Published online Dec 27, 2023. doi: 10.4254/wjh.v15.i12.1325
Peer-review started: August 29, 2023
First decision: September 14, 2023
Revised: September 27, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: December 27, 2023
Processing time: 117 Days and 17.5 Hours
Periodontitis has been associated with various liver diseases. However, the relevance of periodontitis in the progression of decompensated cirrhosis remains inconclusive. In particular, it is unclear whether the common periodontitis pathogens, Porphyromonas gingivalis (P. gingivalis) and Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans), can be detected not only in the oral mucosa but also in ascites and stool.
To investigate the significance of periodontitis, P. gingivalis, and A. actinomycetemcomitans in cirrhosis patients with ascitic decompensation.
This prospective study was conducted at the University Hospital Hamburg-Eppendorf, a tertiary center in Northern Germany. A cohort of 27 patients with ascitic decompensated liver cirrhosis underwent dental examinations to assess the association between periodontitis and various clinical parameters of cirrhosis, as well as patient outcomes. PCR was used to test gingival samples, ascites, and stool for the presence of P. gingivalis and A. actinomycetemcomitans. Gingival samples were collected by probing the deepest gum pocket of a sextant and wiping them on a cotton swab.
Periodontitis was diagnosed in 22 out of 27 (82%) ascite patients, which is significantly more common than in a control cohort of 100 unselected patients (59%, P = 0.04). P. gingivalis was detected in the gingiva of six patients, and one of them also had P. gingivalis in their stool. However, P. gingivalis was not found in the ascites of any patient. Five out of six patients with P. gingivalis had periodontitis (83%). A. actinomycetemcomitans was not detected in any sample. Patients without periodontitis had a significantly higher mortality rate compared to those with periodontitis, and survival (Kaplan-Meier analysis) was longer in patients with periodontitis (P = 0.02). Transplant-free survival was also more common in patients with periodontitis compared to those without (63% vs 0%, P = 0.02).
Decompensated cirrhotic patients frequently suffer from periodontitis. However, there was no evidence of the translocation of P. gingivalis or A. actinomycetemcomitans into ascites. The survival of cirrhotic patients with periodontitis was not reduced.
Core Tip: In this prospective cohort study, we aimed to assess the prevalence of periodontitis and the potential dissemination of classical periodontitis pathogens into ascites among 27 cirrhotic patients experiencing ascitic decompensation. We also compared this group with 100 unselected patients from a dental practice. Our findings revealed that decompensated cirrhotic patients often experience periodontitis. However, we did not observe any evidence of the translocation of Porphyromonas gingivalis or Actinobacillus actinomycetemcomitans into ascites. Furthermore, the presence of periodontitis did not appear to have a detrimental effect on the survival of cirrhotic patients.
- Citation: Pischke S, Ashouri MM, Peters U, Shiprov A, Schulze Zur Wiesch J, Sterneck M, Fischer F, Huebener P, Mader M, Fischer L, Fründt T, Aarabi G, Beikler T. High incidence of periodontitis in patients with ascitic decompensated cirrhosis. World J Hepatol 2023; 15(12): 1325-1332
- URL: https://www.wjgnet.com/1948-5182/full/v15/i12/1325.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i12.1325
Cirrhosis, the final stage of chronic liver disease, is a very serious condition associated with a significantly reduced life expectancy[1]. One of its most threatening complications is spontaneous bacterial peritonitis (SBP)[1,2]. It is assumed that bacteria from the intestinal flora migrate into the abdominal cavity and multiply in the ascites. In contrast, secondary bacterial peritonitis in patients with portal hypertension is caused by an abdominal source of infection, such as an abscess or perforation. Secondary bacterial peritonitis is much less common than SBP, accounting for approximately 15% of all peritonitis cases. It carries a 20% risk of mortality[3].
SBP is defined as the presence of more than 250 polymorphonuclear cells, specifically neutrophil granulocytes, per mm3 in ascites[4]. Gram-negative bacteria, commonly found in the intestine, are the primary pathogens associated with SBP. The extent to which colonization of the oral flora contributes to the occurrence of SBP and whether bacteria from the oral cavity can enter the ascites through the intestinal tract and migration remain unclear.
Periodontitis, an inflammation of the gingiva, has garnered increasing interest over the past two decades. The presence of periodontitis has been linked to systemic inflammation. Animal experiments in mice have demonstrated a connection between periodontitis and the development of liver fibrosis[5].
Porphyromonas gingivalis (P. gingivalis), one of the most significant periodontal pathogens, is believed to enter the bloodstream through the oral mucosa, potentially leading to the release of various cytokines. Apart from direct translocation of P. gingivalis into the bloodstream through damaged gingiva with reduced barrier function, this bacterium can also easily move from the oral cavity to the intestine. It can be envisioned that the disruption of the intestinal microbiota composition by orally derived P. gingivalis may contribute to the gut-liver axis and the pathogenesis of SBP[6,7]. However, this aspect has not been thoroughly investigated.
The relevance of periodontitis has been studied in various patient cohorts with liver disease, including those with cirrhosis. A 1995 Vienna study involving 97 cirrhotic patients, including 64 with alcoholic cirrhosis and 33 with non-alcoholic cirrhosis, revealed that alcohol-dependent cirrhosis, but not cirrhosis in general, was associated with reduced oral hygiene (P < 0.01), decreased dental care (P < 0.001), the presence of periodontitis, and the need for dental treatment
Additionally, both periodontitis and cirrhosis have the potential to trigger an inflammatory response and generate inflammatory mediators, which may enable them to mutually influence each other. In several patient cohorts, individuals with cirrhosis have been observed to exhibit poorer periodontal clinical parameters compared to those without cirrhosis[6].
Some studies have suggested a potential connection between periodontitis and liver diseases, including cirrhosis. It is theorized that the inflammatory responses associated with periodontitis may contribute to the development of liver diseases like cirrhosis. Chronic inflammation in the body can result in liver cell damage and expedite the progression of liver diseases.
However, it is crucial to emphasize that further research is required to fully comprehend and clarify the relationship between periodontitis and liver diseases such as cirrhosis.
These observations naturally raise the question of whether P. gingivalis also contributes to the development of SBP and whether this pathogen can be detected in ascites.
To investigate whether P. gingivalis and Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans), bacteria typically associated with periodontitis, can be detected in ascites, we conducted a prospective study examining oral mucosal samples, ascites specimens and stool specimens for the presence of P. gingivalis. Mucosal samples were collected by penetrating the deepest gum pocket of a sextant and swabbing the area, followed by PCR testing. Additionally, we explored whether dental factors such as the detection of these bacteria, the presence of periodontitis, the number of teeth, and other variables were associated with the outcomes of patients with end-stage liver cirrhosis.
In this prospective study, we invited all adult cirrhosis patients who required paracentesis at the University Hospital Hamburg-Eppendorf in Hamburg, Germany, between March 2021 and July 2021 to participate. This cohort comprises both inpatients and outpatients experiencing ascitic decompensation. Twenty-seven patients agreed to participate, provided written informed consent, and were subsequently enrolled in this study. Among these participants, 27 were afflicted with ascitic decompensation attributable to liver cirrhosis. The diagnosis of cirrhosis had previously been established based on clinical criteria in conjunction with liver elastography results and biopsies.
To assess patient follow-up, we conducted a review of medical records in January 2023. It is important to note that there were no interventions by the investigators between the time of study inclusion and this evaluation. Given that all patients were well-documented cases of end-stage liver cirrhosis under the care of our university hospital, we had access to comprehensive records maintained by the treating physicians.
An intraoral examination, encompassing a full mouth assessment, including the assessment of bleeding on probing, was conducted by a proficient dental medicine student (Ashouri MM under the supervision of the head of the Department of Periodontics, Preventive and Restorative Dentistry (author Beikler T). This comprehensive intraoral examination encompassed the evaluation of dental status, including the number of teeth, mucosal health, and oral hygiene, as measured by the sulcus bleeding index. The grading of periodontal disease was carried out in accordance with the guidelines and recommendations established by the European Federation of Periodontology/Oral Recon
All patients underwent a standardized interview and completed a questionnaire, which inquired about various parameters, including the frequency of their dental visits and their smoking habits.
To assess the prevalence of periodontitis in a control group unaffected by cirrhosis, we included 100 unselected patients from a typical dental practice as our control cohort. These patients were retrospectively studied and anonymized to protect their privacy. The control group comprised individuals who had undergone standardized periodontitis screening at a regular dental practice in Hamburg. Basic demographic information, such as age, sex, and periodontal status, was retrospectively analyzed for these anonymized patients in accordance with our local regulations and ethical guidelines. It is worth noting that this control cohort has been previously referenced in a prior publication[11].
P. gingivalis was identified using species-specific PCR as previously described[12]. A probe was inserted into the deepest gum pocket of a sextant and then wiped onto a cotton swab, which was subsequently tested by PCR. Laboratory data and baseline patient characteristics, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin levels, age, clinical attachment loss – the most critical parameter for assessing periodontal tissue loss due to periodontal disease – the number of teeth, smoking status, and the patients’ interest in dental medicine, were all recorded.
Continuous variables with a non-normal distribution were presented as the median and interquartile range. The distribution of these parameters among different groups was compared using the Mann-Whitney U-test. Categorical variables were expressed as numbers (%) and compared using Fisher’s exact test. A significance level of P < 0.05 was used to determine statistical significance. Statistical analyses were conducted using SPSS, version 21.0 (IBM Corp., Armonk, NY, United States).
This prospective study underwent review and received approval from the Ethics Committee of the Medical Council of Hamburg (PV-4081 and MC-368/18). The study adhered to the guidelines set forth in the Declaration of Helsinki. The retrospective analysis of the control cohort was conducted with complete anonymization, eliminating the need for further clarification or formal ethics committee approval in accordance with local laws and regulations.
In 22 out of 27 (82%) ascites patients, periodontitis was diagnosed. Characteristics of patients with periodontitis, in comparison with those without it, are presented in Table 1.
| Total cohort (n = 27) | P value | |||
| Periodontitis (n = 22, 81.50%) | No periodontitis (n = 5, 28.50%) | |||
| Male | 12 (55%) | 3 (60%) | 1 | |
| Age, mean in years (range) | 56 (37-76) | 59 (56-68) | 0.61 | |
| Smoker | 17 (77%) | 2 (40%) | 0.13 | |
| Diabetes | 8 (36%) | 1 (20%) | 0.62 | |
| Good oral hygiene | 0 (0%) | 3 (60%) | < 0.01 | |
| Outcome | Alive without transplantation | 14 (63%) | 0 (0%) | 0.02 |
| Transplantation | 5 (23%) | 2 (40%) | 0.61 | |
| Deceased | 3 (14%) | 3 (60%) | <0.05 | |
| Motivated for oral hygiene (exact question was: “are you interested in oral hygiene”) | 12 (55%) | 5 (100)% | 0.11 | |
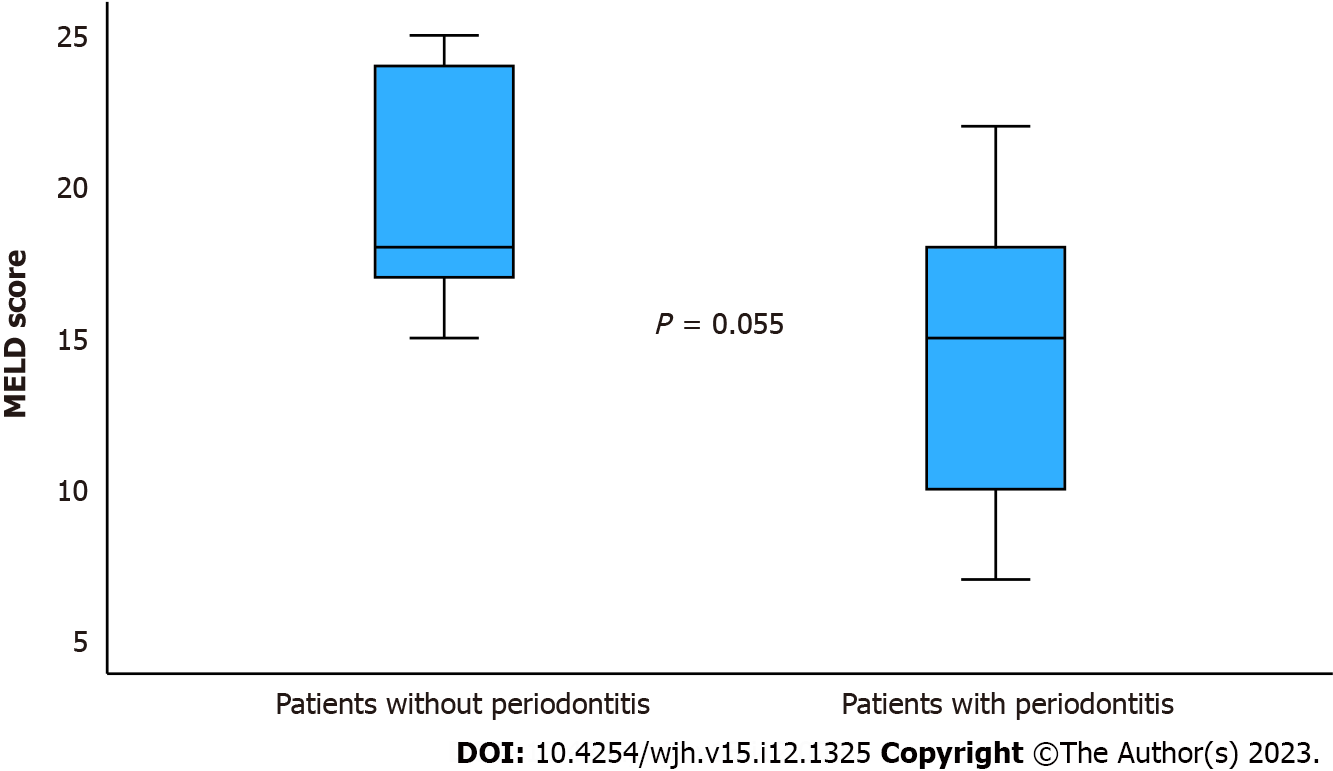
| MELD score, mean (range) | 20 (15-25) | 15 (7-22) | 0.055 | |
| AST U/ml, mean (range) | 75 (52-114) | 109 (9-652) | 0.83 | |
| ALT U/ml, mean (range) | 66 (28-175) | 50 (9-340) | 0.15 | |
| Bilirubin mg/dL, mean (range) | 6.0 (1.4-10.9) | 3.3 (0.4-8.3) | 0.17 | |
The age of the total cohort ranged from 37 to 76 years, with a median age of 57 years. Out of the total cohort, 15 individuals (56%) were male. Among the patients, nine (33%) had diabetes, and four (15%) were taking anticoagulants. Furthermore, 16 patients (59%) were diagnosed with alcoholic cirrhosis, five with non-alcoholic steatohepatitis (NASH) cirrhosis (19%), three (11%) had re-cirrhosis following liver transplantation, and one patient each (4%) had primary biliary cholangitis, hepatitis B virus (HBV), or hepatitis C Virus infections. Additionally, six patients had a Transjugular Intrahepatic portosystemic Shunt, and two patients had hepatocellular carcinoma (two with alcoholic liver cirrhosis, one with HBV, one with NASH). Notably, one of the patients with re-cirrhosis after transplantation had NASH as the underlying disease, another had primary sclerosing cholangitis, and one had alcoholic cirrhosis.
In order to assess the incidence of periodontitis in the cirrhosis cohort compared to the baseline incidence in the general population, we analyzed a retrospective cohort comprising 100 patients from a dental practice in Hamburg. This particular cohort has been previously described. Out of these subjects, 47 were male (47%), and their ages ranged from 17 to 89 years, with a median age of 51 years. Among these patients, 59 (59%) had periodontitis, a significantly lower rate than observed in the cirrhosis cohort, where the incidence was 82% (P = 0.04).
A notable finding was that a greater number of patients without periodontitis experienced mortality compared to those with periodontitis. Survival analysis using Kaplan-Meier methods indicated that patients with periodontitis had a longer survival duration compared to those without (P = 0.02, Figure 1). Additionally, transplant-free survival was more common among patients with periodontitis as opposed to those without (Table 1).
Among the patients, two had a complete set of teeth with no missing teeth (0/32), one patient had one missing tooth, two patients had three missing teeth, one patient had five missing teeth, six patients had six missing teeth, while one patient each had seven, eight, 13, 14, or 16 missing teeth. Additionally, three patients had ten missing teeth, two patients had 12 missing teeth, four patients had partial prostheses, and five patients were completely toothless and had total prostheses. Importantly, no association was found between the number of teeth and survival.
AST, ALT, bilirubin, and model for end-stage liver disease (MELD) scores showed no significant differences between patients with and without periodontitis (Table 1). Nonetheless, it is worth noting that there was a tendency towards a higher MELD score in patients without periodontitis, as depicted in Figure 2.
This study revealed a remarkably elevated incidence of periodontitis among cirrhotic patients (82%) when compared to healthy controls (59%, P = 0.04). This underscores the significance of regular dental check-ups for individuals with cirrhosis. Hepatologists should inquire explicitly about the dental care habits of their cirrhotic patients during medical history assessments and, if necessary, advocate for regular dental visits.
The identification of a substantial prevalence of periodontitis aligns with findings from a Danish study involving 262 cirrhotic patients. In this particular investigation, 46% (n = 66) exhibited severe periodontitis, 39% (n = 55) demonstrated moderate periodontitis, and only 15% (n = 22) displayed no or mild periodontitis[13]. Consequently, it is unequivocal that cirrhotic patients constitute a high-risk group for the development of periodontitis.
However, a far more critical question arises: whether periodontitis is correlated with reduced survival or transplant-free survival. In this regard, our study revealed that 63% of patients with periodontitis (12/22) survived without requiring transplantation, compared to 0% (0/5) of patients without periodontitis (Table 1, P = 0.02). Additionally, overall survival was superior among patients with periodontitis when compared to those without (Table 1, P < 0.05, Figure 1). Furthermore, there was a tendency toward higher MELD-score values in the five patients without periodontitis in contrast to the 22 patients with periodontitis (Figure 2). Hence, our preliminary study does not indicate worsened graft-free survival or a trend toward more severe liver damage (MELD score) in patients with periodontitis; in fact, it suggests slightly better outcomes. These findings diverge from a previously published study from Denmark[14]. In that study involving 184 cirrhotic patients, 44% had severe periodontitis, and unlike our study, there was a poorer survival associated with the presence of severe periodontitis. The reasons behind this discrepancy and the tendency in our study toward better survival and lower MELD scores in cirrhotic patients with periodontitis remain unclear. However, it is crucial not to overinterpret this aspect, given the small sample size of five patients without periodontitis in our pilot study. Larger cohorts are needed to validate these findings, as less than 20% of the cirrhotic patients in our study did not have periodontitis.
A prior study established a link between the severity of NASH and the presence of periodontitis[11], a finding of particular relevance here. From a pathophysiological perspective, it is conceivable that the gingival entry point in periodontitis patients serves as a gateway for bacteria to enter the bloodstream, triggering cytokine release and inflammation. The intriguing aspect is that this inflammation did not appear to negatively impact the survival of our end-stage cirrhosis patients with ascites. Instead, our study revealed that patients without periodontitis had a less favorable survival outcome, which necessitates further investigation through large-scale studies.
Our pilot study holds considerable validity, being based on a well-defined and thoroughly characterized cohort of 27 patients. Nevertheless, larger cohorts are imperative for further exploration of this question. The limitations of our study encompass not only the relatively small patient sample but also the single-center study design and the varied nature of the inquiries we pursued. We simultaneously investigated the prevalence of periodontitis in decompensated cirrhosis patients, the potential translocation of P. gingivalis and A. actinomyctemcomitans into ascites, and the association between periodontitis and survival in these patients. We successfully addressed two of these three questions, specifically the frequency of periodontitis in decompensated cirrhotic patients and the potential bacterial translocation into ascites. However, to elucidate the third question concerning the impact of periodontitis on survival, larger cohorts are indispensable. Additionally, future studies should also prospectively examine whether gingival status and bacterial colonization change over time.
It is especially pertinent in this context to consider whether periodontal therapy can potentially enhance the survival prospects of end-stage liver cirrhosis patients. A recent review article unequivocally demonstrated that periodontal therapy could exert a beneficial influence on the progression of NASH[15]. It is worth exploring to what extent this therapeutic approach might also be applicable to cirrhotic patients. Additionally, a recent study conducted in the United States, involving 442 cirrhosis patients, revealed a significant association between poor oral health and 3-month hospitalizations, irrespective of portal hypertensive complications, minimal hepatic encephalopathy, or frailty[16].
Nonetheless, the primary objective of our study was to investigate whether classical periodontitis pathogens, namely P. gingivalis and A. actinomyctemcomitans, could be detected in decompensated cirrhotic patients through translocation into the ascites. However, we did not observe such translocation in our study.
Based on our small pilot study, it appears that these two bacteria may not play significant roles in the development of ascites or potentially in the occurrence of SBP. This particular question had not been explored previously. While our study did not confirm the hypothesis that these microorganisms could enter ascites from the gingival reservoir in decompensated cirrhotic patients, this finding remains noteworthy because it provides conclusive clarification on the matter.
This pilot study examines the prevalence of periodontitis in cirrhotic patients experiencing ascite decompensation.
Previous studies have not investigated whether bacteria from the oral mucosa associated with periodontitis can be translocated into the ascites of cirrhotic patients.
To investigate the significance of periodontitis in cirrhotic patients with ascites.
This is a prospective cohort study. The oral hygiene and dental status of 27 patients with cirrhosis and ascites decompensation were documented. The prevalence of periodontitis in these patients was compared to that of 100 unselected patients from a standard dental practice. Samples from ascites and gingiva were tested for Porphyromonas gingivalis (P. gingivalis) and Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans) using PCR.
Periodontitis was diagnosed in 22 out of 27 patients (82%) with ascites. This rate is significantly higher than in the control group of 100 unselected patients, where the rate was 59% (P = 0.04). P. gingivalis was identified in the gingiva of six patients and concurrently in the stool of one patient. However, P. gingivalis was not found in the ascites of any patient. Of the patients who tested positive for P. gingivalis, 83% (five out of six) suffered from periodontitis. A. actinomycetemcomitans was not detected in any of the samples. Significantly, a greater number of patients without periodontitis passed away compared to those with periodontitis, and the survival rate (as determined by the Kaplan-Meier analysis) was longer for patients with periodontitis (P = 0.02). Transplant-free survival was observed more often in patients with periodontitis than those without (63% vs 0%, P = 0.02).
Periodontitis is common in cirrhotic patients with ascites.
Hepatologists should recommend regular dental visits for cirrhotic patients. Future studies should assess whether this recommendation improves dental health and reduces the incidence of periodontitis.
| 1. | Premkumar M, Anand AC. Overview of Complications in Cirrhosis. J Clin Exp Hepatol. 2022;12:1150-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: A literature review. World J Hepatol. 2018;10:254-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Solà E, Solé C, Ginès P. Management of uninfected and infected ascites in cirrhosis. Liver Int. 2016;36 Suppl 1:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Căruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. J Gastrointestin Liver Dis. 2006;15:51-56. [PubMed] |
| 5. | Bai L, Wang YL, Chen YL, Li HX, Zhu SW, Liu Y, Song ZC, Duan SZ. The combination of experimental periodontitis and oral microbiota from periodontitis patients aggravates liver fibrosis in mice. J Clin Periodontol. 2022;49:1067-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Rinčić G, Gaćina P, Virović Jukić L, Rinčić N, Božić D, Badovinac A. ASSOCIATION BETWEEN PERIODONTITIS AND LIVER DISEASE. Acta Clin Croat. 2022;60:510-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Åberg F, Helenius-Hietala J. Oral Health and Liver Disease: Bidirectional Associations-A Narrative Review. Dent J (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 8. | Novacek G, Plachetzky U, Pötzi R, Lentner S, Slavicek R, Gangl A, Ferenci P. Dental and periodontal disease in patients with cirrhosis--role of etiology of liver disease. J Hepatol. 1995;22:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, Dalmet SS, Sikaroodi M, Gillevet PM, Sahingur SE. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G824-G837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Jepsen S, Blanco J, Buchalla W, Carvalho JC, Dietrich T, Dörfer C, Eaton KA, Figuero E, Frencken JE, Graziani F, Higham SM, Kocher T, Maltz M, Ortiz-Vigon A, Schmoeckel J, Sculean A, Tenuta LM, van der Veen MH, Machiulskiene V. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44 Suppl 18:S85-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Pischke S, Shiprov A, Peters U, Schulze Zur Wiesch J, Kluwe J, Westphal T, Fischer F, Mader M, Fründt T, Horvatits K, Horvatits T, Aarabi G, Beikler T. High prevalence of periodontal disease in patients with NASH- possible association of poor dental health with NASH severity. Ann Hepatol. 2023;28:100887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Reinhardt B, Klocke A, Neering SH, Selbach S, Peters U, Flemmig TF, Beikler T. Microbiological dynamics of red complex bacteria following full-mouth air polishing in periodontally healthy subjects-a randomized clinical pilot study. Clin Oral Investig. 2019;23:3905-3914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Grønkjær LL, Holmstrup P, Schou S, Kongstad J, Jepsen P, Vilstrup H. Periodontitis in patients with cirrhosis: a cross-sectional study. BMC Oral Health. 2018;18:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Ladegaard Grønkjær L, Holmstrup P, Schou S, Jepsen P, Vilstrup H. Severe periodontitis and higher cirrhosis mortality. United European Gastroenterol J. 2018;6:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kuraji R, Shiba T, Dong TS, Numabe Y, Kapila YL. Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis. World J Gastroenterol. 2023;29:967-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Bajaj JS, Lai JC, Tandon P, O'Leary JG, Wong F, Garcia-Tsao G, Vargas HE, Kamath PS, Biggins SW, Limon-Miro A, Shaw J, Mbachi C, Chew M, Golob Deeb J, Thacker LR, Reddy KR. Role of Oral Health, Frailty, and Minimal Hepatic Encephalopathy in the Risk of Hospitalization: A Prospective Multi-Center Cohort of Outpatients With Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:1864-1872.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao S, China S-Editor: Lin C L-Editor: A P-Editor: Yuan YY