary, hepatic, cerebral or spinal AVMs |
| Family history | A first degree relative with HHT |
| Number of criteria | HHT diagnosis |
| 3-4 | Definite |
| 2 | Possible |
| 0-1 | Unlikely |
Molecular genetic test is useful in order to detect gene mutations. Endoglin (ENG, on chromosome 9) and activin A receptor type II-like 1 (ACVRL1, on chromosome 12) genes are involved in approximately 90% of cases and are responsible of HHT1 and HHT2, respectively. In addition to these two genes, mutation of SMAD4 has been identified in patients with the association of juvenile polyposis and HHT (PJ-HHT syndrome, approximately 2% of cases) in which anemia is the predominant symptom due to gastrointestinal bleeding. Mutations of GDF2 and RASA-1 genes have also been described but they are extremely rare (Table 2)[3,4].
| Gene | Protein | Location | Phenotype | Liver involvement prevalence |
| ENG | Endoglin | 9q34.11 | HHT1 | 7.6%-43.0% |
| ACVLR1 | ALK1 | 12q13.13 | HHT2 | 40.6%-57.6% |
| MADH4 | Smad4 | 18q21.1 | PJ-HHT | 33.3% |
| GDF2 | BMP9 | 10q11.22 | HHT-like | Unknown |
| RASA-1 | p120-RasGAP | 5q14.3 | CM-AVM | Unknown |
Loss of function mutations in ENG and ACVRL1 cause anomalous angiogenesis leading to VMs development[2]. One of the primary mechanisms underlying aberrant vascular endothelial growth factor (VEGF)-related angiogenesis in HHT patients appears to be the overactivation of phosphatidylinositol 3-kinase (PI3K) signaling in endothelial cells[5]. High VEGF levels drive VMs development in mouse models and its normalization suppresses progression of these anomalous vascular structures[6,7].
HHT1 is more frequent in Mediterranean countries and it is characterized by a higher incidence of pulmonary and brain VMs, while HHT2 is more frequent in Northern Europe and North America with a higher incidence of hepatic VMs[8]. No significant difference was found in age at debut of symptoms and the severity of epistaxis between patients with HHT1 and HHT2. On the other hand gastrointestinal bleeding was reported to be more common in patients with HHT1[9]. HHT2 is associated to a higher risk of symptomatic liver disease[10].
HHT represents the most common cause of congenital hepatic vascular malformations in adults, and liver involvement is a commonly observed feature in the disease (Table 2)[11-13]; the mean age at diagnosis is 48 years[14].
Whilst more than 90% of cases do not present any hepatic-related symptoms, patients affected by HHT are susceptible to developing a range of clinical condition with varying presentations depending on the type of hepatic VM[15]. In some cases, the severity of clinical conditions requires liver transplantation. Women seems to have a more frequent (female prevalence 4.5 fold higher than males) and more severe liver involvement in both HHT1 and HHT2[16].
In the following paragraphs we will discuss the diagnostic and therapeutic approach for liver involvement in HHT patients.
Three types of hepatic VMs have been described based on liver vascular anatomy: arteriovenous (the most frequent, between hepatic artery and hepatic vein), arterioportal (between hepatic artery and portal vein) and portovenous (between portal vein and hepatic vein)[15]. These different subtypes of hepatic vascular shunting usually coexist and affect the liver diffusely[17]. HHT liver involvement is a continuous process from small teleangiectases to very large VMs; size change during follow up has been observed in 21% of patients[10].
Arteriovenous shunts could cause high output cardiac failure (HOCF), ischemic cholangitis and mesenteric ischemia. Arterioportal shunts could cause portal hypertension, but also biliary ischemia. Portovenous shunts could cause HOCF, but also portosystemic encephalopathy[15]. Generally, one of them predominates functionally, but fluctuation from a clinical condition to another is common.
HOCF is the most common complication of HHT liver involvement and it generally starts being clinically significant when intrahepatic shunt output is > 20% of cardiac output[18]. HOCF is associated to an increased risk of atrial fibrillation and the associated increased pulmonary blood flow secondary to liver VMs may lead to the development of post-capillary pulmonary arterial hypertension. Less frequently, HHT patients may develop a pre-capillary pulmonary arterial hypertension that seems to be related to the remodeling of small pulmonary arteries caused by ENG and ACVRL1 gene mutations with histologic features broadly similar to those observed in idiopathic pulmonary arterial hypertension. Right heart catheterization is essential to differentiate between the two forms[19].
Arteriovenous shunting can cause a blood steal with secondary bile ducts ischemia; this phenomenon is facilitated by the vascular anatomy of the biliary system, which derives its blood supply solely from the hepatic artery via the peribiliary plexus. Biliary ischemia can subsequently evolve in biliary strictures and dilations (secondary sclerosing cholangitis), secondary infection of the biliary system (infectious cholangitis), bilomas or biliary cysts (mimicking Caroli’s disease), and elevation of serum alkaline phosphatase and gamma glutamyl transpeptidase. In the more severe forms, ischemia also affects hepatocytes causing hepatocellular necrosis leading to hepatic hemorrhage and bile leak[18,20].
Modification of normal liver perfusion may increase hepatocytes regenerative activity leading to development of focal nodular hyperplasia (FNH), 100 times more frequent in HHT patients than in general population, or nodular regenerative hyperplasia (NRH). In NRH the liver parenchyma undergoes a diffuse transformation into multiple regenerative nodules with hepatocytes arranged in plates, without fibrosis separating nodules.
Therefore, portal hypertension in HHT patients may be pre-hepatic, due to the increased blood flow from arterioportal VMs, or pre-sinusoidal, due to NRH (a well-known cause of non-cirrhotic intra
Hepatocellular regeneration nodules may be associated with minimal perisinusoidal and portal fibrosis which can mimic cirrhosis on imaging and lead to being diagnosed incorrectly[18,21].
This appearance is commonly defined “pseudocirrhosis” since there is no significant liver fibrosis, liver function tests are generally normal and the risk of hepatocellular carcinoma is not as increased as for liver cirrhosis[22].
Xu et al[23] reported that hepatic involvement in HHT and Budd-Chiari syndrome (BCS) may be linked, suggesting a shared pathogenetic mechanism characterized by vascular dysplasia and a trombophilic condition induced by HHT that would eventually lead to BCS[24]. Nonetheless further studies are needed to evaluate the possible relationship between these two diseases.
Several disease progression predictors have been identified. Singh et al[25] proposed a clinical scoring system for the estimation of the probability of clinically significant liver disease in HHT patients. This score uses readily available information such as patient gender, age, hemoglobin and alkaline phosphatase at presentation, but is currently not widely recognized and still need to be validated (Table 3).
| Criteria | Points | |
| Age at presentation (yr) | > 47 | 1 |
| ≤ 47 | 0 | |
| Sex | Female | 1 |
| Male | 0 | |
| Hb at presentation (g/dL) | < 8 | 3 |
| 8-12 | 2 | |
| 12-16 | 1 | |
| > 16 | 0 | |
| ALP at presentation (IU/L) | > 300 | 4 |
| 225-300 | 3 | |
| 150-224 | 2 | |
| 75-149 | 1 | |
| > 75 | 0 | |
| Clinical Scoring Index | Clinical probability of significant liver disease | |
| ≤ 2 | Low | (0.4%-3.2%) |
| 3-6 | Intermediate | (8.2%-64.1%) |
| ≥ 7 | High | (82.9%-93.0%) |
Screening for liver VMs should be offered to adults with a definite or suspected diagnosis of HHT[26] and the imaging test of choice for screening is Doppler ultrasound[27] for its accuracy in detecting hepatic VMs[28], its availability, repeatability, low cost and interobserver agreement[29-31]. In addition, Doppler ultrasound allows to establish the grade of severity of liver involvement and therefore correlates with patient outcomes and predictors of clinical outcomes[27].
Regarding the follow-up of hepatic VMs there are no standardized protocols nor consensus; ultrasound is usually repeated every 1 or 2 years according to the severity of liver involvement and is generally determined case by case.
Caselitz et al[28] defined major and minor criteria required for the diagnosis of liver VMs in HHT by Doppler ultrasound: A dilated common hepatic artery (> 7 mm) and intrahepatic arterial hypervascularization are the two major criteria; minor criteria are either Vmax in hepatic artery > 110 cm/s, low resistivity index (RI) of the proper hepatic artery (i.e. < 0.60), Vmax of portal vein > 25 cm/s and/or a tortuous course of extrahepatic hepatic artery. Presence of liver VMs in HHT is defined by two major criteria or one major criterion and two minor criteria[28]. According to Buscarini et al[27] severity grading ranges from 0.5 to 4 (Table 4)[32].
| VMs grade | Doppler US findings |
| 0.5 | HA diameter 5-6 mm and/or |
| PFV > 80 cm/sec and/or | |
| HA RI < 0.55 and/or | |
| Peripheral hepatic hypervascularization | |
| 1 | HA dilation > 6 mm (only extrahepatic) and |
| PFV > 80 cm/sec and/or | |
| HA RI < 0.55 and/or | |
| 2 | HA dilation intra- and extrahepatic and |
| PFV > 80 cm/sec | |
| Possible flow abnormality in portal and/or hepatic veins | |
| 3 | Complex changes in HA and its branches with marked flow abnormalities |
| Flow abnormality in portal and/or hepatic veins | |
| 4 | Decompensation of arteriovenous shunt with dilatation of portal and/or hepatic vein and marked flow abnormalities in both arteries and vein/s |
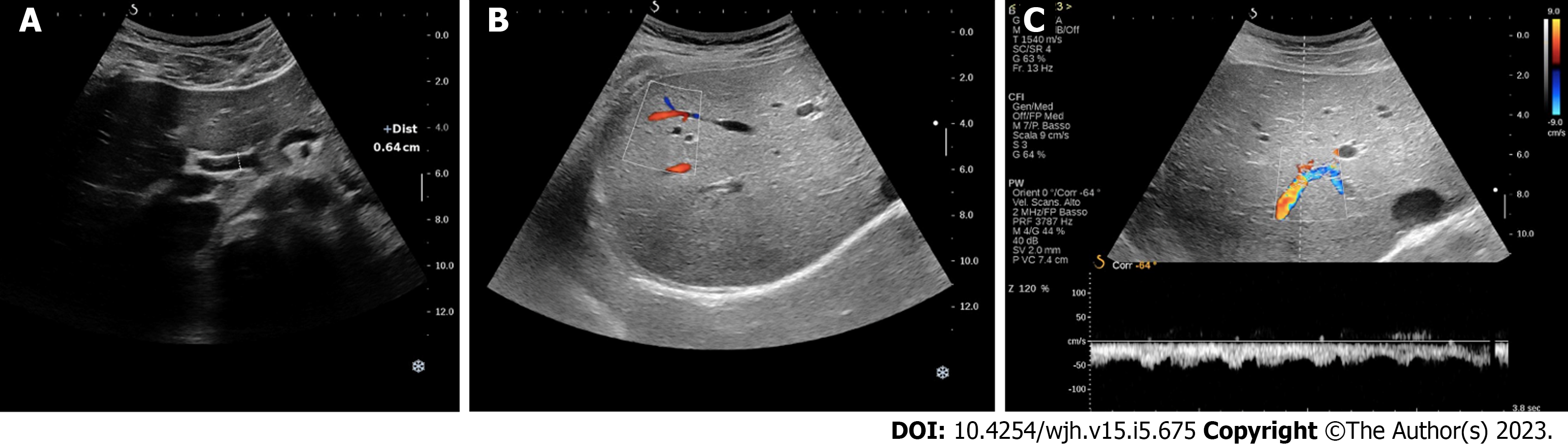
Hepatic artery dilation > 4 mm is a very sensitive parameter to differentiate HHT patients with or without liver involvement from the very early stages (Figure 1A)[32]; despite cirrhosis and hype
Peripheral subcapsular spots (identified by color Doppler) with high-velocity arterial blood flow and low RI are suggestive of small peripheral VMs, which are usually found from early stage in HHT patients with liver involvement (Figure 1B)[27].
Common hepatic artery dilation is also a predictor of HOCF development in patients with liver VMs[33]. A high velocity flow with low RI in intrahepatic branches of hepatic artery is highly suggestive of intrahepatic arterioportal shunt; furthermore, hepatic artery to portal vein shunts commonly cause pulsatility of portal flow with phasic or continuous reversal (Figure 1C). Arteriovenous shunts, on the other hand, usually result in a change in the Doppler waveform of hepatic veins (from triphasic to biphasic or even continuous patterns in severe involvements)[27,32].
FNH is common in HHT patients with liver involvement, and it generally appears as an isoechoic nodular lesion in liver parenchyma.
In those cases where the liver involvement is more severe, common findings are nodular and irregular liver surface with a coarse echo-pattern, previously known as pseudocirrhosis[34], as well as portal vein and hepatic vein dilation[27,32].
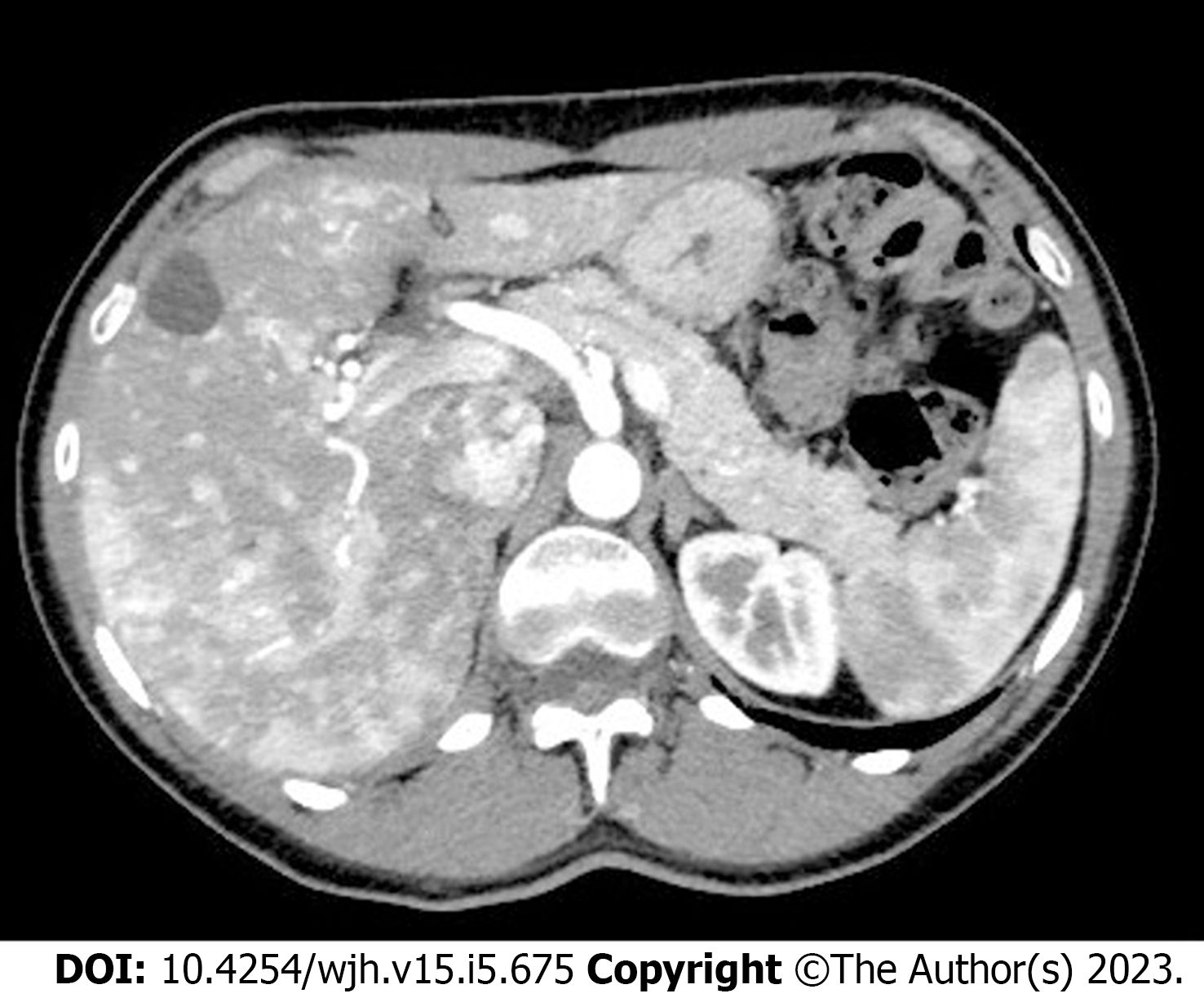
Multiphase contrast-enhanced abdominal computed tomography (CT) has an excellent yield and accuracy in defining liver vascular malformations and it is easily reproducible across different centers (Figure 2), however, it does not correlate with liver VMs severity and clinical presentations and is therefore recommended only if the expertise in detecting liver VMs using Doppler US is unavailable[17,35]. Nonetheless, it is widely used in complicated liver vascular malformation which are considered for liver transplantation[14] as it has the advantage of great accuracy in detecting biliary complications (i.e. necrotizing cholangitis with formation of bilomas)[32]; it is able to characterize the complexity of hepatic vascular alterations, the different types of shunts and parenchymal perfusion disorders[36,37] and it has great accuracy in differentiation between FNH from regenerative nodules[38].
Magnetic resonance imaging (MRI) of the liver shows great accuracy in characterizing focal liver lesions and in detecting liver VMs (they are better depicted on MRI angiograms and dynamic MRI images outlining a map of anomalous vessels)[39]. MRI is as accurate as multirow CT scan, with the advantage of the absence of ionizing radiations; nonetheless due its high cost and low availability it is recommended for diagnosis and follow-up of liver AVMs only when expertise in Doppler US is lacking[26,36].
The role of contrast-enhanced ultrasound (CEUS) with sulfur hexafluoride-filled microbubbles has been recently investigated in a cohort of 18 patients with HHT regarding macro and micro-circulation showing a higher percentage of hepatic VMs (especially of arterioportal shunts) than what is reported in literature[40]. However, CEUS seems to add no further information to Doppler US evaluation that still has great accuracy and sensitivity. It should also be noted that the use of sulfur hexafluoride-filled microbubbles is contraindicated in patients with right-to-left shunts and may result in an unjustified risk considering the high percentage of pulmonary VMs in HHT patients[32].
Liver biopsy is generally not necessary for diagnosis of hepatic VMs due to the increased bleeding risk related to a percutaneous procedure. Therefore, hepatic nodules in HHT patients should be characterized non-invasively when possible. If a biopsy is needed, always consider the increased risk of bleeding in HHT patients[14,26].
The first case of liver transplantation (LT) for HHT was reported in 1995[41]. Nowadays, LT is the recommended surgical option for severe hepatic involvement in HHT patients[26]. The main indication for LT are refractory HOCF and ischemic cholangitis (67.5% and 39.7% of cases, respectively)[42].
A recent systematic review by Riera-Mestre et al[42], reported 83 cases of LT for HHT worldwide. Perioperative complications within 30 days were described in 33.7% of patients (mainly bleeding complications) and a survival rate of 88% at six years has been reported.
While ischemic cholangitis is considered an urgent indication to LT, the best timing for transplantation in a patient with HOCF has not been defined yet.
MELD score was designed for cirrhotic patients and is widely used in defining the LT waitlist priority; HHT patients are exempt from being scored and should be included and prioritized in LT waitlist regardless of MELD score[43]. Right heart catheterization should always be performed in patients with HHT being evaluated for LT, to exclude severe pulmonary hypertension; LT can be undertaken if pulmonary vascular resistance is < 240 dynes· sec· cm-5 (< 3 Woods units)[14].
LT for HHT patients constitutes a more complex surgical procedure compared to other indications for LT and is characterized by higher blood transfusion requirement and more perioperative complications.
The hepatic artery in HHT patients may be dilated, tortuous and/or aneurysmatic and arterial graft anastomosis could be more challenging. Moreover, there is a high incidence of hepatic artery thrombosis after LT (about 7% of cases) that could result in need for re-transplantation. The presence of high-flow extrahepatic arterial teleangiectases may cause an arterial steal, so an attentive stadiation of disease before transplantation and an intraoperatively ultrasound arterial flow measurement through the anastomosis are strongly suggested[42,44].
The hyperdynamic state following recipient hepatic artery dissection constitutes a potential risk of bleeding in any extrahepatic site of VMs. Fatal pulmonary bleeding has been described in two patients, so embolization of pulmonary VMs should be considered before LT[45,46].
Intrahepatic relapse of HHT lesions is a late but common event after LT. The median recurrence time is 127 mo and can occur up to 19 years after LT; the estimated cumulative risk of recurrence at 5, 10, 15 and 20 years is 0%, 16.7%, 47.9% and 87%, respectively[42,47-49]. For this reason, these patients require a life-long follow-up.
The pathophysiology of recurrence in the transplanted liver is still unclear. Presence of endothelial cells of recipient origin in the transplanted liver has been recently described[48]. Microchimerism after LT is a well-known phenomenon, but in this case the liver graft repopulation by patient endothelial cells may lead to an aberrant angiogenesis causing the recurrence of the disease[50].
An mTOR inhibitor-based immunosuppressive regimen after LT may reduce hepatic VMs recurrence by blocking the PI3K signaling pathway[51].
Hepatic VMs are generally considered not suitable for endovascular or surgical approach due to the high morbidity and mortality rates.
Transarterial embolization is generally used for treating HOCF and portal hypertension. This procedure is performed in multiple stages (one to five sessions); among the several protocols proposed, the most used one consists in an initial embolization of vascular bed with a mixture of polyvinyl alcohol followed by embolization with microcoils. Arterial branches of right and left lobe have to be embolized in different sessions[52].
A peri-procedural infusion of analgesics, anti-emetics and steroids is generally advised; some authors also consider a peri- and post-procedural prophylactic antibiotic coverage[53].
The most common complications are biliary or hepatic necrosis that occur in 20%-60% of cases[53]; need for emergent LT and death is reported in up to 10% of cases[54].
Regarding the high risk of ischemic hepatic damage, transarterial embolization is generally contraindicated in patients with signs of biliary involvement[14].
There have been very few published accounts of transjugular intrahepatic portosystemic shunt (TIPS) as portal decompressive intervention. The high risk of worsening the cardiac output and the high bleeding risk related to the puncture lead to consider this treatment largely unsuccessful and so not recommended[55,56].
Hepatic artery banding and/or ligation are other potential approaches for managing HOCF due to hepatic VMs. Banding consists in the diameter reduction by one third to a half of the pre-operative diameter of common hepatic artery and potentially lobar arteries; ligation consists in closure of feeding arteries of the lobe predominantly involved by VMs.
The diameter reduction achieved with arterial banding should be sufficient to reduce liver hyperperfusion, without causing ischemic hepatobiliary damage. Banding should be guided by colorDoppler ultrasound with a desired hepatic artery flow of 330 ± 80 mL/min[57]; another indirect parameter of sufficient arterial banding is the return of arterialized areas of liver surface to normal red color[58].
CT angiography is always recommended before surgery in order to investigate extra-hepatic vascular anatomy. If appropriate, collateral circulation arising from superior mesenteric or left gastric arteries could also be ligated and enlarged gastroduodenal artery banding may also be considered[58].
Based on the risk of hepatic necrosis, these procedures are contraindicated in case of significant portovenous shunting[59].
For a long time, hepatic artery ligation or banding has been used in limited number of cases due to the high rate of ischemic cholangitis and undefined long-term survival[57,60,61]. Lui et al[58] recently reported a series of 13 patients treated with hepatic artery ligation/banding with a low rate of peri- and post-operative complications (only two patients experienced cholangitis, who were treated conservatively), improvement of symptoms and good survival outcome (only one patient died in a median follow-up of 50 mo). Authors advise against dissecting malformed and tortuous vessels around extrahepatic biliary tract in order to reduce the risk of ischemic damage and against dividing perihepatic ligaments in order to preserve arterial flow to the liver.
Conventional hepatic surgery, like segmental resection or hemi-hepatectomy, is anecdotal[62] or reported for hepatic shunting in non-HHT patients[63] and for non VM indications in HHT patients[64,65]. This approach could be considered in very selected patients with symptomatic disease and very large VMs localized in a single segment/lobe, but such kind of indication should be given with caution.
Considering the high complication and mortality rates, together with their palliative role, endovascular and surgical treatments are still generally not recommended and should be proposed only in severely symptomatic patients that are not transplant candidates and have failed medical therapy; these approaches should be deliberated by a multidisciplinary team and should be performed only by expert physicians in referral centers[14].
First-line medical treatment, such as management of anemia with iron replacement therapy or management of mild bleedings with antifibrinolytics, concerns almost all HHT patients but it is not the aim of this paper, so it will not be discussed further. At the same time, first-line medical treatment for hepatic VM-related HOCF should be evaluated and managed by physicians with expertise in that field (such as cardiologists) and it goes beyond the purpose of this paper.
Management of portal hypertension follows the same principles as in patients without HHT[66,67], but non-selective beta-blockers should be used with caution in patients with HOCF, although they still are the drugs of choice[26].
Similarly, the management of portosystemic encephalopathy follows the same principles as in cirrhotic patients without HHT (i.e. lactulose and rifaximin)[26,68].
Infectious complications, such as cholangitis and hepatic abscesses, generally require antibiotic therapy. Large biliary duct obstruction is uncommon in HHT patients, and endoscopic retrograde cholangiopancreatography with stenting is not indicated[26], because it seems to increase the risk of infection in ischemic ducts and the risk of hemobilia[69,70].
Over the last few decades, research has primarily focused on utilizing antiangiogenetic drugs with the aim of targeting the aberrant angiogenesis causing VM formation and endothelial frailty. Several molecules have been investigated and multiple clinical trials are ongoing (such as thalidomide[71,72], tacrolimus[73], sorafenib[74], pazopanib[75,76], doxycycline[77,78] and others) with interesting results on nasal and gastrointestinal bleeding control, but the only molecule that has been studied for HOCF related to hepatic VMs is bevacizumab.
Bevacizumab is a humanized monoclonal antibody which exerts its antiangiogenic activity by inhibiting the VEGF. In 2012, its efficacy has been prospectively investigated in HHT patients with HOCF related to liver VMs resulting in a decrease cardiac output[79]; a reduced or delayed need for transplantation has also been described[80]. Bevacizumab has also demonstrated a reduction in nasal and gastrointestinal bleedings resulting in an improvement of anemia, decrease of blood transfusion need and better quality of life[81,82].
Numerous dosing schedules have been investigated, but the most common dose for initiation was 5 mg/kg every 2 wk for a total of 6 injections; infusion duration should be of at least 30 min (first administration should be given in at least 60 min to assess patient drug tolerance)[79]. Despite a high inter-patient bleeding-free interval, almost all patients relapse after a year of discontinuation of bevacizumab and they may require maintenance therapy or may repeat a new administration cycle that could become lifelong[83]. To date, there are no prospective studies concerning maintenance therapy; the dosing schedule should therefore be determined based on patient response and tolerance[81,83].
Similarly, the safety of long-term bevacizumab administration has not been prospectively evaluated. However, it could be inferred indirectly from prolonged administration of the drug for other indication.
The most frequent adverse events are generally mild and infusion-related, such as headache, nausea and vomiting, asthenia, abdominal pain, muscle pain, diarrhea and rash[79].
A major concern among drug-related adverse events is addressed to arterial hypertension, venous thrombosis and hemoptysis from pulmonary VMs[81,84]. Therefore, it is crucial to assess patients prothrombotic conditions prior to starting therapy with bevacizumab, and pulmonary VMs screening and treatment should be performed according to guidelines as for every HHT patient. Other potentially serious adverse events are gastrointestinal perforation and proteinuria[84]. Since a delay in wound healing has been reported during antiangiogenetic treatment, it is recommended to stop bevacizumab 6-8 wk before surgery and to restart it only if wounds are totally healed.
Bevacizumab is contraindicated in patients with severe arteriopathy, a history of ischemic complications, recent deep vein thrombosis (< 6 mo) or recent severe infection (< 1 mo) and should be used with caution in patients with non-post-capillary pulmonary hypertension[85]. It is also contraindicated in pregnancy, so effective contraceptive measures should be adopted by women in childbearing age during treatment and for six months after discontinuation[85].
A recent international expert consensus paper suggests a monitoring protocol for HHT patients treated with bevacizumab which consists in regular clinical examination (blood pressure measurement, epistaxis monitoring, blood transfusion require recording, adverse events collection) laboratory (blood cell count, liver and kidney function, ferritin, proteinuria) and scheduled echocardiography with cardiac index measurement[85].
To date, there is not sufficient available evidence from randomized control trials and bevacizumab is not market-authorized for HHT, but international expert consensus recommends considering intravenous bevacizumab for severe and refractory nasal and/or gastrointestinal bleeding and for HOCF secondary to hepatic VMs not sufficiently responder to first-line medical therapy[26,85,86]. Based on the rates of minimal or partial response to bevacizumab and the recurrence after drug discontinuation, intravenous bevacizumab should be considered as a potential “bridge” therapy to LT.
Liver involvement is very common in HHT patients and hepatologists should be aware of this condition and the available diagnostic and prognostic tools. Fortunately, clinically significant liver disease is uncommon, but its management could be challenging. Liver transplantation remains the only curative treatment for these patients. Endovascular and surgical approaches should be avoided in patients with liver VMs. Bevacizumab has shown promising results, but it should be used with caution and only in referral centers.
| 1. | Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 2. | McDonald J, Wooderchak-Donahue W, VanSant Webb C, Whitehead K, Stevenson DA, Bayrak-Toydemir P. Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet. 2015;6:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Hernandez F, Huether R, Carter L, Johnston T, Thompson J, Gossage JR, Chao E, Elliott AM. Mutations in RASA1 and GDF2 identified in patients with clinical features of hereditary hemorrhagic telangiectasia. Hum Genome Var. 2015;2:15040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Viteri-Noël A, González-García A, Patier JL, Fabregate M, Bara-Ledesma N, López-Rodríguez M, Gómez Del Olmo V, Manzano L. Hereditary Hemorrhagic Telangiectasia: Genetics, Pathophysiology, Diagnosis, and Management. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 5. | Iriarte A, Figueras A, Cerdà P, Mora JM, Jucglà A, Penín R, Viñals F, Riera-Mestre A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Thalgott JH, Dos-Santos-Luis D, Hosman AE, Martin S, Lamandé N, Bracquart D, Srun S, Galaris G, de Boer HC, Tual-Chalot S, Kroon S, Arthur HM, Cao Y, Snijder RJ, Disch F, Mager JJ, Rabelink TJ, Mummery CL, Raymond K, Lebrin F. Decreased Expression of Vascular Endothelial Growth Factor Receptor 1 Contributes to the Pathogenesis of Hereditary Hemorrhagic Telangiectasia Type 2. Circulation. 2018;138:2698-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Han C, Choe SW, Kim YH, Acharya AP, Keselowsky BG, Sorg BS, Lee YJ, Oh SP. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis. 2014;17:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Sánchez-Martínez R, Iriarte A, Mora-Luján JM, Patier JL, López-Wolf D, Ojeda A, Torralba MA, Juyol MC, Gil R, Añón S, Salazar-Mendiguchía J, Riera-Mestre A; RiHHTa Investigators of the Rare Diseases Working Group from the Spanish Society of Internal Medicine. Current HHT genetic overview in Spain and its phenotypic correlation: data from RiHHTa registry. Orphanet J Rare Dis. 2020;15:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Kjeldsen AD, Møller TR, Brusgaard K, Vase P, Andersen PE. Clinical symptoms according to genotype amongst patients with hereditary haemorrhagic telangiectasia. J Intern Med. 2005;258:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Buscarini E, Leandro G, Conte D, Danesino C, Daina E, Manfredi G, Lupinacci G, Brambilla G, Menozzi F, De Grazia F, Gazzaniga P, Inama G, Bonardi R, Blotta P, Forner P, Olivieri C, Perna A, Grosso M, Pongiglione G, Boccardi E, Pagella F, Rossi G, Zambelli A. Natural history and outcome of hepatic vascular malformations in a large cohort of patients with hereditary hemorrhagic teleangiectasia. Dig Dis Sci. 2011;56:2166-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, Westermann CJ. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Lesca G, Olivieri C, Burnichon N, Pagella F, Carette MF, Gilbert-Dussardier B, Goizet C, Roume J, Rabilloud M, Saurin JC, Cottin V, Honnorat J, Coulet F, Giraud S, Calender A, Danesino C, Buscarini E, Plauchu H; French-Italian-Rendu-Osler Network. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med. 2007;9:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Jelsig AM, Kjeldsen A, Christensen LL, Bertelsen B, Karstensen JG, Brusgaard K, Torring PM. Hereditary haemorrhagic telangiectasia in Danish patients with pathogenic variants in SMAD4: a nationwide study. J Med Genet. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (3)] |
| 15. | Khalid SK, Garcia-Tsao G. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia. Semin Liver Dis. 2008;28:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Mora-Luján JM, Iriarte A, Alba E, Sánchez-Corral MA, Cerdà P, Cruellas F, Ordi Q, Corbella X, Ribas J, Castellote J, Riera-Mestre A. Gender differences in hereditary hemorrhagic telangiectasia severity. Orphanet J Rare Dis. 2020;15:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Wu JS, Saluja S, Garcia-Tsao G, Chong A, Henderson KJ, White RI Jr. Liver involvement in hereditary hemorrhagic telangiectasia: CT and clinical findings do not correlate in symptomatic patients. AJR Am J Roentgenol. 2006;187:W399-W405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Buscarini E, Plauchu H, Garcia Tsao G, White RI Jr, Sabbà C, Miller F, Saurin JC, Pelage JP, Lesca G, Marion MJ, Perna A, Faughnan ME. Liver involvement in hereditary hemorrhagic telangiectasia: consensus recommendations. Liver Int. 2006;26:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Vorselaars VM, Velthuis S, Snijder RJ, Vos JA, Mager JJ, Post MC. Pulmonary hypertension in hereditary haemorrhagic telangiectasia. World J Cardiol. 2015;7:230-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol. 2007;46:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Buscarini E, Danesino C, Plauchu H, de Fazio C, Olivieri C, Brambilla G, Menozzi F, Reduzzi L, Blotta P, Gazzaniga P, Pagella F, Grosso M, Pongiglione G, Cappiello J, Zambelli A. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med Biol. 2004;30:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Cooney T, Sweeney EC, Coll R, Greally M. 'Pseudocirrhosis' in hereditary haemorrhagic telangiectasia. J Clin Pathol. 1977;30:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Xu BG, Liang J, Jia KF, Han T. Liver cirrhosis in a patient with hepatic hereditary hemorrhagic telangiectasia and Budd-Chiari syndrome: a case report. BMC Gastroenterol. 2020;20:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Shovlin CL, Sulaiman NL, Govani FS, Jackson JE, Begbie ME. Elevated factor VIII in hereditary haemorrhagic telangiectasia (HHT): association with venous thromboembolism. Thromb Haemost. 2007;98:1031-1039. [PubMed] |
| 25. | Singh S, Swanson KL, Hathcock MA, Kremers WK, Pallanch JF, Krowka MJ, Kamath PS. Identifying the presence of clinically significant hepatic involvement in hereditary haemorrhagic telangiectasia using a simple clinical scoring index. J Hepatol. 2014;61:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Faughnan ME, Mager JJ, Hetts SW, Palda VA, Lang-Robertson K, Buscarini E, Deslandres E, Kasthuri RS, Lausman A, Poetker D, Ratjen F, Chesnutt MS, Clancy M, Whitehead KJ, Al-Samkari H, Chakinala M, Conrad M, Cortes D, Crocione C, Darling J, de Gussem E, Derksen C, Dupuis-Girod S, Foy P, Geisthoff U, Gossage JR, Hammill A, Heimdal K, Henderson K, Iyer VN, Kjeldsen AD, Komiyama M, Korenblatt K, McDonald J, McMahon J, McWilliams J, Meek ME, Mei-Zahav M, Olitsky S, Palmer S, Pantalone R, Piccirillo JF, Plahn B, Porteous MEM, Post MC, Radovanovic I, Rochon PJ, Rodriguez-Lopez J, Sabba C, Serra M, Shovlin C, Sprecher D, White AJ, Winship I, Zarrabeitia R. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann Intern Med. 2020;173:989-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 27. | Buscarini E, Danesino C, Olivieri C, Lupinacci G, De Grazia F, Reduzzi L, Blotta P, Gazzaniga P, Pagella F, Grosso M, Pongiglione G, Buscarini L, Plauchu H, Zambelli A. Doppler ultrasonographic grading of hepatic vascular malformations in hereditary hemorrhagic telangiectasia -- results of extensive screening. Ultraschall Med. 2004;25:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Caselitz M, Bahr MJ, Bleck JS, Chavan A, Manns MP, Wagner S, Gebel M. Sonographic criteria for the diagnosis of hepatic involvement in hereditary hemorrhagic telangiectasia (HHT). Hepatology. 2003;37:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Buscarini E, Buscarini L, Danesino C, Piantanida M, Civardi G, Quaretti P, Rossi S, Di Stasi M, Silva M. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia: Doppler sonographic screening in a large family. J Hepatol. 1997;26:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Naganuma H, Ishida H, Niizawa M, Igarashi K, Shioya T, Masamune O. Hepatic involvement in Osler-Weber-Rendu disease: findings on pulsed and color Doppler sonography. AJR Am J Roentgenol. 1995;165:1421-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Buscarini E, Gebel M, Ocran K, Manfredi G, Del Vecchio Blanco G, Stefanov R, Olivieri C, Danesino C, Zambelli A. Interobserver agreement in diagnosing liver involvement in hereditary hemorrhagic telangiectasia by Doppler ultrasound. Ultrasound Med Biol. 2008;34:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Buscarini E, Gandolfi S, Alicante S, Londoni C, Manfredi G. Liver involvement in hereditary hemorrhagic telangiectasia. Abdom Radiol (NY). 2018;43:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Cusumano LR, Tesoriero JA, Wilsen CB, Sayre J, Quirk M, McWilliams JP. Predictors of heart failure symptoms in hereditary hemorrhagic telangiectasia patients with hepatic arteriovenous malformations. Orphanet J Rare Dis. 2021;16:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Sharma VK, Howden CW. Gastrointestinal and hepatic manifestations of hereditary hemorrhagic telangiectasia. Dig Dis. 1998;16:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Cavel A, Bleuzen A, Bertrand P, Patat F, Cottier JP. Comparison between Doppler ultrasonography and multiphase multidetector-row computed tomography in the detection of liver involvement in Rendu-Osler disease: An analysis of 62 patients. Diagn Interv Imaging. 2016;97:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Scardapane A, Stabile Ianora A, Sabbà C, Moschetta M, Suppressa P, Castorani L, Angelelli G. Dynamic 4D MR angiography vs multislice CT angiography in the evaluation of vascular hepatic involvement in hereditary haemorrhagic telangiectasia. Radiol Med. 2012;117:29-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Memeo M, Stabile Ianora AA, Scardapane A, Buonamico P, Sabbà C, Angelelli G. Hepatic involvement in hereditary hemorrhagic telangiectasia: CT findings. Abdom Imaging. 2004;29:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Dioguardi Burgio M, Ronot M, Salvaggio G, Vilgrain V, Brancatelli G. Imaging of Hepatic Focal Nodular Hyperplasia: Pictorial Review and Diagnostic Strategy. Semin Ultrasound CT MR. 2016;37:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Milot L, Kamaoui I, Gautier G, Pilleul F. Hereditary-hemorrhagic telangiectasia: one-step magnetic resonance examination in evaluation of liver involvement. Gastroenterol Clin Biol. 2008;32:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Schelker RC, Barreiros AP, Hart C, Herr W, Jung EM. Macro- and microcirculation patterns of intrahepatic blood flow changes in patients with hereditary hemorrhagic telangiectasia. World J Gastroenterol. 2017;23:486-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Bauer T, Britton P, Lomas D, Wight DG, Friend PJ, Alexander GJ. Liver transplantation for hepatic arteriovenous malformation in hereditary haemorrhagic telangiectasia. J Hepatol. 1995;22:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Riera-Mestre A, Cerdà P, Guzmán YC, Iriarte A, Torroella A, Mora-Luján JM, Castellote J, Hessheimer A, Fondevila C, Lladó L. Perioperative Complications and Long-Term Follow-Up of Liver Transplantation in Hemorrhagic Hereditary Telangiectasia: Report of Three Cases and Systematic Review. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Garcia-Tsao G, Gish RG, Punch J. Model for end-stage liver disease (MELD) exception for hereditary hemorrhagic telangiectasia. Liver Transpl. 2006;12:S108-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Maggi U, Conte G, Nita G, Gatti S, Paone G, Caccamo L, Lauro R, Dondossola D, Buscarini E, Rossi G. Arterial anastomosis in liver transplantation for Rendu-Osler-Weber disease: two case reports. Transplant Proc. 2013;45:2689-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Giacomoni A, De Carlis L, Sammartino C, Lauterio A, Osio C, Slim A, Rondinara G, Forti D. Right hemiliver transplants from living donors: report of 10 cases. Transplant Proc. 2004;36:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Aseni P, Vertemati M, Minola E, Bonacina E. Massive haemoptysis after living donor liver transplantation. J Clin Pathol. 2003;56:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Dupuis-Girod S, Chesnais AL, Ginon I, Dumortier J, Saurin JC, Finet G, Decullier E, Marion D, Plauchu H, Boillot O. Long-term outcome of patients with hereditary hemorrhagic telangiectasia and severe hepatic involvement after orthotopic liver transplantation: a single-center study. Liver Transpl. 2010;16:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Dumortier J, Dupuis-Girod S, Valette PJ, Valent A, Guillaud O, Saurin JC, Hervieu V, Robinson P, Plauchu H, Paliard P, Boillot O, Scoazec JY. Recurrence of Hereditary Hemorrhagic Telangiectasia After Liver Transplantation: Clinical Implications and Physiopathological Insights. Hepatology. 2019;69:2232-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Sabbà C, Gallitelli M, Longo A, Cariati M, Angelelli G. Orthotopic liver transplantation and hereditary hemorrhagic telangiectasia: do hepatic vascular malformations relapse? J Hepatol. 2004;41:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Hove WR, van Hoek B, Bajema IM, Ringers J, van Krieken JH, Lagaaij EL. Extensive chimerism in liver transplants: vascular endothelium, bile duct epithelium, and hepatocytes. Liver Transpl. 2003;9:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Castellote J, Mora Luján JM, Riera-Mestre A. Letter to the Editor: mTOR-Inhibitor-Based Immunosuppression Following Liver Transplantation for Hereditary Hemorrhagic Telangiectasia. Hepatology. 2020;71:762-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Chavan A, Galanski M, Wagner S, Caselitz M, Schlitt HJ, Gratz KF, Manns M. Hereditary hemorrhagic telangiectasia: effective protocol for embolization of hepatic vascular malformations--experience in five patients. Radiology. 1998;209:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Chavan A, Luthe L, Gebel M, Barg-Hock H, Seifert H, Raab R, Kirchhoff T, Schmuck B. Complications and clinical outcome of hepatic artery embolisation in patients with hereditary haemorrhagic telangiectasia. Eur Radiol. 2013;23:951-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Whiting JH Jr, Korzenik JR, Miller FJ Jr, Pollack JS, White RI Jr. Fatal outcome after embolotherapy for hepatic arteriovenous malformations of the liver in two patients with hereditary hemorrhagic telangiectasia. J Vasc Interv Radiol. 2000;11:855-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Lee JY, Korzenik JR, DeMasi R, Lih-Brody L, White RI Jr. Transjugular intrahepatic portosystemic shunts in patients with hereditary hemorrhagic telangiectasia: failure to palliate gastrointestinal bleeding. J Vasc Interv Radiol. 1998;9:994-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Cura MA, Postoak D, Speeg KV, Vasan R. Transjugular intrahepatic portosystemic shunt for variceal hemorrhage due to recurrent of hereditary hemorrhagic telangiectasia in a liver transplant. J Vasc Interv Radiol. 2010;21:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Koscielny A, Willinek WA, Hirner A, Wolff M. Treatment of high output cardiac failure by flow-adapted hepatic artery banding (FHAB) in patients with hereditary hemorrhagic telangiectasia. J Gastrointest Surg. 2008;12:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Liu ZC, Lu XF, Yang H, Liu HD, Song X, Ning SL, Xu YF, Chen YX. Clinical Outcomes of Patients with Severe Hepatic Hereditary Hemorrhagic Telangiectasia After Banding of the Hepatic Artery and Banding/Ligation of Branches of the Hepatic Artery. Eur J Vasc Endovasc Surg. 2016;51:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Garcia-Tsao G, Korzenik JR, Young L, Henderson KJ, Jain D, Byrd B, Pollak JS, White RI Jr. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2000;343:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 60. | Song X, Chen HQ, Chen YX, Cheng Y, Qu CQ, Liu EY, Guo S, Xu KS, Niu J, Shou NH. Individualized management of hepatic diseases in hereditary hemorrhagic telangiectasia. Am Surg. 2011;77:281-285. [PubMed] |
| 61. | Zieren J, Büttemeyer R, Müller JM. [Adjustable "banding" of the hepatic artery in treatment of shunt-induced heart failure in Osler-Rendu-Weber disease]. Chirurg. 1998;69:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Xiang J, Xie W, Zhang C, Wang H. Right hemihepatectomy combined with ligation of the common hepatic artery and gastroduodenal artery for the treatment of intrahepatic HHT: A case report. Front Surg. 2022;9:900297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 63. | Schmalz MJ, Radhakrishnan K. Vascular anomalies associated with hepatic shunting. World J Gastroenterol. 2020;26:6582-6598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Petrovic I, Pavlek G, Romic M, Grgic D, Romic I. Rupture of suppurated liver hematoma into the anterior abdominal wall in a patient with Rendu-Osler-Weber syndrome. Cir Cir. 2020;88:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Gaujoux S, Bucau M, Ronot M, Paradis V, Vilgrain V, Belghiti J. Liver resection in patients with hepatic hereditary hemorrhagic telangiectasia. Dig Surg. 2013;30:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1979] [Article Influence: 247.4] [Reference Citation Analysis (2)] |
| 67. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1837] [Article Influence: 459.3] [Reference Citation Analysis (2)] |
| 68. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (1)] |
| 69. | Costa Macedo T, Maldonado R, Valente A, Palma R, Raimundo M, Liberato M, Távora I, Alexandrino P, Carneiro de Moura M. Hemobilia in hereditary hemorrhagic telangiectasia: an unusual complication of endoscopic retrograde cholangiopancreatography. Endoscopy. 2003;35:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Hayashi S, Baba Y, Ueno K, Nakajo M. Small arteriovenous malformation of the common bile duct causing hemobilia in a patient with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2008;31 Suppl 2:S131-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Invernizzi R, Quaglia F, Klersy C, Pagella F, Ornati F, Chu F, Matti E, Spinozzi G, Plumitallo S, Grignani P, Olivieri C, Bastia R, Bellistri F, Danesino C, Benazzo M, Balduini CL. Efficacy and safety of thalidomide for the treatment of severe recurrent epistaxis in hereditary haemorrhagic telangiectasia: results of a non-randomised, single-centre, phase 2 study. Lancet Haematol. 2015;2:e465-e473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Baysal M, Ümit EG, Kırkızlar HO, Özdöver AC, Demir AM. Thalidomide for the Management of Bleeding Episodes in Patients with Hereditary Hemorrhagic Telangiectasia: Effects on Epistaxis Severity Score and Quality of Life. Turk J Haematol. 2019;36:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Hessels J, Kroon S, Boerman S, Nelissen RC, Grutters JC, Snijder RJ, Lebrin F, Post MC, Mummery CL, Mager JJ. Efficacy and Safety of Tacrolimus as Treatment for Bleeding Caused by Hereditary Hemorrhagic Telangiectasia: An Open-Label, Pilot Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Kim YH, Kim MJ, Choe SW, Sprecher D, Lee YJ, P Oh S. Selective effects of oral antiangiogenic tyrosine kinase inhibitors on an animal model of hereditary hemorrhagic telangiectasia. J Thromb Haemost. 2017;15:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Faughnan ME, Gossage JR, Chakinala MM, Oh SP, Kasthuri R, Hughes CCW, McWilliams JP, Parambil JG, Vozoris N, Donaldson J, Paul G, Berry P, Sprecher DL. Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis. 2019;22:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | Parambil JG, Gossage JR, McCrae KR, Woodard TD, Menon KVN, Timmerman KL, Pederson DP, Sprecher DL, Al-Samkari H. Pazopanib for severe bleeding and transfusion-dependent anemia in hereditary hemorrhagic telangiectasia. Angiogenesis. 2022;25:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | McWilliams JP, Majumdar S, Kim GH, Lee J, Seals K, Tangchaiburana S, Gilbert S, Duckwiler GR. North American Study for the Treatment of Recurrent Epistaxis with Doxycycline: The NOSTRIL trial. J Thromb Haemost. 2022;20:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Thompson KP, Sykes J, Chandakkar P, Marambaud P, Vozoris NT, Marchuk DA, Faughnan ME. Randomized, double-blind, placebo-controlled, crossover trial of oral doxycycline for epistaxis in hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis. 2022;17:405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 79. | Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, Roux A, Carette MF, Gilbert-Dussardier B, Hatron PY, Lacombe P, Lorcerie B, Rivière S, Corre R, Giraud S, Bailly S, Paintaud G, Ternant D, Valette PJ, Plauchu H, Faure F. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 80. | Mitchell A, Adams LA, MacQuillan G, Tibballs J, vanden Driesen R, Delriviere L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl. 2008;14:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | Al-Samkari H, Kasthuri RS, Parambil JG, Albitar HA, Almodallal YA, Vázquez C, Serra MM, Dupuis-Girod S, Wilsen CB, McWilliams JP, Fountain EH, Gossage JR, Weiss CR, Latif MA, Issachar A, Mei-Zahav M, Meek ME, Conrad M, Rodriguez-Lopez J, Kuter DJ, Iyer VN. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: the InHIBIT-Bleed study. Haematologica. 2021;106:2161-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 82. | Al-Samkari H. Hereditary hemorrhagic telangiectasia: systemic therapies, guidelines, and an evolving standard of care. Blood. 2021;137:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Albitar HAH, Almodallal Y, Gallo De Moraes A, O'Brien E, Choby GW, Pruthi RK, Stokken JK, Kamath PS, Cajigas HR, DuBrock HM, Krowka MJ, Iyer VN. Intravenous Bevacizumab in Hereditary Hemorrhagic Telangiectasia-Related Bleeding and High-Output Cardiac Failure: Significant Inter-Individual Variability in the Need for Maintenance Therapy. Mayo Clin Proc. 2020;95:1604-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Buscarini E, Botella LM, Geisthoff U, Kjeldsen AD, Mager HJ, Pagella F, Suppressa P, Zarrabeitia R, Dupuis-Girod S, Shovlin CL; VASCERN-HHT. Safety of thalidomide and bevacizumab in patients with hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis. 2019;14:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | Dupuis-Girod S, Shovlin CL, Kjeldsen AD, Mager HJ, Sabba C, Droege F, Fargeton AE, Fialla AD, Gandolfi S, Hermann R, Lenato GM, Manfredi G, Post MC, Rennie C, Suppressa P, Sure U; ePag group, Buscarini E. European Reference Network for Rare Vascular Diseases (VASCERN): When and how to use intravenous bevacizumab in Hereditary Haemorrhagic Telangiectasia (HHT)? Eur J Med Genet. 2022;65:104575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 86. | Stillo F, Mattassi R, Diociaiuti A, Neri I, Baraldini V, Dalmonte P, Amato B, Ametrano O, Amico G, Bianchini G, Campisi C, Cattaneo E, Causin F, Cavalli R, Colletti G, Corbeddu M, Coppo P, DE Fiores A, DI Giuseppe P, El Hachem M, Esposito F, Fulcheri E, Gandolfo C, Grussu F, Guglielmo A, Leuzzi M, Manunza F, Moneghini L, Monzani N, Nicodemi E, Occella C, Orso M, Pagella F, Paolantonio G, Pasetti F, Rollo M, Ruggiero F, Santecchia L, Spaccini L, Taurino M, Vaghi M, Vercellio G, Zama M, Zocca A, Aguglia M, Castronovo EL, DE Lorenzi E, Fontana E, Gusson E, Lanza J, Lizzio R, Mancardi MM, Rosina E. Guidelines for Vascular Anomalies by the Italian Society for the study of Vascular Anomalies (SISAV). Int Angiol. 2022;41:1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baysal M, Turkey; Naganuma H, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Cai YX